Software- and TDM-Guided Dosing of Meropenem Promises High Rates of Target Attainment in Critically Ill Patients
Abstract
1. Introduction
2. Results
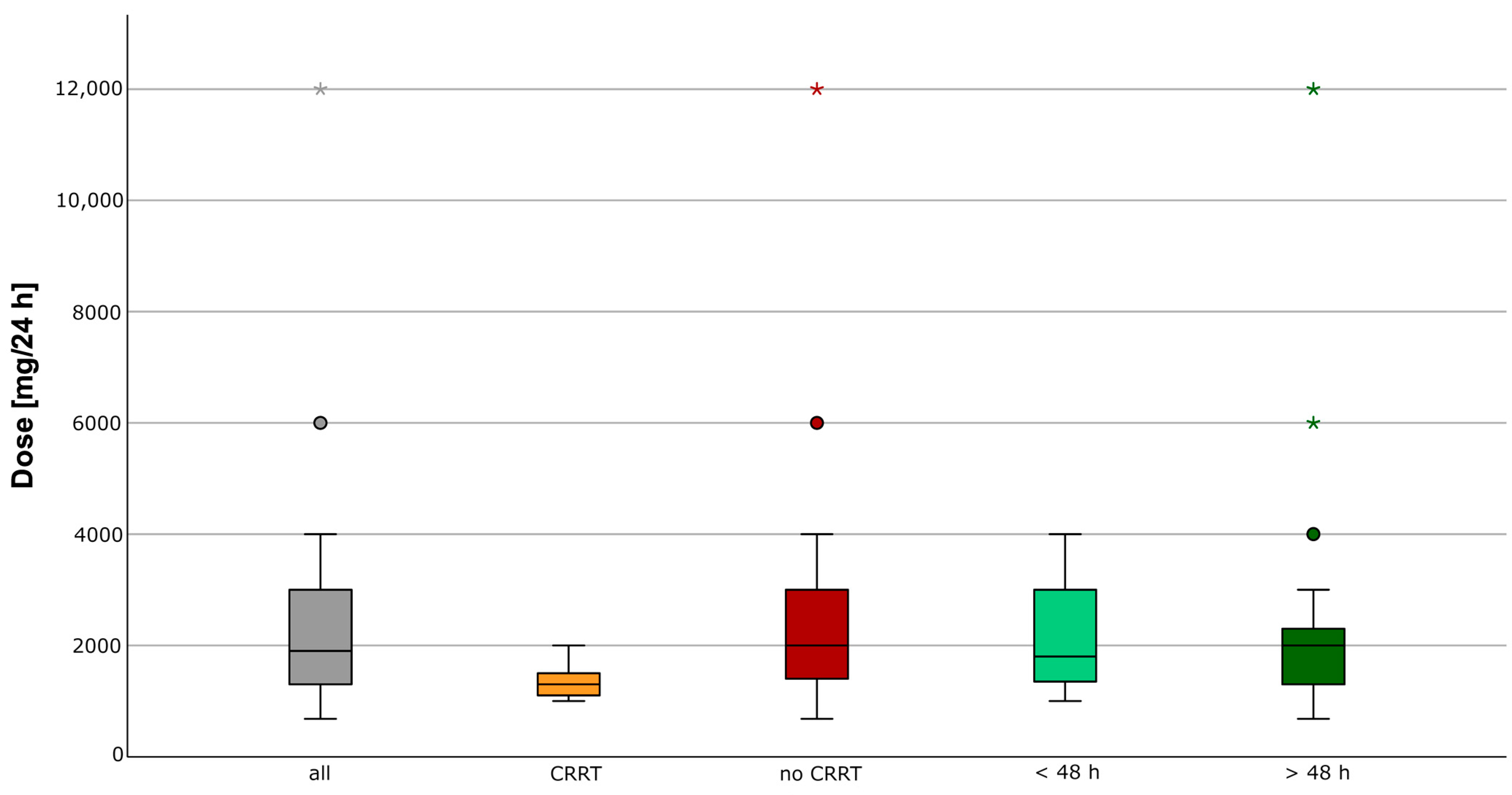
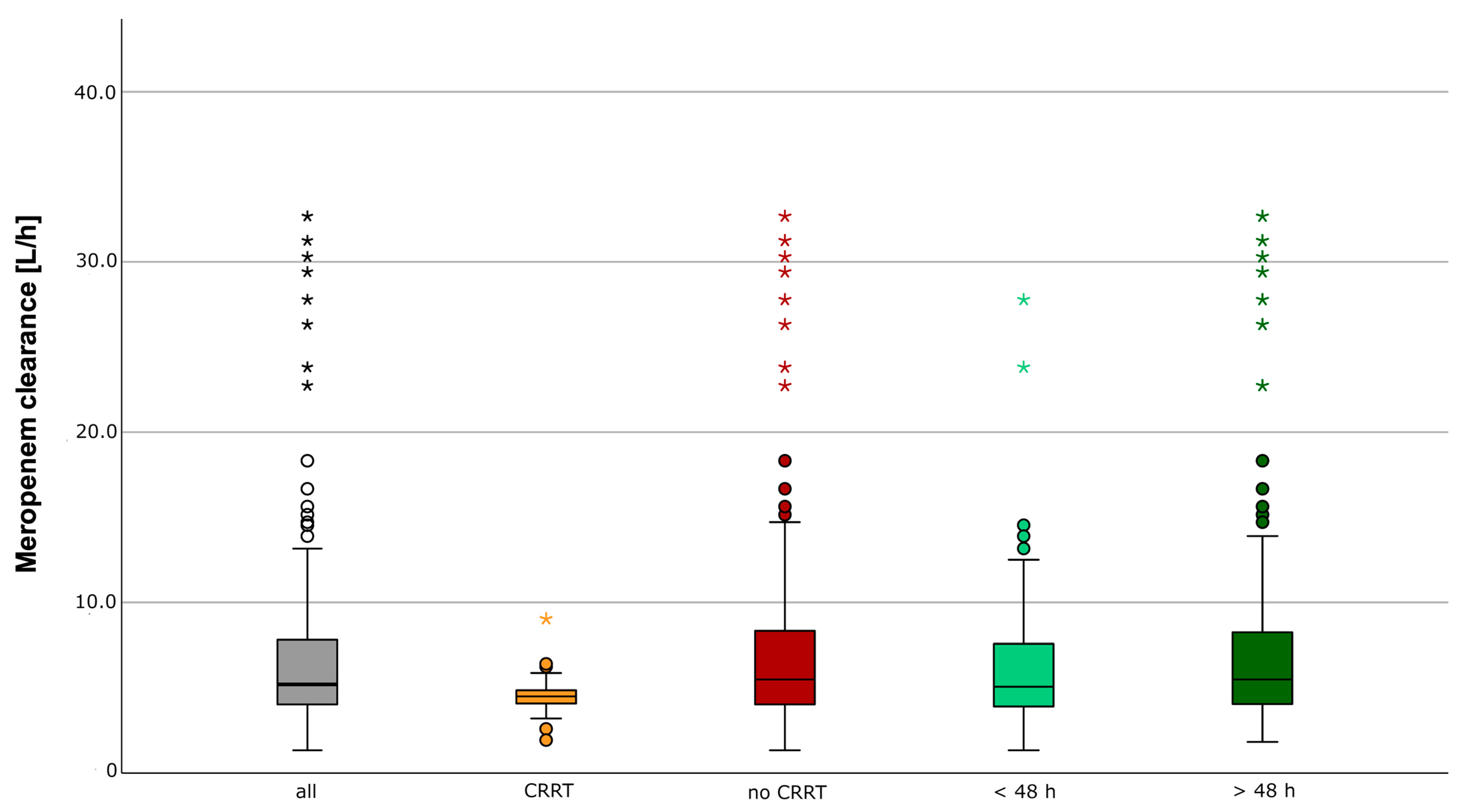
2.1. Therapeutic Exposure
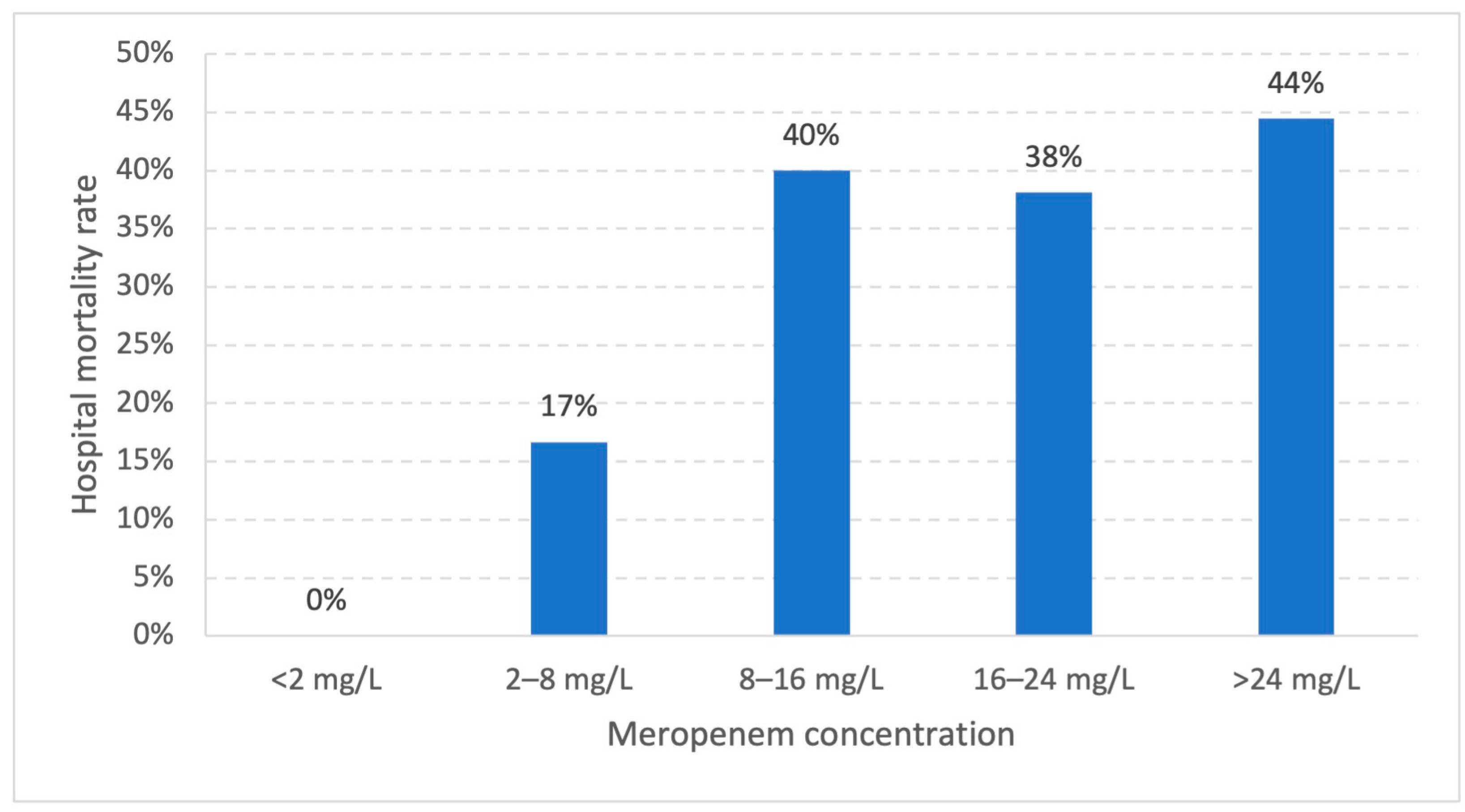
2.2. Predictors for Clinical Outcome
3. Discussion
4. Material and Methods
4.1. Study Design and Population
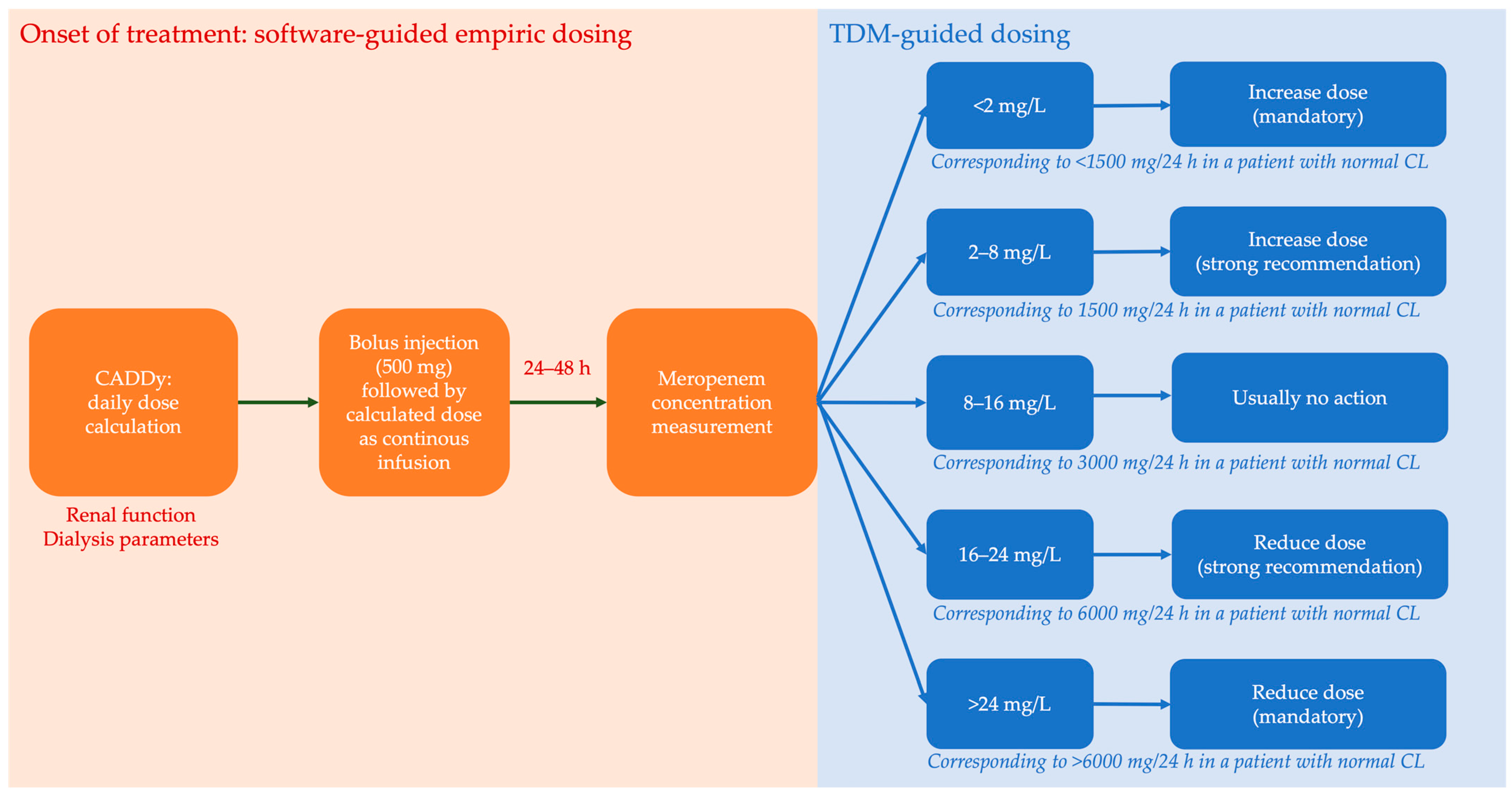
4.2. Therapeutic Drug Monitoring (TDM) Program
4.3. Therapeutic Drug Exposure
4.4. Statistical Analysis
5. Conclusions
6. Key Messages
- Meropenem clearance in critically ill patients with septic patients shows high variability.
- Dosing software (CADDy) and TDM allow an approach based on expert clinical pharmacological interpretation, resulting in efficient serum concentrations.
- In contrast to piperacillin, no association between high serum levels and mortality was observed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinelt, F.A.; Stegemann, M.S.; Theloe, A.; Pfafflin, F.; Achterberg, S.; Weber, F.; Dubel, L.; Mikolajewska, A.; Uhrig, A.; Kiessling, P.; et al. Evaluation of a Meropenem and Piperacillin Monitoring Program in Intensive Care Unit Patients Calls for the Regular Assessment of Empirical Targets and Easy-to-Use Dosing Decision Tools. Antibiotics 2022, 11, 758. [Google Scholar] [CrossRef] [PubMed]
- Ewoldt, T.M.J.; Abdulla, A.; Rietdijk, W.J.R.; Muller, A.E.; de Winter, B.C.M.; Hunfeld, N.G.M.; Purmer, I.M.; van Vliet, P.; Wils, E.J.; Haringman, J.; et al. Model-informed precision dosing of beta-lactam antibiotics and ciprofloxacin in critically ill patients: A multicentre randomised clinical trial. Intensive Care Med. 2022, 48, 1760–1771. [Google Scholar] [CrossRef]
- Scharf, C.; Paal, M.; Schroeder, I.; Vogeser, M.; Draenert, R.; Irlbeck, M.; Zoller, M.; Liebchen, U. Therapeutic Drug Monitoring of Meropenem and Piperacillin in Critical Illness-Experience and Recommendations from One Year in Routine Clinical Practice. Antibiotics 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P. Bench-to-bedside review: Appropriate antibiotic therapy in severe sepsis and septic shock—Does the dose matter? Crit. Care 2009, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Derendorf, H.; Heinrichs, T.; Reimers, T.; Lebert, C.; Brinkmann, A. Calculated initial parenteral treatment of bacterial infections: Pharmacokinetics and pharmacodynamics. GMS Infect. Dis. 2020, 8. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kumar, A.; Lipman, J. Right Dose, Right Now: Customized Drug Dosing in the Critically Ill. Crit. Care Med. 2017, 45, 331–336. [Google Scholar] [CrossRef]
- Wicha, S.G.; Märtson, A.G.; Nielsen, E.I.; Koch, B.C.P.; Friberg, L.E.; Alffenaar, J.W.; Minichmayr, I.K.; International Society of Anti-Infective Pharmacology (ISAP), the PK/PD study group of the European Society of Clinical Microbiology, Infectious Diseases (EPASG). From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef]
- De Waele, J.J.; Lipman, J.; Akova, M.; Bassetti, M.; Dimopoulos, G.; Kaukonen, M.; Koulenti, D.; Martin, C.; Montravers, P.; Rello, J.; et al. Risk factors for target non-attainment during empirical treatment with beta-lactam antibiotics in critically ill patients. Intensive Care Med. 2014, 40, 1340–1351. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Naicker, S.; Islam, K.; Cottrell, K.; Wallis, S.C.; Lipman, J.; Harris, P.N.A.; Sime, F.B.; Roberts, J.A. Pharmacodynamic evaluation of intermittent versus extended and continuous infusions of piperacillin/tazobactam in a hollow-fibre infection model against Klebsiella pneumoniae. J. Antimicrob. Chemother. 2020, 75, 2633–2640. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef]
- Richter, D.C.; Heininger, A.; Chiriac, U.; Frey, O.R.; Rau, H.; Fuchs, T.; Rohr, A.C.; Brinkmann, A.; Weigand, M.A. Antibiotic Stewardship and Therapeutic Drug Monitoring of beta-Lactam Antibiotics: Is There a Link? An Opinion Paper. Ther. Drug. Monit. 2022, 44, 103–111. [Google Scholar] [CrossRef]
- Roger, C.; Louart, B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Liebchen, U.; Paal, M.; Taubert, M.; Vogeser, M.; Irlbeck, M.; Zoller, M.; Schroeder, I. The higher the better? Defining the optimal beta-lactam target for critically ill patients to reach infection resolution and improve outcome. J. Intensive Care 2020, 8, 86. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Kalimeris, G.D.; Triarides, N.A.; Falagas, M.E. An update on adverse drug reactions related to beta-lactam antibiotics. Expert Opin. Drug Saf. 2018, 17, 499–508. [Google Scholar] [CrossRef]
- Imani, S.; Buscher, H.; Marriott, D.; Gentili, S.; Sandaradura, I. Too much of a good thing: A retrospective study of beta-lactam concentration-toxicity relationships. J. Antimicrob. Chemother. 2017, 72, 2891–2897. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Thooft, A.D.J.; Farkas, A.; Lipman, J.; Verstraete, A.G.; Stove, V.; Roberts, J.A.; De Waele, J.J. Early target attainment of continuous infusion piperacillin/tazobactam and meropenem in critically ill patients: A prospective observational study. J. Crit. Care 2019, 52, 75–79. [Google Scholar] [CrossRef]
- Richter, D.C.; Frey, O.; Rohr, A.; Roberts, J.A.; Koberer, A.; Fuchs, T.; Papadimas, N.; Heinzel-Gutenbrunner, M.; Brenner, T.; Lichtenstern, C.; et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: A retrospective analysis of four years of clinical experience. Infection 2019, 47, 1001–1011. [Google Scholar] [CrossRef]
- Hagel, S.; Fiedler, S.; Hohn, A.; Brinkmann, A.; Frey, O.R.; Hoyer, H.; Schlattmann, P.; Kiehntopf, M.; Roberts, J.A.; Pletz, M.W.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin/tazobactam to improve outcome in patients with sepsis (TARGET): A prospective, multi-centre, randomised controlled trial. Trials 2019, 20, 330. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Hoste, E.A.; De Waele, J.J. Why We May Need Higher Doses of Beta-Lactam Antibiotics: Introducing the ‘Maximum Tolerable Dose’. Antibiotics 2022, 11, 889. [Google Scholar] [CrossRef]
- Berry, A.V.; Kuti, J.L. Pharmacodynamic Thresholds for Beta-Lactam Antibiotics: A Story of Mouse Versus Man. Front. Pharmacol. 2022, 13, 833189. [Google Scholar] [CrossRef]
- Pai Mangalore, R.; Ashok, A.; Lee, S.J.; Romero, L.; Peel, T.N.; Udy, A.A.; Peleg, A.Y. Beta-Lactam Antibiotic Therapeutic Drug Monitoring in Critically Ill Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022, 75, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Codina, M.; Bozkir, H.O.; Jorda, A.; Zeitlinger, M. Individualized antimicrobial dose optimization: A systematic review and meta-analysis of randomized controlled trials. Clin. Microbiol. Infect. 2023, 29, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Liebchen, U.; Paal, M.; Jung, J.; Schroeder, I.; Frey, L.; Zoller, M.; Scharf, C. Therapeutic drug monitoring-guided high dose meropenem therapy of a multidrug resistant Acinetobacter baumannii—A case report. Respir. Med. Case Rep. 2020, 29, 100966. [Google Scholar] [CrossRef]
- Gatti, M.; Pea, F. Jumping into the future: Overcoming pharmacokinetic/pharmacodynamic hurdles to optimize the treatment of severe difficult to treat-Gram-negative infections with novel beta-lactams. Expert Rev. Anti Infect. Ther. 2023, 21, 149–166. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Liu, J.; O’Donnell, J.N.; Dulhunty, J.M.; Abdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged Infusion Piperacillin-Tazobactam Decreases Mortality and Improves Outcomes in Severely Ill Patients: Results of a Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.C.; Dietrich, M.; Lalev, L.D.; Schmitt, F.C.F.; Fiedler, M.O.; Bruckner, T.; Stoerzinger, D.; Chiriac, U.; Klein, S.; Hackert, T.; et al. Prolonged Infusion of beta-Lactams Decreases Mortality in Patients with Septic Shock: A Retrospective before-and-after Study. Antibiotics 2021, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent beta-Lactam Infusion in Severe Sepsis. A Meta-analysis of Individual Patient Data from Randomized Trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Liebchen, U.; Weinelt, F.; Scharf, C.; Schroeder, I.; Paal, M.; Zoller, M.; Kloft, C.; Jung, J.; Michelet, R. Combination of Pharmacokinetic and Pathogen Susceptibility Information to Optimize Meropenem Treatment of Gram-Negative Infections in Critically Ill Patients. Antimicrob. Agents Chemother. 2022, 66, e0183121. [Google Scholar] [CrossRef]
- Beumier, M.; Casu, G.S.; Hites, M.; Wolff, F.; Cotton, F.; Vincent, J.L.; Jacobs, F.; Taccone, F.S. Elevated beta-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015, 81, 497–506. [Google Scholar]
- Quinton, M.C.; Bodeau, S.; Kontar, L.; Zerbib, Y.; Maizel, J.; Slama, M.; Masmoudi, K.; Lemaire-Hurtel, A.S.; Bennis, Y. Neurotoxic Concentration of Piperacillin during Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e00654-17. [Google Scholar] [CrossRef]
- Crass, R.L.; Rodvold, K.A.; Mueller, B.A.; Pai, M.P. Renal Dosing of Antibiotics: Are We Jumping the Gun? Clin. Infect. Dis. 2019, 68, 1596–1602. [Google Scholar] [CrossRef]
- Roberts, J.A.; Joynt, G.M.; Lee, A.; Choi, G.; Bellomo, R.; Kanji, S.; Mudaliar, M.Y.; Peake, S.L.; Stephens, D.; Taccone, F.S.; et al. The Effect of Renal Replacement Therapy and Antibiotic Dose on Antibiotic Concentrations in Critically Ill Patients: Data from the Multinational Sampling Antibiotics in Renal Replacement Therapy Study. Clin. Infect. Dis. 2021, 72, 1369–1378. [Google Scholar] [CrossRef]
- Westermann, I.; Gastine, S.; Muller, C.; Rudolph, W.; Peters, F.; Bloos, F.; Pletz, M.; Hagel, S. Population pharmacokinetics and probability of target attainment in patients with sepsis under renal replacement therapy receiving continuous infusion of meropenem: Sustained low-efficiency dialysis and continuous veno-venous haemodialysis. Br. J. Clin. Pharmacol. 2021, 87, 4293–4303. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- Carlier, M.; Stove, V.; Verstraete, A.G.; De Waele, J.J. Stability of generic brands of meropenem reconstituted in isotonic saline. Minerva Anestesiol. 2015, 81, 283–287. [Google Scholar]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Roehr, A.C.; Frey, O.R.; Koeberer, A.; Fuchs, T.; Roberts, J.A.; Brinkmann, A. Anti-infective drugs during continuous hemodialysis—Using the bench to learn what to do at the bedside. Int. J. Artif. Organs 2015, 38, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pfizer. Meronem 1000 mg Pulver zur Herstellung einer Injektions-/Infusionslösung. In Summary of Product Characteristics; Pfizer: New York, NY, USA, 2022. [Google Scholar]
- Chiriac, U.; Richter, D.C.; Frey, O.R.; Rohr, A.C.; Helbig, S.; Preisenberger, J.; Hagel, S.; Roberts, J.A.; Weigand, M.A.; Brinkmann, A. Personalized Piperacillin Dosing for the Critically Ill: A Retrospective Analysis of Clinical Experience with Dosing Software and Therapeutic Drug Monitoring to Optimize Antimicrobial Dosing. Antibiotics 2021, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef]
- Sheiner, L.B.; Beal, S.L. Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 1981, 9, 503–512. [Google Scholar] [CrossRef]

| Median (IQR), no. (%) | |
|---|---|
| Age, years | 73 (18) |
| Weight, kg | 80 (20) |
| Height, cm | 170 (10) |
| BMI, kg/m2 | 27.1 (6.7) |
| Sex, male | 60 (66%) |
| Creatinine, mg/dL | 1.3 (1.6) |
| CrCL, mL/min | 45.3 (53.7) |
| CRRT | 12 (13%) |
| Mechanical ventilation | 54 (59%) |
| SOFA | 8 (8) |
| SAPS | 41 (18) |
| ICU mortality | 28 (31%) |
| Hospital mortality | 35 (39%) |
| Length of hospital stay, days | 26 (28) |
| Length of ICU stay, days | 10 (15) |
| Antimicrobial treatment, days | 7 (4) |
| Diagnosis | No. (%) |
|---|---|
| Sepsis/Severe sepsis | 55 (60%) |
| Septic shock | 36 (40%) |
| Suspected site of infection | |
| Pneumonia | 34 (37%) |
| Abdominal infection, peritonitis | 12 (13%) |
| Soft tissue/bone infection | 17 (19%) |
| Urinary tract infection | 4 (4%) |
| Blood stream infection | 2 (2%) |
| Cholecystitis, cholangitis | 4 (4%) |
| Diverse | 1 (1%) |
| cMER (mg/L) | <2 | 2–8 | 8–16 | 16–24 | >24 |
|---|---|---|---|---|---|
| Software-guided empiric dosing (n = 91) | 0 (0.0%) | 6 (6.6%) | 55 (60.4%) | 21 (23.1%) | 9 (9.9%) |
| TDM-guided dosing (n = 138) | 0 (0.0%) | 13 (9.4%) | 96 (69.6%) | 29 (21.0%) | 0 (0.0%) |
| cMER (mg/L) | <2 | 2–8 | 8–16 | 16–24 | >24 |
|---|---|---|---|---|---|
| Predicted based on standard dosing (Bolus) | 45 (19.7%) | 62 (27.0%) | 61 (26.6%) | 35 (15.3%) | 26 (11.4%) |
| Predicted based on standard dosing (CI) | 0 (0.0%) | 15 (6.6%) | 110 (48.0%) | 60 (26.2%) | 44 (19.2%) |
| Observed individualized dosing (CI) | 0 (0.0%) | 19 (8.3%) | 151 (65.9%) | 50 (21.8%) | 9 (3.9%) |
| cMER (mg/L) | 8–16 | <2 | 2–8 | 16–24 | >24 |
|---|---|---|---|---|---|
| Patients (%) | 55 (60.4%) | 0 (0.0%) | 6 (6.6%) | 21 (23.1%) | 9 (9.9%) |
| Hospital mortality (%) | 22 (40%) | 0 (0) | 1 (17%) * | 8 (38%) | 4 (44%) |
| Median SOFA score (IQR) | 6 (9) | 0 (0) | 1 (5) * | 8 (11) | 8 (7) |
| Median CrCL (mL/min) (IQR) | 52.4 (56.2) | 0.0 (0.0) | 52.6 (103.1) | 35.1 (35.4) * | 39.9 (37.2) |
| Median meropenem clearance (L/h) (IQR) | 5.9 (4.9) | 0.0 (0.0) | 8.9 (15.8) * | 4.9 (3.2) * | 4.3 (1.7) * |
| Median age (years) (IQR) | 72 (18) | 0 (0) | 46 (42) * | 75 (11) | 76 (7) * |
| Median BMI (kg/m2) (IQR) | 28 (8) | 0 (0) | 30 (5) | 26 (5) | 26 (6) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiriac, U.; Richter, D.; Frey, O.R.; Röhr, A.C.; Helbig, S.; Hagel, S.; Liebchen, U.; Weigand, M.A.; Brinkmann, A. Software- and TDM-Guided Dosing of Meropenem Promises High Rates of Target Attainment in Critically Ill Patients. Antibiotics 2023, 12, 1112. https://doi.org/10.3390/antibiotics12071112
Chiriac U, Richter D, Frey OR, Röhr AC, Helbig S, Hagel S, Liebchen U, Weigand MA, Brinkmann A. Software- and TDM-Guided Dosing of Meropenem Promises High Rates of Target Attainment in Critically Ill Patients. Antibiotics. 2023; 12(7):1112. https://doi.org/10.3390/antibiotics12071112
Chicago/Turabian StyleChiriac, Ute, Daniel Richter, Otto R. Frey, Anka C. Röhr, Sophia Helbig, Stefan Hagel, Uwe Liebchen, Markus A. Weigand, and Alexander Brinkmann. 2023. "Software- and TDM-Guided Dosing of Meropenem Promises High Rates of Target Attainment in Critically Ill Patients" Antibiotics 12, no. 7: 1112. https://doi.org/10.3390/antibiotics12071112
APA StyleChiriac, U., Richter, D., Frey, O. R., Röhr, A. C., Helbig, S., Hagel, S., Liebchen, U., Weigand, M. A., & Brinkmann, A. (2023). Software- and TDM-Guided Dosing of Meropenem Promises High Rates of Target Attainment in Critically Ill Patients. Antibiotics, 12(7), 1112. https://doi.org/10.3390/antibiotics12071112








