Bactericidal Activity to Escherichia coli: Different Modes of Action of Two 24-Mer Peptides SAAP-148 and OP-145, Both Derived from Human Cathelicidine LL-37
Abstract
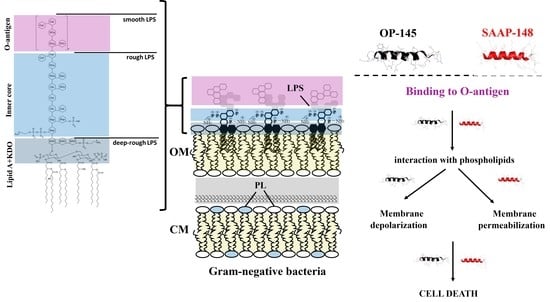
1. Introduction
2. Results
2.1. Effect of the Peptides on Binding to Bacterial Surfaces of E. coli Producing Different LPS Lengths
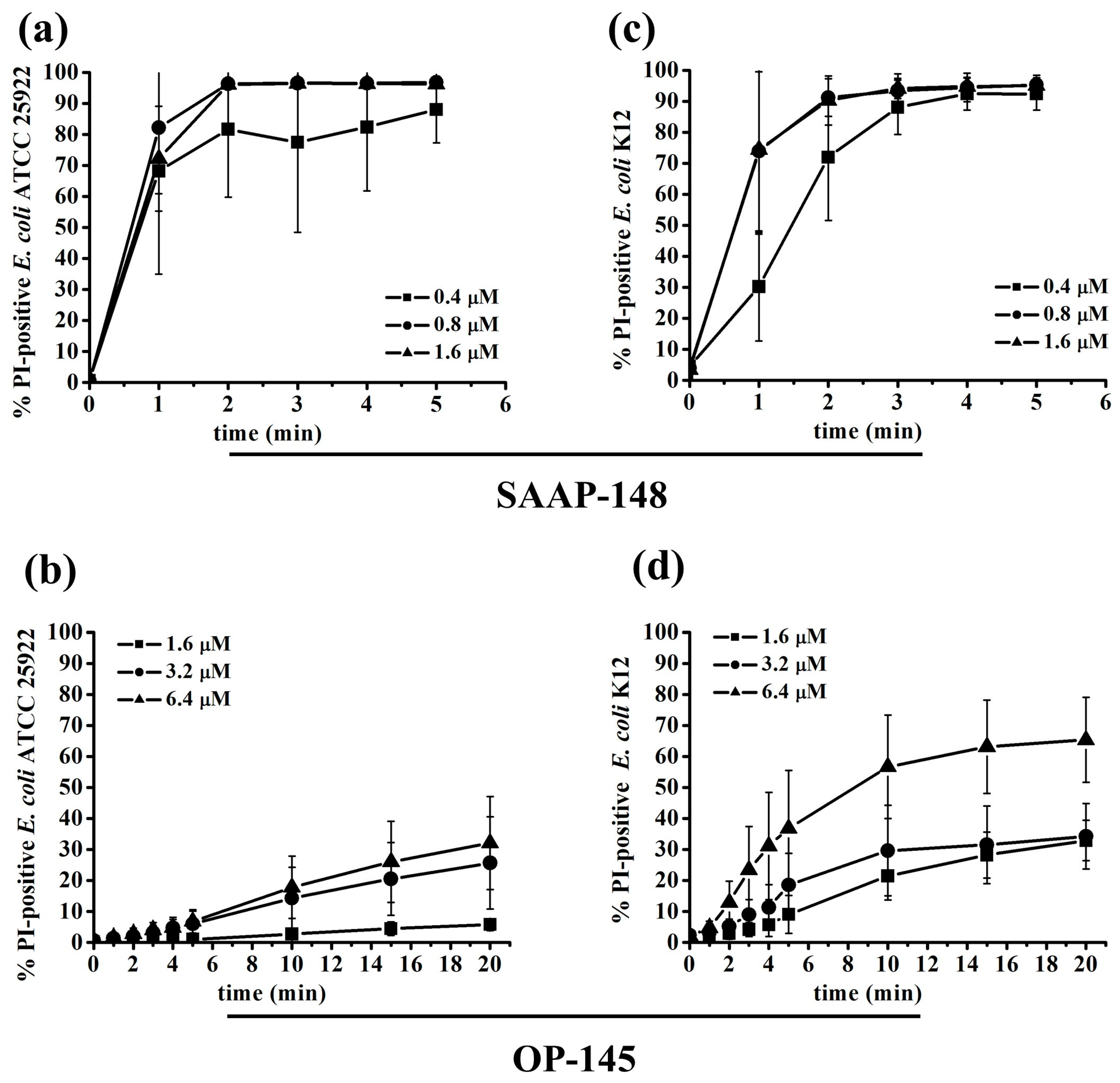
2.2. Effect of the Peptides on Membranes of E. coli Producing Different LPS Length
2.3. Effect of the Peptides on Phospholipids
3. Discussion
4. Materials and Methods
4.1. Peptides
4.2. Lipids
4.3. Microorganisms and Culture
4.4. Antimicrobial Activity
4.5. Zeta Potential Measurements
4.6. Lipid Staining of Bacteria by Fluorescence Microscopy
4.7. Membrane Depolarization Assay and Measurement by Fluorescence Spectroscopy
4.8. Membrane Permeabilization Assay Using Flow Cytometry
4.9. Preparation of Liposomes
4.10. DSC
4.11. Vesicle Leakage Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, N.; Jiang, C. Antimicrobial peptides: Structure, mechanism, and modification. Eur. J. Med. Chem. 2023, 255, 115377. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Vejzovic, D. Novel insights at the crossroads of antibiotic use and cancer risk. Cell Stress 2023, 7, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Sinha, G. Tumors can teem with microbes. But what are they doing there? Science 2022, 378, 693–694. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef]
- Galeano Niño, J.L.; Wu, H.; LaCourse, K.D.; Kempchinsky, A.G.; Baryiames, A.; Barber, B.; Futran, N.; Houlton, J.; Sather, C.; Sicinska, E.; et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 2022, 611, 810–817. [Google Scholar] [CrossRef]
- Bechinger, B.; Lohner, K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1529–1539. [Google Scholar] [CrossRef]
- Hale, J.D.; Hancock, R.E. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti-Infect. Ther. 2007, 5, 951–959. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Malanovic, N.; Marx, L.; Blondelle, S.E.; Pabst, G.; Semeraro, E.F. Experimental concepts for linking the biological activities of antimicrobial peptides to their molecular modes of action. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183275. [Google Scholar] [CrossRef] [PubMed]
- de Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef]
- Vejzovic, D.; Piller, P.; Cordfunke, R.A.; Drijfhout, J.W.; Eisenberg, T.; Lohner, K.; Malanovic, N. Where Electrostatics Matter: Bacterial Surface Neutralization and Membrane Disruption by Antimicrobial Peptides SAAP-148 and OP-145. Biomolecules 2022, 12, 1252. [Google Scholar] [CrossRef] [PubMed]
- Kakar, A.; Sastré-Velásquez, L.E.; Hess, M.; Galgóczy, L.; Papp, C.; Holzknecht, J.; Romanelli, A.; Váradi, G.; Malanovic, N.; Marx, F. The membrane activity of the amphibian Temporin B peptide analog TB_KKG6K sheds light on the mechanism that kills Candida albicans. Msphere 2022, 7, e0029022. [Google Scholar] [CrossRef] [PubMed]
- Riool, M.; de Breij, A.; Kwakman, P.H.S.; Schonkeren-Ravensbergen, E.; de Boer, L.; Cordfunke, R.A.; Malanovic, N.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Thrombocidin-1-derived antimicrobial peptide TC19 combats superficial multi-drug resistant bacterial wound infections. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183282. [Google Scholar] [CrossRef]
- Malanovic, N.; Buttress, J.A.; Vejzovic, D.; Ön, A.; Piller, P.; Kolb, D.; Lohner, K.; Strahl, H. Disruption of the Cytoplasmic Membrane Structure and Barrier Function Underlies the Potent Antiseptic Activity of Octenidine in Gram-Positive Bacteria. Appl. Environ. Microbiol. 2022, 88, e0018022. [Google Scholar] [CrossRef]
- Malanovic, N.; Ön, A.; Pabst, G.; Zellner, A.; Lohner, K. Octenidine: Novel insights into the detailed killing mechanism of Gram-negative bacteria at a cellular and molecular level. Int. J. Antimicrob. Agents 2020, 56, 106146. [Google Scholar] [CrossRef]
- Piller, P.; Wolinski, H.; Cordfunke, R.A.; Drijfhout, J.W.; Keller, S.; Lohner, K.; Malanovic, N. Membrane Activity of LL-37 Derived Antimicrobial Peptides against Enterococcus hirae: Superiority of SAAP-148 over OP-145. Biomolecules 2022, 12, 523. [Google Scholar] [CrossRef]
- Melo, M.N.; Castanho, M.A. The Mechanism of Action of Antimicrobial Peptides: Lipid Vesicles vs. Bacteria. Front. Immunol. 2012, 3, 236. [Google Scholar] [CrossRef]
- Freire, J.M.; Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. Shifting gear in antimicrobial and anticancer peptides biophysical studies: From vesicles to cells. J. Pept. Sci. 2015, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M. Development of Novel Antimicrobial Agents: Emerging Strategies, Edited by Karl Lohner, Horizon Scientific Press, Norfolk, UK, 2001. Chem. Phys. Lipids 2001, 111, 193. [Google Scholar] [CrossRef]
- Lohner, K. New strategies for novel antibiotics: Peptides targeting bacterial cell membranes. Gen. Physiol. Biophys. 2009, 28, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Lohner, K. Membrane-active Antimicrobial Peptides as Template Structures for Novel Antibiotic Agents. Curr. Top. Med. Chem. 2017, 16, 508–519. [Google Scholar] [CrossRef]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Kramer, U.; et al. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E1409–E1418. [Google Scholar] [CrossRef]
- Scheinpflug, K.; Krylova, O.; Nikolenko, H.; Thurm, C.; Dathe, M. Evidence for a novel mechanism of antimicrobial action of a cyclic R-,W-rich hexapeptide. PLoS ONE 2015, 10, e0125056. [Google Scholar] [CrossRef]
- Omardien, S.; Drijfhout, J.W.; Vaz, F.M.; Wenzel, M.; Hamoen, L.W.; Zaat, S.A.J.; Brul, S. Bactericidal activity of amphipathic cationic antimicrobial peptides involves altering the membrane fluidity when interacting with the phospholipid bilayer. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2404–2415. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Leitgeb, B.; Szekeres, A.; Manczinger, L.; Vagvolgyi, C.; Kredics, L. The history of alamethicin: A review of the most extensively studied peptaibol. Chem. Biodivers. 2007, 4, 1027–1051. [Google Scholar] [CrossRef]
- Jennings, J.; Ašćerić, D.; Semeraro, E.; Lohner, K.; Malanovic, N.; Pabst, G. High-throughput screening of cationic lipidoids reveals how molecular conformation affects membrane-targeting antimicrobial activity. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Nell, M.J.; Tjabringa, G.S.; Wafelman, A.R.; Verrijk, R.; Hiemstra, P.S.; Drijfhout, J.W.; Grote, J.J. Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides 2006, 27, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Leber, R.; Schmuck, M.; Kriechbaum, M.; Cordfunke, R.A.; Drijfhout, J.W.; de Breij, A.; Nibbering, P.H.; Kolb, D.; Lohner, K. Phospholipid-driven differences determine the action of the synthetic antimicrobial peptide OP-145 on Gram-positive bacterial and mammalian membrane model systems. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Prehm, P.; Schmidt, G.; Jann, B.; Jann, K. The cell-wall lipopolysaccharide of Escherichia coli K-12. Structure and acceptor site for O-antigen and other substituents. Eur. J. Biochem. 1976, 70, 171–177. [Google Scholar] [CrossRef]
- Gmeiner, J.; Schlecht, S. Molecular composition of the outer membrane of Escherichia coli and the importance of protein-lipopolysaccharide interactions. Arch. Microbiol. 1980, 127, 81–86. [Google Scholar] [CrossRef]
- Vejzovic, D.; Iftic, A.; Ön, A.; Semeraro, E.F.; Malanovic, N. Octenidine’s Efficacy: A Matter of Interpretation or the Influence of Experimental Setups? Antibiotics 2022, 11, 1665. [Google Scholar] [CrossRef]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef]
- Koraimann, G.; Högenauer, G. A stable core region of the tra operon mRNA of plasmid R1-19. Nucleic Acids Res. 1989, 17, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G.; Monner, D.A. Characterization of lipopolysaccharides from Escherichia coli K-12 mutants. J. Bacteriol. 1975, 121, 455–464. [Google Scholar] [CrossRef]
- Boman, H.G.; Eriksson-Grennberg, K.G.; Normark, S.; Matsson, E. Resistance of Escherichia coli to penicillins: IV. Genetic study of mutants resistant to D,L-ampicillin concentrations of 100 μg/mL. Genet. Res. 1968, 12, 169–185. [Google Scholar] [CrossRef]
- Grundström, T.; Jaurin, B.; Edlund, T.; Normark, S. Physical mapping and expression of hybrid plasmids carrying chromosomal beta-lactamase genes of Escherichia coli K-12. J. Bacteriol. 1980, 143, 1127–1134. [Google Scholar] [CrossRef]
- Farnaud, S.; Spiller, C.; Moriarty, L.C.; Patel, A.; Gant, V.; Odell, E.W.; Evans, R.W. Interactions of lactoferricin-derived peptides with LPS and antimicrobial activity. FEMS Microbiol. Lett. 2004, 233, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Loffredo, M.R.; Troiano, C.; Bobone, S.; Malanovic, N.; Eichmann, T.O.; Caprio, L.; Canale, V.C.; Park, Y.; Mangoni, M.L.; et al. Binding of an antimicrobial peptide to bacterial cells: Interaction with different species, strains and cellular components. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183291. [Google Scholar] [CrossRef] [PubMed]
- Pozo, N.B.; Lohner, K.; Deutsch, G.; Sevcsik, E.; Riske, K.A.; Dimova, R.; Garidel, P.; Pabst, G. Composition dependence of vesicle morphology and mixing properties in a bacterial model membrane system. Biochim. Biophys. Acta (BBA)-Biomembr. 2005, 1716, 40–48. [Google Scholar] [CrossRef]
- Rappolt, M.; Hickel, A.; Bringezu, F.; Lohner, K. Mechanism of the lamellar/inverse hexagonal phase transition examined by high resolution x-ray diffraction. Biophys. J. 2003, 84, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the hydrated phosphatidylethanolamines. Chem. Phys. Lipids 1994, 69, 1–34. [Google Scholar] [CrossRef]
- Rhys, N.H.; Soper, A.K.; Dougan, L. The hydrogen-bonding ability of the amino acid glutamine revealed by neutron diffraction experiments. J. Phys. Chem. B 2012, 116, 13308–13319. [Google Scholar] [CrossRef]
- Haines, T.H.; Dencher, N.A. Cardiolipin: A proton trap for oxidative phosphorylation. FEBS Lett. 2002, 528, 35–39. [Google Scholar] [CrossRef]
- Lohner, K. DSC Studies on the Modulation of Membrane Lipid Polymorphism and Domain Organization by Antimicrobial Peptides. In Biocalorimetry: Foundations and Contemporary Approaches; Bastos, M., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2015; pp. 169–190. ISBN 978-1-4822-4665-0. [Google Scholar]
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Wang, X. Insights into the structure of Escherichia coli outer membrane as the target for engineering microbial cell factories. Microb. Cell Fact. 2021, 20, 73. [Google Scholar] [CrossRef]
- Sabnis, A.; Hagart, K.L.; Klöckner, A.; Becce, M.; Evans, L.E.; Furniss, R.C.D.; Mavridou, D.A.; Murphy, R.; Stevens, M.M.; Davies, J.C.; et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. Elife 2021, 10, e65836. [Google Scholar] [CrossRef]
- Marx, L.; Semeraro, E.F.; Mandl, J.; Kremser, J.; Frewein, M.P.; Malanovic, N.; Lohner, K.; Pabst, G. Bridging the Antimicrobial Activity of Two Lactoferricin Derivatives in E. coli and Lipid-Only Membranes. Front. Med. Technol. 2021, 3, 625975. [Google Scholar] [CrossRef] [PubMed]
- Adélaïde, M.; Salnikov, E.; Ramos-Martín, F.; Aisenbrey, C.; Sarazin, C.; Bechinger, B.; D’Amelio, N. The Mechanism of Action of SAAP-148 Antimicrobial Peptide as Studied with NMR and Molecular Dynamics Simulations. Pharmaceutics 2023, 15, 761. [Google Scholar] [CrossRef] [PubMed]






| E. coli Strain | Relevant Genotype | Source/Reference |
|---|---|---|
| Full length LPS producing strain | ||
| ATCC 25992 | wildtype | LGC Standards GmbH, Germany |
| K-12 derived strains lacking O-antigen | ||
| MG1655 | F-, λ-, ilvG, rfb-50, rph-1 | [36] |
| K-12 5K | tre, thi, rpsl+, kdsR, kdsM+, lac | [37] |
| K12-derived strain with modifications in the outer core | ||
| D12 | F-, proA23, lac-28, tsx-81, trp-30, his-51, rpsL173(strR), ampCp-1 | [38,39,40] |
| D12-derived strain lacking O-antigen, outer and the inner core of LPS | ||
| D12f2 | F-, proA23, lac-28, tsx-81, trp-30, his-51, rpsL173(strR), rfa-31, rfa-1, ampCp-1 | [38,39,40,41] |
| Antimicrobial Activity (µM) | |||||
|---|---|---|---|---|---|
| Peptides | ATCC25992 | K12 | D21 | D21f2 | |
| SAAP-148 | MIC | 0.8 | 0.8 | 0.8 | 0.8 |
| OP-145 | 3.2 | 1.6–3.2 | 1.6–3.2 | 1.6 | |
| SAAP-148 | LC99.9% | 0.4–1.6 | 0.2–0.4 | 0.2–0.4 | 0.4 |
| OP-145 | 3.2–12.8 | 1.6–3.2 | 1.6–3.2 | 1.6–12.8 | |
| Heating Scans | Cooling Scans | |||||
|---|---|---|---|---|---|---|
| Enthalpy * [kcal/mol] | Tm [°C] | ∆T1/2 [°C] | Enthalpy * [kcal/mol] | Tm [°C] | ∆T1/2 [°C] | |
| POPG/POPE | 5.8 | 21.1 | 2.4 | 5.3 | 18.7 | 1.4 |
| +OP-145 | 6.4 | 18.9 | 3.3 | 0.6 3.5 2.0 | 9.5 16.5 19.2 | 3.9 0.9 0.6 |
| +SAAP-148 | 1.5 4.5 | 15.1 20.9 | 4.0 3.4 | 0.9 1.8 2.9 | 12.0 17.0 19.0 | 3.1 0.6 1.2 |
| DPPG/DPPE | 9.2 | 60.4 | 1.6 | 7.9 | 59.3 | 0.4 |
| +OP-145 | 7.5 | 60.6 | 1.9 | 1.0 5.9 | 52.4 59.8 | 2.9 0.2 |
| +SAAP-148 | 0.9 3.0 | 55.1 60.3 | 1.9 1.7 | 0.6 2.9 | 52.4 59.7 | 4.6 0.3 |
| POPG/POPE/TMCL | 7.6 | 26 | 6.2 | 5.7 | 25.4 | 2.6 |
| +OP-145 | 7.2 | 26.4 | 6.2 | 7.3 | 25.1 | 5.6 |
| +SAAP-148 | 1.4 | 24.4 | 5 | 1.6 | 24.6 | 4.1 |
| DPPG/DPPE/TMCL | 11.1 | 56.5 | 3.4 | 10.5 | 55.9 | 1.1 |
| +OP-145 | 9.5 | 59.6 | 2.2 | 0.7 8.8 | 39.0 58.9 | 8.5 1.0 |
| +SAAP-148 | 1.2 4.6 | 49.5 55.9 | 6.1 5.5 | 5.0 | 56.7 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ön, A.; Vejzovic, D.; Jennings, J.; Parigger, L.; Cordfunke, R.A.; Drijfhout, J.W.; Lohner, K.; Malanovic, N. Bactericidal Activity to Escherichia coli: Different Modes of Action of Two 24-Mer Peptides SAAP-148 and OP-145, Both Derived from Human Cathelicidine LL-37. Antibiotics 2023, 12, 1163. https://doi.org/10.3390/antibiotics12071163
Ön A, Vejzovic D, Jennings J, Parigger L, Cordfunke RA, Drijfhout JW, Lohner K, Malanovic N. Bactericidal Activity to Escherichia coli: Different Modes of Action of Two 24-Mer Peptides SAAP-148 and OP-145, Both Derived from Human Cathelicidine LL-37. Antibiotics. 2023; 12(7):1163. https://doi.org/10.3390/antibiotics12071163
Chicago/Turabian StyleÖn, Ayse, Djenana Vejzovic, James Jennings, Lena Parigger, Robert A. Cordfunke, Jan Wouter Drijfhout, Karl Lohner, and Nermina Malanovic. 2023. "Bactericidal Activity to Escherichia coli: Different Modes of Action of Two 24-Mer Peptides SAAP-148 and OP-145, Both Derived from Human Cathelicidine LL-37" Antibiotics 12, no. 7: 1163. https://doi.org/10.3390/antibiotics12071163
APA StyleÖn, A., Vejzovic, D., Jennings, J., Parigger, L., Cordfunke, R. A., Drijfhout, J. W., Lohner, K., & Malanovic, N. (2023). Bactericidal Activity to Escherichia coli: Different Modes of Action of Two 24-Mer Peptides SAAP-148 and OP-145, Both Derived from Human Cathelicidine LL-37. Antibiotics, 12(7), 1163. https://doi.org/10.3390/antibiotics12071163