Abstract
Invasive methicillin-resistant Staphylococcus aureus (MRSA) infections are leading causes of morbidity and mortality that are complicated by increasing resistance to conventional antibiotics. Thus, minimizing virulence and enhancing antibiotic efficacy against MRSA is a public health imperative. We originally demonstrated that diflunisal (DIF; [2-hydroxy-5-(2,4-difluorophenyl) benzoic acid]) inhibits S. aureus virulence factor expression. To investigate pharmacophores that are active in this function, we evaluated a library of structural analogues for their efficacy to modulate virulence phenotypes in a panel of clinically relevant S. aureus isolates in vitro. Overall, the positions of the phenyl, hydroxyl, and carboxylic moieties and the presence or type of halogen (F vs. Cl) influenced the efficacy of compounds in suppressing hemolysis, proteolysis, and biofilm virulence phenotypes. Analogues lacking halogens inhibited proteolysis to an extent similar to DIF but were ineffective at reducing hemolysis or biofilm production. In contrast, most analogues lacking the hydroxyl or carboxylic acid groups did not suppress proteolysis but did mitigate hemolysis and biofilm production to an extent similar to DIF. Interestingly, chirality and the substitution of fluorine with chlorine resulted in a differential reduction in virulence phenotypes. Together, this pattern of data suggests virulence-suppressing pharmacophores of DIF and structural analogues integrate halogen, hydroxyl, and carboxylic acid moiety stereochemistry. The anti-virulence effects of DIF were achieved using concentrations that are safe in humans, do not impair platelet antimicrobial functions, do not affect S. aureus growth, and do not alter the efficacy of conventional antibiotics. These results offer proof of concept for using novel anti-virulence strategies as adjuvants to antibiotic therapy to address the challenge of MRSA infection.
1. Introduction
Staphylococcus aureus is responsible for significant morbidity and mortality worldwide. The ability of S. aureus to cause diverse infections ranging from soft tissue abscesses to life-threatening invasive disease is mediated by the coordinated expression of an extensive repertoire of virulence factors including toxins, proteases, and immune avoidance effectors. Moreover, the rapid emergence of clinical isolates exhibiting multi-drug resistance, including methicillin (MRSA)- and vancomycin (VRSA)-resistant S. aureus, has accelerated the urgent need for novel strategies to counter S. aureus pathogenic mechanisms. Therefore, targeting such virulence determinants with potential novel therapeutics is highly logical and may reveal improved methods for preventing or treating infections caused by this or other human pathogens.
We originally demonstrated that diflunisal (DIF) strongly attenuates S. aureus virulence gene expression and phenotypes [1,2,3,4,5]. This advance extended the observations of our group and others regarding the beneficial effects of aspirin on S. aureus infection [6,7,8,9,10,11,12,13]. Specifically, aspirin and its primary metabolite salicylic acid appear to reduce the severity and progression of clinical and experimental infective endocarditis (IE) [6,7,13,14,15,16,17] and significantly decrease the risk of S. aureus bacteremia in catheterized patients [18,19]. Likewise, meta-analyses revealed a significant beneficial effect of aspirin and reduced risk of patient systemic embolism in IE [20]. Several studies have suggested that salicylates may attenuate virulence through interactions with S. aureus global regulatory systems [1,2,6,11,21,22,23,24]. Others have since validated these findings in a number of experimental models [25,26,27].
The current work builds upon our previous findings to explore bioactive pharmacophores of DIF and structural analogues for their ability to modulate hemolysis, proteolysis, and biofilm formation as prototypic virulence phenotypes in S. aureus. The present findings reveal that specific chemical moieties and their three-dimensional stereochemistries are integral to the suppression of virulence phenotypes in clinically relevant S. aureus isolates. These results substantiate our strategic approach to develop novel therapeutic agents to interfere with essential virulence mechanisms and thereby promote host defenses against S. aureus. This approach is likely to be extendable to other high-priority antibiotic-resistant pathogens and may hold promise for addressing the threat of untreatable infections.
2. Results
In the present study, we investigated the structure–activity relationships of DIF and structural analogues to suppress virulence phenotypes in vitro. The goal of this study was to evaluate compounds for anti-virulence efficacy relative to DIF and discern key pharmacophores that may be correlated with the inhibition of specific virulence phenotypes in S. aureus.
2.1. Selection of Staphylococcus aureus Strains
Prior studies by our group and others showed that DIF, a difluorinated analogue of salicylic acid, can mitigate the expression of virulence factors in S. aureus [14]. DIF and 26 structurally distinct DIF analogues were compared for their inhibition of virulence phenotypes in a panel of highly relevant S. aureus study strains (Table 1).

Table 1.
Staphylococcus aureus strains used in this study.
2.2. Selection of DIF Analogues
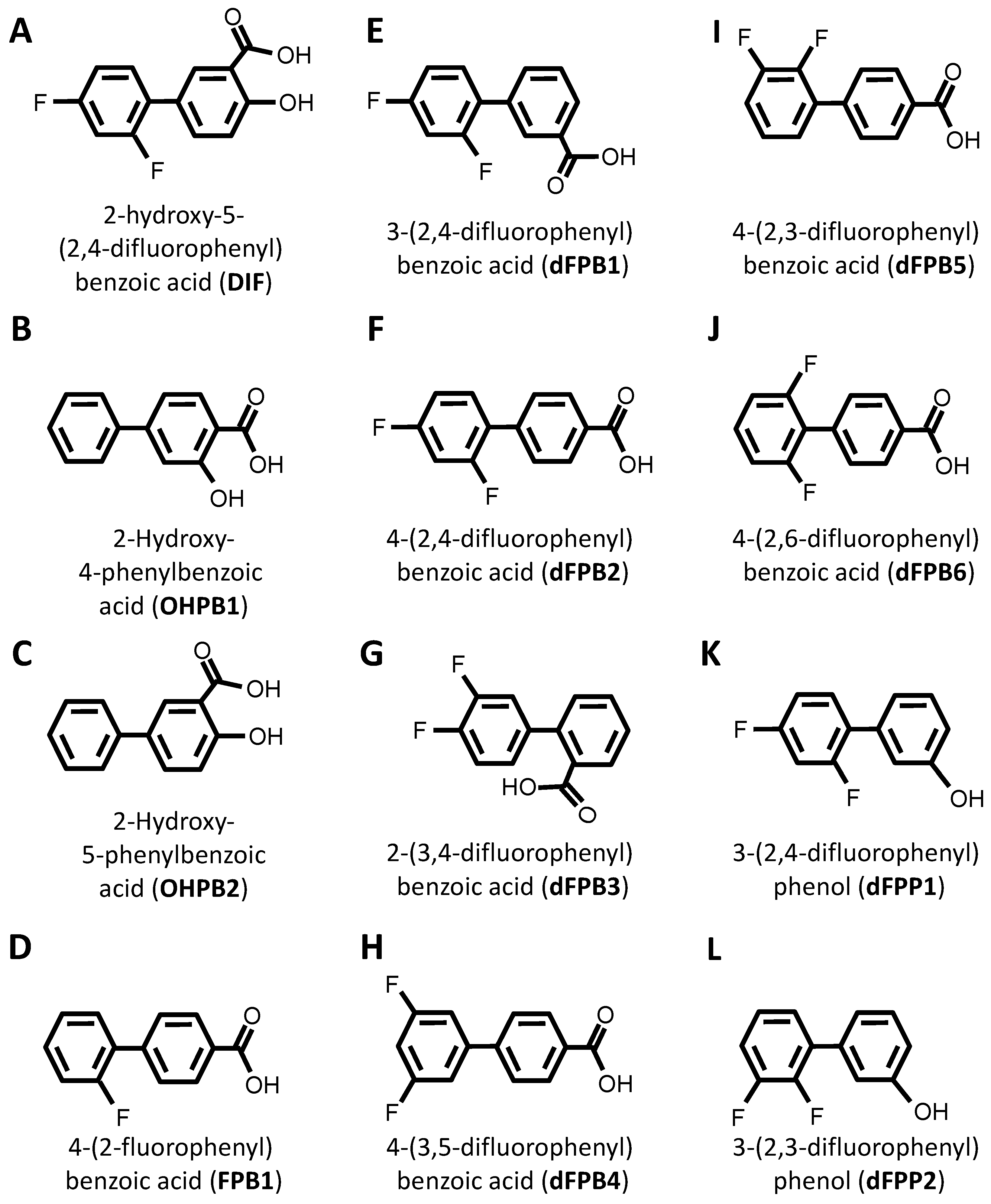
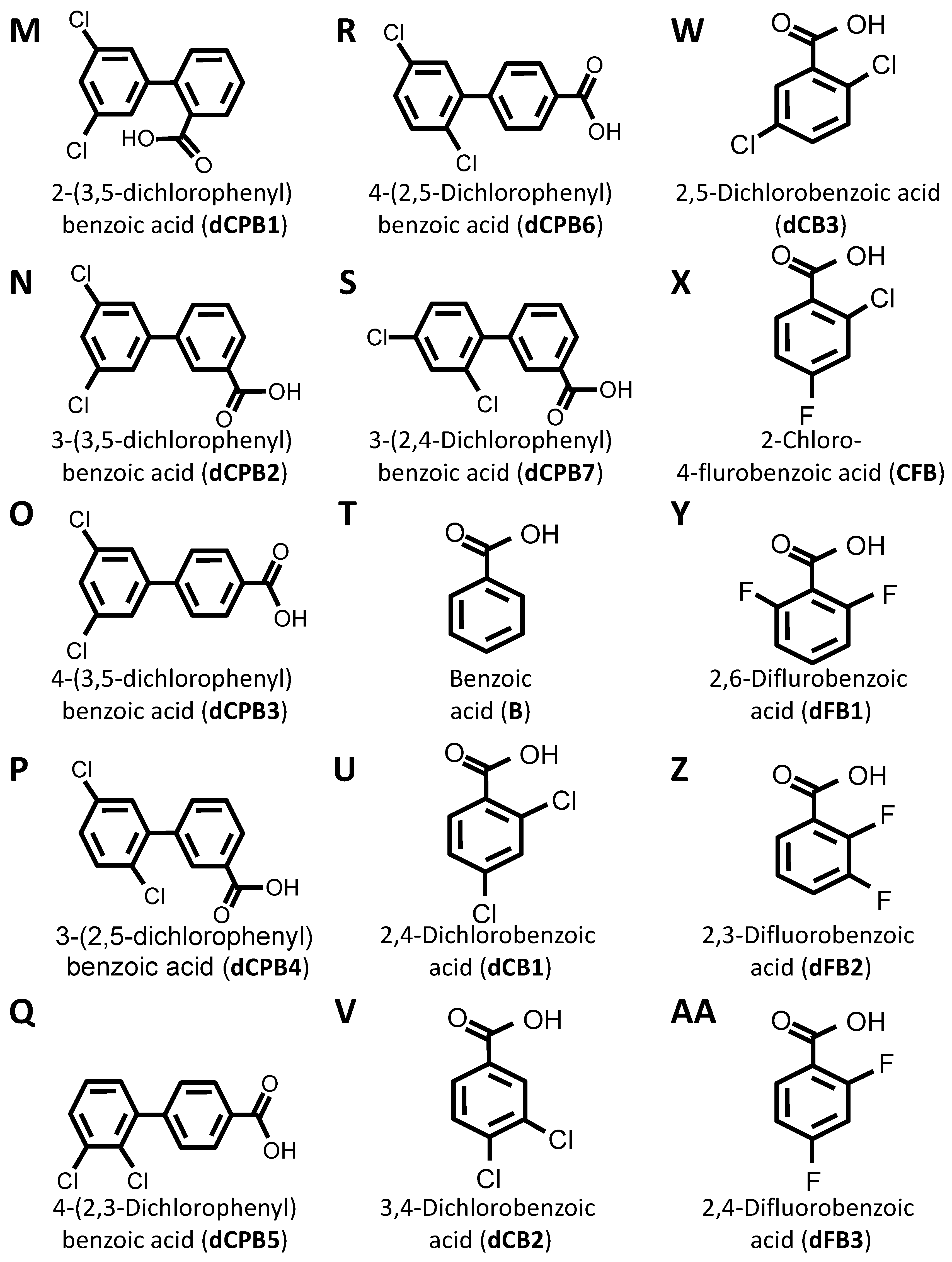
DIF comprises three moieties: (1) a benzoic acid aromatic ring; (2) a difluorophenyl group; and (3) a hydroxyl group (Figure 1A). The commercially available DIF analogues that were selected included representatives with differing halogen, carbonyl, or hydroxyl groups and positioning of chemical moieties (Figure 1). To determine the impact of halogens on DIF activity, hydroxyphenylbenzoic acid analogues were evaluated (Figure 1B,C; OHPB). Monofluorophenyl (Figure 1D; FPB) and difluorophenyl (Figure 1E–J; dFPB) isotypes were selected to assess the importance of the halogen number and positioning on the phenyl group in the absence of a hydroxyl group. Alternatively, difluorophenyl analogues lacking a carboxylic acid group were evaluated to determine the role of benzoic acid versus phenol groups (Figure 1K,L; dFPP). To assess the role of the halogen type in DIF efficacy, dichlorophenyl-substituted analogues (Figure 1M–S; dCPB) were studied. Finally, to assess the impact of the phenyl ring of DIF, halogen-benzoic acid analogues were evaluated.
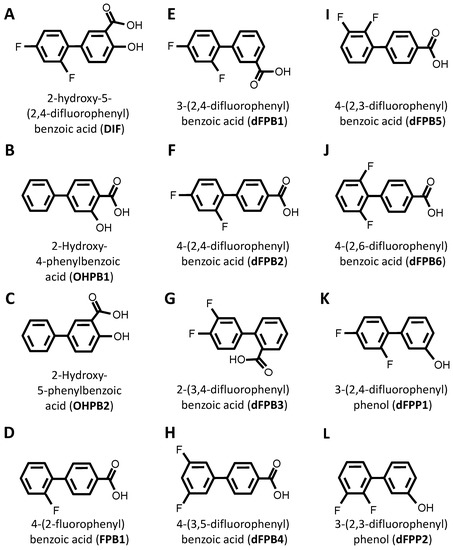
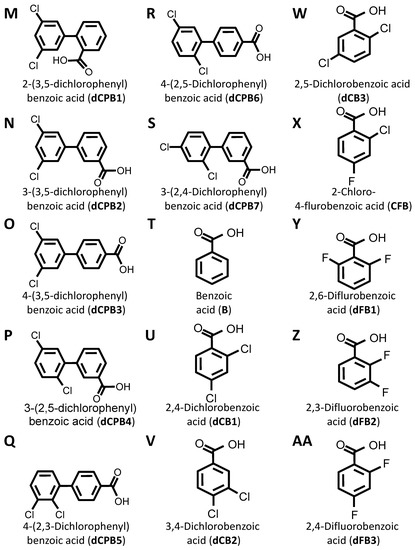
Figure 1.
Diflunisal and comparative analogue chemical structures. Diflunisal (DIF) (A) and its logical structural analogues (B–AA) were compared for efficacies in mitigating virulence phenotypes. Structural analogues were selected based on (1) halogen type (fluorine vs. chlorine); (2) benzoic acid or phenol aromatic rings; (3) presence or absence of carboxyl group; (4) presence or absence of hydroxyl group; and (5) positioning of halogen, carboxyl, or hydroxyl moieties with respect to relational chirality.
The components that may be important for structure–activity relationships are summarized in Table 2. Together, this library of compounds was used to assess the impact on the efficacy of four different anti-staphylococcal antibiotics and the mitigation of three virulence phenotypes in eight highly relevant clinical and laboratory S. aureus strains. Importantly, across the range of concentrations tested in the virulence phenotyping studies, neither DIF nor its analogues alone inhibited the growth of prototypical S. aureus strains in vitro (Supplementary Materials Figure S1).

Table 2.
Compounds used in this study.
2.3. Impact of DIF and Analogues on Antibiotic Susceptibility
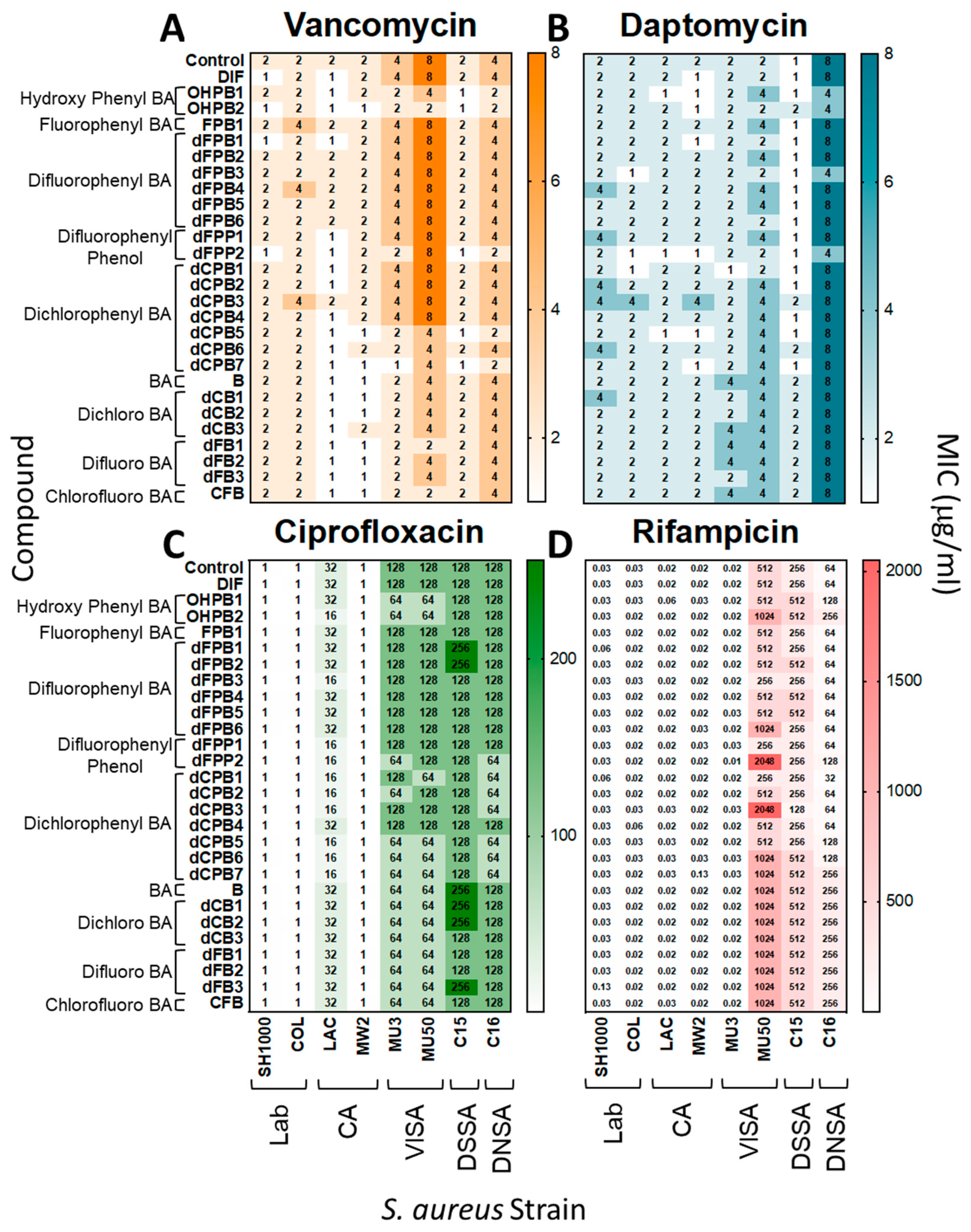
DIF and its analogues were tested with gold-standard antibiotics for interactions that may alter S. aureus susceptibility or resistance. Overall, the MICs of vancomycin (VAN), daptomycin (DAP), ciprofloxacin (CIP), and rifampicin (RIF) were equivalent against S. aureus in the presence or absence of DIF or analogues in vitro (Figure 2). Thus, DIF and its analogues are not antagonistic to traditional antistaphylococcal therapeutics and appear to act independently from their mechanisms.
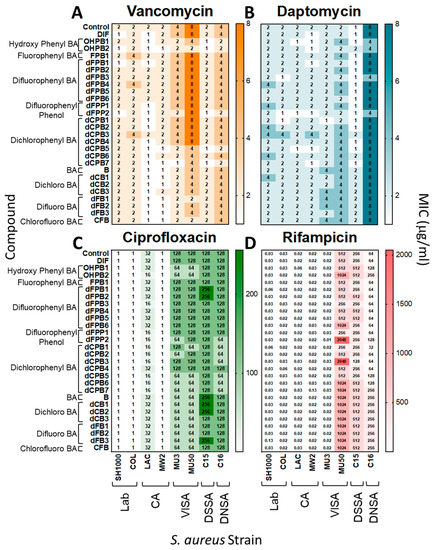
Figure 2.
Diflunisal and structural analogues do not impact antibiotic efficacy against S. aureus. Minimum inhibitory concentrations (MICs) of vancomycin, daptomycin, ciprofloxacin, and rifampicin were determined for S. aureus strains in the presence or absence of DIF or analogues. Overall, the addition of compounds with antibiotics did not significantly alter susceptibility profiles of S. aureus isolates (≤2-fold difference, which is consistent with CLSI guidelines [31,32]). The data shown are representative values of three separate experiments with equivalent outcomes.
2.4. Impact on Virulence Phenotypes
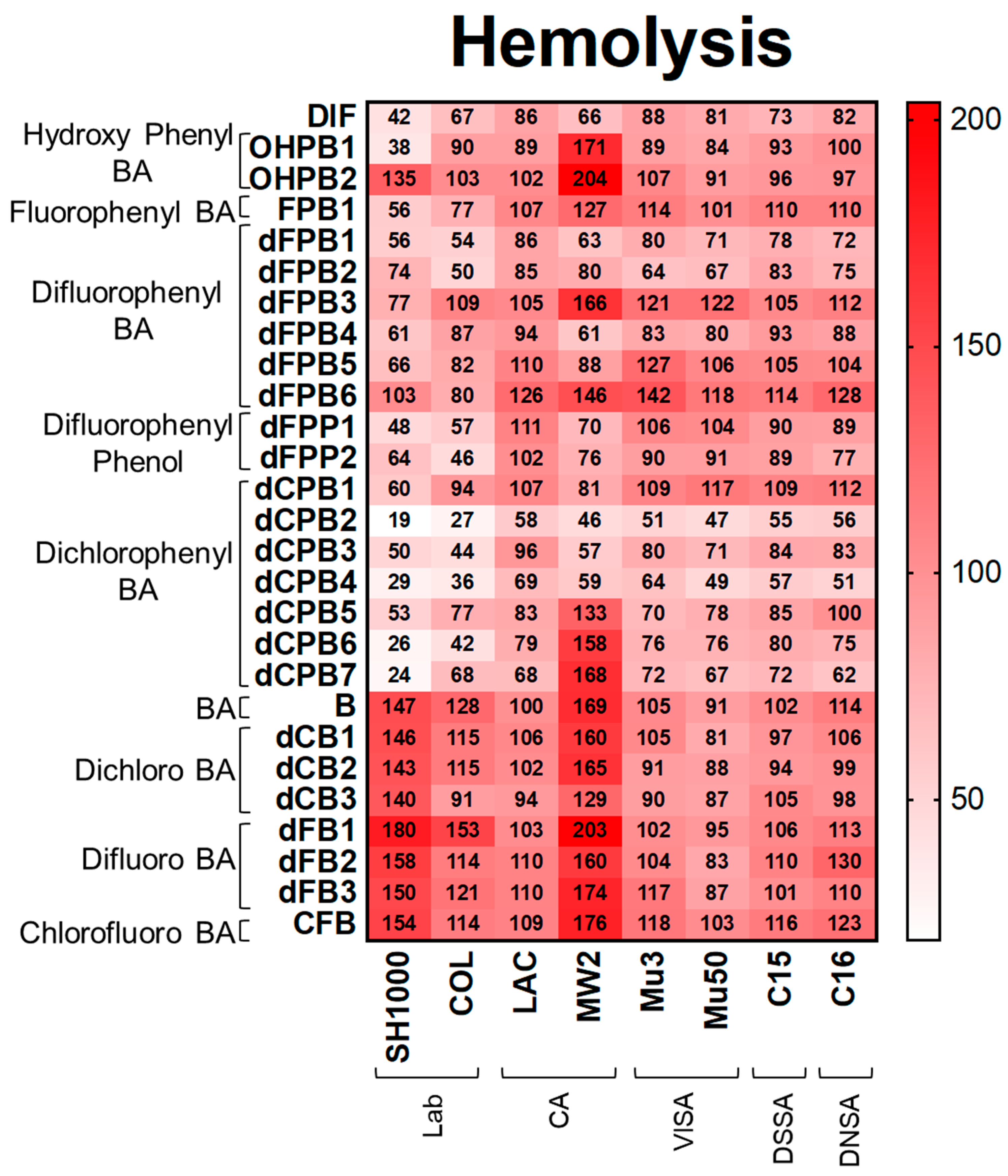
Hemolysis. Consistent with DIF, its structural analogues reduced hemolysis, proteolysis, and biofilm production in S. aureus strains (Figure 3, Figure 4 and Figure 5). DIF inhibited hemolysis in S. aureus strains by 42–88% compared with the no-compound control (Figure 3). The positioning of the phenyl and hydroxyl groups in OHPB1 resulted in similar effects to DIF, but removing the fluorides from DIF abrogated hemolysis inhibition (OHPB2) in most S. aureus strains. Furthermore, the monofluorophenyl analogue (FPB) was less efficacious than DIF. In contrast, the difluorophenyl benzoic acid (dFPB) analogues showed differential effects on hemolysis depending on the localization of fluorides on the phenyl group or carboxylic acid group on the benzene ring moiety. The analogues dFPB1, -2, and -4 showed similar effects on hemolysis compared to DIF (54–90%); however, dFPB3, -5, and -6 were less efficacious. Difluorophenyl phenols (dFPP; DIF analogues lacking the carboxylic acid group) showed similar inhibitory effects to DIF. Likewise, dichlorophenyl benzoic acid (dCPB) isotypes had differential impacts on hemolysis compared with DIF. The analogues dCPB2, -3, and -4 were better at mitigating hemolysis compared with other analogues (19–58%). The analogue dCPB2 exceeded DIF in hemolysis suppression (Figure 3; Supplementary Materials Tables S1 and S4). Results of hemolysis assays performed using sheep vs. rabbit blood were highly concordant (Supplementary Materials Figure S2).
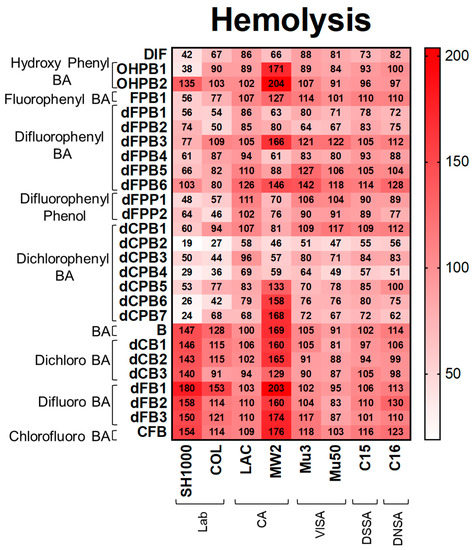
Figure 3.
DIF and structural analogues mitigate hemolysis phenotypes of S. aureus study strains. Hemolysis assays were performed by plating S. aureus on rabbit blood agar. Values are expressed as percentages of control and represented as means of triplicate experiments (n = 3). Statistical analyses (p-value and standard deviation for each value) can be found in Supplementary Materials Tables S1 and S4.
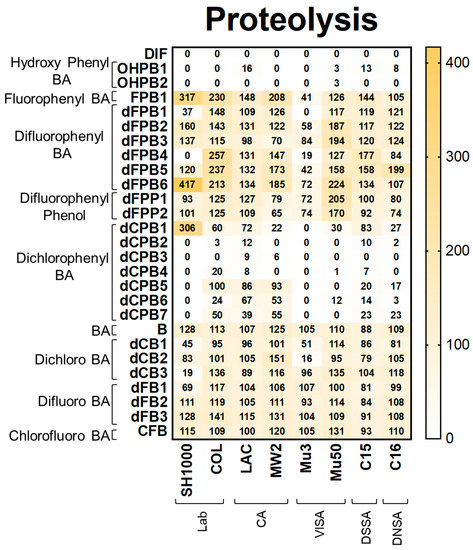
Figure 4.
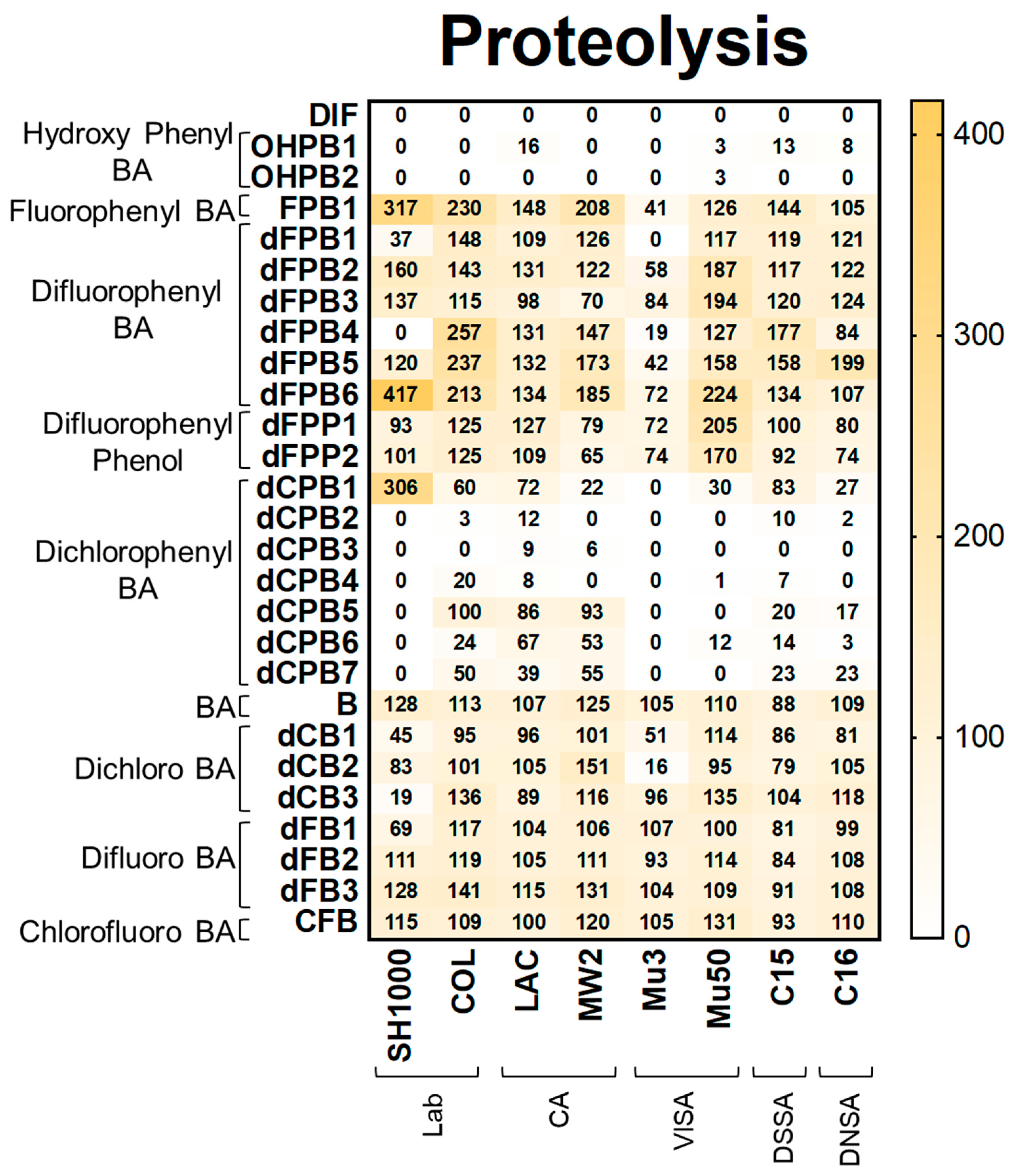
DIF and structural analogues mitigate proteolysis phenotypes in S. aureus study strains. Proteolysis assays were performed by plating log-phase cultures of S. aureus on casein agar. Values are expressed as percentages of control and represented as means of triplicate experiments (n = 3). Statistical analyses (p-value and standard deviation for each value) can be found in Supplementary Materials Tables S1 and S2.
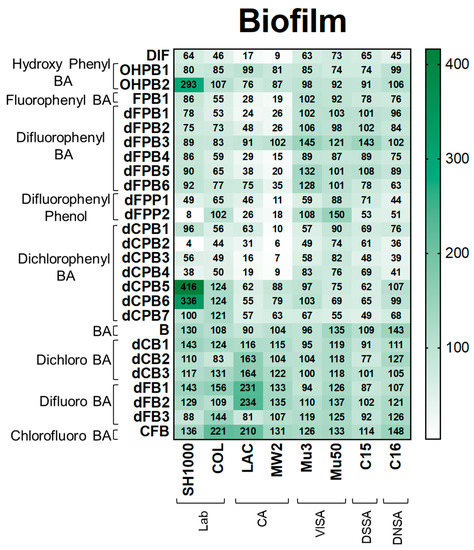
Figure 5.
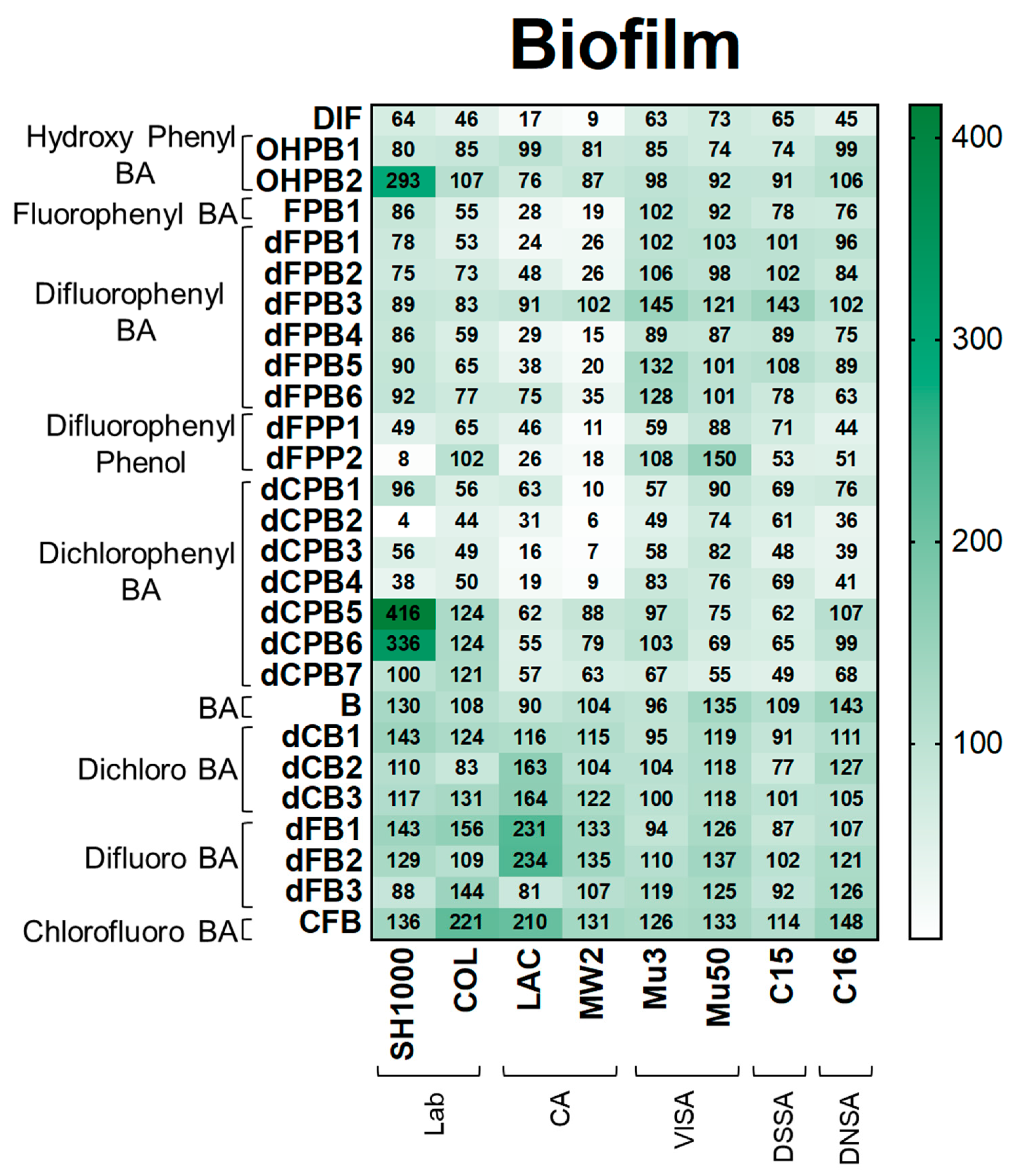
DIF and structural analogues mitigate biofilm production in S. aureus study strains. Biofilm assays were performed by growing log-phase cultures of S. aureus in brain-heart infusion broth with glucose. Cultures were removed and resultant biofilms were stained with safranin. Values are expressed as percentages of control and represented as means of triplicate experiments (n = 3). Statistical analyses (p-value and standard deviation for each value) can be found in Supplementary Materials Tables S1 and S2.
Proteolysis. Proteolysis assays demonstrated that DIF completely inhibited detectable proteolysis production in all strains (Figure 4). Likewise, hydroxyphenyl benzoic acid (OHPB) analogues completely inhibited proteolysis in most study strains. In contrast, fluorophenyl benzoic acid and phenol analogues (FPB; dFPB; and dFPP) were ineffective in suppressing proteolysis production. These results indicate the role of the presence of both hydroxyl and carboxylic acid groups on the benzene ring in anti-proteolysis efficacy. Interestingly, dichlorophenyl benzoic acid analogues (dCPB) exhibited differential effects on proteolysis in S. aureus strains depending on phenyl- and carboxylic acid group localization. For example, dCPB1, dCPB2, and dCPB3 are analogues with the same 3,5-dichlorophenyl group, but this group is in different carbon positions relative to the carboxylic acid group on the benzene ring. Isotype dCPB1 (carbon position 2) was less efficacious than dCPB2 (carbon position 3) or dCPB3 (carbon position 4), suggesting a stereochemical role of the phenyl vs. carboxylic acid groups in the inhibition of S. aureus proteolysis. In comparison, the chlorinated analogues dCPB4 and dCPB5 are similar to dCPB3, whereas dCPB6 is similar to dCPB2 but with different chlorine positions. These analogues were less efficacious than DIF against COL, LAC, MW2, C15, and C16 strains. These findings suggest a stereochemical role of chlorine atoms in mitigating proteolysis in these strains. Similar to hemolysis, halogen-benzoic acid analogues did not reduce proteolysis in any S. aureus strain (Figure 4; Supplementary Materials Tables S1B and S2).
Biofilm. Next, DIF and its analogues were assessed for their ability to mitigate biofilm production as this virulence phenotype is essential for human S. aureus infections, including infective endocarditis, prosthetic joint infection, and device-related infection [33,34,35,36,37,38]. As expected, DIF inhibited biofilm production in S. aureus strains by 9–73% compared with the no-compound control (Figure 5). In contrast, non-halogenated hydroxyphenyl benzoic acid (OHPB) analogues were not as effective in reducing biofilm production compared with DIF. These results suggest an important role of halogen(s) in the suppression of this virulence phenotype. Most fluorophenyl benzoic acid and phenol analogues (FPB; dFPB; and dFPP) exhibited strain-specific efficacy in attenuating biofilm production in laboratory and community S. aureus strains. However, the VISA, DNSA, and DSSA strains were generally not susceptible to biofilm inhibition by these compounds. Moreover, the analogue dFPB3 was ineffective in reducing biofilm production in most study strains (83–145%), and some study compounds (e.g., OHPB2, dCPB5, and dCPB6) induced biofilm formation. Similarly, dichlorophenyl benzoic acid (dCPB) analogues also exhibited strain- and stereo-specific efficacy in reducing biofilm production. The analogues dCPB2, dCPB3, and dCPB4 had similar or better efficacy in mitigating biofilm production than DIF, whereas closely related analogues dCPB1, dCPB5, dCPB6, and dCPB7 were worse than DIF. Similar to other virulence outcomes, halogen-benzoic acid analogues were ineffective in preventing biofilm production (Figure 5; Supplementary Materials Tables S1C and S2).
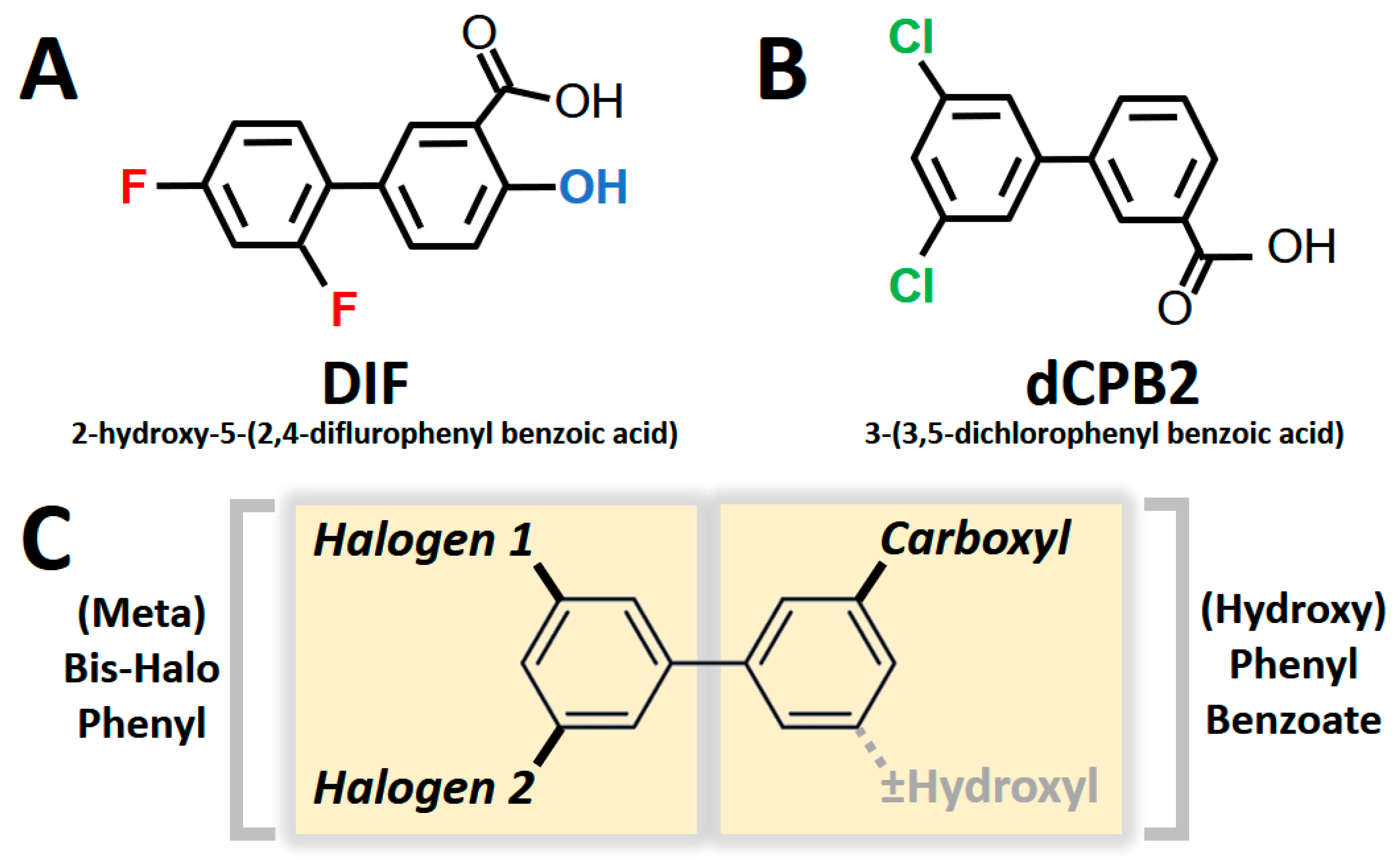
Collectively, the current results reveal structural and chirality patterns in compounds that suppress S. aureus virulence phenotypes. Thematically, the compounds containing a hydroxyl-diflurophenyl motif (e.g., DIF; Figure 6A) or containing a dichlorophenyl motif in the presence or absence of a hydroxyl moiety (e.g., dCPB2; Figure 6B) exhibited the greatest efficacy in suppressing hemolysis, proteolysis, and biofilm production in S. aureus in this study. Integrating these results, the consensus pharmacophores for the broadest anti-virulence efficacy against S. aureus appear to be the [hydroxy]-phenyl-benzoate and [meta]-bis-halo-phenyl moieties, which have conformational degrees of freedom associated with chiral specificity (Figure 6C).
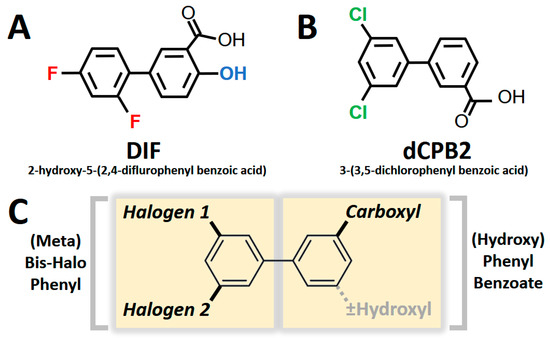
Figure 6.
Pharmacophores correlating with broad anti-virulence efficacy against S. aureus. Difluorinated DIF (A) and a dichlorinated structural analogue dCPB2 (B) exhibited the greatest anti-hemolysis, anti-proteolysis, and anti-biofilm efficacy overall. These compounds are used as representatives of study compounds containing core and variable pharmacophores. Two core structures comprise a consensus pharmacophore signature (C): a [hydroxy]-benzoic acid group (with or without a hydroxyl moiety) and a [meta]-bis-halo-phenyl group.
3. Discussion
Conventional in vitro metrics that are used to determine the efficacy of anti-staphylococcal compounds have historically been based on growth inhibition (e.g., minimum inhibitory concentration (MIC)) or killing (e.g., minimum bactericidal concentration (MBC)) of the target organism. In the present study, we evaluated compounds for their ability to interfere with virulence factor expression, independent of growth inhibition or killing. This strategy has the advantage of avoiding the selection of antibiotic resistance and thus represents an attractive approach to innovative antimicrobial therapy.
Originally, we demonstrated the efficacies of aspirin, salicylates, and DIF against S. aureus in a variety of in vitro and in vivo experimental models [1,2,3,4,5,6,7,14]. Our and other subsequent studies furthered these findings regarding DIF and related compounds [39,40,41,42,43,44,45]. The present study assessed the structural determinants of DIF and its chemical analogues regarding (1) the inhibition of virulence phenotypes and (2) the impact on conventional antibiotic efficacy against relevant MRSA isolates representing diverse genotypes correlated with resistance phenotypes. Analogues differing in structure were found to demonstrate differential efficacy in inhibiting virulence phenotypes of specific S. aureus strains. Consistent with our previous findings, DIF was effective at inhibiting hemolysis, proteolysis, and biofilm formation in a variety of S. aureus strains in vitro.
The current findings verified that DIF inhibits key virulence phenotypes in S. aureus strains with MRSA, VISA, or DNSA resistance phenotypes representing community-acquired and healthcare-associated isolates. Thus, DIF served as a logical standard for the comparative evaluation of structurally related compounds. Relative to DIF, its analogues exerted structure-specific inhibition of the prototypic virulence phenotypes, including hemolysis, proteolysis, and biofilm production. For example, the dichlorophenyl benzoic acid compound (dCPB2) was superior to DIF in mitigating hemolysis but equivalent in its inhibition of proteolysis and biofilm formation in the majority of S. aureus strains tested. Interestingly, other dichlorophenyl analogues (dCPB3 and dCPB4) displayed a pattern of outcomes similar to DIF. This pattern suggested that the stereochemistry and position of the carboxylic acid and halogen moieties contribute to virulence inhibition activity. Moreover, removing halogens (F) from DIF resulted in a hydroxyphenyl benzoate (OHPB) analogue that was equal to DIF in mitigating proteolysis but did not inhibit hemolysis or biofilm production in the S. aureus study strains. This finding points to the importance of fluorine in inhibiting hemolysis and biofilm production but not proteolysis. In comparison, a distinct group of difluorophenyl benzoic acid (dFPB) analogues lacking a hydroxyl moiety exhibited poor inhibition of all virulence phenotypes relative to DIF or dCPB2. This result indicates the importance of the hydroxyl residue in coordination with halogen and carboxylic acid moieties for virulence suppression. The positioning of the halogens on the [meta]-bis-halo-phenyl group in relation to the positioning of the carboxylic acid or hydroxyl moieties of the [hydroxy]-benzoic acid group influences the anti-virulence efficacy of the study compounds. This finding indicates that preferential or isomer-specific stereochemical optima exist for compounds that exhibit anti-hemolysis, anti-proteolysis, and anti-biofilm efficacies. This result further implies that S. aureus has cognate ligands that serve as specific target(s) of these compounds.
The current findings support our hypothesis that pharmacophores of DIF and structurally related analogues interfere with essential virulence factor regulation and effector mechanisms. Interestingly, efficacious compounds consistently contained a combination of carboxylic acid and hydroxyl moieties approximating the lactone ring in autoinducing peptides. For example, the carbonyl-oxygen structure in DIF and dCPB2 appears to be integral to the virulence-suppressing mechanisms of these compounds. This concept is further supported by recent observations that DIF and structurally related hydroxyphenyl benzoate analogues (OHPB) interfere with quorum sensing and attenuate virulence factor expression in S. aureus [25,26,27,46]. Thus, the current findings suggest that structural similarities or common structure–activity relationships exist between chemical moieties in the pharmacophores of the study compounds and those of S. aureus auto-inducing peptides.
We recognize the limitations of this study. First, in vitro experiments do not recapitulate the full spectrum of host–pathogen interactions that occur during infection in vivo. Beyond the scope of the current investigation, we are actively exploring the in vivo efficacies of DIF and selected structural analogues alone as adjuvants to conventional antibiotics in highly innovative experimental models of S. aureus infection. Second, the current study focused only on the inhibitory effects of DIF and analogues on hemolysis, proteolysis, and biofilm formation. We appreciate that other mechanisms of virulence are also important for infection. Finally, this study included eight different S. aureus genetic backgrounds to represent the majority of isolates encountered in clinical infection. However, there may be other backgrounds that respond differently to the study compounds. Studies within our laboratories are ongoing to address these limitations, further define the molecular and cellular mechanisms of virulence inhibition, and evaluate strategic anti-virulence strategies for their ability to benefit conventional antibiotic efficacy in S. aureus infections. Collectively, the present findings and their application may accelerate innovative anti-infective strategies to meet the challenge of antibiotic-resistant MRSA infection. Such strategies may also be generalizable to address the growing threat of other high-priority human pathogens that are increasingly resistant to traditional therapies.
4. Materials and Methods
4.1. Staphylococcus aureus Strains
This study utilized 8 different well-characterized and prototypic clinical and laboratory S. aureus strains representing antibiotic-susceptible and -resistant genotypes and phenotypes (Table 1). Organisms from virulence-validated master cell banks were cultured to log-phase in brain–heart infusion (BHI) medium at 37 °C. The resulting cells were harvested, washed in phosphate-buffered saline (PBS; pH 7.2), sonicated, quantified with spectrophotometry, and diluted to the desired inoculum in PBS buffer.
4.2. Antibiotics and Study Compounds
Vancomycin (VAN), ciprofloxacin (CIP), rifampicin (RIF) (Sigma Aldrich, St. Louis, MO, USA), and daptomycin (DAP) (Merck, Branchburg, NJ, USA) were dissolved in double-distilled water (ddH20). Diflunisal (DIF) (Sigma) and its structural analogues (Combi-Blocks, San Diego, CA, USA; Sigma Aldrich, Rockville, MD, USA) (25 μg/mL; Table 2; Figure 1) were dissolved in DMSO and diluted to appropriate concentrations in aqueous buffer.
4.3. Growth Rate
To assess the impact of the compounds on S. aureus growth, strains were cultured in the presence of a study compound across a logical dose range encompassing that approved for DIF human therapy. As a reference standard for these studies, 106 colony-forming units (CFU) of prototypic strains, SH1000 and LAC, were inoculated into 10 mL of tryptic soy broth, and the optical density (OD600) was measured hourly over 8 h (Supplemental Figure S1). At the concentrations used in virulence phenotyping studies (25 μg/mL), no compound inhibited the growth of any study strain.
4.4. Minimum Inhibitory Concentration
The minimum inhibitory concentrations (MICs) of the antibiotics against the S. aureus strains were determined using the recommended standard Clinical Standards Laboratory Institute (CLSI) broth microdilution protocol [31,32]. The S. aureus strains were cultured in cation-adjusted Mueller–Hinton broth in the presence or absence of an antibiotic and/or study compound. The cultures were incubated overnight at 37 °C, and the lowest concentration inhibiting growth was recorded as the MIC. Assays were repeated a minimum of 3 times (n = 3+) on different days for experimental validation.
4.5. Hemolysis Assay
The strains were grown in brain–heart infusion broth (BHI; Becton Dickenson, Holdrege, NJ, USA) and inoculated (106 CFU/10 μL) via microdrop plating onto tryptic soy agar containing 5% rabbit blood with or without compounds. The plates were incubated at 37 °C for 24 h followed by cold shock at 4 °C for 48 h. The diameters (mm2) of the zones of clearance were measured, and the data are represented as percentages of the control (no compound) (Figure 3; Supplementary Materials Table S4). Significant differences between DIF and the study compounds were analyzed using Student’s t-test (the p-values are presented in Supplementary Materials Table S1). The values highlighted in green indicate significantly decreased (p < 0.05) virulence phenotypes compared with DIF, whereas values highlighted in red indicate significantly increased (p < 0.05) virulence phenotypes. Rabbit blood agar assays were verified in parallel with sheep blood agar plates as described above and showed comparable results (Supplementary Materials Figure S2).
4.6. Proteolysis Assay
The strains were grown in BHI and plated (106 CFU/10 μL) as described above onto casein agar with or without compounds. The plates were grown at 37 °C for 24 h. Zones of clearance (mm2) were measured and normalized to the control as detailed for the hemolysis assays above. The data are represented as percentages of the control (no compound) (Figure 4; Supplementary Materials Table S4). Significant differences between DIF and the study compounds were analyzed using Student’s t-test as detailed above (the p-values are presented in Supplementary Materials Table S2).
4.7. Biofilm Assay
The strains were grown in BHI with 0.5% glucose with or without DIF or an analogue for 18 h at 37 °C in 96-well flat-bottom polystyrene plates (Fisher Scientific, Waltham, MA USA). After removing the culture suspensions, the plates were washed with 1X PBS and dried at 37 °C for 1 h. Biofilms were stained with 0.1% safranin, washed with distilled water, and decolorized with 30% glacial acetic acid. The resulting biofilm densities were then measured using spectrophotometry at OD490 and normalized to the control. The data are represented as percentages of the control (no compound) (Figure 4; Supplementary Materials Table S4). Significant differences between DIF and the study compounds were analyzed using Student’s t-test as detailed in the hemolysis section (the p-values are presented in Supplementary Materials Table S3).
4.8. Statistical Analyses
Bivariate differences in the experimental results were compared using Student’s t-test. The data are represented as means ± standard deviation. p-values of <0.05 were considered statistically significant. Statistical analyses were implemented and graphs were generated using the GraphPad Prism software.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics12071180/s1. Figure S1: DIF and structural analogues do not impact growth in S. aureus strains. Strains were grown in tryptic soy broth with or without DIF or analogues (25 µg/mL). Cultures were analyzed every hour to measure growth. As expected, anti-staphylococcal antibiotics significantly inhibit growth in comparison with DIF or analogues (data not shown). Figure S2: Hemolysis assays performed in rabbit vs. sheep blood agar showed similar reductions when treated with DIF. Tables S1–S3: Student’s t-test p-values reveal compounds that perform significantly better than DIF. p-values of DIF vs. structural analogues in hemolysis (A), proteolysis (B), and biofilm (C) assays were calculated for significantly different impacts. Values highlighted in green indicate significantly decreased (p < 0.05) virulence phenotypes compared with DIF, whereas values highlighted in red indicate significantly increased (p < 0.05) virulence phenotypes. Table S4: Hemolysis (H), proteolysis (P), and biofilm (B) for DIF and structural analogues are represented as percentages of control. Mean values and standard deviation (SD) are shown for DIF and each structural analog. Values of structural analogues that significantly (p < 0.05; Student’s t-test) differed from DIF are indicated in bold font.
Author Contributions
Conceptualization, L.C.C., A.S.B. and M.R.Y.; data curation, L.C.C., H.K.L., L.W., Y.Q.X., S.C. and M.R.Y.; formal analysis, L.C.C., H.K.L., L.W., Y.Q.X., S.C., R.A.P. and M.R.Y.; funding acquisition, L.C.C., A.S.B. and M.R.Y.; methodology, L.C.C., H.K.L., L.W., S.C., Y.Q.X., A.S.B. and M.R.Y.; project administration, M.R.Y.; writing—original draft, L.C.C. and M.R.Y.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by grants from the U.S. National Institutes of Health, including an R21/R33 Innovation Award and a U01 Systems Immunology Award (NIAID AI-111661-01 and U01-AI124319 to M.R.Y.) as well as a UCLA CTSI KL2-TR001882-05 (to L.C.C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within this article (Figure 2, Figure 3, Figure 4 and Figure 5) and the Supplementary Materials.
Conflicts of Interest
M.R.Y. holds patents on novel anti-infective therapy, immunotherapy, and vaccines and is a consultant for Genentech-Roche, Alexion/AstraZeneca, and Horizon Pharmaceuticals, which are involved in the development of immunotherapeutic agents and strategies. A.S.B. holds patents on novel anti-infective therapy. R.A.P. is a consultant for IBT, which is producing a multivalent antitoxin vaccine, and is a member of the review board at the University of Rochester, which is involved in an anti-Gmd vaccine effort. The other authors report no conflicts of interest.
References
- Chan, L.C.; Park, M.; Lee, H.K.; Chaili, S.; Xiong, Y.Q.; Bayer, A.S.; Proctor, R.A.; Yeaman, M.R. Diflunisal Attenuates Virulence Factor Gene Regulation and Phenotypes in Staphylococcus aureus. Antibiotics 2023, 12, 902. [Google Scholar] [CrossRef]
- Park, M.; Bayer, A.S.; Yount, N.; Yang, S.J.; Xiong, Y.Q.; Yeaman, M.R. Modulation of Virulence Gene Expression in Staphylococcus aureus Using Salicylate Analogues. In Proceedings of the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 12–15 September 2009. [Google Scholar]
- Yeaman, M.R.; Bayer, A.S. Anti-Infective Hydroxy-Phenyl-Benzoates and Methods of Use. U.S. Patent 9,205,097 B2, 8 December 2015. LA BioMedical Research Institute at Harbor-UCLA Medical Center: Torrance, CA, USA, 2014. [Google Scholar]
- Yeaman, M.R.; Bayer, A.S. Anti-Infective Hydroxy-Phenyl-Benzoates and Methods of Use. U.S. Patent 8,809,263 B2, 19 August 2014. Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center: Torrance, CA, USA, 2014. [Google Scholar]
- Yeaman, M.R.; Bayer, A.S. Anti-Infective Hydroxy-Phenyl-Benzoates and Methods of Use. U.S. Patent 9,585,897 B2, 7 March 2017. LA BioMedical Research Institute at Harbor-UCLA Medical Center: Torrance, CA, USA, 2017. [Google Scholar]
- Kupferwasser, L.I.; Yeaman, M.R.; Nast, C.C.; Kupferwasser, D.; Xiong, Y.Q.; Palma, M.; Cheung, A.L.; Bayer, A.S. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J. Clin. Investig. 2003, 112, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Kupferwasser, L.I.; Yeaman, M.R.; Shapiro, S.M.; Nast, C.C.; Sullam, P.M.; Filler, S.G.; Bayer, A.S. Acetylsalicylic acid reduces vegetation bacterial density, hematogenous bacterial dissemination, and frequency of embolic events in experimental Staphylococcus aureus endocarditis through antiplatelet and antibacterial effects. Circulation 1999, 99, 2791–2797. [Google Scholar] [CrossRef]
- Muller, E.; Al-Attar, J.; Wolff, A.G.; Farber, B.F. Mechanism of salicylate-mediated inhibition of biofilm in Staphylococcus epidermidis. J. Infect. Dis. 1998, 177, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.P.; Marangos, M.N.; Nightingale, C.H.; Quintiliani, R. Influence of aspirin on development and treatment of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 1995, 39, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.P.; Tessier, P.R.; Nightingale, C.H. Beneficial effect of combination antiplatelet therapy on the development of experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents 1999, 11, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Bayer, A.; Kupferwasser, L.I.; Joska, T.; Yeaman, M.R.; Cheung, A. Salicylic acid activates sigma factor B by rsbU-dependent and -independent mechanisms. J. Bacteriol. 2006, 188, 5896–5903. [Google Scholar] [CrossRef]
- Riordan, J.T.; O’Leary, J.O.; Gustafson, J.E. Contributions of sigB and sarA to distinct multiple antimicrobial resistance mechanisms of Staphylococcus aureus. Int. J. Antimicrob. Agents 2006, 28, 54–61. [Google Scholar] [CrossRef]
- Polzin, A.; Dannenberg, L.; M’Pembele, R.; Mourikis, P.; Naguib, D.; Zako, S.; Helten, C.; Petzold, T.; Levkau, B.; Hohlfeld, T.; et al. Staphylococcus aureus increases platelet reactivity in patients with infective endocarditis. Sci. Rep. 2022, 12, 12933. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.P.; Freeman, C.D.; Nightingale, C.H.; Quintiliani, R.; Coe, C.J.; Maderazo, E.G.; Cooper, B.W. Reduction of bacterial titers by low-dose aspirin in experimental aortic valve endocarditis. Infect. Immun. 1993, 61, 1593–1595. [Google Scholar] [CrossRef]
- Hannachi, N.; Habib, G.; Camoin-Jau, L. Aspirin Effect on Staphylococcus aureus-Platelet Interactions During Infectious Endocarditis. Front. Med. 2019, 6, 217. [Google Scholar] [CrossRef]
- Veloso, T.R.; Que, Y.A.; Chaouch, A.; Giddey, M.; Vouillamoz, J.; Rousson, V.; Moreillon, P.; Entenza, J.M. Prophylaxis of experimental endocarditis with antiplatelet and antithrombin agents: A role for long-term prevention of infective endocarditis in humans? J. Infect. Dis. 2015, 211, 72–79. [Google Scholar] [CrossRef]
- Eisen, D.P.; Corey, G.R.; McBryde, E.S.; Fowler, V.G., Jr.; Miro, J.M.; Cabell, C.H.; Street, A.C.; Paiva, M.G.; Ionac, A.; Tan, R.S.; et al. Reduced valve replacement surgery and complication rate in Staphylococcus aureus endocarditis patients receiving acetyl-salicylic acid. J. Infect. 2009, 58, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, M.; Gemery, J.M.; Cheung, A.L.; Bayer, A.S.; Remillard, B.D. Aspirin treatment is associated with a significantly decreased risk of Staphylococcus aureus bacteremia in hemodialysis patients with tunneled catheters. Am. J. Kidney Dis. 2007, 49, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Osthoff, M.; Sidler, J.A.; Lakatos, B.; Frei, R.; Dangel, M.; Weisser, M.; Battegay, M.; Widmer, A.F. Low-Dose Acetylsalicylic Acid Treatment and Impact on Short-Term Mortality in Staphylococcus aureus Bloodstream Infection: A Propensity Score-Matched Cohort Study. Crit. Care Med. 2016, 44, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.P. Manifold beneficial effects of acetyl salicylic acid and nonsteroidal anti-inflammatory drugs on sepsis. Intensive Care Med. 2012, 38, 1249–1257. [Google Scholar] [CrossRef]
- Dotto, C.; Lombarte Serrat, A.; Ledesma, M.; Vay, C.; Ehling-Schulz, M.; Sordelli, D.O.; Grunert, T.; Buzzola, F. Salicylic acid stabilizes Staphylococcus aureus biofilm by impairing the agr quorum-sensing system. Sci. Rep. 2021, 11, 2953. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, L.; Zhao, B.C.; Deng, X.; Cho, H.; Yi, C.; Jian, X.; Song, C.X.; Luan, C.H.; Bae, T.; et al. Targeting MgrA-mediated virulence regulation in Staphylococcus aureus. Chem. Biol. 2011, 18, 1032–1041. [Google Scholar] [CrossRef]
- Alvarez, L.P.; Barbagelata, M.S.; Gordiola, M.; Cheung, A.L.; Sordelli, D.O.; Buzzola, F.R. Salicylic acid diminishes Staphylococcus aureus capsular polysaccharide type 5 expression. Infect. Immun. 2010, 78, 1339–1344. [Google Scholar] [CrossRef]
- Herrmann, M. Salicylic acid: An old dog, new tricks, and staphylococcal disease. J. Clin. Investig. 2003, 112, 149–151. [Google Scholar] [CrossRef]
- Khodaverdian, V.; Pesho, M.; Truitt, B.; Bollinger, L.; Patel, P.; Nithianantham, S.; Yu, G.; Delaney, E.; Jankowsky, E.; Shoham, M. Discovery of antivirulence agents against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 3645–3652. [Google Scholar] [CrossRef]
- Leonard, P.G.; Bezar, I.F.; Sidote, D.J.; Stock, A.M. Identification of a hydrophobic cleft in the LytTR domain of AgrA as a locus for small molecule interactions that inhibit DNA binding. Biochemistry 2012, 51, 10035–10043. [Google Scholar] [CrossRef]
- Sully, E.K.; Malachowa, N.; Elmore, B.O.; Alexander, S.M.; Femling, J.K.; Gray, B.M.; DeLeo, F.R.; Otto, M.; Cheung, A.L.; Edwards, B.S.; et al. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog. 2014, 10, e1004174. [Google Scholar] [CrossRef]
- Miller, L.G.; Perdreau-Remington, F.; Rieg, G.; Mehdi, S.; Perlroth, J.; Bayer, A.S.; Tang, A.W.; Phung, T.O.; Spellberg, B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 2005, 352, 1445–1453. [Google Scholar] [CrossRef]
- Baba, T.; Takeuchi, F.; Kuroda, M.; Yuzawa, H.; Aoki, K.; Oguchi, A.; Nagai, Y.; Iwama, N.; Asano, K.; Naimi, T.; et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 2002, 359, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Aritaka, N.; Hanaki, H.; Kawasaki, S.; Hosoda, Y.; Hori, S.; Fukuchi, Y.; Kobayashi, I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 1997, 350, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; 27th Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.; Rudkin, J.K.; Black, N.S.; Gallagher, L.; O’Neill, E.; O’Gara, J.P. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Doster, R.S.; Kirk, L.A.; Tetz, L.M.; Rogers, L.M.; Aronoff, D.M.; Gaddy, J.A. Staphylococcus aureus Infection of Human Gestational Membranes Induces Bacterial Biofilm Formation and Host Production of Cytokines. J. Infect. Dis. 2017, 215, 653–657. [Google Scholar] [CrossRef]
- Baudoin, J.P.; Camoin-Jau, L.; Prasanth, A.; Habib, G.; Lepidi, H.; Hannachi, N. Ultrastructure of a late-stage bacterial endocarditis valve vegetation. J. Thromb. Thrombolysis 2021, 51, 821–826. [Google Scholar] [CrossRef]
- Kwiecinski, J.M.; Jacobsson, G.; Horswill, A.R.; Josefsson, E.; Jin, T. Biofilm formation by Staphylococcus aureus clinical isolates correlates with the infection type. Infect Dis. 2019, 51, 446–451. [Google Scholar] [CrossRef]
- Hsu, C.C.; Hsu, R.B.; Ohniwa, R.L.; Chen, J.W.; Yuan, C.T.; Chia, J.S.; Jung, C.J. Neutrophil Extracellular Traps Enhance Staphylococcus aureus Vegetation Formation through Interaction with Platelets in Infective Endocarditis. Thromb. Haemost. 2019, 119, 786–796. [Google Scholar] [CrossRef]
- Spoonmore, T.J.; Ford, C.A.; Curry, J.M.; Guelcher, S.A.; Cassat, J.E. Concurrent Local Delivery of Diflunisal Limits Bone Destruction but Fails to Improve Systemic Vancomycin Efficacy during Staphylococcus aureus Osteomyelitis. Antimicrob. Agents Chemother. 2020, 64, e00182-20. [Google Scholar] [CrossRef]
- Greenberg, G.N. Recurrent sulindac-induced aseptic meningitis in a patient tolerant to other nonsteroidal anti-inflammatory drugs. South. Med. J. 1988, 81, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Carestia, A.; Davis, R.P.; Grosjean, H.; Lau, M.W.; Jenne, C.N. Acetylsalicylic acid inhibits intravascular coagulation during Staphylococcus aureus-induced sepsis in mice. Blood 2020, 135, 1281–1286. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Borges, A.; Borges, F.; Simões, M. Repurposing ibuprofen to control Staphylococcus aureus biofilms. Eur. J. Med. Chem. 2019, 166, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Varma, G.Y.N.; Kummari, G.; Paik, P.; Kalle, A.M. Celecoxib potentiates antibiotic uptake by altering membrane potential and permeability in Staphylococcus aureus. J. Antimicrob. Chemother. 2019, 74, 3462–3472. [Google Scholar] [CrossRef]
- Carta, D.; Brun, P.; Dal Pra, M.; Bernabè, G.; Castagliuolo, I.; Ferlin, M.G. Synthesis and preliminary anti-inflammatory and anti-bacterial evaluation of some diflunisal aza-analogs. Medchemcomm 2018, 9, 1017–1032. [Google Scholar] [CrossRef]
- Ford, C.A.; Spoonmore, T.J.; Gupta, M.K.; Duvall, C.L.; Guelcher, S.A.; Cassat, J.E. Diflunisal-loaded poly(propylene sulfide) nanoparticles decrease S. aureus-mediated bone destruction during osteomyelitis. J. Orthop. Res. 2021, 39, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, B.; Solomon, A.P. Targeting AgrA quorum sensing regulator by bumetanide attenuates virulence in Staphylococcus aureus—A drug repurposing approach. Life Sci. 2021, 273, 119306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).