Assessment of Cold Atmospheric Pressure Plasma (CAPP) Treatment for Degradation of Antibiotic Residues in Water
Abstract
:1. Introduction
2. Results and Discussion
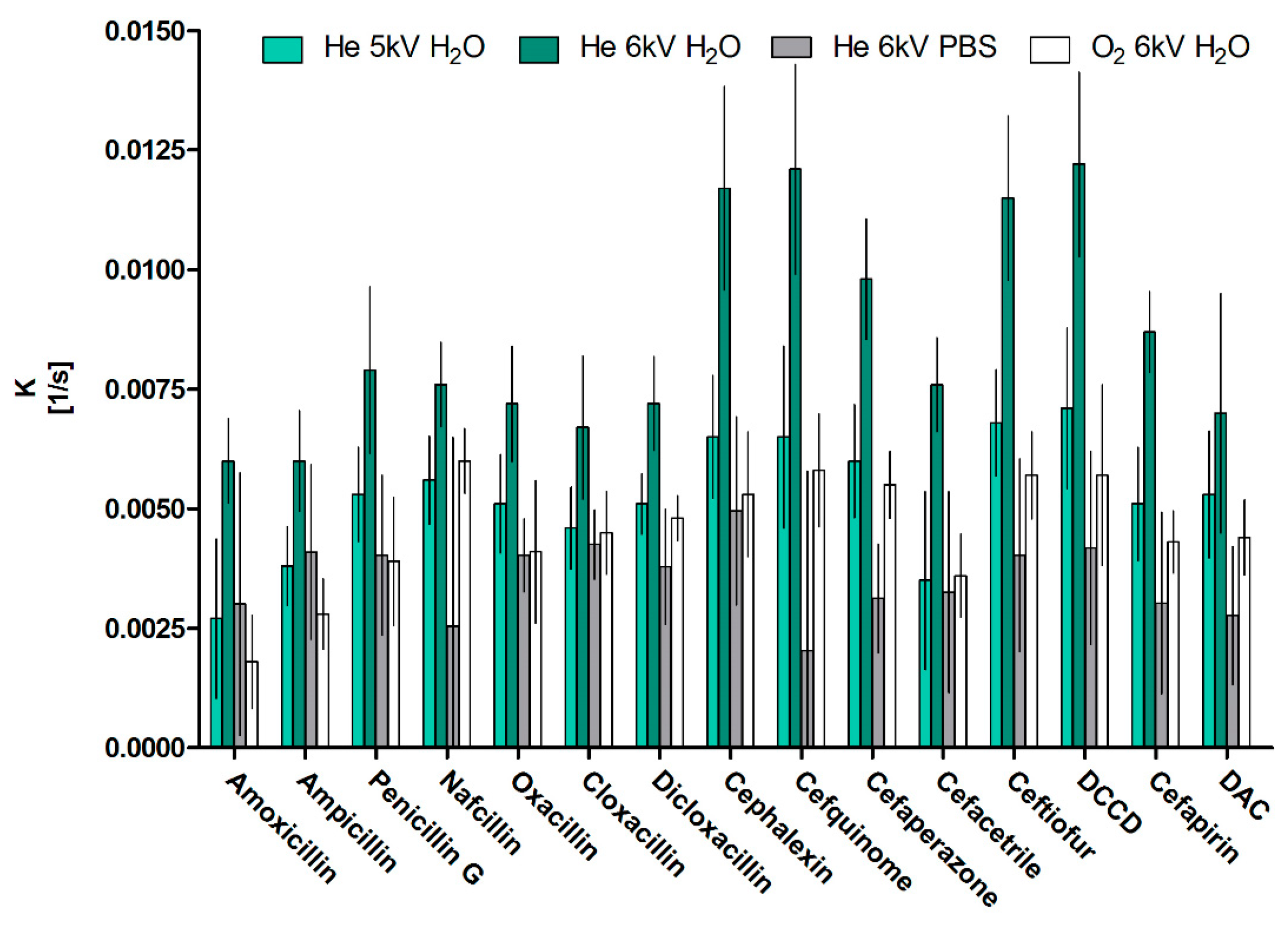
2.1. Decontamination Mechanism and Efficiency in Solvent
2.2. By-Product Formation
2.3. Toxicological and Antibiotic-Resistance Assessment
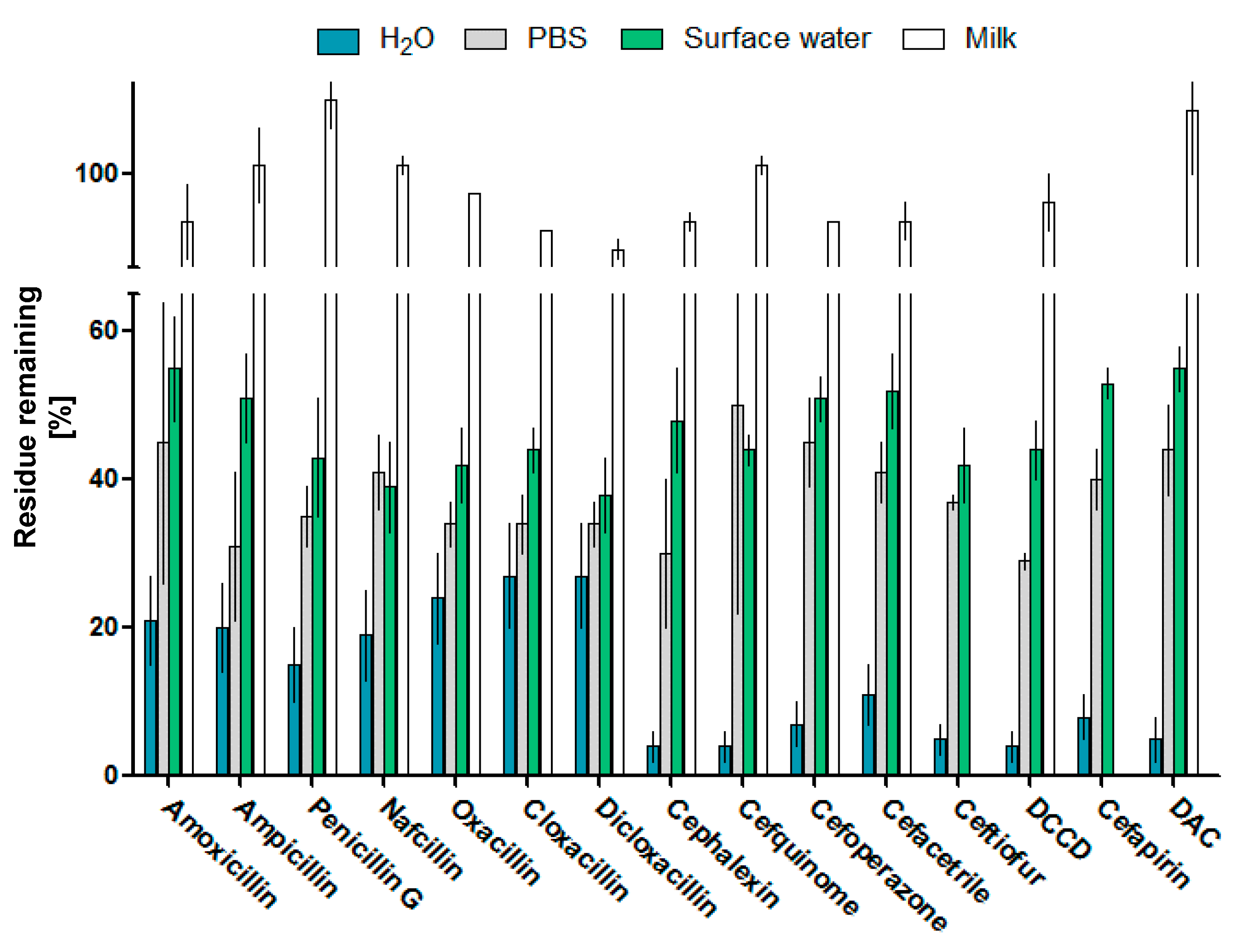
2.4. Plasma Efficiency in Residue Removal in the Matrix
3. Materials and Methods
3.1. Milk and Surface Water Samples
3.2. Sample Preparation
3.3. Atmospheric Pressure Plasma System
3.4. Plasma Exposure of Solvent Solutions, Surface Water and Milk
3.5. UPLC-MS Instrumental Set-Up
3.6. Preparation of Standard Solutions and Quality Control Samples
3.7. LC-MS/MS Method Validation
3.8. High Content Analysis
3.9. Resistance-Evolution Study and Antimicrobial Assessment of CAPP-Treated Amoxicillin
3.10. Computational Chemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenzweig, C.; Rind, D.; Lacis, A.; Peters, D. Our Warming Planet: Topics in Climate Dynamics; World Scientific: Singapore, 2018; Volume 1. [Google Scholar]
- Parris, K. Sustainable Management of Water Resources in Agriculture; OECD Publishing: Paris, France, 2010. [Google Scholar]
- Campanhola, C.; Pandey, S. Sustainable Food and Agriculture: An Integrated Approach; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Robert, L.; Raquel, N.D.C.; Sara, C.; Sofia, C.A.D.; Michela, G.; Teresa, L.; Giovanni, L.; Bruno, P.; Simona, T.; Bernd, G. EU Wide Monitoring Survey on Waste Water Treatment Plant Effluents; EU: Brussels, Belgium, 2012.
- European Comission. RASFF—Food and Safety Alerts. Available online: https://ec.europa.eu/food/safety/rasff_en (accessed on 5 September 2022).
- Jeong, S.-H.; Kang, D.; Lim, M.-W.; Kang, C.S.; Sung, H.J. Risk assessment of growth hormones and antimicrobial residues in meat. Toxicol. Res. 2010, 26, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Review on Antimicrobial Resistance; Wellcome Trust and UK Government: London, UK, 2014.
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antibiotic Resistance. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 5 September 2022).
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.L.; Boxall, A.B.; Kolpin, D.W.; Leung, K.M.; Lai, R.W.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [Green Version]
- Grenni, P. Antimicrobial resistance in rivers: A review of the genes detected and new challenges. Environ. Toxicol. Chem. 2022, 41, 687–714. [Google Scholar] [CrossRef]
- Nnadozie, C.; Kumari, S.; Bux, F. Status of pathogens, antibiotic resistance genes and antibiotic residues in wastewater treatment systems. Rev. Environ. Sci. Bio/Technol. 2017, 16, 491–515. [Google Scholar] [CrossRef]
- Casado, J.; Brigden, K.; Santillo, D.; Johnston, P. Screening of pesticides and veterinary drugs in small streams in the European Union by liquid chromatography high resolution mass spectrometry. Sci. Total Environ. 2019, 670, 1204–1225. [Google Scholar] [CrossRef]
- Amador, P.P.; Fernandes, R.M.; Prudêncio, M.C.; Barreto, M.P.; Duarte, I.M. Antibiotic resistance in wastewater: Occurrence and fate of Enterobacteriaceae producers of Class A and Class C β-lactamases. J. Environ. Sci. Health Part A 2015, 50, 26–39. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, L15, 1–72.
- European Food Safety Authority. Report for 2016 on the Results from the Monitoring of Veterinary Medicinal Product Residues and Other Substances in Live Animals and Animal Products; 2397-8325; Wiley Online Library: Hoboken, NJ, USA, 2018.
- Chowdhury, S.; Hassan, M.M.; Alam, M.; Sattar, S.; Bari, M.S.; Saifuddin, A.; Hoque, M.A. Antibiotic residues in milk and eggs of commercial and local farms at Chittagong, Bangladesh. Vet. World 2015, 8, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogurschi, E.; Ciric, A.; Zugrav, C.; Patrascu, D. Identification of antibiotic residues in raw milk samples coming from the metropolitan area of Bucharest. Agric. Agric. Sci. Procedia 2015, 6, 242–245. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic residues in food: Extraction, analysis, and human health concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Batista, L.; Prudêncio, C. Antimicrobial residues in milk: A food policy problem in an ethical framework. In Food Futures: Ethics, Science and Culture; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 137–142. [Google Scholar]
- Li, L.; Wei, D.; Wei, G.; Du, Y. Transformation of cefazolin during chlorination process: Products, mechanism and genotoxicity assessment. J. Hazard. Mater. 2013, 262, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, X.; Du, P.; Zhang, T.; Cai, M.; Sun, P.; Huang, C.-H. Oxidation of β-lactam antibiotics by peracetic acid: Reaction kinetics, product and pathway evaluation. Water Res. 2017, 123, 153–161. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, W.G.; Yoon, Y.; Kang, J.-W.; Hong, Y.M.; Kim, H.W. Removal of amoxicillin by UV and UV/H2O2 processes. Sci. Total Environ. 2012, 420, 160–167. [Google Scholar] [CrossRef]
- Yargeau, V.; Leclair, C. Potential of ozonation for the degradation of antibiotics in wastewater. Water Sci. Technol. 2007, 55, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Mutiyar, P.K.; Mittal, A.K. Occurrences and fate of an antibiotic amoxicillin in extended aeration-based sewage treatment plant in Delhi, India: A case study of emerging pollutant. Desalination Water Treat. 2013, 51, 6158–6164. [Google Scholar] [CrossRef]
- Le, T.-H.; Ng, C.; Tran, N.H.; Chen, H.; Gin, K.Y.-H. Removal of antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in municipal wastewater by membrane bioreactor systems. Water Res. 2018, 145, 498–508. [Google Scholar] [CrossRef]
- Liu, M.-K.; Liu, Y.-Y.; Bao, D.-D.; Zhu, G.; Yang, G.-H.; Geng, J.-F.; Li, H.-T. Effective removal of tetracycline antibiotics from water using hybrid carbon membranes. Sci. Rep. 2017, 7, 43717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamovich, I.; Baalrud, S.; Bogaerts, A.; Bruggeman, P.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.; Favia, P. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Samukawa, S.; Hori, M.; Rauf, S.; Tachibana, K.; Bruggeman, P.; Kroesen, G.; Whitehead, J.C.; Murphy, A.B.; Gutsol, A.F.; Starikovskaia, S. The 2012 plasma roadmap. J. Phys. D Appl. Phys. 2012, 45, 253001. [Google Scholar] [CrossRef]
- Stratton, G.R.; Bellona, C.L.; Dai, F.; Holsen, T.M.; Thagard, S.M. Plasma-based water treatment: Conception and application of a new general principle for reactor design. Chem. Eng. J. 2015, 273, 543–550. [Google Scholar] [CrossRef]
- Thagard, S.M.; Stratton, G.R.; Dai, F.; Bellona, C.L.; Holsen, T.M.; Bohl, D.G.; Paek, E.; Dickenson, E.R. Plasma-based water treatment: Development of a general mechanistic model to estimate the treatability of different types of contaminants. J. Phys. D Appl. Phys. 2016, 50, 014003. [Google Scholar] [CrossRef]
- Magureanu, M.; Bradu, C.; Parvulescu, V. Plasma processes for the treatment of water contaminated with harmful organic compounds. J. Phys. D Appl. Phys. 2018, 51, 313002. [Google Scholar] [CrossRef]
- Magureanu, M.; Piroi, D.; Mandache, N.; David, V.; Medvedovici, A.; Bradu, C.; Parvulescu, V. Degradation of antibiotics in water by non-thermal plasma treatment. Water Res. 2011, 45, 3407–3416. [Google Scholar] [CrossRef]
- Smith, J.B.; Adams, I.; Ji, H.-F. Mechanism of ampicillin degradation by non-thermal plasma treatment with FE-DBD. Plasma 2018, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sarangapani, C.; Ziuzina, D.; Behan, P.; Boehm, D.; Gilmore, B.F.; Cullen, P.; Bourke, P. Degradation kinetics of cold plasma-treated antibiotics and their antimicrobial activity. Sci. Rep. 2019, 9, 3955. [Google Scholar] [CrossRef] [Green Version]
- Joh, H.M.; Choi, J.Y.; Kim, S.J.; Chung, T.; Kang, T.-H. Effect of additive oxygen gas on cellular response of lung cancer cells induced by atmospheric pressure helium plasma jet. Sci. Rep. 2014, 4, 6638. [Google Scholar] [CrossRef] [Green Version]
- Flynn, P.B.; Busetti, A.; Wielogorska, E.; Chevallier, O.P.; Elliott, C.T.; Laverty, G.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Non-thermal plasma exposure rapidly attenuates bacterial AHL-dependent quorum sensing and virulence. Sci. Rep. 2016, 6, 26320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algwari, Q.T.; O’Connell, D. Electron dynamics and plasma jet formation in a helium atmospheric pressure dielectric barrier discharge jet. Appl. Phys. Lett. 2011, 99, 121501. [Google Scholar] [CrossRef]
- Wielogorska, E.; Ahmed, Y.; Meneely, J.; Graham, W.G.; Elliott, C.T.; Gilmore, B.F. A holistic study to understand the detoxification of mycotoxins in maize and impact on its molecular integrity using cold atmospheric plasma treatment. Food Chem. 2019, 301, 125281. [Google Scholar] [CrossRef]
- Berendsen, B.; Elbers, I.; Stolker, A. Determination of the stability of antibiotics in matrix and reference solutions using a straightforward procedure applying mass spectrometric detection. Food Addit. Contam. Part A 2011, 28, 1657–1666. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Carmichael, G.R. Kinetics of oxidation of dimethyl sulfide by hydrogen peroxide in acidic and alkaline medium. Environ. Sci. Technol. 1986, 20, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef] [Green Version]
- Teschke, M.; Kedzierski, J.; Finantu-Dinu, E.; Korzec, D.; Engemann, J. High-speed photographs of a dielectric barrier atmospheric pressure plasma jet. IEEE Trans. Plasma Sci. 2005, 33, 310–311. [Google Scholar] [CrossRef]
- Di Rocco, M.; Moloney, M.; O’Beirne, T.; Earley, S.; Berendsen, B.; Furey, A.; Danaher, M. Development and validation of a quantitative confirmatory method for 30 β-lactam antibiotics in bovine muscle using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2017, 1500, 121–135. [Google Scholar] [CrossRef]
- Riediker, S.; Rytz, A.; Stadler, R.H. Cold-temperature stability of five β-lactam antibiotics in bovine milk and milk extracts prepared for liquid chromatography–electrospray ionization tandem mass spectrometry analysis. J. Chromatogr. A 2004, 1054, 359–363. [Google Scholar] [CrossRef]
- Gordon, R.C.; Regamey, C.; Kirby, W.M. Comparative clinical pharmacology of amoxicillin and ampicillin administered orally. Antimicrob. Agents Chemother. 1972, 1, 504–507. [Google Scholar] [CrossRef] [Green Version]






| Compound | Retention Time | Experimental m/z | Theoretical m/z | Mass Error [ppm] | DBE | Formula | Modification |
|---|---|---|---|---|---|---|---|
| Amoxicillin | 5.54 | 366.1113 | 366.1118 | −1.4 | 9 | C16H19N3O5S | |
| 1.74/4.13/4.78 | 382.1070 | 382.1067 | 0.7 | 10 | C16H19N3O6S | +O | |
| 3.13 | 398.1019 | 398.1016 | 0.6 | 11 | C16H19N3O7S | +2O | |
| Cloxacillin | 13.81 | 436.0737 | 436.0728 | 2 | 12 | C19H18ClN3O5S | |
| 11.28/12.33 | 452.0709 | 452.0678 | 6.9 | 13 | C19H18ClN3O6S | +O | |
| 9.62 | 454.0859 | 454.0834 | 5.5 | 11 | C19H20ClN3O6S | +H2O | |
| 9.36 | 438.0913 | 438.0885 | 6.4 | 12 | C19H20ClN3O5S | +2H | |
| Cefazolin | 8.5 | 455.0406 | 455.0373 | 7.3 | 12 | C14H14N8O4S3 | |
| 7.89 | 210.9692 | 210.9664 | 13.2 | 3 | C4H6N2O2S3 | ||
| 6.41/6.53 | 471.0327 | 471.0322 | 1.1 | 13 | C14H14N8O5S3 | +O | |
| Cefaperazone | 8.98 | 646.1490 | 646.1497 | −1 | 17 | C25H27N9O8S2 | |
| 7.97/8.13 | 662.1453 | 662.1446 | 1.1 | 18 | C25H27N9O9S2 | +O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wielogorska, E.; Flynn, P.B.; Meneely, J.; Thompson, T.P.; Graham, W.G.; Gilmore, B.F.; Elliott, C.T. Assessment of Cold Atmospheric Pressure Plasma (CAPP) Treatment for Degradation of Antibiotic Residues in Water. Antibiotics 2023, 12, 1115. https://doi.org/10.3390/antibiotics12071115
Wielogorska E, Flynn PB, Meneely J, Thompson TP, Graham WG, Gilmore BF, Elliott CT. Assessment of Cold Atmospheric Pressure Plasma (CAPP) Treatment for Degradation of Antibiotic Residues in Water. Antibiotics. 2023; 12(7):1115. https://doi.org/10.3390/antibiotics12071115
Chicago/Turabian StyleWielogorska, Ewa, Padrig B. Flynn, Julie Meneely, Thomas P. Thompson, William G. Graham, Brendan F. Gilmore, and Christopher T. Elliott. 2023. "Assessment of Cold Atmospheric Pressure Plasma (CAPP) Treatment for Degradation of Antibiotic Residues in Water" Antibiotics 12, no. 7: 1115. https://doi.org/10.3390/antibiotics12071115
APA StyleWielogorska, E., Flynn, P. B., Meneely, J., Thompson, T. P., Graham, W. G., Gilmore, B. F., & Elliott, C. T. (2023). Assessment of Cold Atmospheric Pressure Plasma (CAPP) Treatment for Degradation of Antibiotic Residues in Water. Antibiotics, 12(7), 1115. https://doi.org/10.3390/antibiotics12071115







