Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review
Abstract
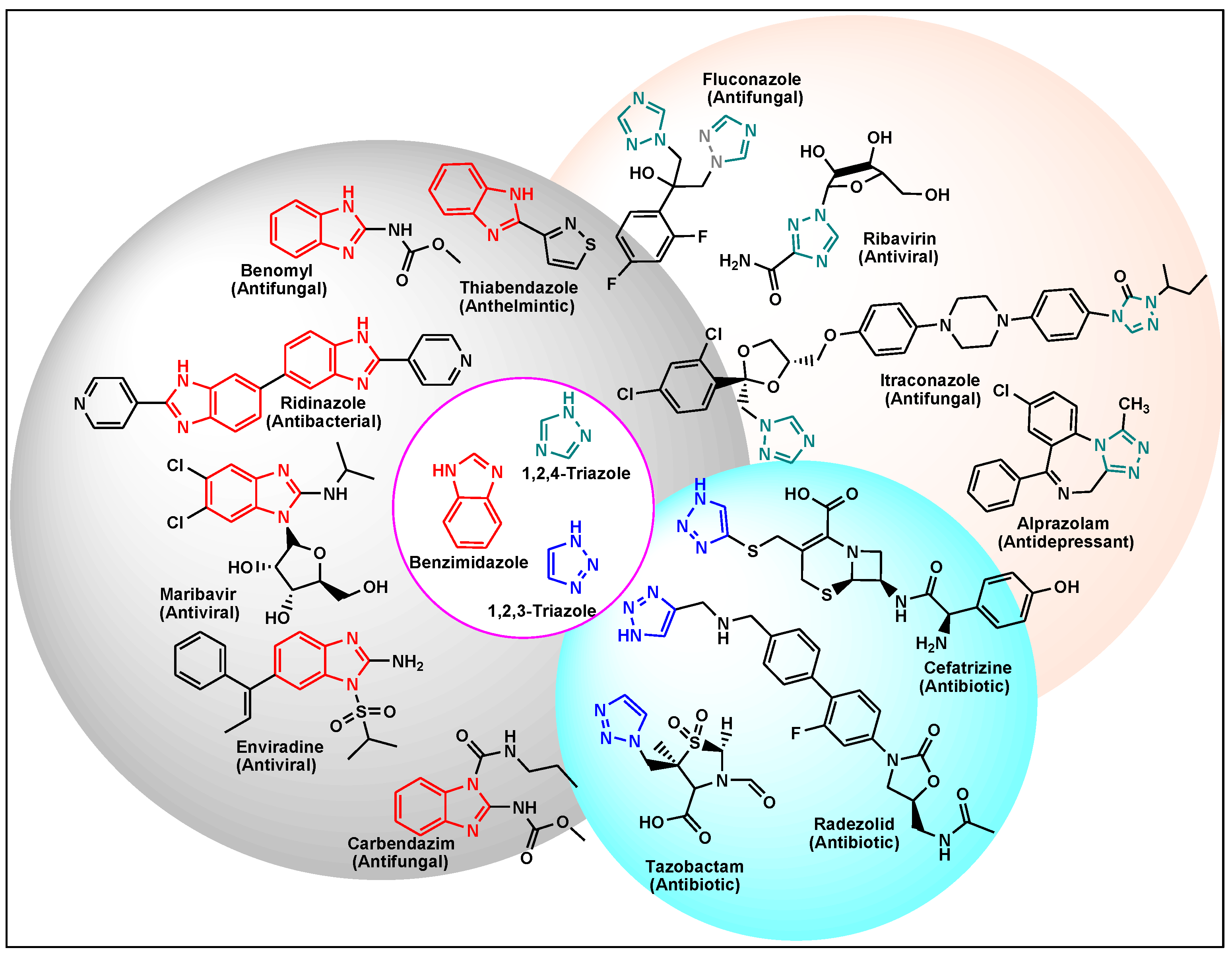
:1. Introduction
2. Synthesis and Antimicrobial Activities of Benzimidazole-1,2,3-Triazoles
2.1. 2-Benzimidazole-R(Ar)-1,4-Disubstituted-1,2,3-Triazole Hybrids
2.2. 1-Benzimidazole-R(Ar)-1,4-Disubstituted-1,2,3-Triazole Hybrids
2.3. 1,2-Bis-Substitutedbenzimidazoles-R(Ar)-1,4-Disubstituted-1,2,3-Triazole
2.4. Benzimidazole-R(Ar)-1,2,3-Triazole Hybrids as Antitubecular Agents
3. Synthesis and Antimicrobial Activities of Benzimidazole-1,2,4-Triazoles
3.1. 2-Benzimidazole-R(Ar)-1-(1,2,4-Triazole)
3.2. 1-Benzimidazole-R(Ar)-2-1,2,3-Triazole
3.3. 2-Benzimidazole-R(Ar)-2-1,2,4-Triazole
3.4. 6-Substituted-Benzimidazole-R(Ar)-1-1,2,4-Triazole
4. Synthesis and Antiviral Activities of Benzimidazole-Triazoles
5. Conclusions
- -
- The presence of substituents in the “4” or “5” positions of the benzimidazole nucleus can increase the antimicrobial activity of the benzimidazole-triazole hybrids (compounds 12, 13, 19, 20, 35).
- -
- The presence of the ortho- or para-substituted phenyl substituent in the “1” position of 1,2,3-triazoles in benzimidazole-triazole hybrids can increase their antimicrobial activity.
- -
- In the case of benzimidazoles substituted in the “1” position with triazoles, the presence of an aliphatic or aromatic radical substituent increases the antimicrobial activity of the hybrids.
- -
- The presence of the oxygen atom in the bridge that connects the benzimidazole and triazole rings is favorable to the antimicrobial activity of the hybrids (compounds 19, 20, 21, 29, 30).
- -
- The presence of the sulfur atom in the bridge that connects the benzimidazole and triazole rings is favorable to the antimicrobial activity of the hybrids and even to the antitubercular activity (95–97, 105, 107).
- -
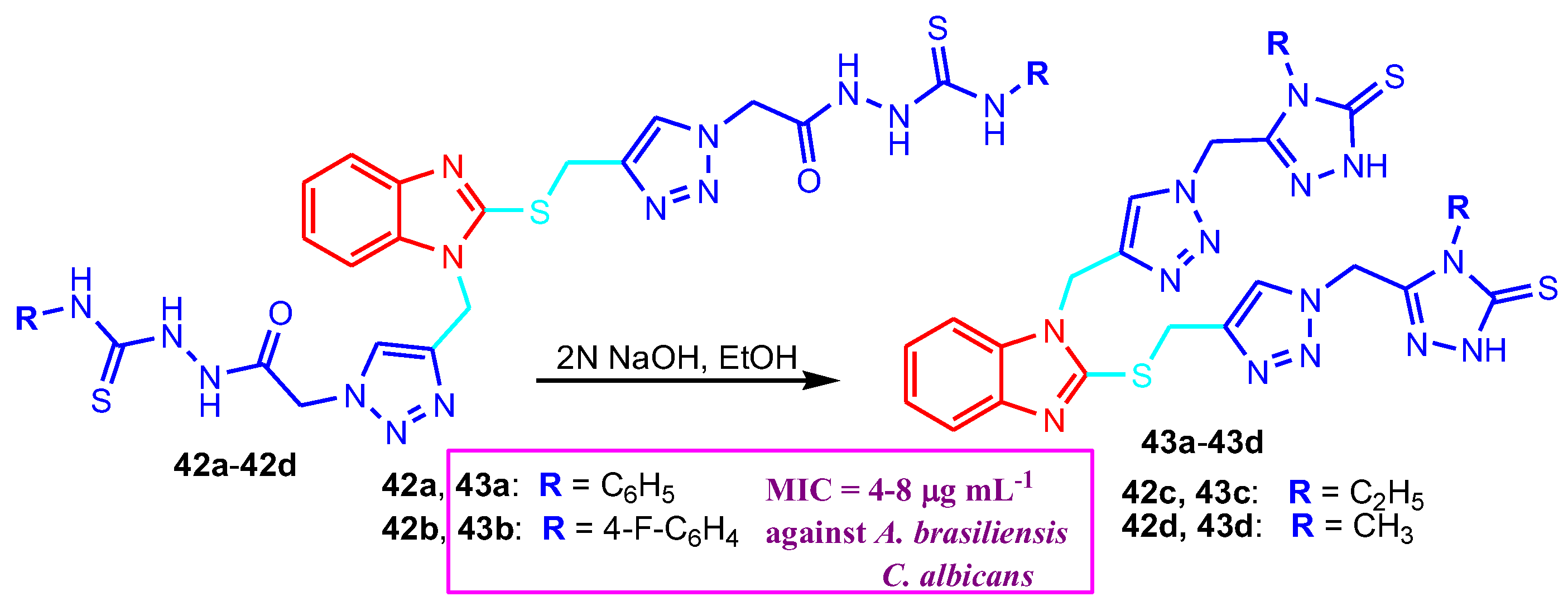
- The presence of a supplementary triazole ring in benzimidazole-triazole hybrids improves their antimicrobial activity (compounds 43, 45, 47).
- -
- The presence of the benzoyl substituent in the “5” position of the benzimidazole in the benzimidazole-1,2,4-triazole hybrids clearly improves their antimicrobial activity (compounds 85a–85e).
- -
- The phenyl nucleus as a spacer between the “1” position of 1,2,4-triazole and the “2” position of benzimidazole favors the formation of antimicrobial compounds, and the substituents in the “5” position of the benzimidazole nucleus increase the antimicrobial activity (compounds 79, 111, 112, 113).
- -
- Only benzimidazole-1,2,3-triazole hybrids are mentioned in the literature as having antiviral properties.
- -
- 2-Substituted or 1,2-disubstituted benzimidazoles with 1,2,3-triazoles are mentioned as antiviral compounds, and the presence of an additional triazole ring improves the antiviral activity (compound 140).
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Nishanth Rao, R.; Jena, S.; Mukherjee, M.; Maiti, B.; Chanda, K. Green synthesis of biologically active heterocycles of medicinal importance: A review. Environ. Chem. Lett. 2021, 19, 3315–3358. [Google Scholar] [CrossRef]
- Ebenezer, O.; Oyetunde-Joshua, F.; Omotoso, O.D.; Shapi, M. Benzimidazole and its derivatives: Recent Advances (2020–2022). Results Chem. 2023, 5, 100925. [Google Scholar] [CrossRef]
- Bansal, Y.; Kaur, M.; Bansal, G. Antimicrobial Potential of Benzimidazole Derived Molecules. Mini Rev. Med. Chem. 2019, 19, 624–646. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M. Synthesis of Antimicrobial Benzimidazole–Pyrazole Compounds and Their Biological Activities. Antibiotics 2021, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Deswal, L.; Verma, V.; Kumar, D.; Deswal, Y.; Kumar, A.; Kumar, R.; Parshad, M.; Bhatia, M. Synthesis, antimicrobial and α-glucosidase inhibition of new benzimidazole-1,2,3-triazole-indoline derivatives: A combined experimental and computational venture. Chem. Pap. 2022, 76, 7607–7622. [Google Scholar] [CrossRef]
- Raducka, A.; Świątkowski, M.; Korona-Głowniak, I.; Kaproń, B.; Plech, T.; Szczesio, M.; Gobis, K.; Szynkowska-Jóźwik, M.I.; Czylkowska, A. Zinc Coordination Compounds with Benzimidazole Derivatives: Synthesis, Structure, Antimicrobial Activity and Potential Anticancer Application. Int. J. Mol. Sci. 2022, 23, 6595. [Google Scholar] [CrossRef] [PubMed]
- Zalaru, C.; Dumitrascu, F.; Draghici, C.; Tarcomnicu, I.; Marinescu, M.; Nitulescu, G.M.; Tatia, R.; Moldovan, L.; Popa, M.; Chifiriuc, M.C. New Pyrazolo-Benzimidazole Mannich Bases with Antimicrobial and Antibiofilm Activities. Antibiotics 2022, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Su, S.; Zhou, Q.; Tang, X.; Liu, T.; Peng, F.; He, M.; Luo, H.; Xue, W. Antibacterial and antiviral activities and action mechanism of flavonoid derivatives with a benzimidazole moiety. J. Saudi Chem. Soc. 2021, 25, 101194. [Google Scholar] [CrossRef]
- Kanwal, A.; Ahmad, M.; Aslam, S.; Naqvi, S.A.R.; Jawwad Saif, M.J. Molecular-biological prolems of drug design and mehanism of drug action. Recent advances in antiviral benyimidayole derivatives: A mini review. Pharm. Chem. J. 2019, 53, 179–187. [Google Scholar] [CrossRef]
- Brishty, S.R.; Hossain, M.J.; Khandaker, M.U.; Faruque, M.R.I.; Osman, H.; Rahman, S.M.A. A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives. Front. Pharmacol. 2021, 12, 762807. [Google Scholar] [CrossRef] [PubMed]
- Yhou, S.; Huang, G. Synthesis of anti-allergic drugs. RSC Adv. 2020, 10, 5874. [Google Scholar] [CrossRef]
- Vasil’ev, P.M.; Kalitin, K.Y.; Spasov, A.A.; Grechko, O.Y.; Poroikov, V.V.; Filimonov, D.A.; Anisimova, V.A. Search for new dugs prediction and study of anticonvulsant properties of benzimidazole derivatives. Pharm. Chem. J. 2017, 50, 775–780. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; OOn, C.E. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta Pharm. Sin. B 2023, 13, 478–497. [Google Scholar] [CrossRef] [PubMed]
- Satija, G.; Sharma, B.; Madan, A.; Iqubal, A.; Shaquiquzzaman, M.; Akhter, M.; Parvez, S.; Khan, M.A.; Alam, M.M. Benzimidazole based derivatives as anticancer agents: Structure activity relationship analysis for various targets. J. Heterocycl. Chem. 2021, 59, 22–66. [Google Scholar] [CrossRef]
- Song, B.; Park, E.Y.; Kim, K.J.; Ki, S.H. Repurposing of Benzimidazole Anthelmintic Drugs as Cancer Therapeutics. Cancers 2022, 14, 4601. [Google Scholar] [CrossRef]
- Chen, W.L.; Li, D.D.; Chen, X.; Wang, Y.Z.; Xu, J.-J.; Jiang, Z.-Y.; You, Q.-D.; Guo, X.-K. Proton pump inhibitors selectively suppress MLL rearranged leukemia cells via disrupting MLL1-WDR5 protein-protein interaction. Eur. J. Med. Chem. 2020, 188, 112027. [Google Scholar] [CrossRef]
- Argirova, M.A.; Georgieva, M.K.; Hristova-Avakumova, N.G.; Vuchev, D.I.; Popova-Daskalova, G.V.; Anichina, K.K.; Yancheva, D.Y. New 1H-benzimidazole-2-yl hydrazones with combined antiparasitic and antioxidant activity. RSC Adv. 2021, 11, 39848–39868. [Google Scholar] [CrossRef]
- Valderas-García, E.; Häberli, C.; Álvarez-Bardón, M.; Escala, N.; Castilla-Gómez de Agüero, V.; de la Vega, J.; del Olmo, E.; Balaña-Fouce, R.; Keiser, J.; Martínez-Valladares, M. Benzimidazole and aminoalcohol derivatives show in vitro anthelmintic activity against Trichuris muris and Heligmosomoides polygyrus. Parasit. Vectors 2022, 15, 243. [Google Scholar] [CrossRef]
- Kamat, V.; Yallur, B.C.; Poojary, B.; Patil, V.B.; Nayak, S.P.; Krishna, P.M.; Joshi, S.D. Synthesis, molecular docking, antibacterial, and anti-inflammatory activities of benzimidazole-containing tricyclic systems. J. Chin. Chem. Soc. 2021, 68, 1055–1066. [Google Scholar] [CrossRef]
- Moharana, A.K.; Dash, R.N.; Mahanandia, N.C.; Subudhi, B.B. Synthesis and anti-inflammatory activity evaluation of some benzimidazoled erivatives. Pharm. Chem. J. 2022, 56, 1070–1074. [Google Scholar] [CrossRef]
- Veerasamy, R.; Roy, A.; Karunakaran, R.; Rajak, H. Structure–Activity Relationship Analysis of Benzimidazoles as Emerging Anti-Inflammatory Agents: An Overview. Pharmaceuticals 2021, 14, 663. [Google Scholar] [CrossRef]
- Iqbal, H.; Verma, A.K.; Yadav, P.; Alam, S.; Shafiq, M.; Mishra, D.; Khan, F.; Hanif, K.; Negi, A.S.; Chanda, D. Antihypertensive Effect of a Novel Angiotensin II Receptor Blocker Fluorophenyl Benzimidazole: Contribution of cGMP, Voltage-dependent Calcium Channels, and BKCa Channels to Vasorelaxant Mechanisms. Front. Pharmacol. 2021, 30, 611109. [Google Scholar] [CrossRef]
- Tajane, P.S.; Sawant, R.L. An updated review on benzimidazole derivatives as potential antihypertensive agents. Int. J. Health Sci. 2022, 6 (Suppl. S1), 7169–7179. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; El Rashedy, A.A. Benzimidazole Derivatives as Antidiabetic Agents. Med. Chem. 2015, 5, 318–325. [Google Scholar] [CrossRef]
- Dik, B.; Coşkun, D.; Bahcivan, E.; Unez, K. Potential antidiabetic activity of benzimidazole derivative albendazole and lansoprazole drugs in different doses in experimental type 2 diabetic rats. Turk. J. Med. Sci. 2021, 51, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.M.; Noori, M.; Montazer, M.N.; Ghomi, M.K.; Mollazadeh, M.; Dastyafteh, N.; Irajie, C.; Zomorodian, K.; Mirfazli, S.S.; Mojtabavi, S.; et al. Synthesis and structure–activity relationship studies of benzimidazole-thioquinoline derivatives as α-glucosidase inhibitors. Sci. Rep. 2023, 13, 4392. [Google Scholar] [CrossRef]
- Stanton, J.B.; Schneider, D.A.; Dinkel, K.D.; Balmer, B.F.; Baszler, T.V.; Mathison, B.A.; Boykin, D.W.; Kumar, A. Discovery of a Novel, Monocationic, Small-Molecule Inhibitor of Scrapie Prion Accumulation in Cultured Sheep Microglia and Rov Cells. PLoS ONE 2012, 7, e0119084. [Google Scholar] [CrossRef]
- Dinparast, L.; Zengin, G.; Bahadori, M.B. Cholinesterases Inhibitory Activity of 1H-benzimidazole Derivatives. Biointerface Res. Appl. Chem. 2021, 11, 10739–10745. [Google Scholar] [CrossRef]
- Adalat, B.; Rahim, F.; Taha, M.; Alshamrani, F.J.; Anouar, E.H.; Uddin, N.; Shah, S.A.A.; Ali, Z.; Zakaria, Z.A. Synthesis of Benzimidazole–Based Analogs as Anti Alzheimer’s Disease Compounds and Their Molecular Docking Studies. Molecules 2020, 25, 4828. [Google Scholar] [CrossRef]
- Cheretaev, I.V.; Korenyuk, I.I.; Nozdrachev, A.D. Neurotropic, Psychoactive, and Analgesic Properties of Benzimidazole and Its Derivatives: Physiological Mechanisms. Neurosci. Behav. Physiol. 2018, 48, 848–853. [Google Scholar] [CrossRef]
- Maltsev, D.V.; Spasov, A.A.; Vassiliev, P.M.; Skripka, M.O.; Miroshnikov, M.V.; Kochetkov, A.N.; Eliseeva, N.V.; Lifanova, Y.V.; Kuzmenko, T.A.; Divaeva, L.N.; et al. Synthesis and Pharmacological Evaluation of Novel 2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole Derivatives as Promising Anxiolytic and Analgesic Agents. Molecules 2021, 26, 6049. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.L.; Wu, P.; Chng, C.L.; Yang, W.B.; Jayakumar, T.; Geraldine, P.; Chou, C.M.; Chang, C.Y.; Lu, W.J.; Sheu, J.R. Novel synthetic benzimidazole-derived oligosaccharide, M3BIM, prevents ex vivo platelet aggregation and in vivo thromboembolism. J. Biomed. Sci. 2016, 23, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Liu, Q.; Ren, Y. Design, synthesis and biological activity evaluation of novel methyl substituted benzimidazole derivatives. Tetrahedron 2020, 76, 131027. [Google Scholar] [CrossRef]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Strzelecka, M.; Świątek, P. 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals 2021, 14, 224. [Google Scholar] [CrossRef]
- Patil, S.A.; Nesaragi, A.R.; Rodríguez-Berrios, R.R.; Hampton, S.M.; Bugarin, A.; Patil, S.A. Coumarin Triazoles as Potential Antimicrobial Agents. Antibiotics 2023, 12, 160. [Google Scholar] [CrossRef]
- Kazeminejad, Z.; Marzi, M.; Shiroudi, A.; Kouhpayeh, S.A.; Farjam, M.; Zarenezhad, E. Novel 1,2,4-Triazoles as Antifungal Agents. BioMed Res. Int. 2022, 2022, 4584846. [Google Scholar] [CrossRef]
- Sharma, A.; Agrahari, A.K.; Rajkhowa, S.; Tiwari, V.K. Emerging impact of triazoles as anti-tubercular agent. Eur. J. Med. Chem. 2022, 238, 114454. [Google Scholar] [CrossRef]
- El-Shoukrofy, M.S.; Atta, A.; Fahmy, S.; Sriram, D.; Mahran, M.A.; Labouta, I.M. New tetrahydropyrimidine-1,2,3-triazole clubbed compounds: Antitubercular activity and Thymidine Monophosphate Kinase (TMPKmt) inhibition. Bioorg. Chem. 2023, 131, 106312. [Google Scholar] [CrossRef]
- Ravisankar, N.; Sarathi, N.; Maruthavanan, T.; Ramasundaram, S.; Ramesh, M.; Sankar, C.; Umamatheswari, S.; Kanthimathi, G.; Oh, T.H. Synthesis, antimycobacterial screening, molecular docking, ADMET prediction and pharmacological evaluation on novel pyran-4-one bearing hydrazone, triazole and isoxazole moieties: Potential inhibitors of SARS-CoV-2. Synthesis, antimycobacterial screening, molecular docking, ADMET prediction and pharmacological evaluation on novel pyran-4-one bearing hydrazone, triazole and isoxazole moieties: Potential inhibitors of SARS-CoV-2. J. Mol. Struct. 2023, 1285, 135461. [Google Scholar] [CrossRef]
- Musa, A.; Abulkhair, H.S.; Aljuhani, A.; Rezki, N.; Abdelgawad, M.A.; Shalaby, K.; El-Ghorab, A.H.; Aouad, M.R. Phenylpyrazolone-1,2,3-triazole Hybrids as Potent Antiviral Agents with Promising SARS-CoV-2 Main Protease Inhibition Potential. Pharmaceuticals 2023, 16, 463. [Google Scholar] [CrossRef]
- Seliem, I.A.; Panda, S.S.; Girgis, A.S.; Moatasim, Y.; Kandeil, A.; Mostafa, A.; Ali, M.A.; Nossier, E.S.; Rasslan, F.; Srour, A.M.; et al. New quinoline-triazole conjugates: Synthesis, and antiviral properties against SARS-CoV-2. Bioorg. Chem. 2021, 114, 105117. [Google Scholar] [CrossRef]
- Venkatesham, P.; Schols, D.; Persoons, L.; Claes, S.; Sangolkar, A.A.; Chedupaka, R.; Vedula, R.R. Synthesis of novel thioalkylated triazolothiazoles and their promising in-vitro antiviral activity. J. Mol. Struct. 2023, 1286, 135573. [Google Scholar] [CrossRef]
- Pinheiro, N.G.; Gonzaga, D.T.G.; da Silva, A.R.; Fuly, A.C.; von Ranke, N.L.; Rodrigues, C.R.; Magalhães, B.Q.; Pereira, J.S.; Pacheco, P.A.F.; Silva, A.C.; et al. Triazoles with inhibitory action on P2X7R impaired the acute inflammatory response in vivo and modulated the hemostatic balance in vitro and ex vivo. Inflamm. Res. 2023, 72, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, S.; Lesyk, R.; Yadlovskyi, O.; Holota, S.; Yarmoluk, S.; Tsyhankov, S.; Demchenko, A. Fused Triazole-Azepine Hybrids as Potential Non-Steroidal Antiinflammatory Agents. Sci. Pharm. 2023, 91, 26. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Hashem, H.E.; Amr, A.E.-G.E.; Nossier, E.S.; Anwar, M.M.; Azmy, E.M. New Benzimidazole-1,2,4-Triazole-, and 1,3,5-Triazine-Based Derivatives as Potential EGFRWT and EGFRT790M Inhibitors: Microwave-Assisted Synthesis, Anticancer Evaluation, and Molecular Docking Study. ACS Omega 2022, 7, 7155–7171. [Google Scholar] [CrossRef]
- Othman, D.I.A.; Hamdi, A.; Tawfik, S.S.; Elgazar, A.A.; Mostafa, A.S. Identification of new benzimidazole-triazole hybrids as anticancer agents: Multi-target recognition, in vitro and in silico studies. J. Enzym. Inhib. Med. Chem. 2023, 38, 2166037. [Google Scholar] [CrossRef]
- Gupta, O.; Pradhan, G.T.; Chawla, G. An updated review on diverse range of biological activities of 1,2,4-triazole derivatives: Insight into structure activity relationship. J. Mol. Struct. 2023, 1274 Pt 2, 134487. [Google Scholar] [CrossRef]
- Abu-Melha, S.; Azher, O.A.; Alaysuy, O.; Alnoman, R.B.; Abualnaja, M.M.; Althagafi, I.; El-Metwaly, N.M. Synthesis, molecular modeling and antioxidant activity of new thiadiazolyl-triazole analogues. J. Saudi Chem. Soc. 2023, 27, 101596. [Google Scholar] [CrossRef]
- Dawbaa, S.; Nuha, D.; Evren, A.E.; Cankiliç, M.Y.; Yurttaş, L.; Turan, G. New oxadiazole/triazole derivatives with antimicrobial and antioxidant properties. J. Mol. Struct. 2023, 1282, 135213. [Google Scholar] [CrossRef]
- Zhao, W.; Song, M.; Hua, Y.; Zhu, Y.; Liu, W.; Xia, Q.; Deng, X.; Huang, Y. Design, Synthesis, and Pharmacology of New Triazole-Containing Quinolinones as CNS Active Agents. Molecules 2023, 28, 1987. [Google Scholar] [CrossRef] [PubMed]
- Dixit, D.; Verma, P.K.; Marwaha, R.K. A review on ‘triazoles’: Their chemistry, synthesis and pharmacological potentials. J. Iran Chem. Soc. 2021, 18, 2535–2565. [Google Scholar] [CrossRef]
- Fallah, Z.; Tajbakhsh, M.; Alikhani, M.; Larijani, B.; Faramarzi, M.A.; Hamedifar, H.; Mohammadi-Khanaposhtani, M.; Mahdavi, M. A review on synthesis, mechanism of action, and structure-activity relationships of 1,2,3-triazole-based α-glucosidase inhibitors as promising anti-diabetic agents. J. Mol. Struct. 2022, 1255, 132469. [Google Scholar] [CrossRef]
- Rahim, F.; Ullah, H.; Hussain, R.; Taha, M.; Khan, S.; Nawaz, M.; Nawaz, F.; Gilani, S.J.; Bin Jumah, M.N. Thiadiazole based triazole/hydrazone derivatives: Synthesis, in vitro α-glucosidase inhibitory activity and in silico molecular docking study. J. Mol. Struct. 2023, 1287, 135619. [Google Scholar] [CrossRef]
- Kumar, S.; Khokra, S.L.; Yadav, A. Triazole analogues as potential pharmacological agents: A brief review. Future J. Pharm. Sci. 2021, 7, 106. [Google Scholar] [CrossRef]
- Kaproń, B.; Łuszczki, J.J.; Siwek, A.; Karcz, T.; Nowak, G.; Zagaja, M.; Andres-Mach, M.; Stasiłowicz, A.; Cielecka-Piontek, J.; Kocki, J.; et al. Preclinical evaluation of 1,2,4-triazole-based compounds targeting voltage-gated sodium channels (VGSCs) as promising anticonvulsant drug candidates. Bioorg. Chem. 2020, 94, 103355. [Google Scholar] [CrossRef]
- Chu, X.M.; Wang, C.; Wang, W.-L.; Liang, L.L.; Liu, W.; Gong, K.K.; Sun, K.L. Triazole derivatives and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2019, 166, 206–223. [Google Scholar] [CrossRef]
- Xu, M.; Peng, Y.; Zhu, L.; Wang, S.; Ji, J.; Rakesh, K.P. Triazole derivatives as inhibitors of Alzheimer’s disease: Current developments and structure-activity relationships. Eur. J. Med. Chem. 2019, 180, 656–672. [Google Scholar] [CrossRef]
- Khan, S.A.; Akhtar, M.J.; Gogoi, U.; Meenakshi, D.U.; Das, A. An Overview of 1,2,3-triazole-Containing Hybrids and Their Potential Anticholinesterase Activities. Pharmaceuticals 2023, 16, 179. [Google Scholar] [CrossRef]
- Sooknual, P.; Pingaew, R.; Phopin, K.; Ruankham, W.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and neuroprotective effects of novel chalcone-triazole hybrids. Bioorg. Chem. 2020, 105, 104384. [Google Scholar] [CrossRef]
- Manzoor, S.; Almarghalani, D.A.; James, A.W.; Raza, M.K.; Kausar, T.; Nayeem, S.M.; Hoda, N.; Shah, Z.A. Synthesis and Pharmacological Evaluation of Novel Triazole-Pyrimidine Hybrids as Potential Neuroprotective and Anti-neuroinflammatory Agents. Pharm. Res. 2023, 40, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug. Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Gorabi, A.M.; Talaei, S.; Beiraghdar, F.; Akbarzadeh, A.; Tarhriz, V.; Mellatyar, H. An overview on the treatments and prevention against COVID-19. Virol. J. 2023, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, T.; Takano, T.; Matsui, H.; Kobayashi, N.; Ōmura, S.; Hanaki, H. Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model. J. Antibiot. 2023, 76, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Misra, S. Antimicrobials in COVID-19: Strategies for treating a COVID-19 pandemic. J. Basic Clin. Physiol. Pharmacol. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W. Current and emerging immunomodulators for treatment of SARS-CoV-2 infection (COVID-19). Expert Opin. Pharmacother. 2022, 23, 623–628. [Google Scholar] [CrossRef]
- Fazio, S.; Bellavite, P. Early Multi-Target Treatment of Mild-to-Moderate COVID-19, Particularly in Terms of Non-Steroidal Anti-Inflammatory Drugs and Indomethacin. BioMed 2023, 3, 177–194. [Google Scholar] [CrossRef]
- Mallikanti, V.; Thumma, V.; Matta, R.; Valluru, K.R.; Sharma Konidena, L.N.; Boddu, L.S.; Pochampally, J. Synthesis, antimicrobial activity and molecular docking of novel benzimidazole conjugated 1,2,3-triazole analogues. Chem. Data Collect. 2023, 45, 101034. [Google Scholar] [CrossRef]
- Güzel, E.; Çevik, U.A.; Evren, A.E.; Bostancı, H.E.; Gül, U.D.; Kayış, U.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of Benzimidazole-1,2,4-triazole Derivatives as Potential Antifungal Agents Targeting 14α-Demethylase. ACS Omega 2023, 8, 4369–4384. [Google Scholar] [CrossRef]
- Ghobadi, E.; Hashemi, S.M.; Fakhim, H.; Hosseini-khah, Z.; Badali, H.; Emami, S. Design, synthesis and biological activity of hybrid antifungals derived from fluconazole and mebendazole. Eur. J. Med. Chem. 2023, 249, 115146. [Google Scholar] [CrossRef]
- Youssif, B.G.M.; Mohamed, Y.A.M.; Salim, M.T.A.; Inagaki, F.; Mukai, C.; Abdu-Allah, H.H.M. Synthesis of some benzimidazole derivatives endowed with 1,2,3-triazole as potential inhibitors of hepatitis C virus. Acta Pharm. 2016, 66, 219–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Humaidi, J.Y.; Shaaban, M.M.; Rezki, N.; Aouad, M.R.; Zakaria, M.; Jaremko, M.; Hagar, M.; Elwakil, B.H. 1,2,3-Triazole-Benzofused Molecular Conjugates as Potential Antiviral Agents against SARS-CoV-2 Virus Variants. Life 2022, 12, 1341. [Google Scholar] [CrossRef] [PubMed]
- Harkala, K.J.; Eppakayala, L.; Maringanti, T.C. Synthesis and biological evaluation of benzimidazole-linked 1,2,3-triazole congeners as agents. Org. Med. Chem. Lett. 2014, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, K.; Winska, P.; Skierka, K.; Wielechowska, M.; Bretner, M. Synthesis, in vitro antiproliferative activity and kinase profile of new benzimidazole and benzotriazole derivatives. Bioorg. Chem. 2017, 72, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sahay, I.I.; Ghalsasi, P.S. Synthesis of New 1,2,3-triazole Linked Benzimidazole Molecules as Anti-Proliferative Agents. Synth. Commun. 2017, 47, 825–834. [Google Scholar] [CrossRef]
- Aouad, M.R.; Soliman, M.A.; Alharbi, M.O.; Bardaweel, S.K.; Sahu, P.K.; Ali, A.A.; Messali, M.; Rezki, N.; Al-Soud, Y.A. Design, Synthesis and Anticancer Screening of Novel Benzothiazole-Piperazine-1,2,3-Triazole Hybrids. Molecules 2018, 23, 2788. [Google Scholar] [CrossRef] [Green Version]
- Bistrović, A.; Krstulović, L.; Harej, A.; Grbčić, P.; Sedić, M.; Koštrun, S.; Kraljević, S.P.; Bajić, M.; Raić-Malić, S. Design, synthesis and biological evaluation of novel benzimidazole amidines as potent multi-target inhibitors for the treatment of non-small cell lung cancer. Eur. J. Med. Chem. 2018, 143, 1616–1634. [Google Scholar] [CrossRef]
- Aouad, M.R.; Almehmadi, M.A.; Rezki, N.; Al-blewi, F.F.; Messali, M.; Ali, I. Design, click synthesis, anticancer screening and docking studies of novel benzothiazole-1,2,3-triazoles appended with some bioactive benzofused heterocycles. J. Mol. Struct. 2019, 1188, 153–164. [Google Scholar] [CrossRef]
- Meščić Macan, A.; Perin, N.; Jakopec, S.; Mioč, M.; Radić Stojković, M.; Kralj, M.; Hranjec, M.; Raić-Malić, S. Synthesis, antiproliferative activity and DNA/RNA-binding properties of mono- and bis-(1,2,3-triazolyl)-appended benzimidazo[1,2-a]quinoline derivatives. Eur. J. Med. Chem. 2020, 185, 111845. [Google Scholar] [CrossRef]
- Ashok, D.; Reddy, M.R.; Nagaraju, N.; Dharavath, R.; Ramakrishna, K.; Gundu, S.; Shravani, P.; Sarasija, M. Microwave-assisted synthesis and in vitro antiproliferative activity of some novel 1,2,3-triazole-based pyrazole aldehydes and their benzimidazole derivatives. Med. Chem. Res. 2020, 29, 699–706. [Google Scholar] [CrossRef]
- Djemoui, A.; Naouri, A.; Ridha Ouahrani, M.; Djemoui, D.; Lahcene, S.; Boualem Lahrech, M.; Boukenna, L.; Albuquerque, H.M.T.; Saher, L.; Rocha, D.H.A.; et al. A step-by-step synthesis of triazole-benzimidazole-chalcone hybrids: Anticancer activity in human cells+. J. Mol. Struct. 2020, 1204, 127487. [Google Scholar] [CrossRef]
- Sridhar Goud, N.; Pooladanda, V.; Chandra, K.M.; Soukya, P.S.L.; Alvala, R.; Kumar, P.; Nagaraj, C.; Dawn Bharath, R.; Qureshi, I.A.; Godugu, C.; et al. Novel benzimidazole-triazole hybrids as apoptosis inducing agents in lung cancer: Design, synthesis, 18F-radiolabeling & galectin-1 inhibition studies. Bioorg. Chem. 2020, 102, 104125. [Google Scholar] [CrossRef]
- Singu, P.S.; Chilakamarthi, U.; Mahadik, N.S.; Keerti, B.; Valipenta, N.; Mokale, S.N.; Nagesh, N.; Kumbhare, R.M. Benzimidazole-1,2,3-triazole hybrid molecules: Synthesis and study of their interaction with G-quadruplex DNA. RSC Med. Chem. 2021, 12, 416. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Mahboob Alam, M.; Elhenawy, A.A.; Malebari, A.M.; Nazreen, S. Synthesis, antiproliferative, docking and DFT studies of benzimidazole derivatives as EGFR inhibitors. J. Mol. Struct. 2022, 1253, 132265. [Google Scholar] [CrossRef]
- Husain, A.; Rashid, M.; Mishra, R.; Parveen, S.; Shin, D.-S.; Kumar, D. Benzimidazole bearing oxadiazole and triazolo-thiadiazoles nucleus: Design and synthesis as anticancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 5438–5444. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Rashid, M.; Shaharyar, M.; Siddiqui, A.A.; Mishra, R. Benzimidazole clubbed with triazolo-thiadiazoles and triazolo-thiadiazines: New anticancer agents. Eur. J. Med. Chem. 2013, 62, 785–798. [Google Scholar] [CrossRef]
- Temirak, A.; Shaker, Y.M.; Ragab, F.A.F.; Ali, M.M.; Ali, H.I.; El Diwani, H.I. Part I. Synthesis, biological evaluation and docking studies of new 2-furylbenzimidazoles as antiangiogenic agents. Eur. J. Med. Chem. 2014, 87, 868–880. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Awadallah, F.M.; Refaat, H.M.; Amin, K.M. Molecular docking simulation, synthesis and 3D pharmacophore studies of novel 2-substituted-5-nitro-benzimidazole derivatives as anticancer agents targeting VEGFR-2 and c-Met. Bioorg. Chem. 2018, 77, 457–470. [Google Scholar] [CrossRef]
- Celik, I.; Ayhan-Kılcıgil, G.; Guven, B.; Kara, Z.; Gurkan-Alp, A.S.; Karayel, A.; Onay-Besikci, A. Design, synthesis and docking studies of benzimidazole derivatives as potential EGFR inhibitors. Eur. J. Med. Chem. 2019, 173, 240–249. [Google Scholar] [CrossRef]
- Mancini, R.S.; Barden, C.J.; Weaver, D.F.; Reed, M.A. Furazans in Medicinal Chemistry. J. Med. Chem. 2021, 64, 1786–1815. [Google Scholar] [CrossRef] [PubMed]
- Kus, C.; Ayhan-Kilcigil, G.; Can Eke, B.; IŞcan, M. Synthesis and antioxidant properties of some novel benzimidazole derivatives on lipid peroxidation in the rat liver. Arch. Pharm. Res. 2004, 27, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ayhan-Kilcigil, G.; Kus, C.; Çoban, T.; Can-Eke, B.; Iscan, M. Synthesis and Antioxidant Properties of Novel Benzimidazole Derivatives. J. Enzyme Inhib. Med. Chem. 2004, 19, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Karaali, N. Synthesis of Some New Benzimidazole Derivatives Containing Chlorine and Investigation of Their Antioxidant and Anti-urease Activities. JOTCSA 2018, 5, 971–980. [Google Scholar] [CrossRef] [Green Version]
- Faraji, L.; Shahkarami, S.; Nadri, H.; Moradi, A.; Saeedi, M.; Foroumadi, A.; Ramazani, A.; Haririan, I.; Reza Ganjali, M.; Shafiee, A.; et al. Synthesis of novel benzimidazole and benzothiazole derivatives bearing a 1,2,3-triazole ring system and their acetylcholinesterase inhibitory activity. J. Chem. Res. 2017, 41, 30–35. [Google Scholar] [CrossRef]
- Unsal-Tan, O.; Ozadali-Sari, K.; Ayazgok, B.; Küçükkılınç, T.T.; Balkan, A. Novel 2-Arylbenzimidazole derivatives as multi-targeting agents to treat Alzheimer’s disease. Med. Chem. Res. 2017, 26, 1506–1515. [Google Scholar] [CrossRef]
- Acar Cevik, U.; Saglik, B.N.; Levent, S.; Osmaniye, D.; Kaya Cavuşoglu, B.; Ozkay, Y.; Kaplancikli, Z.A. Synthesis and AChE-Inhibitory Activity of New Benzimidazole Derivatives. Molecules 2019, 24, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Y.; Rehman, W.; Hussain, R.; Khan, S.; Malik, A.; Khan, M.; Liaqat, A.; Rasheed, L.; Begum, F.; Fazil, S.; et al. New biologically potent benzimidazole-based-triazole derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors along with molecular docking study. J. Heterocycl. Chem. 2022, 52, 2225–2239. [Google Scholar] [CrossRef]
- Özil, M.; Emirik, M.; Etlik, S.Y.; Kahveci, B. A simple and efficient synthesis of novel inhibitors of alpha-glucosidase based on benzimidazole skeleton and molecular docking studies. Bioorg. Chem. 2016, 68, 226–235. [Google Scholar] [CrossRef]
- Deswal, L.; Verma, V.; Kumar, D.; Kaushik, C.P.; Kumar, A.; Deswal, Y.; Punia, S. Synthesis and antidiabetic evaluation of benzimidazole-tethered 1,2,3-triazoles. Arch. Pharm. 2020, 353, 2000090. [Google Scholar] [CrossRef] [PubMed]
- Asemanipoor, N.; Mohammadi-Khanaposhtani, M.; Moradi, S.; Vahidi, M.; Asadi, M.; Faramarzi, M.A.; Mahdavi, M.; Biglar, M.; Larijani, B.; Hamedifar, H.; et al. Synthesis and biological evaluation of new benzimidazole-1,2,3-triazole hybrids as potential α-glucosidase inhibitors. Bioorg. Chem. 2020, 95, 103482. [Google Scholar] [CrossRef]
- Esfahani, A.N.; Iraji, A.; Alamir, A.; Moradi, S.; Sadegh Asgari, M.; Hosseini, S.; Mojtabavi, S.; Nasli-Esfahani, E.; Faramarzi, M.A.; Bandarian, F.; et al. Design and synthesis of phenoxymethybenzoimidazole incorporating different aryl thiazole-triazole acetamide derivatives as α-glycosidase inhibitors. Mol. Divers. 2022, 26, 1995–2009. [Google Scholar] [CrossRef]
- Devi, M.; Kumar, P.; Singh, R.; Sindhu, J.; Kataria, R. Design, synthesis, spectroscopic characterization, single crystal X-ray analysis, in vitro α-amylase inhibition assay, DPPH free radical evaluation and computational studies of naphtho [2,3-d]imidazole-4,9-dione appended 1,2,3-triazoles. Eur. J. Med. Chem. 2023, 250, 115230. [Google Scholar] [CrossRef] [PubMed]
- Nandha, B.; Ramareddy, S.A.; Kuntal, H. Synthesis of substituted fluorobenzimidazoles as inhibitors of 5-lipoxygenase and soluble epoxide hydrolase for anti-inflammatory activity. Arch. Pharm. 2018, 351, 1800030. [Google Scholar] [CrossRef] [PubMed]
- Kondengadan, S.M.; Bansal, S.; Yang, C.; Liu, D.; Fultz, Z.; Wang, B. Click chemistry and drug delivery: A bird’s-eye view. Acta Pharm. Sin. B 2023, 13, 1990–2016. [Google Scholar] [CrossRef]
- de Souza, R.O.M.A.; de Mariz Miranda, L.S. Strategies towards the Synthesis of N2-Substituted 1,2,3-Triazoles. An. Acad. Bras. Ciênc. 2019, 91 (Suppl. S1), e20180751. [Google Scholar] [CrossRef]
- Dai, J.; Tian, S.; Yang, X.; Liu, Z. Synthesis methods of 1,2,3-/1,2,4-triazoles: A review. Front. Chem. 2022, 10, 891484. [Google Scholar] [CrossRef]
- De Nino, A.; Maiuolo, L.; Costanzo, P.; Algieri, V.; Jiritano, A.; Olivito, F.; Tallarida, M.A. Recent Progress in Catalytic Synthesis of 1,2,3-Triazoles. Catalysts 2021, 11, 1120. [Google Scholar] [CrossRef]
- Koranne, A.; Kurrey, K.; Kumar, P.; Gupta, S.; Jha, K.V.; Ravi, R.; Sahu, K.P.; Anamika; Jha, A.K. Metal catalyzed C–H functionalization on triazole rings. RSC Adv. 2022, 12, 27534. [Google Scholar] [CrossRef]
- Marinescu, M. Chemistry and Applications of Benzimidazole and Its Derivatives; IntechOpen: London, UK, 2019; pp. 1–213. [Google Scholar]
- Marinescu, M. Chiral benzimidazoles in medicinal chemistry: Syntheses and applications. In Benzimidazole: Preparation and Applications; Vestergaard, A.A., Ed.; Nova Science Publishers: New York, NY, USA, 2020; pp. 87–112. [Google Scholar]
- Zalaru, C.-M.; Marinescu, M. Benzimidazole compounds with anti-tumor and antibacterial activities. In Benzimidazole: Preparation and Applications; Vestergaard, A.A., Ed.; Nova Science Publishers: New York, NY, USA, 2020; pp. 221–250. [Google Scholar]
- Marinescu, M.; Tudorache, D.G.; Marton, G.I.; Zalaru, C.M.; Popa, M.; Chifiriuc, M.C.; Stavarache, C.E.; Constantinescu, C. Density functional theory molecular modeling, chemical synthesis, and antimicrobial behaviour of selected benzimidazole derivatives. J. Mol. Struct. 2017, 1130, 463–471. [Google Scholar] [CrossRef]
- Marinescu, M.; Cinteza, L.O.; Marton, G.I.; Chifiriuc, M.C.; Popa, M.; Stanculescu, I.; Zalaru, C.M.; Stavarache, C.E. Synthesis, density functional theory study and in vitro antimicrobial evaluation of new benzimidazole Mannich bases. BMC Chem. 2020, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M. Nitrogen-containing heterocycles as corrosion inhibitors. In Corrosion Inhibitors: An Overview; Wilkerson, R., Ed.; Nova Science Publishers: New York, NY, USA, 2021; pp. 161–201. [Google Scholar]
- Qiu, J.; Zou, Y.; Li, S.; Yang, L.; Qiu, Z.; Kong, F.; Gu, X. Discovery of benzimidazole substituted 1, 2, 4-oxadiazole compounds as novel anti-HBV agents with TLR8-agonistic activities. Eur. J. Med. Chem. 2022, 244, 114833. [Google Scholar] [CrossRef] [PubMed]
- Youssif, B.G.M.; Abdel-Moty, S.G.; Sayed, Y.B. Synthesis and biologicalevaluation of some novel 1,2,3-triazol-N-arylidene acetohydrazide incorporating benzimidazole ring moiety as potential antimicrobial agents. J. Curr. Chem. Pharm. Sc. 2014, 4, 54–64. [Google Scholar]
- Al-blewi, F.F.; Almehmadi, M.A.; Aouad, M.R.; Bardaweel, S.K.; Sahu, P.K.; Messali, M.; Rezki, N.; El Ashry, L.S.H. Design, synthesis, ADME prediction and pharmacological evaluation of novel benzimidazole-1,2,3-triazole-sulfonamide hybrids as antimicrobial and antiproliferative agents. Chem. Cent. J. 2018, 12, 110. [Google Scholar] [CrossRef] [Green Version]
- Biswas, D.; Roy, S.; Sen, S. A simple approach for indexing the oral drug likeness of a compound: Discriminating drug like compounds from nondrug like ones. J. Chem. Inf. Model. 2006, 46, 1394–1401. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feene, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Abdelmonsef, A.H.; Abou-Krisha, M.M.; Yousef, T.A. Synthesis, Identification, Computer-Aided Docking Studies, and ADMET Prediction of Novel Benzimidazo-1,2,3-triazole Based Molecules as Potential Antimicrobial Agents. Molecules 2021, 26, 7119. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. In Chemical Biology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1263, pp. 243–250. ISBN 9780123944474. [Google Scholar]
- Ouahrouch, A.; Ighachane, H.; Taourirte, M.; Engels, J.W.; Sedra, M.H.; Lazrek, H.B. Benzimidazole-1,2,3-triazole Hybrid Molecules: Synthesis and Evaluation for Antibacterial/Antifungal Activity. Arch. Pharm. Chem. Life Sci. 2014, 347, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Bistrović, A.; Krstulović, L.; Stolić, I.; Drenjancević, D.; Talapko, J.; Taylor, M.C.; Kelly, J.M.; Bajić, M.; Raic-Malić, S. Synthesis, anti-bacterial and anti-protozoal activities of amidinobenzimidazole derivatives and their interactions with DNA and RNA. J. Enzyme Inhib. Med. Chem. 2018, 33, 1323–1334. [Google Scholar] [CrossRef]
- Rao, Y.J.; Sowjanya, T.; Thirupathi, G.; Murthy, N.Y.S.; Kotapalli, S.S. Synthesis and biological evaluation of novel flavone/triazole/benzimidazole hybrids and flavone/isoxazole-annulated heterocycles as antiproliferative and antimycobacterial agents. Mol. Div. 2018, 22, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Ashok, D.; Gundu, S.; Aamate, V.K.; Devulapally, M.G. Conventional andmicrowave-assisted synthesis of new indole-tethered benzimidazole-based 1,2,3-triazoles and evaluation of their antimycobacterial, antioxidant and antimicrobial activities. Mol. Div. 2018, 22, 769–778. [Google Scholar] [CrossRef]
- Actelion Pharmaceuticals Ltd. OSIRIS Property Explorer Software, Version 2; Actelion Pharmaceuticals Ltd.: Allschwil, Switzerland, 2021; Available online: http://www.organic-chemistry.org/prog/peo/ (accessed on 29 June 2023).
- Kurczab, R.; Kucwaj-Brysz, K.; Śliwa, P. The Significance of Halogen Bonding in Ligand–Receptor Interactions: The Lesson Learned from Molecular Dynamic Simulations of the D4 Receptor. Molecules 2020, 25, 91. [Google Scholar] [CrossRef] [Green Version]
- Chandrika, N.T.; Shrestha, S.K.; Ranjan, N.; Sharma, A.; Arya, D.P.; Garneau-Tsodikova, S. New Application of Neomycin B−Bisbenzimidazole Hybrids as Antifungal Agents. ACS Infect. Dis. 2018, 4, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Anouar, E.H.; Sebbar, G.; El Ibrahimi, B.; Srhir, M.; Hökelek, T.; Mague, J.T.; El Ghayati, L.; Sebbar, N.K.; Essassi, E.M. New 1,2,3-triazole containing benzimidazolone derivatives: Syntheses, crystal structures, spectroscopic characterizations, Hirshfeld surface analyses, DFT calculations, anti-corrosion property anticipation, and antibacterial activities. J. Mol. Struct. 2021, 1242, 130719. [Google Scholar] [CrossRef]
- Mohsen, D.H.; Radhi, A.J.; Shaheed, D.Q.; Abbas, H.K. Synthesis New Benzimidazole Derivatives as Antibacterial. J. Pharm. Negat. Results 2022, 13, 893–898. [Google Scholar] [CrossRef]
- Rezki, N. Green Microwave Synthesis and Antimicrobial Evaluation of Novel Triazoles. Org. Prep. Proc. Int. 2017, 49, 525–541. [Google Scholar] [CrossRef]
- Milite, C.; Amendola, G.; Nocentini, A.; Bua, S.; Cipriano, A.; Barresi, E.; Feoli, A.; Novellino, E.; Da Settimo, F.; Supuran, C.T.; et al. Novel 2-substituted-benzimidazole-6-sulfonamides as carbonic anhydrase inhibitors: Synthesis, biological evaluation against isoforms I, II, IX and XII and molecular docking studies. J. Enzyme Inhib. Med. Chem. 2019, 34, 1697–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparna, Y.; Nirmala, G.; Subhashini, N.J.P.; Sharada, L.N.; Sreekanth, S. Synthesis and Antimicrobial Activity of Novel Bis-1,2,3-triazol-1H-4-yl-substituted Aryl Benzimidazole-2-thiol Derivatives. Russ. J. Gen. Chem. 2020, 90, 1501–1506. [Google Scholar] [CrossRef]
- Gill, C.; Jadhav, G.; Shaikh, M.; Kale, R.; Ghawalkar, A.; Nagargoje, D.; Shiradkar, M. Clubbed [1,2,3] triazoles by fluorine benzimidazole: A novel approach to H37Rv inhibitors as a potential treatment for tuberculosis. Bioorg. Med. Chem. Lett. 2008, 18, 6244–6247. [Google Scholar] [CrossRef]
- Anand, A.; Kulkarni, M.V.; Joshi, S.D.; Dixit, S.R. One pot Click chemistry: A three component reaction for the synthesis of 2-mercaptobenzimidazole linked coumarinyl triazoles as anti-tubercular agents. Bioorg. Med. Chem. Lett. 2016, 26, 4709–4713. [Google Scholar] [CrossRef] [PubMed]
- Khanapurmath, N.; Kulkarni, M.V.; Joshi, S.D.; Kumar, G.N.A. A click chemistry approach for the synthesis of cyclic ureido tethered coumarinyl and 1-aza coumarinyl 1,2,3-triazoles as inhibitors of Mycobacterium tuberculosis H37Rv and their in silico studies. Bioorg. Med. Chem. 2019, 27, 115054. [Google Scholar] [CrossRef]
- Pandey, V.K.; Upadhyay, M.; Upadhyay, M.; Gupta, V.D.; Tandon, M. Benzimidazolyl quinolinyl mercaptotriazoles as potential antimicrobial and antiviral agents. Acta Pharm. 2005, 55, 47–56. [Google Scholar] [PubMed]
- Jadhav, G.R.; Shaikh, M.U.; Kale, R.P.; Shiradkar, M.R.; Gill, C.H. SAR study of clubbed [1,2,4]-triazolyl with fluorobenzimidazoles as antimicrobial and antituberculosis agents. Eur. J. Med. Chem. 2009, 44, 2930–2935. [Google Scholar] [CrossRef]
- Barot, K.P.; Manna, K.S.; Ghate, M.D. Design, synthesis and antimicrobial activities of some novel 1,3,4-thiadiazole, 1,2,4-triazole-5-thione and 1,3-thiazolan-4-one derivatives of benzimidazole. J. Saudi Chem. Soc. 2017, 21, S35–S43. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Wang, Y.; Wang, W.; Wang, S.; Xu, B.; Fan, G.; Dong, G.; Liu, Y.; Yao, J.; Miao, Z.; et al. Discovery of highly potent triazole antifungal derivatives by heterocycle-benzene bioisosteric replacement. Eur. J. Med. Chem. 2013, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-L.; Baathulaa, K.; Kannekanti, V.K.; Zhou, C.-H.; Cai, G.-X. Novel benzimidazole derived naphthalimide triazoles: Synthesis, antimicrobial activity and interactions with calf thymus DNA. Sci. China Chem. 2015, 58, 483–494. [Google Scholar] [CrossRef]
- Ahmadi, A. Synthesis an antibacterial evaluation of ome novel Mannich bases of benzimidazole derivatives. Bull. Chem. Soc. Ethiop. 2016, 30, 421–425. [Google Scholar] [CrossRef] [Green Version]
- Kankate, R.S.; Gide, P.S.; Belsare, D.P. Design, synthesis and antifungal evaluation of novel benzimidazole tertiary amine type of fluconazole analogues. Arab. J. Chem. 2019, 12, 2224–2235. [Google Scholar] [CrossRef] [Green Version]
- Pham, E.C.; Vi Le Thi, T.; Ly Hong, H.H.; Vo Thi, B.N.; Vong, L.B.; Vu, T.T.; Vo, D.D.; Nguyen, N.V.T.; Bao Le, K.N.; Truong, T.N. N,2,6-Trisubstituted 1H-benzimidazole derivatives as a new scaffold of antimicrobial and anticancer agents: Design, synthesis, in vitro evaluation, and in silico studies. RSC Adv. 2023, 13, 399–420. [Google Scholar] [CrossRef]
- Ahuja, R.; Sidhu, A.; Bala, A.; Arora, D.; Sharma, P. Structure based approach for twin-enzyme targeted benzimidazolyl-1,2,4-triazole molecular hybrids as antifungal agents. Arab. J. Chem. 2020, 13, 5832–5848. [Google Scholar] [CrossRef]
- Evren, A.E.; Celik, I.; Akar Cevik, U. Synthesis, molecular docking, in silico ADME and antimicrobial activity studies of some new benzimidazole-triazole derivatives. Cumhur. Sci. J. 2021, 42, 795–805. [Google Scholar]
- Ansari, K.F.; Lal, C.; Khitoliya, R.K. Synthesis and biological activity of some triazole-bearing benzimidazole derivatives. J. Serb. Chem. Soc. 2011, 76, 341–352. [Google Scholar] [CrossRef]
- Tien, C.N.; Cam, D.T.T.; Manh, H.B.; Dang, D.N. Synthesis and Antibacterial Activity of Some Derivatives of 2-Methylbenzimidazole Containing 1,3,4-Oxadiazole or 1,2,4-Triazole Heterocycle. J. Chem. 2016, 2016, 1507049. [Google Scholar] [CrossRef]
- Kantar, G.K.; Mentese, E.; Beris, F.S.; Şasmaz, S.; Kahveci, B. Synthesis and antimicrobial activity of some new triazole bridged benzimidazole substituted phthalonitrile and phthalocyanines. Rev. Roum. Chim. 2018, 63, 59–65. [Google Scholar]
- Nandwana, N.K.; Singh, R.P.; Patel, O.P.S.; Dhiman, S.; Saini, H.K.; Jha, P.N.; Kumar, A. Design and Synthesis of Imidazo/Benzimidazo[1,2-c] quinazoline Derivatives and Evaluation of Their Antimicrobial Activity. ACS Omega 2018, 3, 16338–16346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Majidi, S.M.H.; Ibrahim, H.A.R.; AL-issa, A.H. Synthesis and Identification of Some New Derivatives of ([Benzyl Thio) Benzimidazole N-(Methylene-5-Yl)]-4,5-Di Substituted 1,2,4-Triazole and Evaluation of Their Activity as Antimicrobial and Anti-Inflammatory Agents. Iraqi J. Sci. 2021, 62, 1054–1065. [Google Scholar] [CrossRef]
- El-masry, A.H.; Fahmy, H.H.; Ali Abdelwahed, S.H. Synthesis and Antimicrobial Activity of Some New Benzimidazole Derivatives. Molecules 2000, 5, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Menteşe, E.; Ülker, S.; Kahveci, B. Synthesis and study of α-glucosidase inhibitory, antimicrobial and antioxidant activities of some benzimidazole derivatives containing triazole, thiadiazole, oxaiazole and morpholine rings. Chem. Heterocycl. Comp. 2015, 50, 1671–1682. [Google Scholar] [CrossRef]
- Karale, B.K.; Nirmal, P.R.; Akolkar, H.N. Synthesis and in vitro biological screening of some benzimidazolyl anchored azoles. Ind. J. Chem. 2015, 54, 399–405. [Google Scholar]
- Madawali, I.M.; Gaviraj, E.N.; Kalyane, N.V.; Shivakumar, B. A Review On Substituted Benzimidazoles: Biologically Active Compounds. Am. J. Pharm. Tech. Res. 2019, 9, 256–274. [Google Scholar] [CrossRef]
- Eisa, H.M.; Barghash, A.-E.M.; Badr, S.M.; Farahat, A.A. Synthesis and antimicrobial activity of certain benzimidazole and fused benzimidazole derivatives. Ind. J. Chem. 2010, 49, 1515–1525. [Google Scholar]
- Nevade, S.A.; Lokapure, S.G.; Kalyane, N.V. Synthesis and Pharmacological Evaluation of Some Novel 2-Mercapto Benzimidazole Derivatives. Kor. Che. Soc. 2013, 57, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Can, N.O.; Çevik, U.A.; Saglik, B.N.; Levent, S.; Korkut, B.; Özkay, Y.; Kaplancikli, Z.A.; Koparal, A.S. Synthesis, Molecular Docking Studies, and Antifungal Activity Evaluation of New Benzimidazole-Triazoles as Potential Lanosterol 14α-Demethylase Inhibitors. I. Chem. 2017, 2017, 9387102. [Google Scholar] [CrossRef] [Green Version]
- Karaca Gençer, H.; Acar Çevik, U.; Levent, S.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgın, S.; Öztürk, Y. New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation. Molecules 2017, 22, 507. [Google Scholar] [CrossRef] [Green Version]
- Omar, R.A.; Koparir, P.; Sarac, K.; Koparir, M.; Safin, D. A novel coumarin-triazole-thiophene hybrid: Synthesis, characterization, ADMET prediction, molecular docking and molecular dynamics studies with a series of SARS-CoV-2 proteins. J. Chem. Sci. 2023, 135, 6. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.A.; Fahim, A.M.; Rizk, S.A. Microwave-assisted synthesis, antioxidant activity, docking simulation, and DFT analysis of diferent heterocyclic compounds. Sci. Rep. 2023, 13, 4999. [Google Scholar] [CrossRef] [PubMed]
- Chahat; Bhatia, R.; Kumar, B. p53 as a potential target for treatment of cancer: A perspective on recent advancements in small molecules with structural insights and SAR studies. Eur. J. Med. Chem. 2023, 247, 115020. [Google Scholar] [CrossRef] [PubMed]
- Aatif, M.; Raza, M.A.; Javed, K.; Nashre-ul-Islam, S.M.; Farhan, M.; Alam, M.W. Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review. Antibiotics 2022, 11, 1750. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Design and Synthesis of Novel Antimicrobial Agents. Antibiotics 2023, 12, 628. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Bakheit, A.H.; Al-Agamy, M.H.; Rashid, H.; Mostafa, G.A.E.; Al-Salahi, R. Benzo[g]quinazolines as antifungal against candidiasis: Screening, molecular docking, and QSAR investigations. Saudi Pharm. J. 2023, 31, 815–823. [Google Scholar] [CrossRef]
- Aryal, P.; Shakya, B.J. Synthesis, Cytotoxicity, Antibacterial and Antioxidant Activity of New 2-Substituted Benzimidazole Containing 1,2,4-Triazoles. Nepal Chem. Soc. 2023, 43, 34–45. [Google Scholar] [CrossRef]
- Nandha, B.; Nargund, L.V.G.; Nargund, S.L. Design and synthesis of some new imidazole and 1,2,4-triazole substituted fluorobenzimidazoles for antitubercular and antifungal activity. Pharma Chem. 2013, 5, 317–327. [Google Scholar]
- Karim, M.; Lo, V.-W.; Einav, S. Preparing for the next viral threat with broad-spectrum antivirals. J. Clin. Investig. 2023, 133, e170236. [Google Scholar] [CrossRef]
- Roy, A.; Roy, M.; Gacem, A.; Datta, S.; Zeyaullah, M.; Muzammil, K.; Farghaly, T.A.; Abdellattif, M.H.; Yadav, K.K.; Simal-Gandara, J. Role of bioactive compounds in the treatment of hepatitis: A review. Front. Pharmacol. 2022, 13, 1051751. [Google Scholar] [CrossRef]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Cox, R.M.; Wolf, J.D.; Plemper, R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat. Microbiol. 2021, 6, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Paglietti, G.; Boido, V.; Sparatore, F.; Marongiu, F.; Marongiu, E.; La Colla, P.; Loddo, R. Antiviral Activity of Benzimidazole Derivatives. I. Antiviral Activity of1-Substituted-2-[(Benzotriazol-1/2-yl)methyl]benzimidazoles). Chem. Biodivers. 2008, 5, 2386–2401. [Google Scholar] [CrossRef]
- Hillary, V.E.; Ceasar, S.A. An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines. Heliyon 2023, 9, e13952. [Google Scholar] [CrossRef] [PubMed]
- Polatoğlu, I.; Oncu-Oner, T.; Dalman, I.; Ozdogan, S. COVID-19 in early 2023: Structure, replication mechanism, variants of SARS-CoV-2, diagnostic tests, and vaccine & drug development studies. MedComm 2023, 4, e228. [Google Scholar] [CrossRef]
- El-Sebaey, S.A. Recent Advances in 1,2,4-Triazole Scaffolds as Antiviral Agents. Chem. Sel. 2020, 5, 11654–11680. [Google Scholar] [CrossRef]
- Ion, V.; Matei, A.; Constantinescu, C.; Ionita, I.; Marinescu, M.; Dinescu, M.; Emandi, A. Octahydroacridine thin films grown by matrix-assisted pulsed laser evaporation for non linear optical applications. Mater. Sci. Semicond. Process. 2015, 36, 78–83. [Google Scholar] [CrossRef]
- Zalaru, C.; Dumitrascu, F.; Draghici, C.; Tarcomnicu, I.; Tatia, R.; Moldovan, L.; Chifiriuc, M.C.; Lazar, V.; Marinescu, M.; Nitulescu, M.G.; et al. Synthesis, spectroscopic characterization, DFT study and antimicrobial activity of novel alkylaminopyrazole derivatives. J. Mol. Struct. 2018, 1156, 12–21. [Google Scholar] [CrossRef]
- Zalaru, C.; Dumitrascu, F.; Draghici, C.; Iovu, M.; Marinescu, M.; Tarcomnicu, I.; Nitulescu, G.M. Synthesis and biological screening of some novel 2-(1H-pyrazol-1-yl)-acetamides as lidocaine analogue. Ind. J. Chem. B 2014, 53, 733–739. [Google Scholar]





















































| Compound | Gram-Positive Organisms | Gram-Negative Organisms | Fungi Organisms | |||
|---|---|---|---|---|---|---|
| B.c. | S.a. | P.a. | E.c. | A.b. | C.a. | |
| 6a | 64 | 64 | 256 | 128 | 128 | 128 |
| 6b | 128 | 128 | 128 | 128 | 256 | 256 |
| 6c | 256 | 128 | 256 | 64 | 256 | 156 |
| 6d | 256 | 128 | 256 | 64 | 256 | 256 |
| 6e | 256 | 128 | 256 | 64 | 256 | 256 |
| 6f | 512 | 512 | 256 | 256 | 512 | 512 |
| Ciprofloxacin | 8 | 4 | 8 | 4 | - | - |
| Hybrids | Inhibition Zone Diameters Using the Agar Diffusion Method (mm) | ||||
|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | A. niger | C. albicans | |
| 8a | 15 ± 0.14 | 12 ± 1.08 | 22 ± 1.01 | - | - |
| 8b | - | 5 ± 0.2 | - | 30 ± 1.16 | 27 ± 1.1 |
| 9a | 23 ± 0.8 | - | 13 ± 0.65 | - | - |
| 9b | - | - | 12 ± 0.8 | 14 ± 0.15 | 19 ± 1.04 |
| 10a | 24 ± 0.6 | 25 ± 0.9 | 17 ± 0.75 | 20 ± 0.9 | 16 ± 0.89 |
| 10b | 29 ± 1.2 | 21 ± 1.14 | 19 ± 0.79 | 18 ± 0.12 | 14 ± 0.58 |
| Ciprofloxacin | 20 ± 0.9 | 23 ± 1.02 | 21 ± 0.9 | - | - |
| Nystatin | - | - | - | 22 ± 0.18 | 23 ± 1.15 |
| Compound | S. aureus | E. coli | B. subtilis | S. epidermitis | A. niger | C. albicans |
|---|---|---|---|---|---|---|
| 35a | 0.028 | 0.056 | 0.056 | 0.056 | 0.056 | 0.056 |
| 35b | 0.031 | 0.062 | 0.062 | 0.062 | 0.062 | 0.062 |
| 35c | 0.029 | 0.058 | 0.058 | 0.058 | 0.058 | 0.058 |
| 35d | 0.060 | 0.030 | 0.060 | 0.030 | 0.060 | 0.060 |
| 35e | 0.029 | 0.056 | 0.056 | 0.056 | 0.056 | 0.056 |
| 35f | 0.026 | 0.052 | 0.052 | 0.052 | 0.052 | 0.052 |
| 35g | 0.031 | 0.026 | 0.052 | 0.026 | 0.026 | 0.026 |
| Norfloxacin | 0.020 | 0.039 | 0.039 | 0.039 | - | - |
| Fluconazole | - | - | - | - | 0.04 | 0.020 |
| Compound | Inhibition Zone Diameters Using the Agar Diffusion Method (mm) | |||
|---|---|---|---|---|
| S. aureus | P. aeruginosa | E. coli | S. typhosa | |
| 63a | 28 | 26 | 21 | 19 |
| 63b | 23 | 18 | 16 | 14 |
| 63c | 21 | 23 | 18 | 19 |
| 63d | 20 | 22 | 23 | 23 |
| 63e | 25 | 23 | 21 | 24 |
| 63f | 27 | 26 | 24 | 20 |
| 63g | 19 | 20 | 15 | 13 |
| 63h | 29 | 26 | 22 | 24 |
| 63i | 26 | 22 | 19 | 18 |
| 63j | 14 | 12 | 16 | 16 |
| 63k | 22 | 21 | 20 | 18 |
| 63l | 25 | 23 | 19 | 21 |
| 63m | 21 | 18 | 18 | 16 |
| 63n | 24 | 22 | 22 | 21 |
| 63o | 19 | 21 | 18 | 14 |
| Gentamycin | 34 | 35 | 31 | 30 |
| Compound | F. verticillioides | D. oryzae | C. lunata | F. fujikuroi |
|---|---|---|---|---|
| 74a | 35 | 50 | 28 | 45 |
| 74b | 30 | 25 | 18 | 30 |
| 74c | 16 | 12 | 10 | 15 |
| Carbendazim | 230 | - | - | 150 |
| Propiconazole | 20 | 25 | 22 | 21 |
| Compound | S. aureus | B. subtilis | S. mutans | P. aeruginosa | C. albicans |
|---|---|---|---|---|---|
| 88a | NT | NT | 16 | 16 | 32 |
| 88b | 8 | 16 | 16 | 16 | NT |
| 88c | 8 | 16 | 32 | 32 | 32 |
| Ampicillin | 2 | 2 | <1 | 4 | NT |
| Kanamycin | 2 | <1 | 4 | 2 | NT |
| Compound | Concentration (µg mL−1) | Aspergillus niger | Fusarium oxysporum |
|---|---|---|---|
| 89a | 50 | 50 | - |
| 89b | 50 | 50 | 50 |
| 89c | 50 | 50 | - |
| 89d | 50 | 50 | - |
| Compound (800 µg mL−1) | S. aureus | P. aerugnosa | B. subtilis | A. baumannii | C. albicans |
|---|---|---|---|---|---|
| 95 | 18 | 14 | 15 | - | 10 |
| 96 | 19 | 11 | 12 | - | 11 |
| 97 | 17 | 15 | 14 | 12 | - |
| Amoxicillin | 33 | 32 | 33 | - | - |
| Fluconazole | - | - | - | - | 25 |
| Compound | Minimum Inhibitory Concentrations (μg mL−1) | |||
|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | |||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | |
| 105a | 98 | - | 52 | - |
| 105b | - | - | 65 | - |
| 107a | 75 | 105 | 62 | - |
| 107b | 79 | - | 72 | - |
| Gentamycin * | 64 | 56 | 72 | 48 |
| No | Compound | Zone of Inhibition (mm) | ||
|---|---|---|---|---|
| E. coli | S. aureus | C. albicans | ||
| 1 | 109a | 15 | 13 | 18 |
| 2 | 109b | 13 | 11 | 12 |
| 3 | 109c | 17 | 16 | 14 |
| 4 | 109d | 12 | 13 | 16 |
| 5 | 109e | 13 | 17 | 9 |
| 6 | 109f | 10 | 8 | 11 |
| 7 | 109g | 8 | 11 | 12 |
| 8 | 109h | 12 | 7 | 10 |
| 9 | Ampicilline | 24 | 25 | - |
| 10 | Ketokonazole | - | - | 20 |
| Compound | C. albicans | G. glabrata | C. krusei | C. parapsilosis |
|---|---|---|---|---|
| 111a | 12.5 | 6.25 | 6.25 | 12.5 |
| 111b | 6.25 | 3.12 | 6.25 | 6.25 |
| 111c | 12.5 | 6.25 | 6.25 | 12.5 |
| 111d | 6.25 | 12.5 | 6.25 | 6.25 |
| 111e | 12.5 | 6.25 | 12.5 | 12.5 |
| 111f | 6.25 | 3.12 | 3.12 | 6.25 |
| 111g | 3.12 | 6.25 | 6.25 | 6.25 |
| 111h | 12.5 | 6.25 | 12.5 | 6.25 |
| 111i | 0.78 | 1.56 | 1.56 | 0.78 |
| 111j | 12.5 | 6.25 | 12.5 | 12.5 |
| 111k | 12.5 | 6.25 | 12.5 | 12.5 |
| 111l | 6.25 | 12.5 | 6.25 | 12.5 |
| 111m | 3.12 | 3.12 | 3.12 | 6.25 |
| 111n | 3.12 | 3.12 | 1.56 | 3.12 |
| 111o | 3.12 | 3.12 | 6.25 | 6.25 |
| 111p | 12.5 | 12.52 | 6.25 | 6.25 |
| 111r | 6.25 | 3.12 | 3.12 | 3.12 |
| 111s | 0.78 | 1.56 | 1.56 | 0.78 |
| Ketokonazole | 0.78 | 1.56 | 1.56 | 1.56 |
| Fluconazole | 0.78 | 1.56 | 1.56 | 0.78 |
| Compd. | In Vitro | In Vivo | |||||
|---|---|---|---|---|---|---|---|
| CT50 (µg mL–1) | EC50 (µg mL–1) | TI | CPE Inhibition (%) | Dose (µg per Mouse per Day) | MST (days) | Protection (%) | |
| Anti-JEV | |||||||
| 59a | 125 | 4 | 31 | 30 | 200 | - | - |
| 59b | 125 | 8 | 16 | 90 | 200 | 4 | 16 |
| 59c | - | - | - | - | - | - | - |
| 59d | 125 | 4 | 31 | 30 | 200 | - | - |
| 59e | 250 | 62.5 | 4 | 50 | 200 | 2 | 10 |
| Anti-HSV | |||||||
| 59a | 125 | 62.5 | 2 | 33 | - | - | - |
| 59b | 125 | 62.5 | 2 | 46 | - | - | - |
| 59c | - | - | - | - | - | - | - |
| 59d | 125 | 31.25 | 4 | 53 | 200 | - | - |
| 59e | 250 | 7.8 | 32 | 64 | 200 | - | - |
| Compound | Anti-RSV Activity | Anti-BVDV Activity | Anti-YFV Activity | Anti-CVB2 Activity |
|---|---|---|---|---|
| 118 | 0.7 | - | - | - |
| 119 | 2.3 | - | - | - |
| 120 | 0.7 | >100 | 80 | >100 |
| 121 | 0.7 | 63 | >90 | >100 |
| 122 | 0.3 | 53 | >70 | >100 |
| 123 | 0.15 | 51 | >60 | >100 |
| 124 | 0.03 | - | - | - |
| 125 | 0.7 | - | - | - |
| 126 | 0.06 | 90 | >100 | >100 |
| 127 | 0.1 | 72 | >54 | >100 |
| 128 | 0.9 | 15 | 6 | 40 |
| 129 | 0.05 | 19 | >21 | >88 |
| 130 | 0.02 | 14 | >20 | 26 |
| 131 | 10.0 | - | - | - |
| 132 | 7.0 | - | - | - |
| 133 | 1.9 | 67 | >36 | >100 |
| 134 | >36 | 15 | >18 | >36 |
| 135 | 9 | - | - | - |
| 136 | 11 | 80 | >45 | >100 |
| 137 | 23.0 | 80 | 27 | >83 |
| 6-Azaurine | 1.2 | >100 | 26 | >100 |
| Compound | CC50 (µg mL−1) | EC50 (µg mL−1) | Selectivity Index (SI) |
|---|---|---|---|
| Ceftazidime | 1045.53 | 85.07 | 12.29 |
| 138 | 1065.51 | 155.05 | 6.87 |
| 139 | 1530.5 | 306.1 | 5.0 |
| 140 | 1028.28 | 80.4 | 12.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinescu, M. Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review. Antibiotics 2023, 12, 1220. https://doi.org/10.3390/antibiotics12071220
Marinescu M. Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review. Antibiotics. 2023; 12(7):1220. https://doi.org/10.3390/antibiotics12071220
Chicago/Turabian StyleMarinescu, Maria. 2023. "Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review" Antibiotics 12, no. 7: 1220. https://doi.org/10.3390/antibiotics12071220
APA StyleMarinescu, M. (2023). Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review. Antibiotics, 12(7), 1220. https://doi.org/10.3390/antibiotics12071220






