Anticariogenic Activity of Celastrol and Its Enhancement of Streptococcal Antagonism in Multispecies Biofilm
Abstract
:1. Introduction
2. Results
2.1. The Cytotoxicity and Antibacterial Properties of Celastrol against Oral Streptococci
2.2. Celastrol, at Relatively Low Concentrations, Inhibited Lactic Acid Production and Water-Insoluble Glucan Formation in Multispecies Biofilm
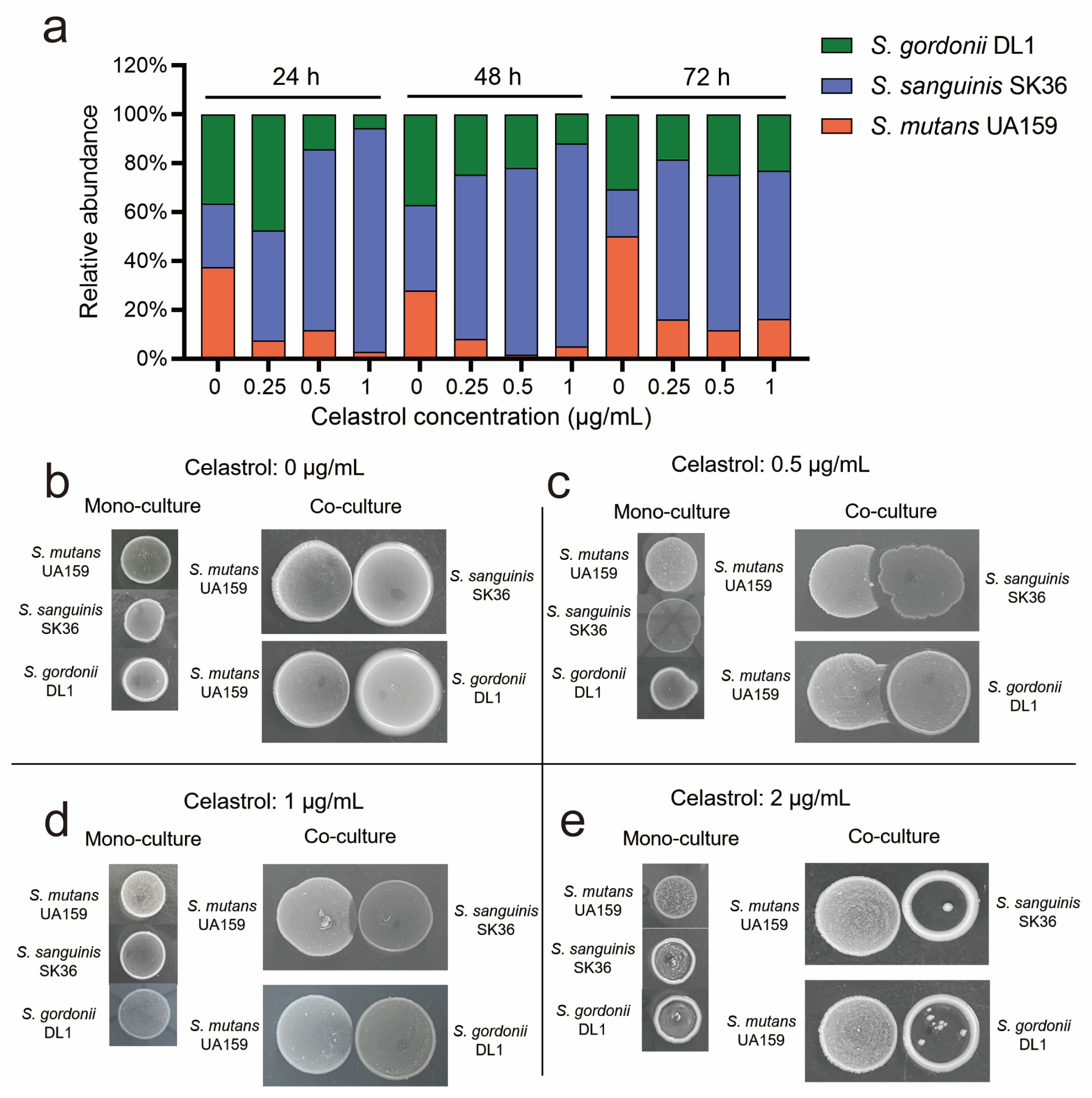
2.3. Celastrol Reduced S. mutans Proportion in Multispecies Biofilm by Promoting Streptococcal Antagonism
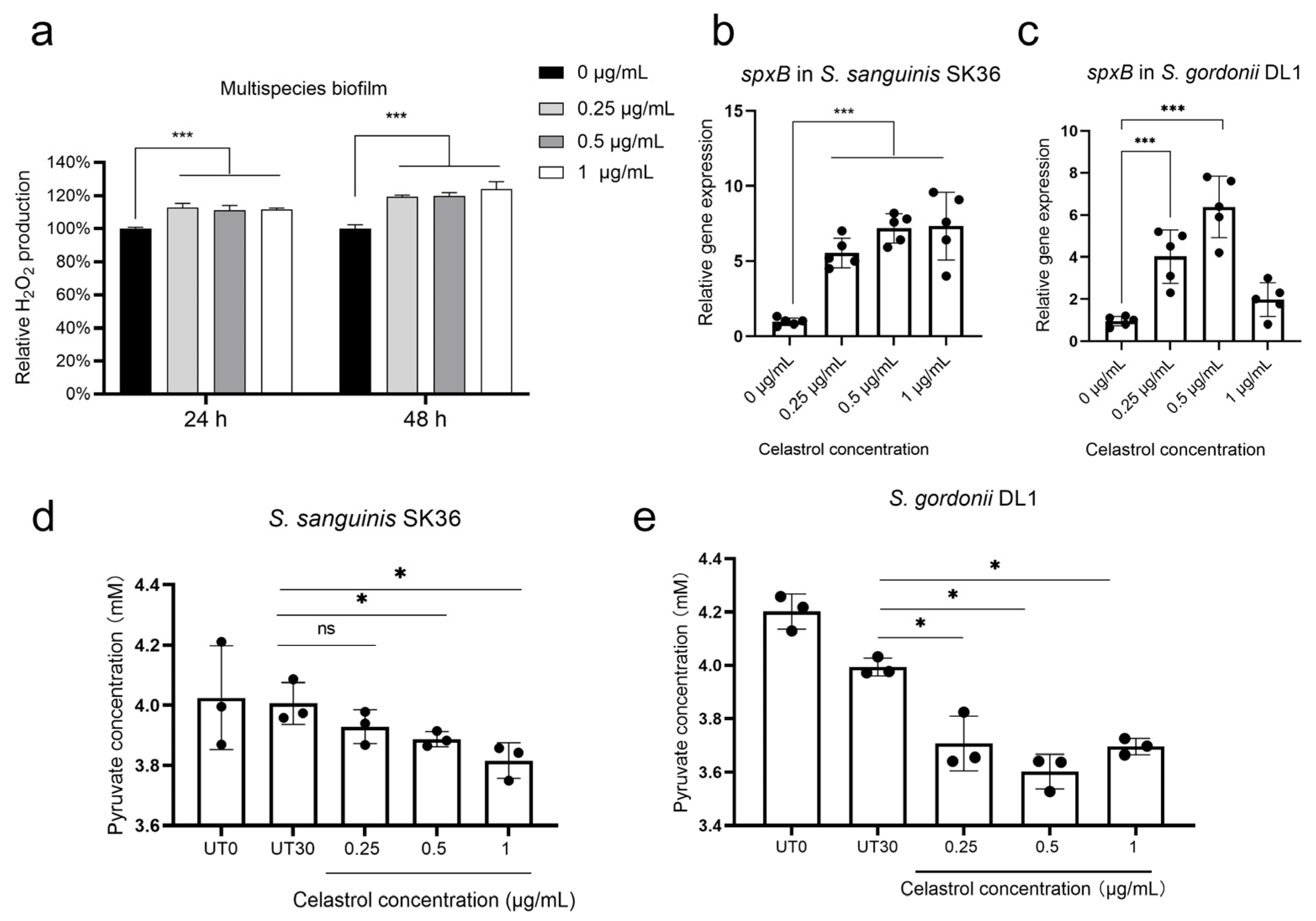
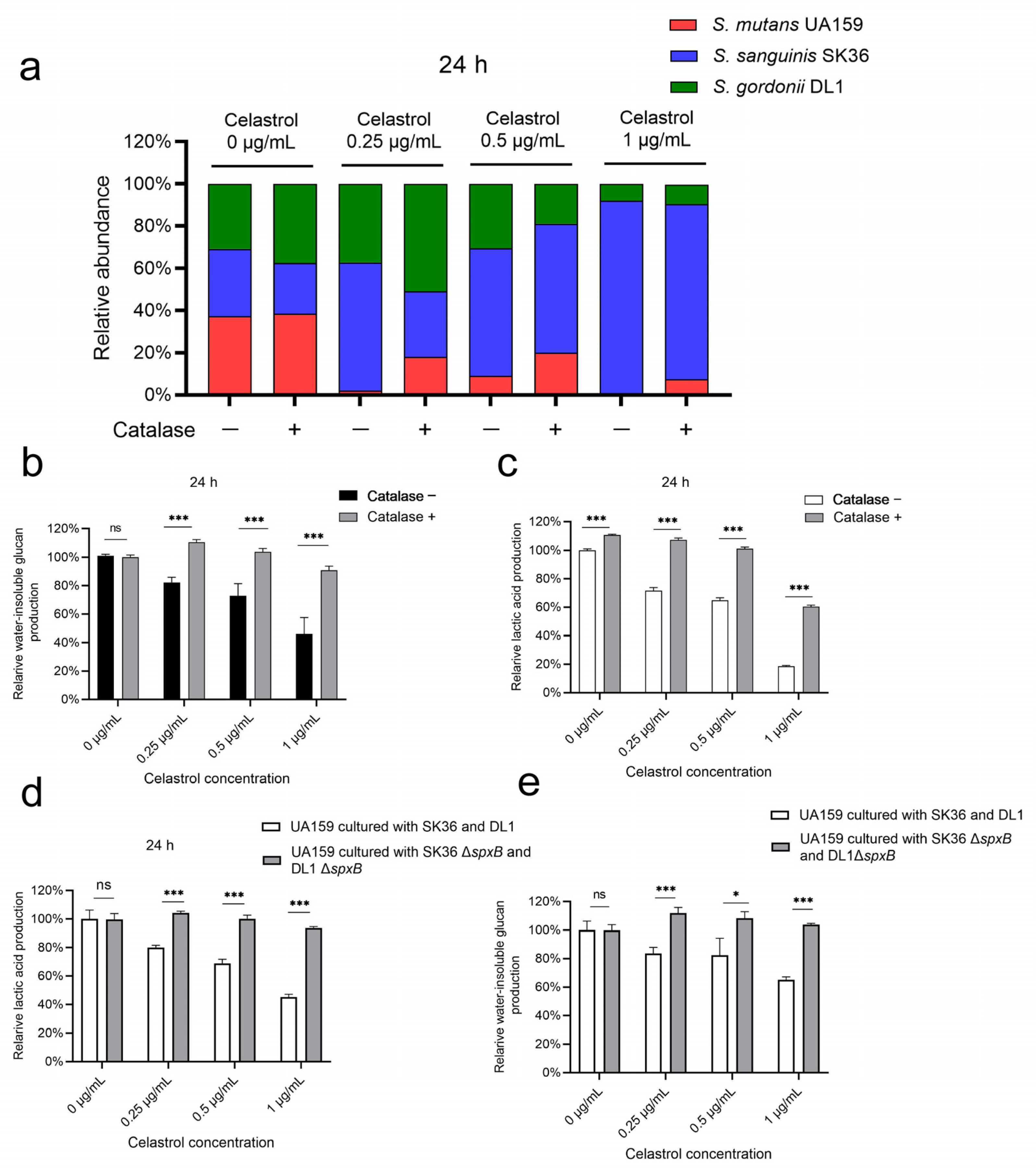
2.4. Celastrol Thwarted S. mutans Outgrowth by Promoting H2O2-Dependent Antagonism Mediated by SpxB
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Strains and Chemicals
5.2. Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Test of Celastrol
5.3. Cell Cytotoxicity Test
5.4. Measurement of Bacterial Growth Curves
5.5. Colony-Forming Unit (CFU) Counts
5.6. Lactic Acid and Water-Insoluble Glucan Measurement
5.7. SEM Observations of Biofilm Treated with Celastrol
5.8. DNA Isolation and Real-Time Polymerase Chain Reaction
5.9. Competition Assays on Agar Plate
5.10. H2O2 Measurements
5.11. Real-Time Quantitative PCR (qPCR) of spxB
5.12. Pyruvate Measurements
5.13. spxB Knockout Mutant Construction
5.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S94–S105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L. Rebalancing the Caries Microbiome Dysbiosis: Targeted Treatment and Sugar Alcohols. Adv. Dent. Res. 2018, 29, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Redanz, S.; Treerat, P.; Mu, R.; Redanz, U.; Zou, Z.; Koley, D.; Merritt, J.; Kreth, J. Pyruvate secretion by oral streptococci modulates hydrogen peroxide dependent antagonism. ISME J. 2020, 14, 1074–1088. [Google Scholar] [CrossRef]
- Zhu, B.; Macleod, L.C.; Kitten, T.; Xu, P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 2018, 13, 915–932. [Google Scholar]
- Jakubovics, N.S.; Gill, S.R.; Vickerman, M.M.; Kolenbrander, P.E. Role of hydrogen peroxide in competition and cooperation between Streptococcus gordonii and Actinomyces naeslundii. FEMS Microbiol. Ecol. 2008, 66, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S12–S22. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, Z.; Hwang, G.; Koo, H. Therapeutic Strategies Targeting Cariogenic Biofilm Microenvironment. Adv. Dent. Res. 2018, 29, 86–92. [Google Scholar] [CrossRef]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [Green Version]
- Jijakli, K.; Jensen, P.A. Metabolic Modeling of Streptococcus mutans Reveals Complex Nutrient Requirements of an Oral Pathogen. mSystems 2019, 4, e00529-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [PubMed]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar]
- Kim, D.; Sengupta, A.; Niepa, T.H.R.; Lee, B.-H.; Weljie, A.; Freitas-Blanco, V.S.; Murata, R.M.; Stebe, K.J.; Lee, D.; Koo, H. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci. Rep. 2017, 7, 41332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Chu, W.; Luo, J.; Yang, J.; He, L.; Li, J. Dental Materials for Oral Microbiota Dysbiosis: An Update. Front. Cell. Infect. Microbiol. 2022, 12, 900918. [Google Scholar] [PubMed]
- Diaz, P.I.; Valm, A.M. Microbial Interactions in Oral Communities Mediate Emergent Biofilm Properties. J. Dent. Res. 2020, 99, 18–25. [Google Scholar]
- Qiu, W.; Zhou, Y.; Li, Z.; Huang, T.; Xiao, Y.; Cheng, L.; Peng, X.; Zhang, L.; Ren, B. Application of Antibiotics/Antimicrobial Agents on Dental Caries. Biomed. Res. Int. 2020, 2020, 5658212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Zhang, K.; Zhang, N.; Melo, M.A.S.; Weir, M.D.; Zhou, X.D.; Bai, Y.X.; Reynolds, M.A.; Xu, H.H.K. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. J. Dent. Res. 2017, 96, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Feng, Z.; Lyu, X.; Zhang, L. Novel Lactotransferrin-Derived Antimicrobial Peptide LF-1 Inhibits the Cariogenic Virulence Factors of Streptococcus mutans. Antibiotics 2023, 12, 563. [Google Scholar]
- Brookes, Z.L.S.; Belfield, L.A.; Ashworth, A.; Casas-Agustench, P.; Raja, M.; Pollard, A.J.; Bescos, R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021, 113, 103768. [Google Scholar]
- Bottery, M.J.; Pitchford, J.W.; Friman, V.P. Ecology and evolution of antimicrobial resistance in bacterial communities. ISME J. 2021, 15, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Chatzigiannidou, I.; Teughels, W.; Van de Wiele, T.; Boon, N. Oral biofilms exposure to chlorhexidine results in altered microbial composition and metabolic profile. NPJ Biofilms Microbiomes 2020, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Cascao, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 2017, 4, 69. [Google Scholar]
- Paris, D.; Ganey, N.J.; Laporte, V.; Patel, N.S.; Beaulieu-Abdelahad, D.; Bachmeier, C.; March, A.; Ait-Ghezala, G.; Mullan, M.J. Reduction of β-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J. Neuroinflamm. 2010, 7, 17. [Google Scholar]
- Venkatesha, S.H.; Dudics, S.; Astry, B.; Moudgil, K.D. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog. Dis. 2016, 74, ftw059. [Google Scholar] [PubMed] [Green Version]
- Shi, J.; Li, J.; Xu, Z.; Chen, L.; Luo, R.; Zhang, C.; Gao, F.; Zhang, J.; Fu, C. Celastrol: A Review of Useful Strategies Overcoming its Limitation in Anticancer Application. Front. Pharmacol. 2020, 11, 558741. [Google Scholar]
- Kyriakou, E.; Schmidt, S.; Dodd, G.T.; Pfuhlmann, K.; Simonds, S.E.; Lenhart, D.; Geerlof, A.; Schriever, S.C.; De Angelis, M.; Schramm, K.W.; et al. Celastrol Promotes Weight Loss in Diet-Induced Obesity by Inhibiting the Protein Tyrosine Phosphatases PTP1B and TCPTP in the Hypothalamus. J. Med. Chem. 2018, 61, 11144–11157. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Montano, N.; de Leon Guerra, L.; Moujir, L. Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone Isolated from Natural Sources. Foods 2021, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Guo, W.; Zhang, Z.; Hu, X.; Nie, T.; Yang, X.; Li, C.; Wang, X.; Li, X.; et al. Antibacterial Activity of an FtsZ Inhibitor Celastrol and Its Synergistic Effect with Vancomycin against Enterococci In Vitro and In Vivo. Microbiol. Spectr. 2023, 11, e03699-22. [Google Scholar] [CrossRef] [PubMed]
- Yehia, F.A.A.; Yousef, N.; Askoura, M. Celastrol mitigates staphyloxanthin biosynthesis and biofilm formation in Staphylococcus aureus via targeting key regulators of virulence; in vitro and in vivo approach. BMC Microbiol. 2022, 22, 106. [Google Scholar]
- Featherstone, J.D.; Fontana, M.; Wolff, M. Novel Anticaries and Remineralization Agents: Future Research Needs. J. Dent. Res. 2018, 97, 125–127. [Google Scholar] [CrossRef]
- Relucenti, M.; Familiari, G.; Donfrancesco, O.; Taurino, M.; Li, X.; Chen, R.; Artini, M.; Papa, R.; Selan, L. Microscopy Methods for Biofilm Imaging: Focus on SEM and VP-SEM Pros and Cons. Biology 2021, 10, 51. [Google Scholar] [CrossRef]
- Li, Z.R.; Sun, J.; Du, Y.; Pan, A.; Zeng, L.; Maboudian, R.; Burne, R.A.; Qian, P.Y.; Zhang, W. Mutanofactin promotes adhesion and biofilm formation of cariogenic Streptococcus mutans. Nat. Chem. Biol. 2021, 17, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Redanz, S.; Cheng, X.; Giacaman, R.A.; Pfeifer, C.S.; Merritt, J.; Kreth, J. Live and let die: Hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol. Oral Microbiol. 2018, 33, 337–352. [Google Scholar] [PubMed]
- Philip, N.; Suneja, B.; Walsh, L.J. Ecological Approaches to Dental Caries Prevention: Paradigm Shift or Shibboleth? Caries Res. 2018, 52, 153–165. [Google Scholar] [CrossRef]
- Bertolini, M.; Costa, R.C.; Barão, V.A.R.; Villar, C.C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J.G. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [PubMed]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Cheng, X.; Wang, L.; Qiu, W.; Wang, S.; Zhou, Y.; Li, M.; Li, Y.; Cheng, L.; Li, J.; et al. Combinatorial effects of arginine and fluoride on oral bacteria. J. Dent. Res. 2015, 94, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Yang, Y.; Weir, M.D.; Dai, Q.; Lei, L.; Homayounfar, N.; Oates, T.W.; Yang, K.; Zhang, K.; Hu, T.; et al. Regulating Oral Biofilm from Cariogenic State to Non-Cariogenic State via Novel Combination of Bioactive Therapeutic Composite and Gene-Knockout. Microorganisms 2020, 8, 1410. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Browngardt, C.M.; Jiang, M.; Ahn, S.J.; Burne, R.A.; Nascimento, M.M. Diversity in Antagonistic Interactions between Commensal Oral Streptococci and Streptococcus mutans. Caries Res. 2018, 52, 88–101. [Google Scholar] [CrossRef]
- Baty, J.J.; Stoner, S.N.; Scoffield, J.A. Oral Commensal Streptococci: Gatekeepers of the Oral Cavity. J. Bacteriol. 2022, 204, e00257-22. [Google Scholar] [CrossRef] [PubMed]
- De León, L.; López, M.R.; Moujir, L. Antibacterial properties of zeylasterone, a triterpenoid isolated from Maytenus blepharodes, against Staphylococcus aureus. Microbiol. Res. 2010, 165, 617–626. [Google Scholar] [CrossRef]
- Wolska, K.; Grudniak, A.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Open Life Sci. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Singh, A. COVID-19: Disinfectants and sanitisers are changing microbiomes. BMJ 2020, 370, m2795. [Google Scholar]
- Naumova, E.A.; Gaengler, P.; Zimmer, S.; Arnold, W.H. Influence of individual saliva secretion on fluoride bioavailability. Open Dent. J. 2010, 4, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Rao, G.S. Dietary intake and bioavailability of fluoride. Ann. Rev. Nutr. 1984, 4, 115–136. [Google Scholar] [CrossRef]
- Cairns, J.; Ruokolainen, L.; Hultman, J.; Tamminen, M.; Virta, M.; Hiltunen, T. Ecology determines how low antibiotic concentration impacts community composition and horizontal transfer of resistance genes. Commun. Biol. 2018, 1, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisher, J.P.; Tsui, H.T.; Ramos-Montañez, S.; Hentchel, K.L.; Martin, J.E.; Trinidad, J.C.; Winkler, M.E.; Giedroc, D.P. Biological and Chemical Adaptation to Endogenous Hydrogen Peroxide Production in Streptococcus pneumoniae D39. mSphere 2017, 2, e00291-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anil, A.; Apte, S.; Joseph, J.; Parthasarathy, A.; Madhavan, S.; Banerjee, A. Pyruvate Oxidase as a Key Determinant of Pneumococcal Viability during Transcytosis across Brain Endothelium. J. Bacteriol. 2021, 203, e0043921. [Google Scholar] [CrossRef]
- Pericone, C.D.; Park, S.; Imlay, J.A.; Weiser, J.N. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J. Bacteriol. 2003, 185, 6815–6825. [Google Scholar] [CrossRef] [Green Version]
- Moreira, H.; Szyjka, A.; Paliszkiewicz, K.; Barg, E. Prooxidative Activity of Celastrol Induces Apoptosis, DNA Damage, and Cell Cycle Arrest in Drug-Resistant Human Colon Cancer Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6793957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Zhao, H.; Ding, C.; Jiang, D.; Zhao, Z.; Li, Y.; Ding, X.; Gao, J.; Zhou, H.; Luo, C.; et al. Celastrol suppresses colorectal cancer via covalent targeting peroxiredoxin 1. Signal Transduct. Target. Ther. 2023, 8, 51. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Luo, W.; Chen, S.; Lin, F.; Zhang, X.; Fan, S.; Shen, X.; Wang, Y.; Liang, G. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics 2020, 10, 10290–10308. [Google Scholar] [CrossRef]
- Gross, E.L.; Leys, E.J.; Gasparovich, S.R.; Firestone, N.D.; Schwartzbaum, J.A.; Janies, D.A.; Asnani, K.; Griffen, A.L. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J. Clin. Microbiol. 2010, 48, 4121–4128. [Google Scholar] [CrossRef] [Green Version]
- Tanzer, J.M.; Thompson, A.; Sharma, K.; Vickerman, M.M.; Haase, E.M.; Scannapieco, F.A. Streptococcus mutans out-competes Streptococcus gordonii in vivo. J. Dent. Res. 2012, 91, 513–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.-W.; Feng, J.-H.; Hu, X.-L.; Long, H.; Huang, X.-F.; Jiang, Z.-Z.; Zhang, X.-Q.; Ye, W.-C.; Wang, H. Synthesis and Biological Evaluation of Celastrol Derivatives as Potential Immunosuppressive Agents. J. Nat. Prod. 2020, 83, 2578–2586. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Kanabar, D.D.; Patel, K.; Ayehunie, S.; Muth, A.; Gupta, V. Enhanced solubility, stability, permeation and anti-cancer efficacy of Celastrol-β-cyclodextrin inclusion complex. J. Mol. Liq. 2020, 318, 113936. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Ren, B.; Li, H.; Weir, M.D.; Zhou, X.; Oates, T.W.; Cheng, L.; Xu, H.H.K. Do quaternary ammonium monomers induce drug resistance in cariogenic, endodontic and periodontal bacterial species? Dent. Mater. 2017, 33, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, F.; Zou, J.; Xu, H.H.K.; Han, Q.; Wang, Z.; Li, B.; Yang, B.; Ren, B.; Li, M.; et al. pH-Responsive Antibacterial Resin Adhesives for Secondary Caries Inhibition. J. Dent. Res. 2020, 99, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Y.; Zhou, X.; Zhu, C.; Han, Q.; Wang, H.; Xu, H.H.K.; Ren, B.; Cheng, L. Intelligent pH-responsive dental sealants to prevent long-term microleakage. Dent. Mater. 2021, 37, 1529–1541. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, L.; Huang, Y.; Jiang, Y.; Chu, C.H.; Peng, X.; Li, M.; Xu, H.H.K.; Zhou, X.; Ren, B. Resumptive Streptococcus mutans Persisters Induced From Dimethylaminododecyl Methacrylate Elevated the Cariogenic Virulence by Up-Regulating the Quorum-Sensing and VicRK Pathway Genes. Front. Microbiol. 2019, 10, 3102. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, A.; Suzuki, N.; Nakano, Y.; Kawada, M.; Oho, T.; Koga, T. Development of a 5′ nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens Streptococcus mutans and Streptococcus sobrinus. J. Clin. Microbiol. 2003, 41, 4438–4441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Nakano, Y.; Yoshida, A.; Yamashita, Y.; Kiyoura, Y. Real-time TaqMan PCR for quantifying oral bacteria during biofilm formation. J. Clin. Microbiol. 2004, 42, 3827–3830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Redanz, S.; Treerat, P.; Qin, H.; Choi, D.; Zhou, X.; Xu, X.; Merritt, J.; Kreth, J. Magnesium-Dependent Promotion of H2O2 Production Increases Ecological Competitiveness of Oral Commensal Streptococci. J. Dent. Res. 2020, 99, 847–854. [Google Scholar] [CrossRef]
- Cheng, X.; Redanz, S.; Cullin, N.; Zhou, X.; Xu, X.; Joshi, V.; Koley, D.; Merritt, J.; Kreth, J. Plasticity of the Pyruvate Node Modulates Hydrogen Peroxide Production and Acid Tolerance in Multiple Oral Streptococci. Appl. Environ. Microbiol. 2018, 84, e01697-17. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Okinaga, T.; Qi, F.; Zhang, Z.; Merritt, J. Cloning-independent and counterselectable markerless mutagenesis system in Streptococcus mutans. Appl. Environ. Microbiol. 2011, 77, 8025–8033. [Google Scholar] [CrossRef] [Green Version]



| Primers/Probes | Primer Sequence (5′–3′) | References |
|---|---|---|
| Primers: | ||
| UA159-F | GCCTACAGCTCAGAGATGCTATTCT | Yoshida et al., 2003 [66] |
| UA159-R | GCCATACACCACTCATGAATTGA | |
| SK36-F | GAGCGGATGGCCAATTATATCT | Zhang et al., 2015 [41] |
| SK36-R | CCGGATGATGTCGGCAATA | |
| DL1-F | GGTGTTGTTTGACCCGTTCAG | Suzuki et al., 2004 [67] |
| DL1-R | AGTCCATCCCACGAGCACAG | |
| Probes: | ||
| UA159 | FAM-TGGAAATGACGGTCGCCGTTATGAA-TAMRA | Yoshida et al., 2003 [66] |
| SK36 | FAM-TGTTCGGGCTCATGATA-Eclipse | Zhang et al., 2015 [41] |
| DL1 | FAM-AACCTTGACCCGCTCATTACCAGCTAGTATG- TAMRA | Suzuki et al., 2004 [67] |
| Genes | Primer Sequence (Forward and Reverse) |
|---|---|
| spxB SK36 | F: AATTCGGCGGCTCAATCG |
| R: AAGGATAGCAAGGAATGGAGTG | |
| spxB DL1 | F: TTGCAGTAGGTTCAGGTGGT |
| R: GGCAAGCTTCGTCAATCACT | |
| 16S rDNA SK36 | F: AAGCAACGCGAAGAACCTTA |
| R: GTCTCGCTAGAGTGCCCAAC | |
| 16S rDNA DL1 | F: GCTTGCTACACCATAGACTG |
| R: TCCTCATCACCATCCATAAAG |
| Primers | Primer Sequence |
|---|---|
| SK36: | |
| spxB-upF | ATACTTGAGCAATACTTAG |
| spxB-upR | GAGTGTTATTGTTGCTCGGCATAATAACTCTCCTTCAATA |
| spxB-dnF | GGTATACTACTGACAGCTTCGGATTGCAATCACGCGCAA |
| spxB-dnR | CCATCTTCAGTATCATAC |
| checkF | CAGAAGCCGGTGTTTTAC |
| checkR | ATATTCACTTTCCGTTGT |
| DL1: | |
| spxB-upF | ATAATGAACGGGTGGCCCAAG |
| spxB-upR | GAGTGTTATTGTTGCTCGGAAGAATAACTCTCCTTCAA |
| spxB-dnF | GGTATACTACTGACAGCTTCTTCTTCTCGTCGAAAATCAA |
| spxB-dnR | CGCTACCATCTTCTGTGTCG |
| checkF | GGCCAGCTCAAAAGAAGC |
| checkR | GTTGAAATACACGCTACCATC |
| ermF | CCGAGCAACAATAACACTC |
| ermR | GAAGCTGTCAGTAGTATACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Niu, C.; Luo, J.; Huang, Z.; Zhou, W. Anticariogenic Activity of Celastrol and Its Enhancement of Streptococcal Antagonism in Multispecies Biofilm. Antibiotics 2023, 12, 1245. https://doi.org/10.3390/antibiotics12081245
Li H, Niu C, Luo J, Huang Z, Zhou W. Anticariogenic Activity of Celastrol and Its Enhancement of Streptococcal Antagonism in Multispecies Biofilm. Antibiotics. 2023; 12(8):1245. https://doi.org/10.3390/antibiotics12081245
Chicago/Turabian StyleLi, Hao, Chenguang Niu, Junyuan Luo, Zhengwei Huang, and Wei Zhou. 2023. "Anticariogenic Activity of Celastrol and Its Enhancement of Streptococcal Antagonism in Multispecies Biofilm" Antibiotics 12, no. 8: 1245. https://doi.org/10.3390/antibiotics12081245






