Long-Term Use of Amoxicillin Is Associated with Changes in Gene Expression and DNA Methylation in Patients with Low Back Pain and Modic Changes
Abstract
:1. Introduction
2. Results
2.1. Study Cohort Characteristics
2.2. Long-Term Amoxicillin Treatment Did Not Alter Blood Cell-Type Composition
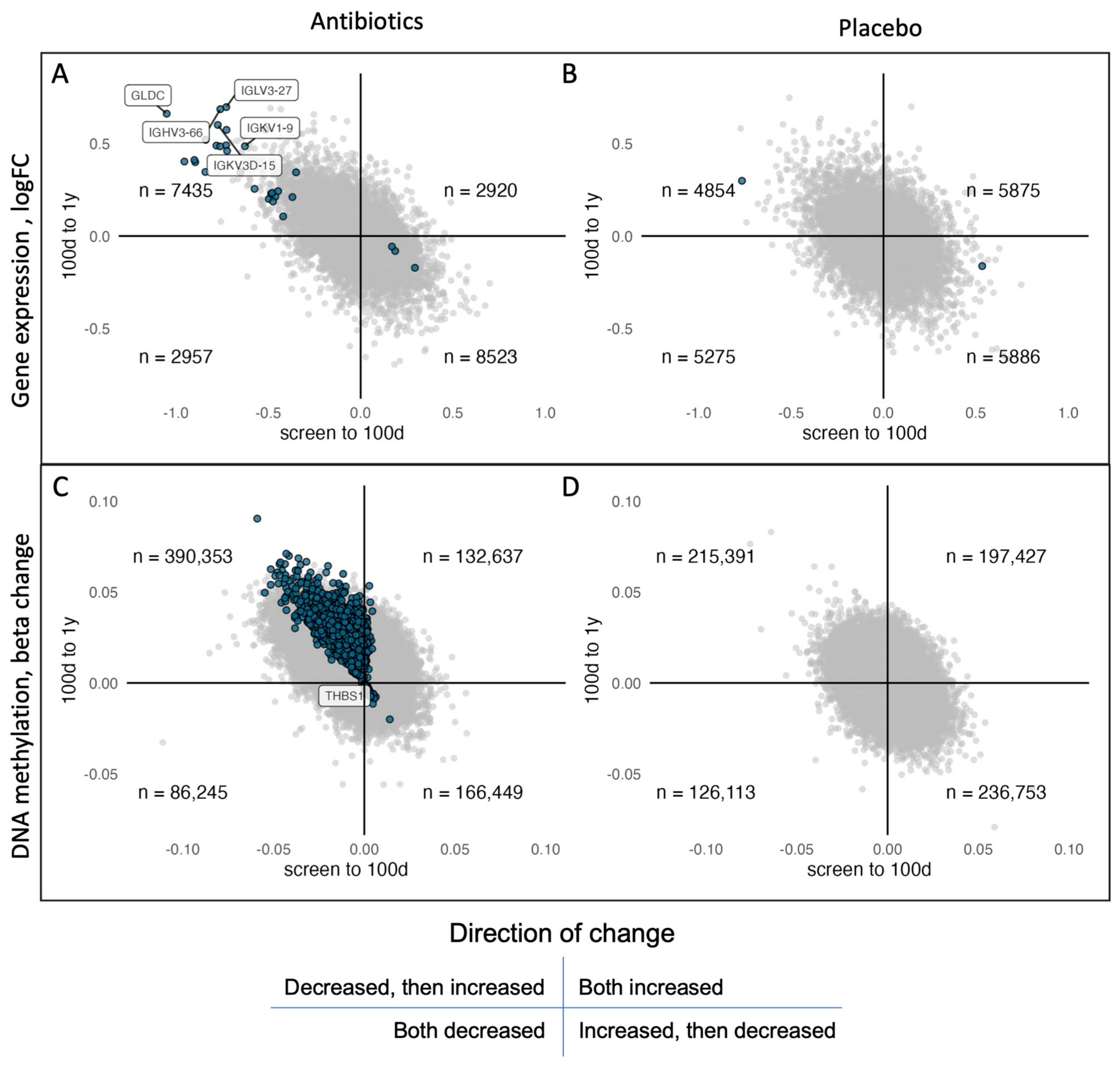
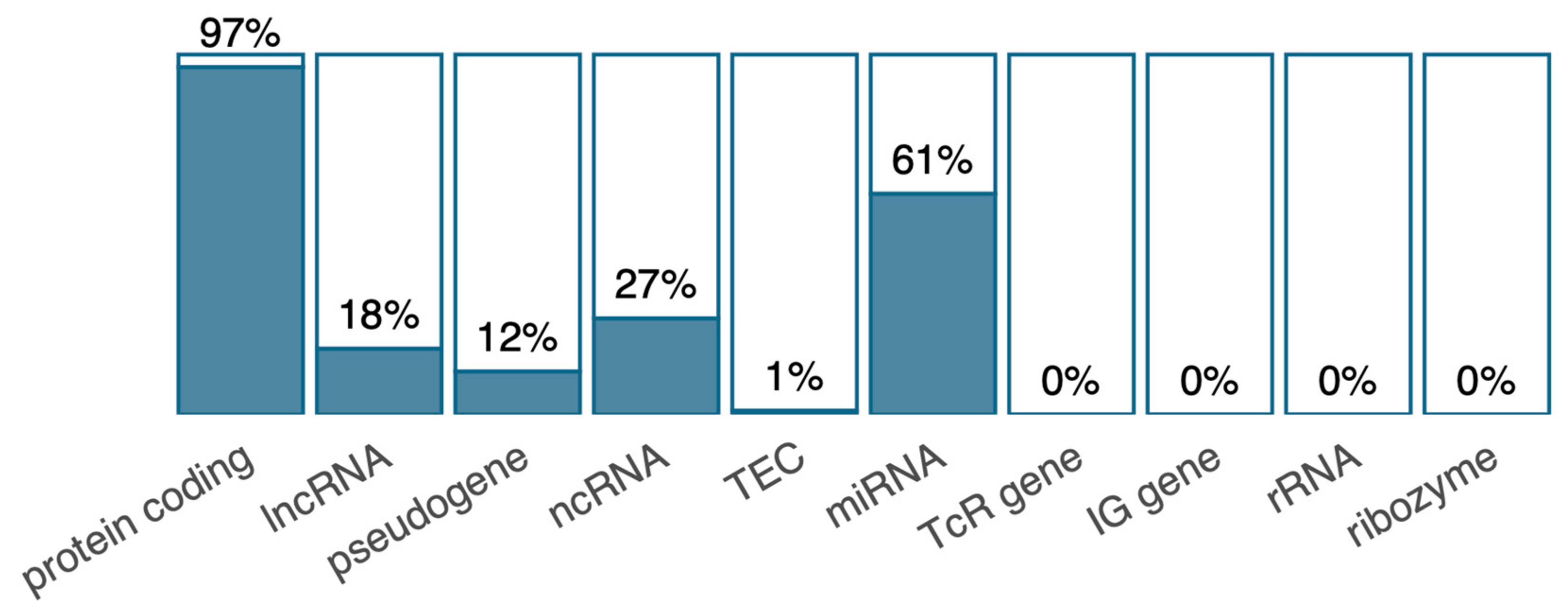
2.3. Altered Gene Expression in Amoxicillin Treatment Group
2.4. Altered DNA Methylation in Amoxicillin Treatment Group
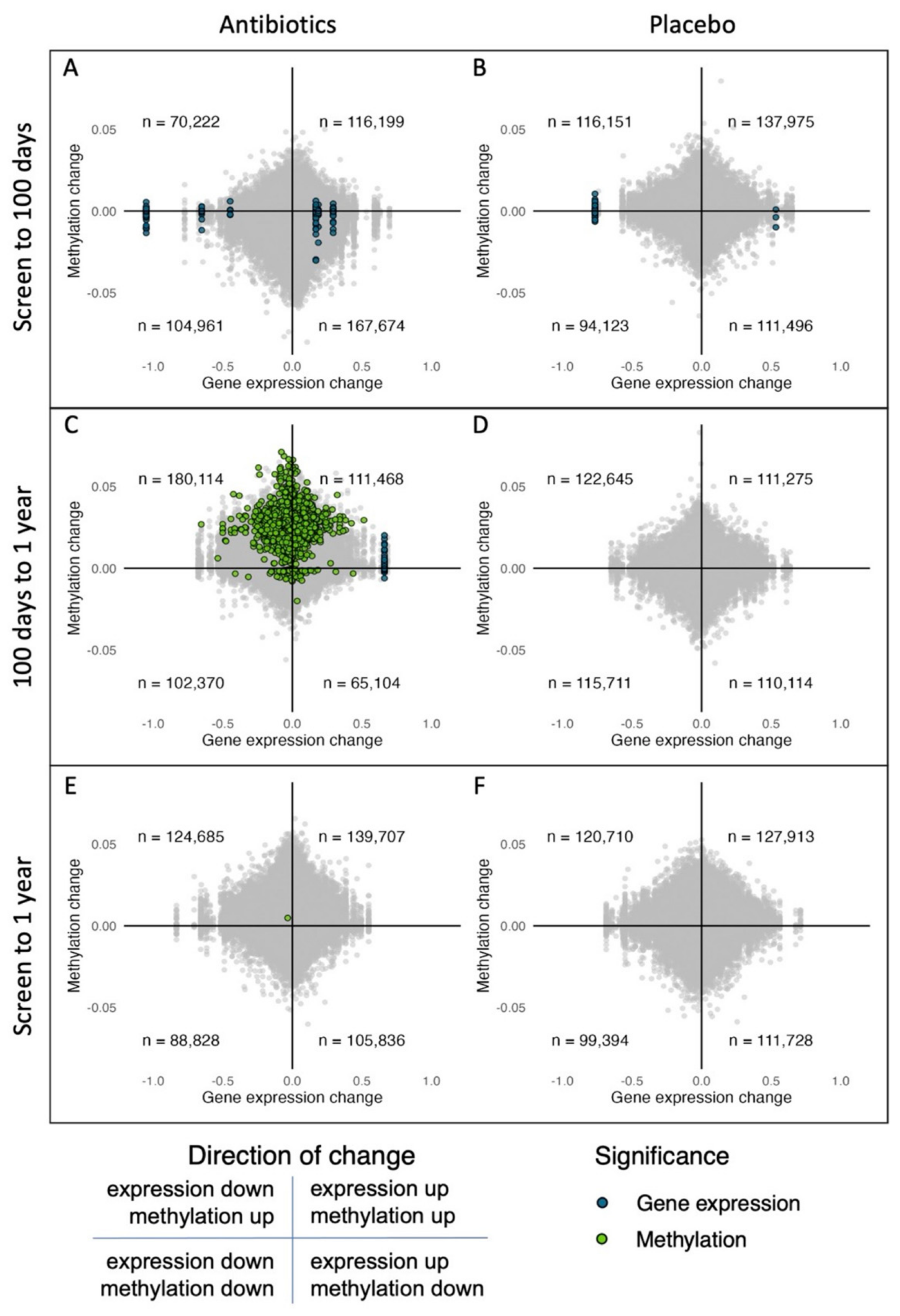
2.5. Low Overlap of Genes in the Gene Expression and DNA Methylation Data
3. Discussion
4. Materials and Methods
4.1. Study Cohort
4.2. Isolation and Preparation of RNA and DNA Samples
4.3. Generation, Preprocessing and Quality Control of Data
4.4. Cell Deconvolution
4.5. Identification of Covariates and Batch Effects
4.6. Differential Gene Expression and DNA Methylation Analysis
4.7. Integration of Gene Expression and DNA Methylation Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UpToDate. Amoxicillin: Drug Information. Available online: www.uptodate.com/contents/amoxicillin-drug-information (accessed on 16 September 2022).
- World Health Organization. World Health Organization Model List of Essential Medicines for Children: 7th List 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Lau, J.S.Y.; Korman, T.M.; Woolley, I. Life-long antimicrobial therapy: Where is the evidence? J. Antimicrob. Chemother. 2018, 73, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Global Health Metrics. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, H.B.; Sorensen, J.S.; Christensen, B.S.; Manniche, C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): A double-blind randomized clinical controlled trial of efficacy. Eur. Spine J. 2013, 22, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Bråten, L.C.H.; Rolfsen, M.P.; Espeland, A.; Wigemyr, M.; Assmus, J.; Froholdt, A.; Haugen, A.J.; Marchand, G.H.; Kristoffersen, P.M.; Lutro, O.; et al. Efficacy of antibiotic treatment in patients with chronic low back pain and Modic changes (the AIM study): Double blind, randomised, placebo controlled, multicentre trial. BMJ (Clin. Res. Ed.) 2019, 367, l5654. [Google Scholar] [CrossRef] [Green Version]
- Herlin, C.; Kjaer, P.; Espeland, A.; Skouen, J.S.; Leboeuf-Yde, C.; Karppinen, J.; Niinimaki, J.; Sorensen, J.S.; Storheim, K.; Jensen, T.S. Modic changes-Their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS ONE 2018, 13, e0200677. [Google Scholar] [CrossRef] [Green Version]
- Albert, H.B.; Lambert, P.; Rollason, J.; Sorensen, J.S.; Worthington, T.; Pedersen, M.B.; Norgaard, H.S.; Vernallis, A.; Busch, F.; Manniche, C.; et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur. Spine J. 2013, 22, 690–696. [Google Scholar] [CrossRef] [Green Version]
- Viuff, A.C.; Pedersen, L.H.; Kyng, K.; Staunstrup, N.H.; Børglum, A.; Henriksen, T.B. Antidepressant medication during pregnancy and epigenetic changes in umbilical cord blood: A systematic review. Clin. Epigenetics 2016, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Lötsch, J.; Schneider, G.; Reker, D.; Parnham, M.J.; Schneider, P.; Geisslinger, G.; Doehring, A. Common non-epigenetic drugs as epigenetic modulators. Trends Mol. Med. 2013, 19, 742–753. [Google Scholar] [CrossRef]
- Bridgeman, S.C.; Ellison, G.C.; Melton, P.E.; Newsholme, P.; Mamotte, C.D.S. Epigenetic effects of metformin: From molecular mechanisms to clinical implications. Diabetes Obes. Metab. 2018, 20, 1553–1562. [Google Scholar] [CrossRef] [Green Version]
- Gervin, K.; Nordeng, H.; Ystrom, E.; Reichborn-Kjennerud, T.; Lyle, R. Long-term prenatal exposure to paracetamol is associated with DNA methylation differences in children diagnosed with ADHD. Clin. Epigenet. 2017, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Cardenas, A.; Faleschini, S.; Cortes Hidalgo, A.; Rifas-Shiman, S.L.; Baccarelli, A.A.; DeMeo, D.L.; Litonjua, A.A.; Neumann, A.; Felix, J.F.; Jaddoe, V.W.V.; et al. Prenatal maternal antidepressants, anxiety, and depression and offspring DNA methylation: Epigenome-wide associations at birth and persistence into early childhood. Clin. Epigenet. 2019, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, M.C.T.; Meier, M.J.; Asensio, J.O.; Gant, T.W.; Tong, W.; Yauk, C.L.; Caiment, F. R-ODAF: Omics data analysis framework for regulatory application. Regul. Toxicol. Pharmacol. 2022, 131, 105143. [Google Scholar] [CrossRef]
- Hodgson, K.; Tansey, K.E.; Powell, T.R.; Coppola, G.; Uher, R.; Zvezdana Dernovšek, M.; Mors, O.; Hauser, J.; Souery, D.; Maier, W.; et al. Transcriptomics and the mechanisms of antidepressant efficacy. Eur. Neuropsychopharmacol. 2016, 26, 105–112. [Google Scholar] [CrossRef]
- Zhou, W.; Laird, P.W.; Shen, H. Comprehensive characterization, annotation and innovative use of Infinium DNA methylation BeadChip probes. Nucleic Acids Res. 2017, 45, e22. [Google Scholar] [CrossRef]
- Westermann, J.; Pabst, R. Lymphocyte subsets in the blood: A diagnostic window on the lymphoid system? Immunol. Today 1990, 11, 406–410. [Google Scholar] [CrossRef]
- Whitney, A.R.; Diehn, M.; Popper, S.J.; Alizadeh, A.A.; Boldrick, J.C.; Relman, D.A.; Brown, P.O. Individuality and variation in gene expression patterns in human blood. Proc. Natl. Acad. Sci. USA 2003, 100, 1896–1901. [Google Scholar] [CrossRef]
- Salas, L.A.; Zhang, Z.; Koestler, D.C.; Butler, R.A.; Hansen, H.M.; Molinaro, A.M.; Wiencke, J.K.; Kelsey, K.T.; Christensen, B.C. Enhanced cell deconvolution of peripheral blood using DNA methylation for high-resolution immune profiling. Nat. Commun. 2022, 13, 761. [Google Scholar] [CrossRef]
- Bouquet, J.; Soloski, M.J.; Swei, A.; Cheadle, C.; Federman, S.; Billaud, J.N.; Rebman, A.W.; Kabre, B.; Halpert, R.; Boorgula, M.; et al. Longitudinal Transcriptome Analysis Reveals a Sustained Differential Gene Expression Signature in Patients Treated for Acute Lyme Disease. mBio 2016, 7, e00100-16. [Google Scholar] [CrossRef] [Green Version]
- Morgun, A.; Dzutsev, A.; Dong, X.; Greer, R.L.; Sexton, D.J.; Ravel, J.; Schuster, M.; Hsiao, W.; Matzinger, P.; Shulzhenko, N. Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut 2015, 64, 1732–1743. [Google Scholar] [CrossRef] [Green Version]
- Dunn-Walters, D.; Townsend, C.; Sinclair, E.; Stewart, A. Immunoglobulin gene analysis as a tool for investigating human immune responses. Immunol. Rev. 2018, 284, 132–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Robak, O.H.; Heimesaat, M.M.; Kruglov, A.A.; Prepens, S.; Ninnemann, J.; Gutbier, B.; Reppe, K.; Hochrein, H.; Suter, M.; Kirschning, C.J.; et al. Antibiotic treatment-induced secondary IgA deficiency enhances susceptibility to Pseudomonas aeruginosa pneumonia. J. Clin. Investig. 2018, 128, 3535–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, V.; Millon, L.; Faucher, J.F.; Bard, E.; Robinet, E.; Piarroux, R.; Vuitton, D.A.; Meillet, D. Effects of a short-course of amoxicillin/clavulanic acid on systemic and mucosal immunity in healthy adult humans. Int. Immunopharmacol. 2005, 5, 917–928. [Google Scholar] [CrossRef]
- Dhariwal, A.; Haugli Bråten, L.C.; Sturød, K.; Salvadori, G.; Bargheet, A.; Åmdal, H.; Junges, R.; Berild, D.; Zwart, J.A.; Storheim, K.; et al. Differential response to prolonged amoxicillin treatment: Long-term resilience of the microbiome versus long-lasting perturbations in the gut resistome. Gut Microbes 2023, 15, 2157200. [Google Scholar] [CrossRef]
- Lefranc, G.; Lefranc, M.P. Regulation of the immunoglobulin gene transcription. Biochimie 1990, 72, 7–17. [Google Scholar] [CrossRef]
- Komaki, S.; Ohmomo, H.; Hachiya, T.; Sutoh, Y.; Ono, K.; Furukawa, R.; Umekage, S.; Otsuka-Yamasaki, Y.; Tanno, K.; Sasaki, M.; et al. Longitudinal DNA methylation dynamics as a practical indicator in clinical epigenetics. Clin. Epigenet. 2021, 13, 219. [Google Scholar] [CrossRef]
- Furukawa, R.; Hachiya, T.; Ohmomo, H.; Shiwa, Y.; Ono, K.; Suzuki, S.; Satoh, M.; Hitomi, J.; Sobue, K.; Shimizu, A. Intraindividual dynamics of transcriptome and genome-wide stability of DNA methylation. Sci. Rep. 2016, 6, 26424. [Google Scholar] [CrossRef] [Green Version]
- Manchikanti, L.; Singh, V.; Falco, F.J.; Benyamin, R.M.; Hirsch, J.A. Epidemiology of low back pain in adults. Neuromodul. J. Int. Neuromodul. Soc. 2014, 17 (Suppl. 2), 3–10. [Google Scholar] [CrossRef]
- Bråten, L.C.H.; Schistad, E.I.; Espeland, A.; Kristoffersen, P.M.; Haugen, A.J.; Marchand, G.H.; Vetti, N.; Pripp, A.H.; Kadar, T.I.; Skouen, J.S.; et al. Association of Modic change types and their short tau inversion recovery signals with clinical characteristics—A cross sectional study of chronic low back pain patients in the AIM-study. BMC Musculoskelet. Disord. 2020, 21, 368. [Google Scholar] [CrossRef]
- Raju, S.C.; Viljakainen, H.; Figueiredo, R.A.O.; Neuvonen, P.J.; Eriksson, J.G.; Weiderpass, E.; Rounge, T.B. Antimicrobial drug use in the first decade of life influences saliva microbiota diversity and composition. Microbiome 2020, 8, 121. [Google Scholar] [CrossRef]
- Storheim, K.; Espeland, A.; Grøvle, L.; Skouen, J.S.; Aßmus, J.; Anke, A.; Froholdt, A.; Pedersen, L.M.; Haugen, A.J.; Fors, T.; et al. Antibiotic treatment In patients with chronic low back pain and Modic changes (the AIM study): Study protocol for a randomised controlled trial. Trials 2017, 18, 596. [Google Scholar] [CrossRef] [Green Version]
- Okonechnikov, K.; Conesa, A.; Garcia-Alcalde, F. Qualimap 2: Advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics 2016, 32, 292–294. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 10 December 2020).
- Vigeland, M.D.; Flåm, S.T.; Vigeland, M.D.; Espeland, A.; Kristoffersen, P.M.; Vetti, N.; Wigemyr, M.; Bråten, L.C.H.; Gjefsen, E.; Schistad, E.I.; et al. Correlation between gene expression and MRI STIR signals in patients with chronic low back pain and Modic changes indicates immune involvement. Sci. Rep. 2022, 12, 215. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, F.; Achuthan, P.; Akanni, W.; Allen, J.; Amode, M.R.; Armean, I.M.; Bennett, R.; Bhai, J.; Billis, K.; Boddu, S.; et al. Ensembl 2019. Nucleic Acids Res. 2018, 47, D745–D751. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Teschendorff, A.E.; Marabita, F.; Lechner, M.; Bartlett, T.; Tegner, J.; Gomez-Cabrero, D.; Beck, S. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 2013, 29, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Niu, L.; Li, L.; Taylor, J.A. ENmix: A novel background correction method for Illumina HumanMethylation450 BeadChip. Nucleic Acids Res. 2016, 44, e20. [Google Scholar] [CrossRef]
- Müller, F.; Scherer, M.; Assenov, Y.; Lutsik, P.; Walter, J.; Lengauer, T.; Bock, C. RnBeads 2.0: Comprehensive analysis of DNA methylation data. Genome Biol. 2019, 20, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pidsley, R.; Zotenko, E.; Peters, T.J.; Lawrence, M.G.; Risbridger, G.P.; Molloy, P.; Van Djik, S.; Muhlhausler, B.; Stirzaker, C.; Clark, S.J. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016, 17, 208. [Google Scholar] [CrossRef] [Green Version]
- McCartney, D.L.; Walker, R.M.; Morris, S.W.; McIntosh, A.M.; Porteous, D.J.; Evans, K.L. Identification of polymorphic and off-target probe binding sites on the Illumina Infinium MethylationEPIC BeadChip. Genom. Data 2016, 9, 22–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, M.D.; He, Y.; Whitaker, J.W.; Hariharan, M.; Mukamel, E.A.; Leung, D.; Rajagopal, N.; Nery, J.R.; Urich, M.A.; Chen, H.; et al. Human body epigenome maps reveal noncanonical DNA methylation variation. Nature 2015, 523, 212–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://github.com/immunomethylomics/FlowSorted.BloodExtended.EPIC (accessed on 1 October 2021).
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [Green Version]
- Smyth, G.K. limma: Linear Models for Microarray Data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Gentleman, R., Carey, V.J., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Springer: New York, NY, USA, 2005; pp. 397–420. [Google Scholar] [CrossRef] [Green Version]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef] [Green Version]
- van Iterson, M.; van Zwet, E.W.; Heijmans, B.T.; BIOS Consortium. Controlling bias and inflation in epigenome- and transcriptome-wide association studies using the empirical null distribution. Genome Biol. 2017, 18, 19. [Google Scholar] [CrossRef] [Green Version]
- Hansen, K.D. IlluminaHumanMethylationEPICanno.ilm10b4.hg19: Annotation for Illumina’s EPIC Methylation Arrays. R Package Version 0.6.0. 2017. Available online: https://bitbucket.com/kasperdanielhansen/Illumina_EPIC (accessed on 12 June 2023).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Phipson, B.; Maksimovic, J.; Oshlack, A. missMethyl: An R package for analyzing data from Illumina’s HumanMethylation450 platform. Bioinformatics 2016, 32, 286–288. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; 260p. [Google Scholar]
| Treatment Group | ||
|---|---|---|
| Placebo (n = 48) | Amoxicillin (n = 52) | |
| Female | 60% | 63% |
| Age (mean, SD) | 45.7 (8.9) | 45.9 (7.6) |
| BMI (median †, IQR) | 24.6 (5.1) | 25.4 (5.2) |
| Smoking, n = 98 | 20% | 31% |
| Disability, RMDQ (mean, SD), n = 93 | 12.5 (3.8) | 13.2 (4.9) |
| LBP intensity, NRS (mean, SD), n = 99 | 6.6 (1.2) | 6.6 (1.1) |
| Previously operated for disc herniation | 33% | 29% |
| LBP duration in years (mean, SD), n = 99 | 6.3 (5.5) | 4.9 (5.2) |
| Glucose, mmol/L (mean, SD), n = 84 | 5.10 (0.6) | 5.10 (0.7) |
| Thrombocytes, ×109/L (mean, SD) | 259 (78) | 268 (53) |
| Haemoglobin, g/100 mL (mean, SD), n = 99 | 14.2 (1.2) | 14.2 (1.2) |
| Hematocrit, % (mean, SD) | 40 (4) | 40 (3) |
| Creatinine, µmol/L (mean, SD) | 70.8 (14.6) | 69.8 (12.8) |
| ASAT, U/L (mean, SD), n = 99 | 22.7 (5.3) | 24.5 (7.8) |
| CRP, mg/L (mean, SD), n = 99 | 1.5 (1.9) | 2.0 (4.0) |
| WBC, ×109/L (mean, SD) | 6.5 (1.8) | 6.5 (1.7) |
| Gene Expression | DNA Methylation | |||
|---|---|---|---|---|
| Time Interval | Amoxicillin | Placebo | Amoxicillin | Placebo |
| screening—100 d | ↑ 3 | ↑ 1 * | ||
| ↓ 25 | ↓ 1 | |||
| 100 d—1 y | ↑ 7 | ↑ 4442 | ||
| ↓ 105 | ||||
| screening—1 y | ↑ 1 | |||
| (A) Antibiotic group, screening—100 days | |||
| Ensembl ID | Gene name | LogFC | Padj |
| ENSG00000178445 | GLDC | −1.05 | 7.6 × 10−6 |
| ENSG00000211669 | IGLV3-10 | −0.896 | 1.7 × 10−4 |
| ENSG00000211964 | IGHV3-48 | −0.575 | 1.7 × 10−4 |
| ENSG00000133328 | HRASLS2 | −0.651 | 2.5 × 10−4 |
| ENSG00000242766 | IGKV1 D-17 | −0.955 | 3.6 × 10−4 |
| ENSG00000224041 | IGKV3D-15 | −0.774 | 1.6 × 10−3 |
| ENSG00000165617 | DACT1 | 0.294 | 3.3 × 10−3 |
| ENSG00000211662 | IGLV3-21 | −0.781 | 2.8 × 10−3 |
| ENSG00000211937 | IGHV2-5 | −0.497 | 4.0 × 10−3 |
| ENSG00000249173 | LINC01093 | −0.447 | 4.2 × 10−3 |
| ENSG00000211611 | IGKV6-21 | −0.901 | 4.2 × 10−3 |
| ENSG00000251546 | IGKV1D-39 | −0.839 | 4.2 × 10−3 |
| ENSG00000211625 | IGKV3D-20 | −0.729 | 6.0 × 10−3 |
| ENSG00000241755 | IGKV1-9 | −0.627 | 6.0 × 10−3 |
| ENSG00000233030 | RP11-196G18.3 | −0.462 | 8.2 × 10−3 |
| ENSG00000005194 | CIAPIN1 | 0.187 | 2.1 × 10−2 |
| ENSG00000211942 | IGHV3-13 | −0.474 | 1.4 × 10−2 |
| ENSG00000211941 | IGHV3-11 | −0.42 | 1.9 × 10−2 |
| ENSG00000124570 | SERPINB6 | 0.169 | 3.0 × 10−2 |
| ENSG00000211655 | IGLV1-36 | −0.761 | 2.3 × 10−2 |
| ENSG00000224220 | AC104699.1 | −0.727 | 2.9 × 10−2 |
| ENSG00000211659 | IGLV3-25 | −0.724 | 3.2 × 10−2 |
| ENSG00000211658 | IGLV3-27 | −0.728 | 3.3 × 10−2 |
| ENSG00000211972 | IGHV3-66 | −0.76 | 4.3 × 10−2 |
| ENSG00000211943 | IGHV3-15 | −0.369 | 4.5 × 10−2 |
| ENSG00000243290 | IGKV1-12 | −0.485 | 4.5 × 10−2 |
| ENSG00000211663 | IGLV3-19 | −0.479 | 4.6 × 10−2 |
| ENSG00000230709 | AC104024.1 | −0.841 | 4.8 × 10−2 |
| (B) Antibiotic group, 100 days—one year | |||
| Ensembl ID | Gene name | LogFC | Padj |
| ENSG00000276566 | IGKV1D-13 | 0.577 | 7.2 × 10−3 |
| ENSG00000178445 | GLDC | 0.662 | 1.0 × 10−2 |
| ENSG00000211658 | IGLV3-27 | 0.698 | 1.0 × 10−2 |
| ENSG00000224041 | IGKV3D-15 | 0.601 | 1.0 × 10−2 |
| ENSG00000211972 | IGHV3-66 | 0.686 | 3.5 × 10−2 |
| ENSG00000232216 | IGHV3-43 | 0.345 | 3.5 × 10−2 |
| ENSG00000241755 | IGKV1-9 | 0.486 | 4.2 × 10−2 |
| (C) Placebo group, screening—100 days | |||
| Ensembl ID | Gene name | LogFC | Padj |
| ENSG00000213934 | HBG1 | 0.534 | 1.5 × 10−3 |
| ENSG00000117707 | PROX1 | −0.766 | 3.3 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigeland, M.D.; Flåm, S.T.; Vigeland, M.D.; Espeland, A.; Zucknick, M.; Wigemyr, M.; Bråten, L.C.H.; Gjefsen, E.; Zwart, J.-A.; Storheim, K.; et al. Long-Term Use of Amoxicillin Is Associated with Changes in Gene Expression and DNA Methylation in Patients with Low Back Pain and Modic Changes. Antibiotics 2023, 12, 1217. https://doi.org/10.3390/antibiotics12071217
Vigeland MD, Flåm ST, Vigeland MD, Espeland A, Zucknick M, Wigemyr M, Bråten LCH, Gjefsen E, Zwart J-A, Storheim K, et al. Long-Term Use of Amoxicillin Is Associated with Changes in Gene Expression and DNA Methylation in Patients with Low Back Pain and Modic Changes. Antibiotics. 2023; 12(7):1217. https://doi.org/10.3390/antibiotics12071217
Chicago/Turabian StyleVigeland, Maria Dehli, Siri Tennebø Flåm, Magnus Dehli Vigeland, Ansgar Espeland, Manuela Zucknick, Monica Wigemyr, Lars Christian Haugli Bråten, Elisabeth Gjefsen, John-Anker Zwart, Kjersti Storheim, and et al. 2023. "Long-Term Use of Amoxicillin Is Associated with Changes in Gene Expression and DNA Methylation in Patients with Low Back Pain and Modic Changes" Antibiotics 12, no. 7: 1217. https://doi.org/10.3390/antibiotics12071217
APA StyleVigeland, M. D., Flåm, S. T., Vigeland, M. D., Espeland, A., Zucknick, M., Wigemyr, M., Bråten, L. C. H., Gjefsen, E., Zwart, J.-A., Storheim, K., Pedersen, L. M., Selmer, K., Lie, B. A., Gervin, K., & The AIM Study Group. (2023). Long-Term Use of Amoxicillin Is Associated with Changes in Gene Expression and DNA Methylation in Patients with Low Back Pain and Modic Changes. Antibiotics, 12(7), 1217. https://doi.org/10.3390/antibiotics12071217






