Electrochemical Assessment of Mitigation of Desulfovibrio ferrophilus IS5 Corrosion against N80 Carbon Steel and 26Cr3Mo Steel Using a Green Biocide Enhanced by a Nature-Mimicking Biofilm-Dispersing Peptide
Abstract
:1. Introduction
2. Results and Discussion
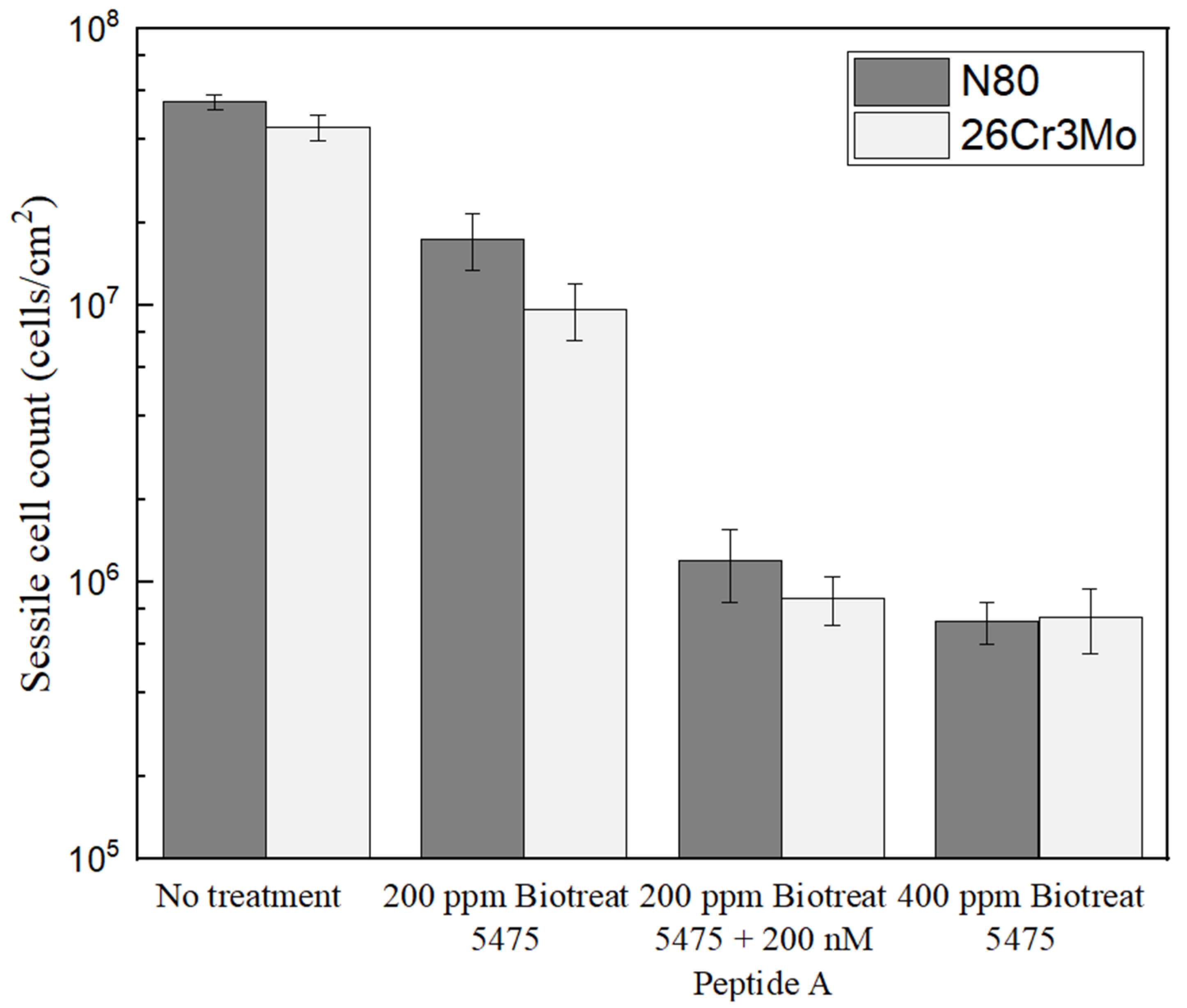
2.1. Enumeration of Sessile Cells on Coupons Soaked in Petri Dishes with and without Biocide
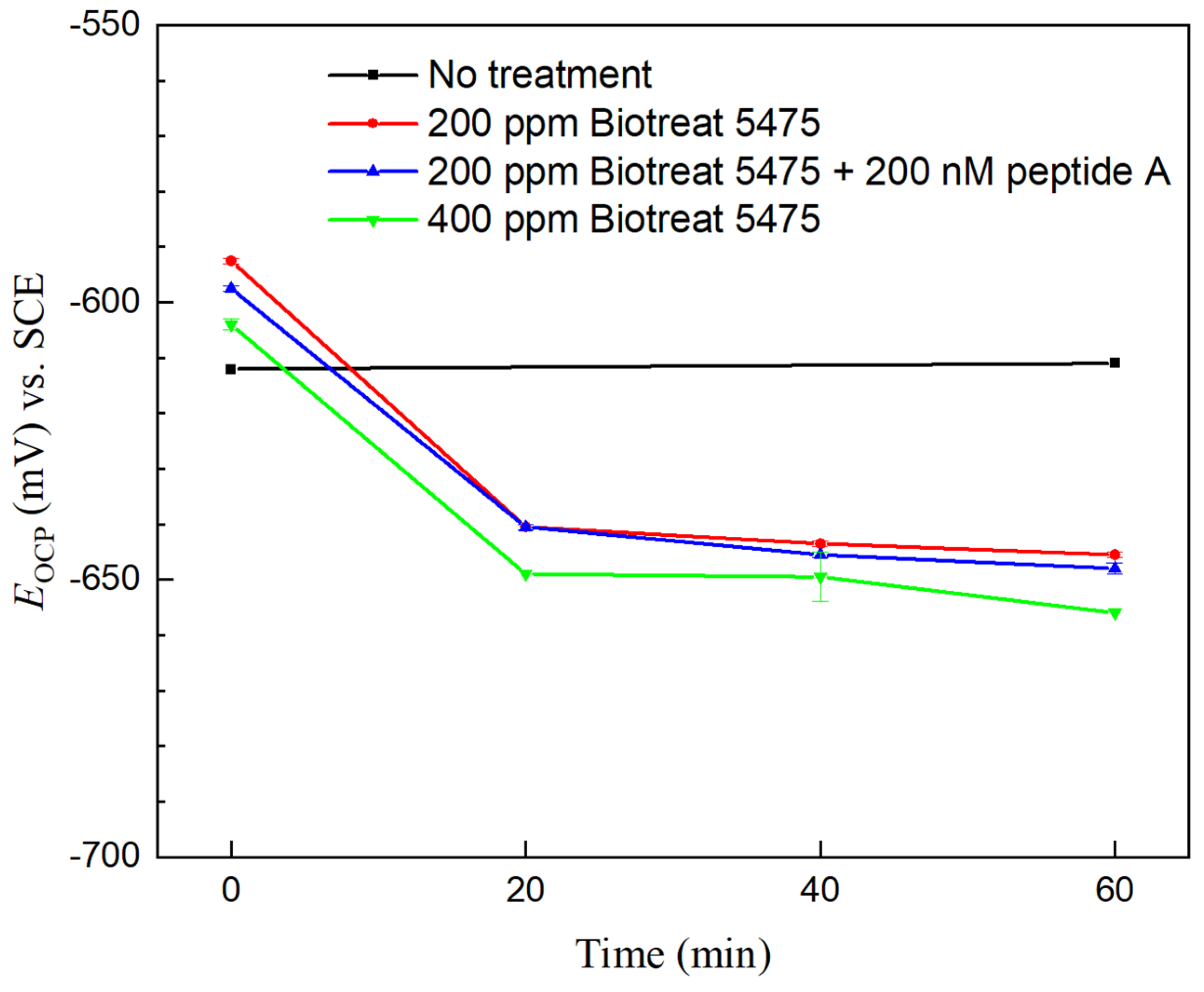
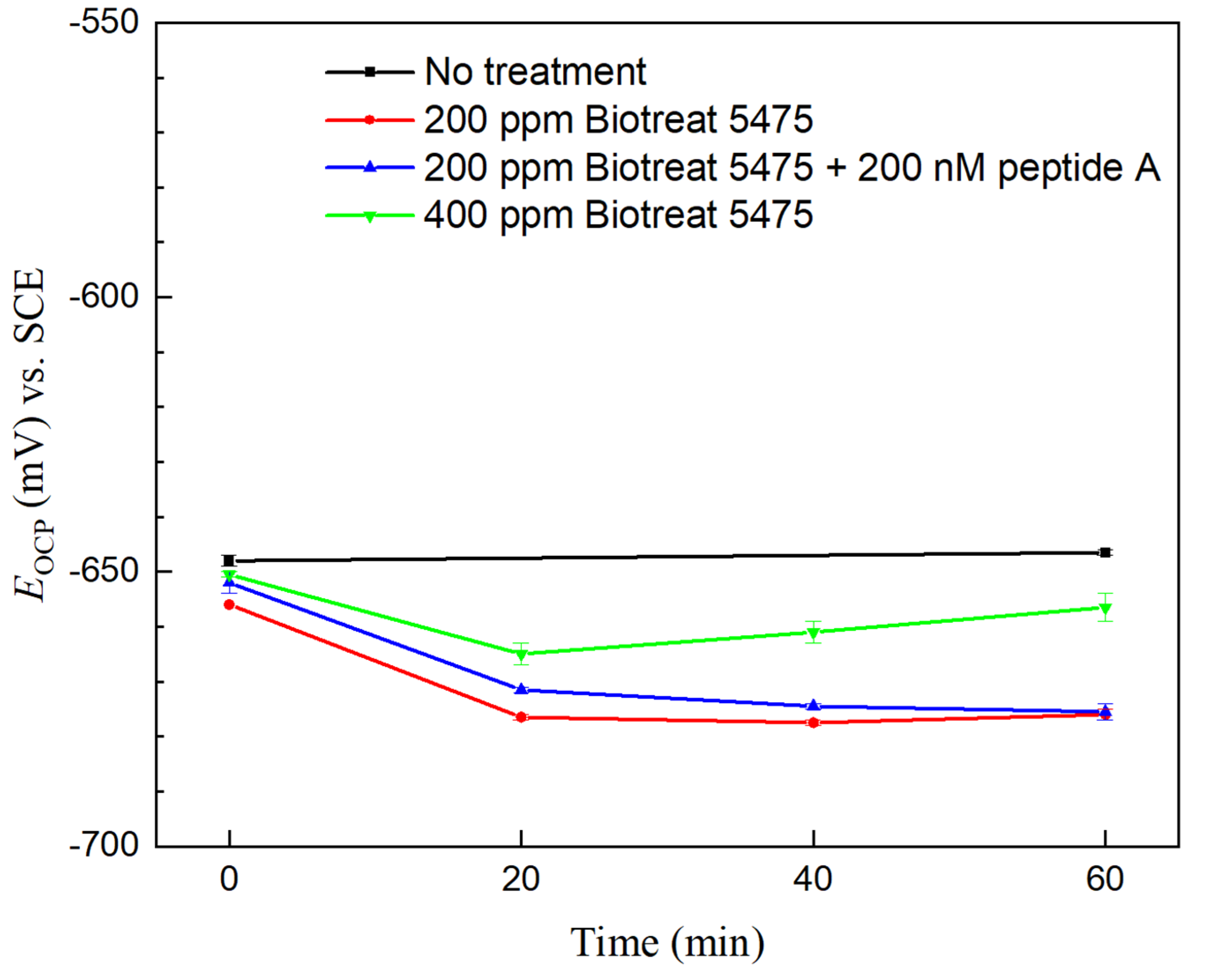
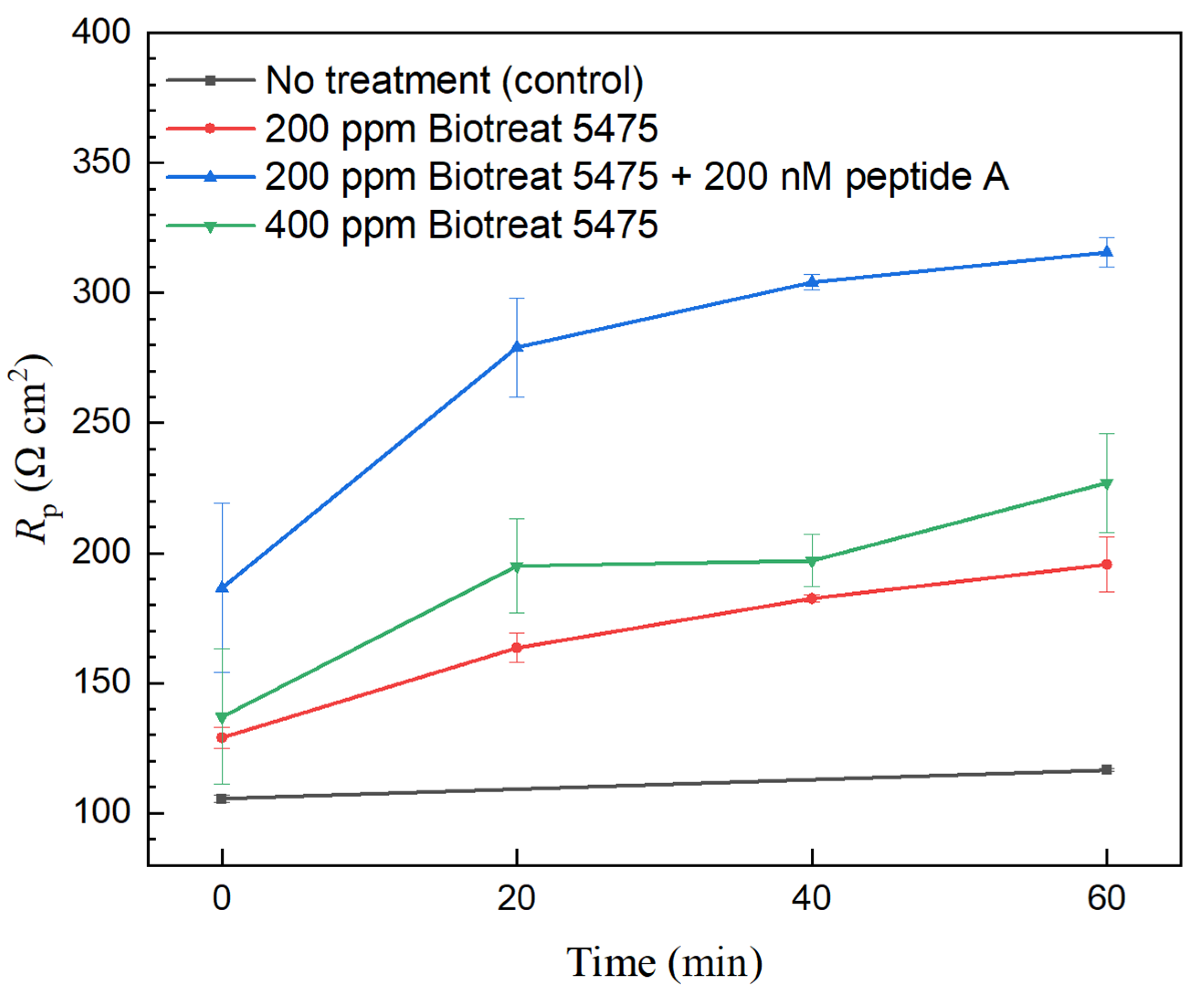
2.2. Electrochemical Test Results
3. Materials and Methods
3.1. Bacterium, Metal, and Chemicals
3.2. Sessile Cell Counts
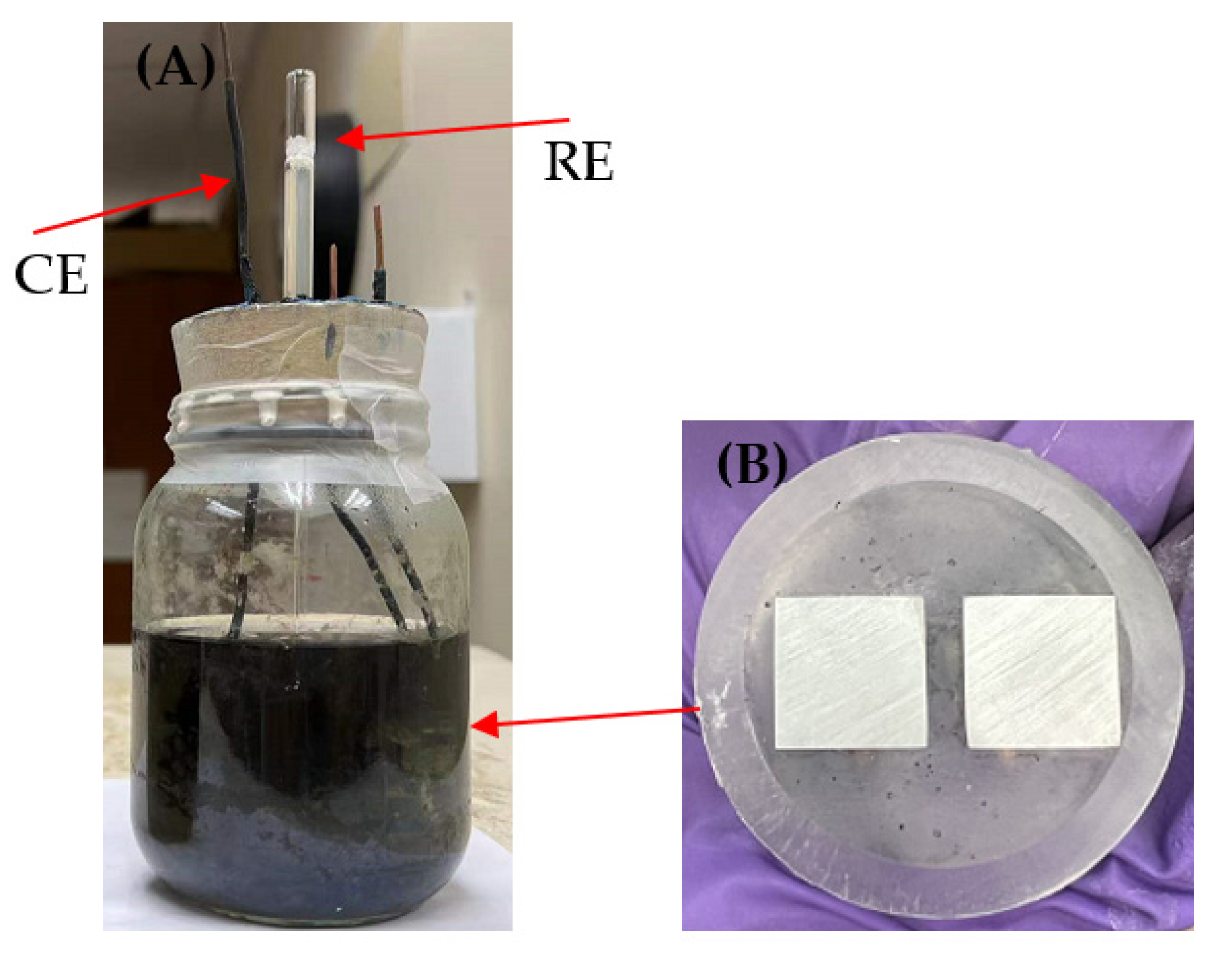
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, D.; Gu, T.; Lovley, D.R. Microbially Mediated Metal Corrosion. Nat. Rev. Microbiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.D.; Fu, A.Q.; Miao, J.; Yin, Z.F.; Zhou, G.S.; Wei, J.F. Corrosion of N80 Carbon Steel in Oil Field Formation Water Containing CO2 in the Absence and Presence of Acetic Acid. Corros. Sci. 2011, 53, 3156–3165. [Google Scholar] [CrossRef]
- Ura-Bińczyk, E.; Banaś, J.; Mazurkiewicz, B.; Solarski, W.; Lewandowska, M.; Roguska, A.; Andrzejczuk, M.; Balcer, M.; Kulik, S.; Żarnowiec, P.; et al. On-Site Monitoring and Laboratory Characterization of Corrosion Processes in the Geothermal Water of Polish Lowland. Geothermics 2019, 77, 267–277. [Google Scholar] [CrossRef]
- Senthilmurugan, B.; Radhakrishnan, J.S.; Poulsen, M.; Tang, L.; AlSaber, S. Assessment of Microbiologically Influenced Corrosion in Oilfield Water Handling Systems Using Molecular Microbiology Methods. Upstream Oil Gas Technol. 2021, 7, 100041. [Google Scholar] [CrossRef]
- Sun, J.; Sun, C.; Wang, Y. Effect of Cr Content on the Electrochemical Behavior of Low- Chromium X65 Steel in CO2 Environment. Int. J. Electrochem. Sci. 2016, 11, 8599–8611. [Google Scholar] [CrossRef]
- Xu, L.; Wang, B.; Zhu, J.; Li, W.; Zheng, Z. Effect of Cr Content on the Corrosion Performance of Low-Cr Alloy Steel in a CO2 Environment. Appl. Surf. Sci. 2016, 379, 39–46. [Google Scholar] [CrossRef]
- Hua, Y.; Mohammed, S.; Barker, R.; Neville, A. Comparisons of Corrosion Behaviour for X65 and Low Cr Steels in High Pressure CO2-Saturated Brine. J. Mater. Sci. Technol. 2020, 41, 21–32. [Google Scholar] [CrossRef]
- Lin, X.; Liu, W.; Wu, F.; Xu, C.; Dou, J.; Lu, M. Effect of O2 on Corrosion of 3Cr Steel in High Temperature and High Pressure CO2–O2 Environment. Appl. Surf. Sci. 2015, 329, 104–115. [Google Scholar] [CrossRef]
- Guo, S.; Xu, L.; Zhang, L.; Chang, W.; Lu, M. Corrosion of Alloy Steels Containing 2% Chromium in CO2 Environments. Corros. Sci. 2012, 63, 246–258. [Google Scholar] [CrossRef]
- Kamimura, T.; Stratmann, M. The Influence of Chromium on the Atmospheric Corrosion of Steel. Corros. Sci. 2001, 43, 429–447. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Zhang, R.; Guan, F.; Hou, B.; Duan, J. Microbiologically Influenced Corrosion of Marine Steels within the Interaction between Steel and Biofilms: A Brief View. Appl. Microbiol. Biotechnol. 2020, 104, 515–525. [Google Scholar] [CrossRef] [PubMed]
- L80-3Cr Tubing Pup Joint EUE PIN X BOX. Available online: http://www.forwellequip.com/products/L80-3Cr-Tubing-Pup-Joint-EUE-PIN-X-BOX.html (accessed on 12 May 2023).
- Gaines, R.H. Bacterial Activity as a Corrosive Influence in the Soil. J. Ind. Eng. Chem. 1910, 2, 128–130. [Google Scholar] [CrossRef]
- Williamson, C.H.D.; Jain, L.A.; Mishra, B.; Olson, D.L.; Spear, J.R. Microbially Influenced Corrosion Communities Associated with Fuel-Grade Ethanol Environments. Appl. Microbiol. Biotechnol. 2015, 99, 6945–6957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton, N.; Critchley, M.; Fabien, R.; Cromar, N.; Fallowfield, H. Microbially Influenced Corrosion of Galvanized Steel Pipes in Aerobic Water Systems. J. Appl. Microbiol. 2010, 109, 239–247. [Google Scholar] [CrossRef]
- Dong, Y.; Lekbach, Y.; Li, Z.; Xu, D.; El Abed, S.; Ibnsouda Koraichi, S.; Wang, F. Microbiologically Influenced Corrosion of 304L Stainless Steel Caused by an Alga Associated Bacterium Halomonas titanicae. J. Mater. Sci. Technol. 2020, 37, 200–206. [Google Scholar] [CrossRef]
- Koch, G.H.; Brongers, M.P.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Cost and Preventive Strategies in the United States; United States Federal Highway Administration: Washington, DC, USA, 2002. [Google Scholar]
- Fu, Q.; Wei, B.; Xu, J.; Qin, Q.; Bai, Y.; Yu, C.; Sun, C. Corrosion Mechanism of Pseudomonas Stutzeri on X80 Steel Subjected to Desulfovibrio Desulfuricans under Elastic Stress and Yield Stress. Corros. Sci. 2023, 216, 111084. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Microbiologically Influenced Corrosion: Looking to the Future. Int. Microbiol. 2005, 8, 169. [Google Scholar]
- Abedi, S.S.; Abdolmaleki, A.; Adibi, N. Failure Analysis of SCC and SRB Induced Cracking of a Transmission Oil Products Pipeline. Eng. Fail. Anal. 2007, 14, 250–261. [Google Scholar] [CrossRef]
- Cai, Z.; Xu, J.; Wei, B.; Sun, C. A Comparative Study of Sulfate-Reducing Desulfovibrio desulfuricans Induced Corrosion Behaviors in Q235, X65, X70, and X80 Pipeline Steels. Int. J. Press. Vessels Pip. 2022, 195, 104599. [Google Scholar] [CrossRef]
- Scarascia, G.; Wang, T.; Hong, P.-Y. Quorum Sensing and the Use of Quorum Quenchers as Natural Biocides to Inhibit Sulfate-Reducing Bacteria. Antibiotics 2016, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Little, B.; Lee, J.; Ray, R. A Review of ‘Green’ Strategies to Prevent or Mitigate Microbiologically Influenced Corrosion. Biofouling 2007, 23, 87–97. [Google Scholar] [CrossRef]
- Md Zain, W.S.; Hairul Salleh, N.I.; Abdullah, A. Natural Biocides for Mitigation of Sulphate Reducing Bacteria. Int. J. Corros. 2018, 2018, 3567569. [Google Scholar] [CrossRef]
- Wang, D.; Kijkla, P.; Mohamed, M.E.; Saleh, M.A.; Kumseranee, S.; Punpruk, S.; Gu, T. Aggressive Corrosion of Carbon Steel by Desulfovibrio ferrophilus IS5 Biofilm Was Further Accelerated by Riboflavin. Bioelectrochemistry 2021, 142, 107920. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Thakur, P.; Saxena, P.; Rauniyar, S.; Gopalakrishnan, V.; Singh, R.N.; Gadhamshetty, V.; Gnimpieba, E.Z.; Jasthi, B.K.; Sani, R.K. Gene Sets and Mechanisms of Sulfate-Reducing Bacteria Biofilm Formation and Quorum Sensing With Impact on Corrosion. Front. Microbiol. 2021, 12, 754140. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef]
- Viera, M.R.; Guiamet, P.S.; De Mele, M.F.L.; Videla, H.A. Biocidal Action of Ozone against Planktonic and Sessile Pseudomonas fluorescens. Biofouling 1999, 14, 131–141. [Google Scholar] [CrossRef]
- Grande Burgos, M.J.; Lucas López, R.; López Aguayo, M.D.C.; Pérez Pulido, R.; Gálvez, A. Inhibition of Planktonic and Sessile Salmonella Enterica Cells by Combinations of Enterocin AS-48, Polymyxin B and Biocides. Food Control 2013, 30, 214–221. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Gutiérrez-Lomelí, M.; Avila-Novoa, M.G. Removal of Mixed-Species Biofilms Developed on Food Contact Surfaces with a Mixture of Enzymes and Chemical Agents. Antibiotics 2021, 10, 931. [Google Scholar] [CrossRef]
- Flemming, H.-C. Biofouling and Microbiologically Influenced Corrosion (MIC)-an Economical and Technical Overview. In Microbial Deterioration of Materials; Heitz, E., Sand, W., Flemming, H.C., Eds.; Springer: Berlin, Gernamy; New York, NY, USA, 1996; pp. 5–14. [Google Scholar]
- Zuo, R. Biofilms: Strategies for Metal Corrosion Inhibition Employing Microorganisms. Appl. Microbiol. Biotechnol. 2007, 76, 1245–1253. [Google Scholar] [CrossRef]
- Kampf, G. Antibiotic Resistance Can Be Enhanced in Gram-Positive Species by Some Biocidal Agents Used for Disinfection. Antibiotics 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampf, G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Unsal, T.; Kumseranee, S.; Punpruk, S.; Saleh, M.A.; Alotaibi, M.D.; Xu, D.; Gu, T. Mitigation of Carbon Steel Biocorrosion Using a Green Biocide Enhanced by a Nature-Mimicking Anti-Biofilm Peptide in a Flow Loop. Bioresour. Bioprocess. 2022, 9, 67. [Google Scholar] [CrossRef]
- Di Martino, P. Ways to Improve Biocides for Metalworking Fluid. AIMS Microbiol. 2021, 7, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Liu, H.; Chen, S.; Cheng, F.; Voordouw, G.; Gieg, L. Effect of Selected Biocides on Microbiologically Influenced Corrosion Caused by Desulfovibrio ferrophilus IS5. Sci. Rep. 2018, 8, 16620. [Google Scholar] [CrossRef] [Green Version]
- Conlette, O. Impacts of Tetrakis-Hydroxymethyl Phosphonium Sulfate (THPS) Based Biocides on the Functional Group Activities of Some Oil Field Microorganisms Associated with Corrosion and Souring. Br. Microbiol. Res. J. 2014, 4, 1463–1475. [Google Scholar] [CrossRef]
- Silva, P.; Oliveira, S.H.; Vinhas, G.M.; Carvalho, L.J.; Barauna, O.S.; Urtiga Filho, S.L.; Lima, M.A.G. Tetrakis Hydroxymethyl Phosphonium Sulfate (THPS) with Biopolymer as Strategy for the Control of Microbiologically Influenced Corrosion in a Dynamic System. Chem. Eng. Process. Process Intensif. 2021, 160, 108272. [Google Scholar] [CrossRef]
- Sharma, M.; Menon, P.; Voordouw, J.; Shen, Y.; Voordouw, G. Effect of Long Term Application of Tetrakis (Hydroxymethyl) Phosphonium Sulfate (THPS) in a Light Oil-Producing Oilfield. Biofouling 2018, 34, 605–617. [Google Scholar] [CrossRef]
- Rüegg, U.T.; Rudinger, J. Reductive Cleavage of Cystine Disulfides with Tributylphosphine. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1977; Volume 47, pp. 111–116. ISBN 978-0-12-181947-7. [Google Scholar]
- Parker, A.J.; Kharasch, N. The Scission of the Sulfur-Sulfur Bond. Chem. Rev. 1959, 59, 583–628. [Google Scholar] [CrossRef]
- Okoro, C.C. The Biocidal Efficacy of Tetrakis-Hydroxymethyl Phosphonium Sulfate (THPS) Based Biocides on Oil Pipeline PigRuns Liquid Biofilms. Pet. Sci. Technol. 2015, 33, 1366–1372. [Google Scholar] [CrossRef]
- Talbot, R.E.; Larsen, J.; Sanders, P.F. Experience with the Use of Tetrakishydroxymethylphosphonium Sulfate (THPS) for the Control of Downhole Hydrogen Sulfide. In Proceedings of the CORROSION/2000 Conference, Paper Number NACE-00123, Orlando, FL, USA, 26–31 March 2000. [Google Scholar]
- Jia, R.; Li, Y.; Al-Mahamedh, H.H.; Gu, T. Enhanced Biocide Treatments with D-Amino Acid Mixtures against a Biofilm Consortium from a Water Cooling Tower. Front. Microbiol. 2017, 8, 1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Ramadan, M.; Kumseranee, S.; Punpruk, S.; Gu, T. Mitigating Microbiologically Influenced Corrosion of an Oilfield Biofilm Consortium on Carbon Steel in Enriched Hydrotest Fluid Using 2,2-Dibromo-3-Nitrilopropionamide (DBNPA) Enhanced by a 14-Mer Peptide. J. Mater. Sci. Technol. 2020, 57, 146–152. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Dou, W.; Liu, J.; Zlotkin, A.; Kumseranee, S.; Punpruk, S.; Li, X.; Gu, T. A Sea Anemone-Inspired Small Synthetic Peptide at Sub-Ppm Concentrations Enhanced Biofilm Mitigation. Int. Biodeterior. Biodegrad. 2019, 139, 78–85. [Google Scholar] [CrossRef]
- Xu, L.; Kijkla, P.; Kumseranee, S.; Punpruk, S.; Gu, T. “Corrosion-Resistant” Chromium Steels for Oil and Gas Pipelines Can Suffer from Very Severe Pitting Corrosion by a Sulfate-Reducing Bacterium. J. Mater. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Astuti, D.; Purwasena, I.A.; Putri, F.Z. Potential of Biosurfactant as an Alternative Biocide to Control Biofilm Associated Biocorrosion. J. Environ. Sci. Technol. 2018, 11, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Bardouniotis, E.; Ceri, H.; Olson, M.E. Biofilm Formation and Biocide Susceptibility Testing of Mycobacterium Fortuitum and Mycobacterium Marinum. Curr. Microbiol. 2003, 46, 0028–0032. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Mohamed, M.E.-S.; Saleh, M.A.; Gu, T. Mitigation of Sulfate Reducing Desulfovibrio Ferrophilus Microbiologically Influenced Corrosion of X80 Using THPS Biocide Enhanced by Peptide A. J. Mater. Sci. Technol. 2022, 107, 43–51. [Google Scholar] [CrossRef]
- Ding, Z.; Tang, Y.; Liu, L.; Ding, Z.; Tan, Y.; He, Q. Improving the Adhesive, Mechanical, Tribological Properties and Corrosion Resistance of Reactive Sputtered Tantalum Oxide Coating on Ti6Al4V Alloy via Introducing Multiple Interlayers. Ceram. Int. 2022, 48, 5983–5994. [Google Scholar] [CrossRef]
- Lokesh, K.S.; De Keersmaecker, M.; Elia, A.; Depla, D.; Dubruel, P.; Vandenabeele, P.; Van Vlierberghe, S.; Adriaens, A. Adsorption of Cobalt (II) 5,10,15,20-Tetrakis(2-Aminophenyl)-Porphyrin onto Copper Substrates: Characterization and Impedance Studies for Corrosion Inhibition. Corros. Sci. 2012, 62, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.T.; Kannoorpatti, K.; Padovan, A.; Thennadil, S. A Study of Bacteria Adhesion and Microbial Corrosion on Different Stainless Steels in Environment Containing Desulfovibrio vulgaris. R. Soc. Open Sci. 2021, 8, 201577. [Google Scholar] [CrossRef]
- Adama, K.; Onyeachu, I. The Corrosion Characteristics of SS316L Stainless Steel in a Typical Acid Cleaning Solution and Its Inhibition by 1-Benzylimidazole: Weight Loss, Electrochemical and SEM Characterizations. J. Niger. Soc. Phys. Sci. 2022, 4, 214–222. [Google Scholar] [CrossRef]
- Jin, S.; Amira, S.; Ghali, E. Electrochemical Impedance Spectroscopy Evaluation of the Corrosion Behavior of Die Cast and Thixocast AXJ530 Magnesium Alloy in Chloride Solution. Adv. Eng. Mater. 2007, 9, 75–83. [Google Scholar] [CrossRef]
- Dinh, H.T.; Kuever, J.; Mußmann, M.; Hassel, A.W.; Stratmann, M.; Widdel, F. Iron Corrosion by Novel Anaerobic Microorganisms. Nature 2004, 427, 829–832. [Google Scholar] [CrossRef] [PubMed]
- API 5CT N80; (N80-1 and N80-Q Types) Casing Pipe—WLD Steel. API Steel Oilfield Pipeline Casing Pipe OCTG Manufacturer Supplier. Available online: https://www.wldsteel.com/product/api-5ct-n80-casing-pipe/ (accessed on 14 July 2023).



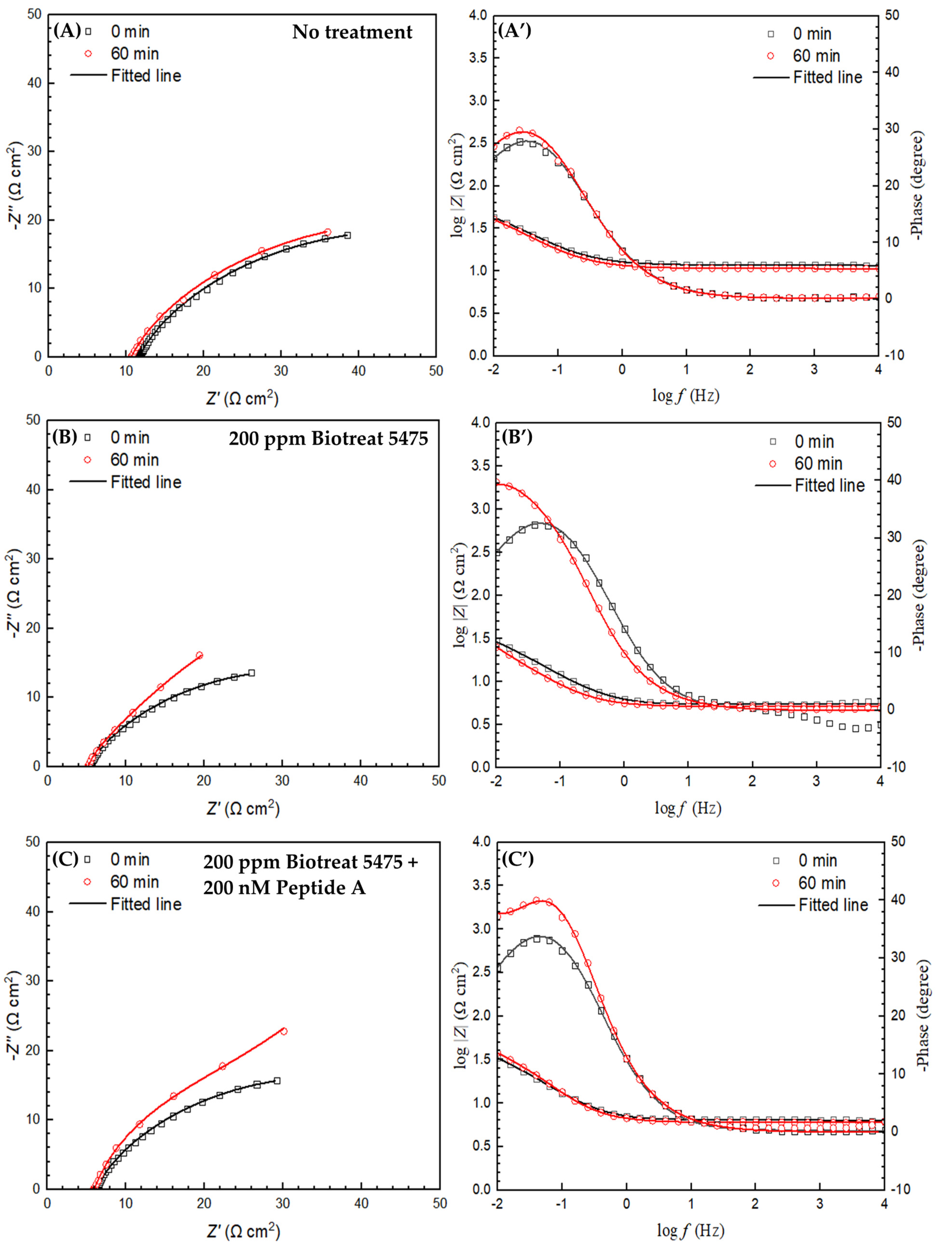
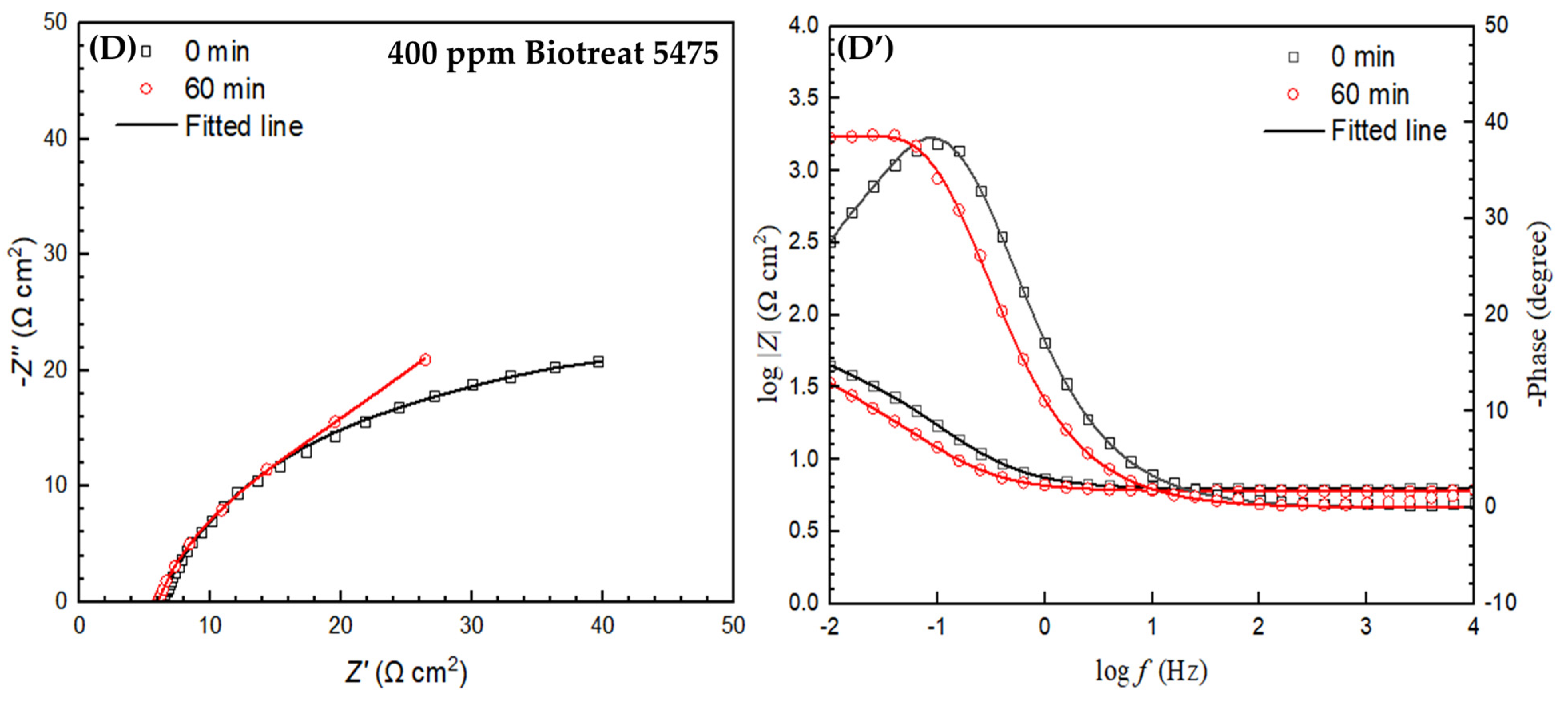
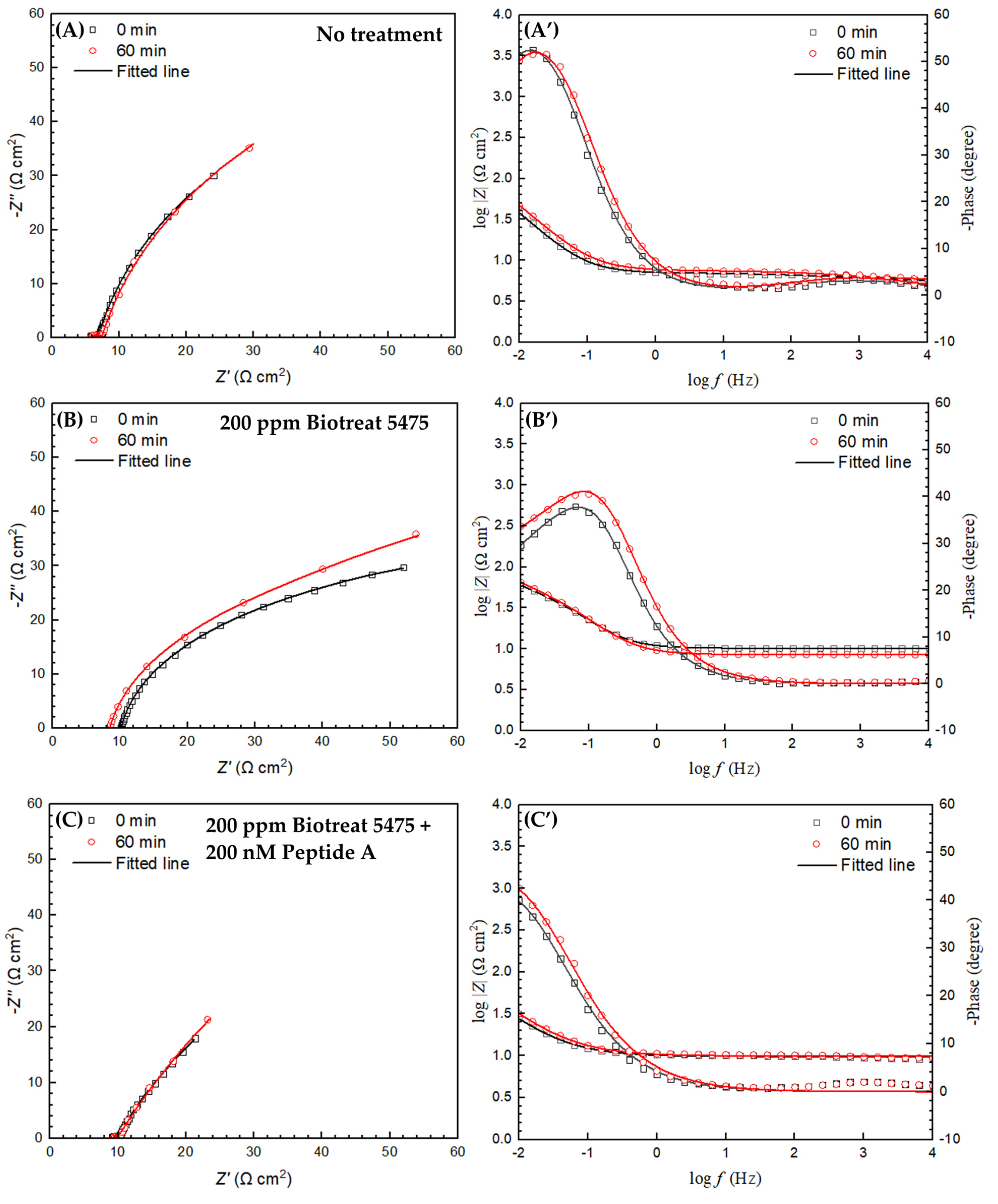
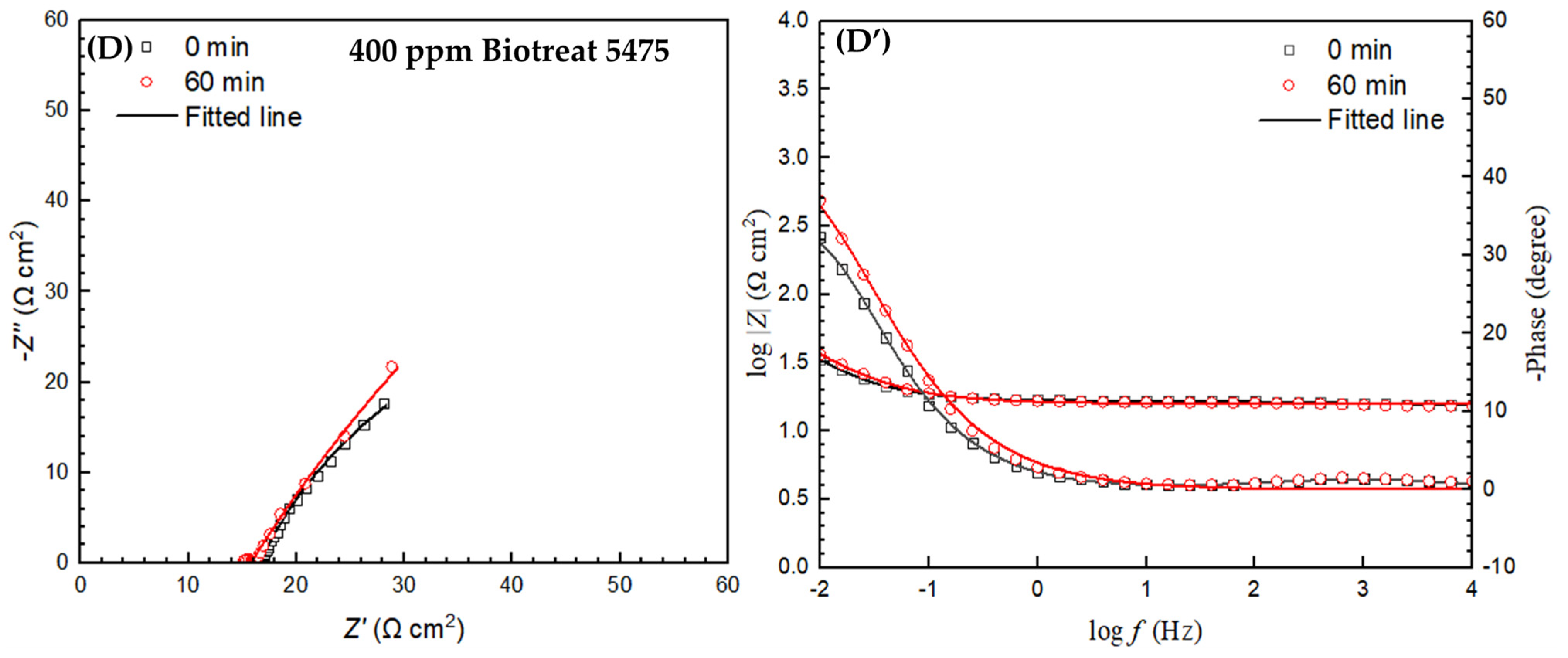
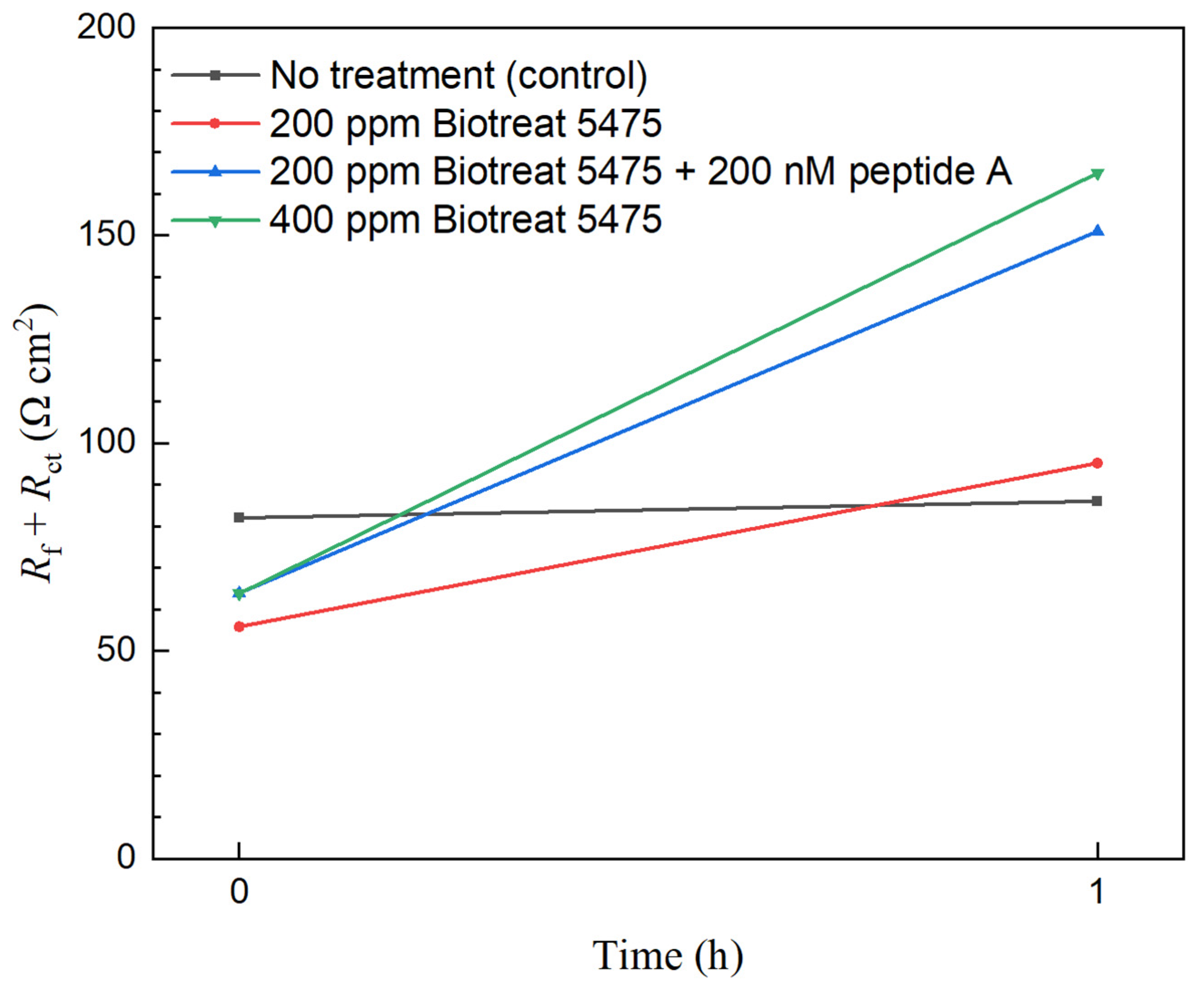
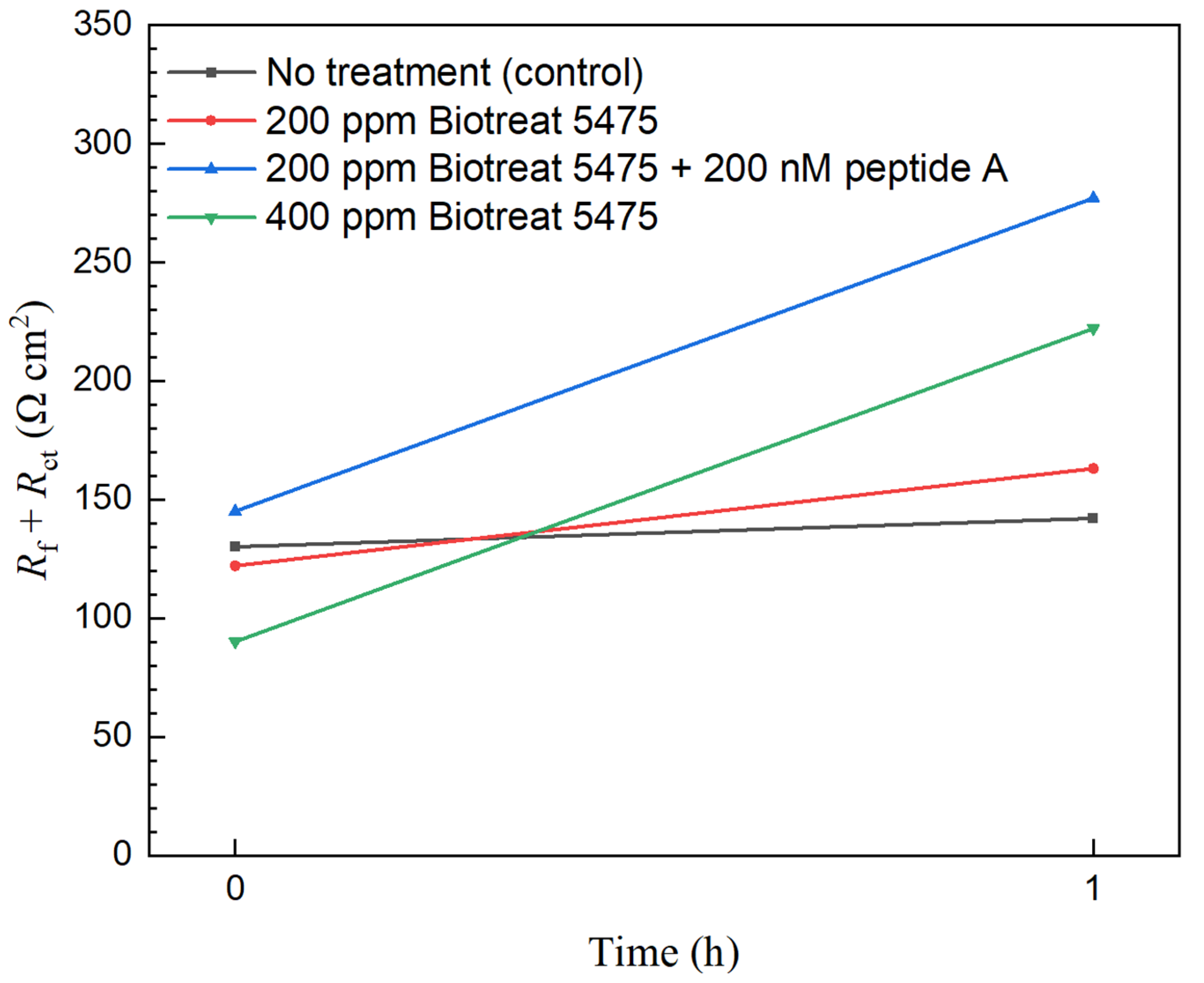
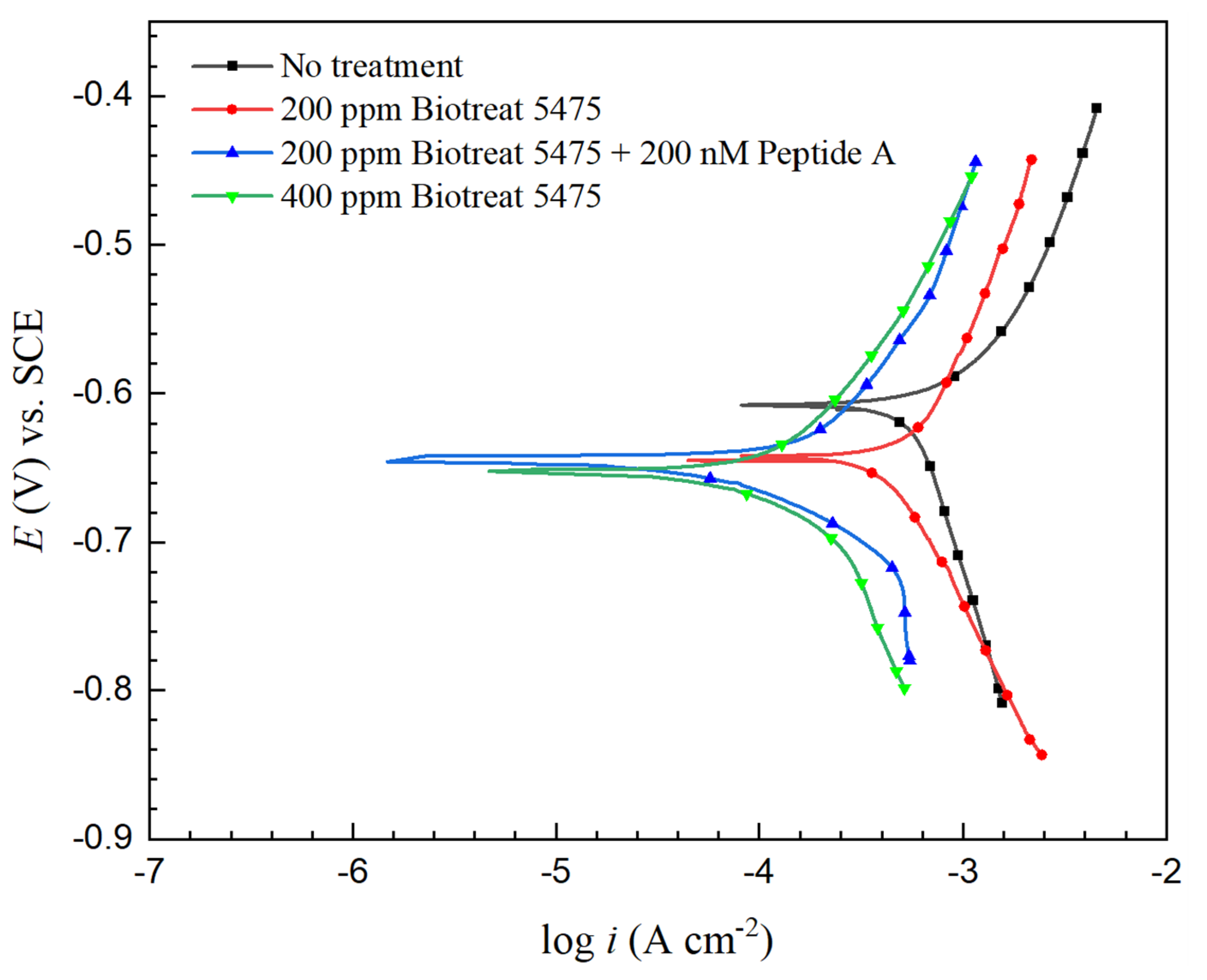
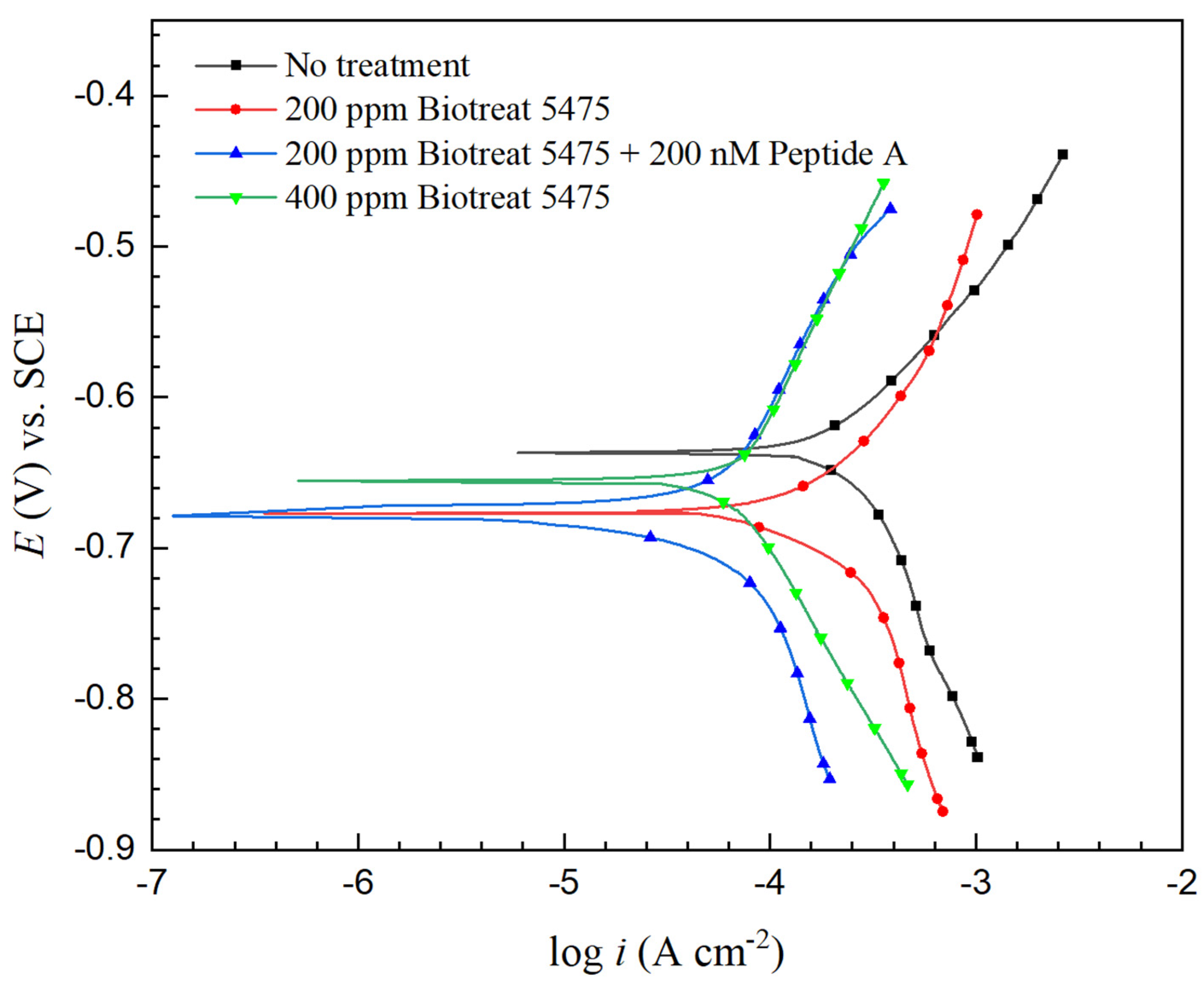
| t (h) | Rs (Ω cm2) | Qf (Ω−1 sn cm−2) | n1 | Rf (Ω cm2) | Qdl (Ω−1 sn cm−2) | n2 | Rct (Ω cm2) | Rf + Rct (Ω cm2) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No treatment | 0 | 11.5 | 4.1 × 10−2 | 0.41 | 0.28 | 8.6 × 10−2 | 0.79 | 82.0 | 82.0 | |
| 1 | 10.5 | 4.9 × 10−2 | 0.44 | 0.25 | 9.3 × 10−2 | 0.80 | 86.0 | 86.0 | ||
| 200 ppm Biotreat 5475 | 0 | 5.52 | 7.2 × 10−2 | 0.98 | 1.69 | 0.12 | 0.55 | 54.1 | 55.8 | |
| 1 | 5.16 | 0.22 | 0.79 | 15.2 | 0.18 | 0.64 | 80.0 | 95.2 | ||
| 200 ppm Biotreat 5475 + 200 nM Peptide A | 0 | 6.32 | 0.10 | 0.55 | 0.17 | 6.0 × 10−2 | 0.98 | 63.7 | 63.9 | |
| 1 | 5.98 | 0.14 | 0.80 | 42.3 | 0.43 | 0.83 | 109 | 151 | ||
| 400 ppm Biotreat 5475 | 0 | 6.24 | 9.6 × 10−2 | 0.79 | 44.9 | 0.87 | 0.98 | 18.9 | 63.8 | |
| 1 | 6.01 | 0.16 | 0.81 | 31.4 | 0.27 | 0.70 | 134 | 165 | ||
| t (h) | Rs (Ω cm2) | Qf (Ω−1 sn cm−2) | n1 | Rf (Ω cm2) | Qdl (Ω−1 sn cm−2) | n2 | Rct (Ω cm2) | Rf + Rct (Ω cm2) | |
|---|---|---|---|---|---|---|---|---|---|
| No treatment | 0 | 5.38 | 9.9 × 10−3 | 0.49 | 1.66 | 0.27 | 0.87 | 128 | 130 |
| 1 | 5.59 | 6.5 × 10−3 | 0.53 | 1.93 | 0.20 | 0.85 | 140 | 142 | |
| 200 ppm Biotreat 5475 | 0 | 10.2 | 8.0 × 10−2 | 0.89 | 36.9 | 0.13 | 0.61 | 84.9 | 122 |
| 1 | 8.46 | 7.1 × 10−2 | 0.87 | 44.1 | 0.12 | 0.65 | 119 | 163 | |
| 200 ppm Biotreat 5475 + 200 nM Peptide A | 0 | 15.3 | 1.3 × 10−6 | 0.39 | 12.1 | 0.32 | 0.72 | 133 | 145 |
| 1 | 15.8 | 0.25 | 0.69 | 0.01 | 8.2 × 10−13 | 0.79 | 277 | 277 | |
| 400 ppm Biotreat 5475 | 0 | 9.73 | 0.35 | 0.77 | 88.6 | 3.9 × 10−3 | 0.61 | 1.37 | 90 |
| 1 | 9.87 | 0.26 | 0.70 | 222 | 0.16 | 0.76 | 0.01 | 222 |
| βa (V/dec) | βc (V/dec) | Ecorr (V) vs. SCE | icorr (mA/cm2) | |
|---|---|---|---|---|
| No treatment | 0.103 | −0.440 | −0.611 | 0.54 |
| 200 ppm Biotreat 5475 | 0.205 | −0.287 | −0.645 | 0.46 |
| 200 ppm Biotreat 5475 + 200 nM Peptide A | 0.161 | −0.148 | −0.649 | 0.16 |
| 400 ppm Biotreat 5475 | 0.200 | −0.192 | −0.656 | 0.15 |
| βa (V/dec) | βc (V/dec) | Ecorr (V) vs. SCE | icorr (mA/cm2) | |
|---|---|---|---|---|
| No treatment | 0.191 | −0.271 | −0.647 | 0.26 |
| 200 ppm Biotreat 5475 | 0.208 | −0.301 | −0.675 | 0.19 |
| 200 ppm Biotreat 5475 + 200 nM Peptide A | 0.286 | −0.242 | −0.677 | 0.055 |
| 400 ppm Biotreat 5475 | 0.287 | −0.245 | −0.659 | 0.066 |
| Corrosion Inhibition Efficiency | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sessile Cell Count Reduction | LPR 1/Rp Reduction (ηi,LPR) | EIS 1/(Rct + Rf) Reduction (ηi,EIS) | PDP icorr Reduction (ηi) | |||||
| N80 | 26Cr3Mo | N80 | 26Cr3Mo | N80 | 26Cr3Mo | N80 | 26Cr3Mo | |
| 200 ppm Biotreat 5475 | 68.2% | 78.0% | 24% | 34% | 41% | 25% | 15% | 27% |
| 200 ppm Biotreat 5475 + 200 nM Peptide A | 97.8% | 98.0% | 31% | 41% | 58% | 48% | 70% | 79% |
| 400 ppm Biotreat 5475 | 98.7% | 98.3% | 43% | 40% | 61% | 60% | 72% | 75% |
| Metal | C | Mn | Cr | Ni | Cu | P | S | Si | Mo | V |
|---|---|---|---|---|---|---|---|---|---|---|
| N80 | 0.34–0.38 | 1.45–1.70 | 0.15 | 0.020 | 0.015 | 0.20–0.35 | ||||
| 26Cr3Mo | 0.25 | 0.49 | 2.99 | 0.02 | 0.02 | 0.008 | 0.001 | 0.26 | 0.12 | 0.015 |
| Parameter | Condition |
|---|---|
| Microbe | D. ferrophilus IS5 |
| Cultural medium | EASW |
| Coupon material | N80 and 26Cr3Mo |
| Liquid volume | 50 mL in 125 mL anaerobic vials |
| Inoculum size | 0.5 mL in anaerobic vials |
| Treatment method | No treatment control 200 ppm Biotreat 5475 200 ppm Biotreat 5475 + 200 nM Peptide A 400 ppm Biotreat 5475 1 cm2 coupon surface/25 mL liquid |
| Temperature | 28 °C |
| Initial pH | 7.0 ± 0.2 |
| Incubation time before biocide soaking | 3 d |
| Biocide treatment time | 1 h in Petri dish in an anaerobic chamber |
| Assay | Sessile cell counts on coupons |
| Parameter | Condition |
|---|---|
| Microbe | D. ferrophilus IS5 |
| Cultural medium | EASW |
| Coupon material | N80 and 26Cr3Mo |
| Liquid volume | 250 mL in 450 mL glass cells |
| Inoculum size | 2.5 mL in glass cells |
| Treatment method | No treatment control 200 ppm Biotreat 5475 200 ppm Biotreat 5475 + 200 nM Peptide A 400 ppm Biotreat 5475 4 glass cells used. Injection was followed by 3 min gentle shaking to dispere biocide. |
| Temperature | 28 °C |
| Initial pH | 7.0 ± 0.2 |
| Incubation time before biocide injection | 3 d |
| Biocide treatment time | 1 h |
| Assay | OCP, LPR, EIS, and Tafel scans using glass cells |
| Chemical | Amount |
|---|---|
| NaCl | 23.476 g |
| Na2SO4 | 3.917 g |
| NaHCO3 | 0.192 g |
| KCl | 0.664 g |
| KBr | 0.096 g |
| H3BO3 | 0.026 g |
| MgCl2 | 4.965 g |
| SrCl2·6H2O | 0.04 g |
| CaCl2·2H2O | 1.469 g |
| Sodium lactate | 3.5 g |
| Yeast extract | 1.0 g |
| Sodium citrate | 0.5 g |
| MgSO4·7H2O | 0.71 g |
| CaSO4·2H2O | 0.1 g |
| NH4Cl | 0.1 g |
| K2HPO4 | 0.05 g |
| Fe(NH4)2(SO4)2·6H2O | 1.37 g |
| DI water | 1 L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Kijkla, P.; Kumseranee, S.; Punpruk, S.; Gu, T. Electrochemical Assessment of Mitigation of Desulfovibrio ferrophilus IS5 Corrosion against N80 Carbon Steel and 26Cr3Mo Steel Using a Green Biocide Enhanced by a Nature-Mimicking Biofilm-Dispersing Peptide. Antibiotics 2023, 12, 1194. https://doi.org/10.3390/antibiotics12071194
Xu L, Kijkla P, Kumseranee S, Punpruk S, Gu T. Electrochemical Assessment of Mitigation of Desulfovibrio ferrophilus IS5 Corrosion against N80 Carbon Steel and 26Cr3Mo Steel Using a Green Biocide Enhanced by a Nature-Mimicking Biofilm-Dispersing Peptide. Antibiotics. 2023; 12(7):1194. https://doi.org/10.3390/antibiotics12071194
Chicago/Turabian StyleXu, Lingjun, Pruch Kijkla, Sith Kumseranee, Suchada Punpruk, and Tingyue Gu. 2023. "Electrochemical Assessment of Mitigation of Desulfovibrio ferrophilus IS5 Corrosion against N80 Carbon Steel and 26Cr3Mo Steel Using a Green Biocide Enhanced by a Nature-Mimicking Biofilm-Dispersing Peptide" Antibiotics 12, no. 7: 1194. https://doi.org/10.3390/antibiotics12071194







