Returning to Nature for the Next Generation of Antimicrobial Therapeutics
Abstract
1. Introduction
2. Natural Product Antibiotic Discovery
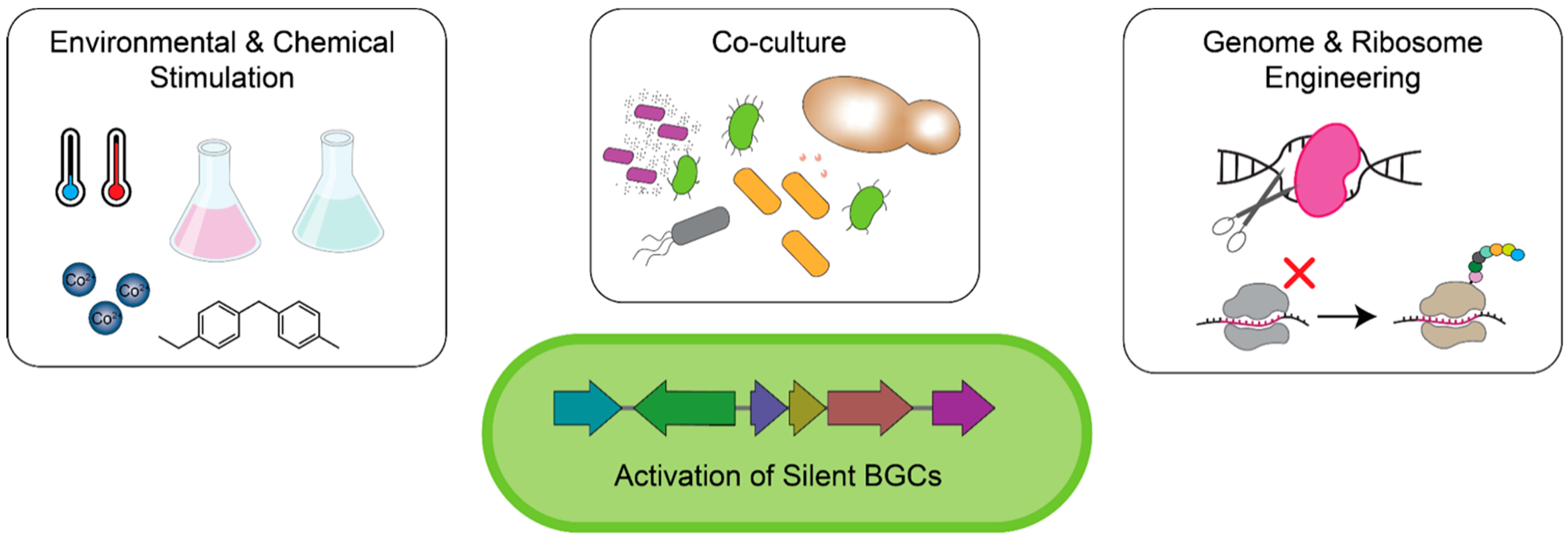
2.1. Reviving Natural Product Antibiotic Discovery in Traditional Antibiotic Producers
2.2. In Situ Cultivation of Previously Unculturable Microbes
2.3. Culture-Independent Mining for Natural Products
| Compound | Structure | Target | Discovery Approach | Comment | Ref. |
|---|---|---|---|---|---|
| Pestalone |  | Unknown | Produced by a marine fungus when co-cultured with a marine bacterium. | Potent activity against MRSA and VRE. | [36,40] |
| Corbomycin |  | Autolysin inhibition | Phylogenetic analysis of BGCs and resistance determinants predicted production of this novel glycopeptide. | Activity against Gram-positive bacteria. Low levels of resistance development. | [50,51] |
| Teixobactin |  | Lipid II Lipid III | In situ cultivation of a previously unculturable microbe. | Activity against Gram-positive bacteria. Low frequency of resistance. | [96] |
| Darobactin |  | BamA | In situ cultivation of a previously unculturable microbe. | Isolated from a nematode symbiont. Activity against Gram-negative bacteria. | [52,53] |
| Lassomycin |  | ClpC 1P1P2 | In situ cultivation of a previously unculturable microbe. | Narrow spectrum M. tuberculosis activity. | [67] |
| Malacidin A |  | Lipid II | Sequence-guided and culture-independent mining of BGCs. | Structurally distinct calcium-dependent antibiotic. Activity against Gram-positive bacteria. | [82] |
| MBA6 |  | Menaquinone | Sequence-guided and culture-independent mining of BGCs. | Menaquinone-targeting antimicrobials contain a conserved binding motif. Activity against Gram-positive bacteria. | [86] |
3. Microbiota-Based Therapeutics
3.1. Colonization Resistance
3.2. Fecal Microbiota Transplantation and Bacterial Consortia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial Competition: Surviving and Thriving in the Microbial Jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Aminov, R.I. The Role of Antibiotics and Antibiotic Resistance in Nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar] [CrossRef]
- Abdelghani, Z.; Hourani, N.; Zaidan, Z.; Dbaibo, G.; Mrad, M.; Hage-Sleiman, R. Therapeutic Applications and Biological Activities of Bacterial Bioactive Extracts. Arch. Microbiol. 2021, 203, 4755–4776. [Google Scholar] [CrossRef]
- Ramírez-Rendon, D.; Passari, A.K.; Ruiz-Villafán, B.; Rodríguez-Sanoja, R.; Sánchez, S.; Demain, A.L. Impact of Novel Microbial Secondary Metabolites on the Pharma Industry. Appl. Microbiol. Biotechnol. 2022, 106, 1855–1878. [Google Scholar] [CrossRef]
- Lewis, K. Recover the Lost Art of Drug Discovery. Nature 2012, 485, 439–440. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial Drug Discovery in the Resistance Era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Lewis, K. Platforms for Antibiotic Discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar]
- Baltz, R.H. Antimicrobials from Actinomycetes:Back to the Future. Microbe 2007, 2, 125–131. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for Bad Bugs: Confronting the Challenges of Antibacterial Discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of Antibacterial Discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for Emerging Pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Smith, P.A.; Koehler, M.F.T.; Girgis, H.S.; Yan, D.; Chen, Y.; Chen, Y.; Crawford, J.J.; Durk, M.R.; Higuchi, R.I.; Kang, J.; et al. Optimized Arylomycins Are a New Class of Gram-Negative Antibiotics. Nature 2018, 561, 189–194. [Google Scholar] [CrossRef]
- Roberts, K.D.; Zhu, Y.; Azad, M.A.K.; Han, M.-L.; Wang, J.; Wang, L.; Yu, H.H.; Horne, A.S.; Pinson, J.-A.; Rudd, D.; et al. A Synthetic Lipopeptide Targeting Top-Priority Multidrug-Resistant Gram-Negative Pathogens. Nat. Commun. 2022, 13, 1625. [Google Scholar] [CrossRef]
- Silver, L.L. Multi-Targeting by Monotherapeutic Antibacterials. Nat. Rev. Drug Discov. 2007, 6, 41–55. [Google Scholar] [CrossRef]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef]
- Silver, L.L. A Gestalt Approach to Gram-Negative Entry. Bioorganic Med. Chem. 2016, 24, 6379–6389. [Google Scholar] [CrossRef]
- Hughes, D.; Karlén, A. Discovery and Preclinical Development of New Antibiotics. Upsala J. Med. Sci. 2014, 119, 162–169. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-Mediated Colonization Resistance against Intestinal Pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, M.; Raffatellu, M. No Vacancy: How Beneficial Microbes Cooperate with Immunity To Provide Colonization Resistance to Pathogens. J. Immunol. 2015, 194, 4081–4087. [Google Scholar] [CrossRef]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007-19. [Google Scholar] [CrossRef] [PubMed]
- Simeis, D.D.; Serra, S. Actinomycetes: A Never-Ending Source of Bioactive Compounds—An Overview on Antibiotics Production. Antibiotics 2021, 10, 483. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0: Updates to the Secondary Metabolite Genome Mining Pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hwang, S.; Kim, J.; Cho, S.; Palsson, B.; Cho, B.-K. Mini Review: Genome Mining Approaches for the Identification of Secondary Metabolite Biosynthetic Gene Clusters in Streptomyces. Comput. Struct. Biotechnol. J. 2020, 18, 1548–1556. [Google Scholar] [CrossRef]
- Watve, M.G.; Tickoo, R.; Jog, M.M.; Bhole, B.D. How Many Antibiotics Are Produced by the Genus Streptomyces? Arch. Microbiol. 2001, 176, 386–390. [Google Scholar] [CrossRef]
- Cibichakravarthy, B.; Jose, P.A. Biosynthetic Potential of Streptomyces Rationalizes Genome-Based Bioprospecting. Antibiotics 2021, 10, 873. [Google Scholar] [CrossRef]
- Pauli, G.F.; Chen, S.-N.; Friesen, J.B.; McAlpine, J.B.; Jaki, B.U. Analysis and Purification of Bioactive Natural Products: The AnaPurNa Study. J. Nat. Prod. 2012, 75, 1243–1255. [Google Scholar] [CrossRef]
- Culp, E.J.; Yim, G.; Waglechner, N.; Wang, W.; Pawlowski, A.C.; Wright, G.D. Hidden Antibiotics in Actinomycetes Can Be Identified by Inactivation of Gene Clusters for Common Antibiotics. Nat. Biotechnol. 2019, 37, 1149–1154. [Google Scholar] [CrossRef]
- Sharma, R.; Jamwal, V.; Singh, V.P.; Wazir, P.; Awasthi, P.; Singh, D.; Vishwakarma, R.A.; Gandhi, S.G.; Chaubey, A. Revelation and Cloning of Valinomycin Synthetase Genes in Streptomyces Lavendulae ACR-DA1 and Their Expression Analysis under Different Fermentation and Elicitation Conditions. J. Biotechnol. 2017, 253, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Kim, J.; Oh, C.-H. Different Effects of Acidic PH Shock on the Prodiginine Production in Streptomyces Coelicolor M511 and SJM1 Mutants. J. Microbiol. Biotech. 2013, 23, 1454–1459. [Google Scholar] [CrossRef]
- Ripa, F.A.; Nikkon, F.; Zaman, S.; Khondkar, P. Optimal Conditions for Antimicrobial Metabolites Production from a New Streptomyces sp. RUPA-08PR Isolated from Bangladeshi Soil. Mycobiology 2009, 37, 211–214. [Google Scholar] [CrossRef]
- Tomm, H.A.; Ucciferri, L.; Ross, A.C. Advances in Microbial Culturing Conditions to Activate Silent Biosynthetic Gene Clusters for Novel Metabolite Production. J. Ind. Microbiol. Biot. 2019, 46, 1381–1400. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Edwards, C. Effects of Metals on a Range of Streptomyces Species. Appl. Environ. Microb. 1989, 55, 2030–2035. [Google Scholar] [CrossRef]
- Akhter, N.; Liu, Y.; Auckloo, B.N.; Shi, Y.; Wang, K.; Chen, J.; Wu, X.; Wu, B. Stress-Driven Discovery of New Angucycline-Type Antibiotics from a Marine Streptomyces Pratensis NA-ZhouS1. Mar. Drugs 2018, 16, 331. [Google Scholar] [CrossRef]
- Auckloo, B.N.; Pan, C.; Akhter, N.; Wu, B.; Wu, X.; He, S. Stress-Driven Discovery of Novel Cryptic Antibiotics from a Marine Fungus Penicillium sp. BB1122. Front. Microbiol. 2017, 8, 1450. [Google Scholar] [CrossRef] [PubMed]
- Okada, B.K.; Seyedsayamdost, M.R. Antibiotic Dialogues: Induction of Silent Biosynthetic Gene Clusters by Exogenous Small Molecules. FEMS Microbiol. Rev. 2017, 41, 19–33. [Google Scholar] [CrossRef]
- Craney, A.; Ozimok, C.; Pimentel-Elardo, S.M.; Capretta, A.; Nodwell, J.R. Chemical Perturbation of Secondary Metabolism Demonstrates Important Links to Primary Metabolism. Chem. Biol. 2012, 19, 1020–1027. [Google Scholar] [CrossRef]
- Pimentel-Elardo, S.M.; Sørensen, D.; Ho, L.; Ziko, M.; Bueler, S.A.; Lu, S.; Tao, J.; Moser, A.; Lee, R.; Agard, D.; et al. Activity-Independent Discovery of Secondary Metabolites Using Chemical Elicitation and Cheminformatic Inference. ACS Chem. Biol. 2015, 10, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Wang, X. Use of Bacterial Co-Cultures for the Efficient Production of Chemicals. Curr. Opin. Biotech. 2018, 53, 33–38. [Google Scholar] [CrossRef]
- Ueda, K.; Beppu, T. Antibiotics in Microbial Coculture. J. Antibiot. 2017, 70, 361–365. [Google Scholar] [CrossRef]
- Park, H.B.; Kwon, H.C.; Lee, C.-H.; Yang, H.O. Glionitrin A, an Antibiotic−Antitumor Metabolite Derived from Competitive Interaction between Abandoned Mine Microbes. J. Nat. Prod. 2009, 72, 248–252. [Google Scholar] [CrossRef]
- Sugiyama, R.; Nishimura, S.; Ozaki, T.; Asamizu, S.; Onaka, H.; Kakeya, H. Discovery and Total Synthesis of Streptoaminals: Antimicrobial (5,5)-Spirohemiaminals from the Combined-Culture of Streptomyces nigrescens and Tsukamurella pulmonis. Angew. Chem. Int. Ed. 2016, 55, 10278–10282. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, S.; Onaka, H.; Abe, I. Activation of Silent Biosynthetic Pathways and Discovery of Novel Secondary Metabolites in Actinomycetes by Co-Culture with Mycolic Acid-Containing Bacteria. J. Ind. Microbiol. Biot. 2019, 46, 363–374. [Google Scholar] [CrossRef]
- Augner, D.; Krut, O.; Slavov, N.; Gerbino, D.C.; Sahl, H.-G.; Benting, J.; Nising, C.F.; Hillebrand, S.; Kronke, M.; Schmalz, H.-G. On the Antibiotic and Antifungal Activity of Pestalone, Pestalachloride A, and Structurally Related Compounds. J. Nat. Prod. 2013, 76, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Oikawa, H.; Ogawa, H.; Hosono, K.; Shinmachi, F.; Takano, H.; Sakuda, S.; Beppu, T.; Ueda, K. Desferrioxamine E Produced by Streptomyces Griseus Stimulates Growth and Development of Streptomyces tanashiensis. Microbiology 2005, 151, 2899–2905. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a New Antibiotic Produced by a Marine Fungus in Response to Bacterial Challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Eto, D.; Watanabe, K.; Saeki, H.; Oinuma, K.; Otani, K.; Nobukuni, M.; Shiratori-Takano, H.; Takano, H.; Beppu, T.; Ueda, K. Divergent Effects of Desferrioxamine on Bacterial Growth and Characteristics. J. Antibiot. 2013, 66, 199–203. [Google Scholar] [CrossRef]
- Lee, N.; Kim, W.; Chung, J.; Lee, Y.; Cho, S.; Jang, K.-S.; Kim, S.C.; Palsson, B.; Cho, B.-K. Iron Competition Triggers Antibiotic Biosynthesis in Streptomyces Coelicolor during Coculture with Myxococcus Xanthus. ISME J. 2020, 14, 1111–1124. [Google Scholar] [CrossRef]
- Amano, S.; Morota, T.; Kano, Y.; Narita, H.; Hashidzume, T.; Yamamoto, S.; Mizutani, K.; Sakuda, S.; Furihata, K.; Takano-Shiratori, H.; et al. Promomycin, a Polyether Promoting Antibiotic Production in Streptomyces spp. J. Antibiot. 2010, 63, 486–491. [Google Scholar] [CrossRef]
- Amano, S.; Sakurai, T.; Endo, K.; Takano, H.; Beppu, T.; Furihata, K.; Sakuda, S.; Ueda, K. A Cryptic Antibiotic Triggered by Monensin. J. Antibiot. 2011, 64, 703. [Google Scholar] [CrossRef]
- Baltz, R.H. Marcel Faber Roundtable: Is Our Antibiotic Pipeline Unproductive Because of Starvation, Constipation or Lack of Inspiration? J. Ind. Microbiol. Biotechnol. 2006, 33, 507–513. [Google Scholar] [CrossRef]
- Caesar, L.K.; Montaser, R.; Keller, N.P.; Kelleher, N.L. Metabolomics and Genomics in Natural Products Research: Complementary Tools for Targeting New Chemical Entities. Nat. Prod. Rep. 2021, 38, 2041–2065. [Google Scholar] [CrossRef]
- Grienke, U.; Foster, P.A.; Zwirchmayr, J.; Tahir, A.; Rollinger, J.M.; Mikros, E. 1H NMR-MS-Based Heterocovariance as a Drug Discovery Tool for Fishing Bioactive Compounds out of a Complex Mixture of Structural Analogues. Sci. Rep. 2019, 9, 11113. [Google Scholar] [CrossRef]
- Volynkina, I.A.; Zakalyukina, Y.V.; Alferova, V.A.; Belik, A.R.; Yagoda, D.K.; Nikandrova, A.A.; Buyuklyan, Y.A.; Udalov, A.V.; Golovin, E.V.; Kryakvin, M.A.; et al. Mechanism-Based Approach to New Antibiotic Producers Screening among Actinomycetes in the Course of the Citizen Science Project. Antibiotics 2022, 11, 1198. [Google Scholar] [CrossRef]
- Martinez-Fructuoso, L.; Arends, S.J.R.; Freire, V.F.; Evans, J.R.; DeVries, S.; Peyser, B.D.; Akee, R.K.; Thornburg, C.C.; Kumar, R.; Ensel, S.; et al. Screen for New Antimicrobial Natural Products from the NCI Program for Natural Product Discovery Prefractionated Extract Library. ACS Infect. Dis. 2023, 9, 1245–1256. [Google Scholar] [CrossRef]
- Medema, M.H.; Fischbach, M.A. Computational Approaches to Natural Product Discovery. Nat. Chem. Biol. 2015, 11, 639–648. [Google Scholar] [CrossRef]
- Culp, E.J.; Waglechner, N.; Wang, W.; Fiebig-Comyn, A.A.; Hsu, Y.-P.; Koteva, K.; Sychantha, D.; Coombes, B.K.; Nieuwenhze, M.S.V.; Brun, Y.V.; et al. Evolution-Guided Discovery of Antibiotics That Inhibit Peptidoglycan Remodelling. Nature 2020, 578, 582–587. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, J.; Qiao, Y.; Lin, B.; Deng, Z.; Kong, L.; You, D. Genome Mining and Metabolic Profiling Reveal Cytotoxic Cyclodipeptides in Streptomyces Hygrospinosus Var. Beijingensis. Antibiotics 2022, 11, 1463. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Vizcaíno, A.; González, V.; Braña, A.F.; Palacios, J.J.; Otero, L.; Fernández, J.; Molina, A.; Kulik, A.; Vázquez, F.; Acuña, J.L.; et al. Pharmacological Potential of Phylogenetically Diverse Actinobacteria Isolated from Deep-Sea Coral Ecosystems of the Submarine Avilés Canyon in the Cantabrian Sea. Microb. Ecol. 2017, 73, 338–352. [Google Scholar] [CrossRef]
- Hui, M.L.-Y.; Tan, L.T.-H.; Letchumanan, V.; He, Y.-W.; Fang, C.-M.; Chan, K.-G.; Law, J.W.-F.; Lee, L.-H. The Extremophilic Actinobacteria: From Microbes to Medicine. Antibiotics 2021, 10, 682. [Google Scholar] [CrossRef]
- Imai, Y.; Meyer, K.J.; Iinishi, A.; Favre-Godal, Q.; Green, R.; Manuse, S.; Caboni, M.; Mori, M.; Niles, S.; Ghiglieri, M.; et al. A New Antibiotic Selectively Kills Gram-Negative Pathogens. Nature 2019, 459–464. [Google Scholar] [CrossRef]
- Kaur, H.; Jakob, R.P.; Marzinek, J.K.; Green, R.; Imai, Y.; Bolla, J.R.; Agustoni, E.; Robinson, C.V.; Bond, P.J.; Lewis, K.; et al. The Antibiotic Darobactin Mimics a β-Strand to Inhibit Outer Membrane Insertase. Nature 2021, 593, 125–129. [Google Scholar] [CrossRef]
- Ghequire, M.G.K.; Swings, T.; Michiels, J.; Buchanan, S.K.; Mot, R. de Hitting with a BAM: Selective Killing by Lectin-like Bacteriocins. mBio 2018, 9, e02138-17. [Google Scholar] [CrossRef]
- Storek, K.M.; Auerbach, M.R.; Shi, H.; Garcia, N.K.; Sun, D.; Nickerson, N.N.; Vij, R.; Lin, Z.; Chiang, N.; Schneider, K.; et al. Monoclonal Antibody Targeting the β-Barrel Assembly Machine of Escherichia Coli Is Bactericidal. Proc. Natl. Acad. Sci. USA 2018, 115, 3692–3697. [Google Scholar] [CrossRef]
- Hart, E.M.; Mitchell, A.M.; Konovalova, A.; Grabowicz, M.; Sheng, J.; Han, X.; Rodriguez-Rivera, F.P.; Schwaid, A.G.; Malinverni, J.C.; Balibar, C.J.; et al. A Small-Molecule Inhibitor of BamA Impervious to Efflux and the Outer Membrane Permeability Barrier. Proc. Natl. Acad. Sci. USA 2019, 116, 201912345. [Google Scholar] [CrossRef]
- Keseler, I.M.; Gama-Castro, S.; Mackie, A.; Billington, R.; Bonavides-Martínez, C.; Caspi, R.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Muñiz-Rascado, L.; et al. The EcoCyc Database in 2021. Front. Microbiol. 2021, 12, 711077. [Google Scholar] [CrossRef]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.-M.; Fournier, P.-E.; Raoult, D. Culturing the Human Microbiota and Culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef]
- Browne, H.P.; Forster, S.C.; Anonye, B.O.; Kumar, N.; Neville, B.A.; Stares, M.D.; Goulding, D.; Lawley, T.D. Culturing of ‘Unculturable’ Human Microbiota Reveals Novel Taxa and Extensive Sporulation. Nature 2016, 533, 543–546. [Google Scholar] [CrossRef]
- Ueda, K.; Yamashita, A.; Ishikawa, J.; Shimada, M.; Watsuji, T.; Morimura, K.; Ikeda, H.; Hattori, M.; Beppu, T. Genome Sequence of Symbiobacterium Thermophilum, an Uncultivable Bacterium That Depends on Microbial Commensalism. Nucleic Acids Res. 2004, 32, 4937–4944. [Google Scholar] [CrossRef]
- Bae, J.-W.; Rhee, S.-K.; Park, J.R.; Kim, B.-C.; Park, Y.-H. Isolation of Uncultivated Anaerobic Thermophiles from Compost by Supplementing Cell Extract of Geobacillus Toebii in Enrichment Culture Medium. Extremophiles 2005, 9, 477–485. [Google Scholar] [CrossRef]
- Nichols, D.; Lewis, K.; Orjala, J.; Mo, S.; Ortenberg, R.; O’Connor, P.; Zhao, C.; Vouros, P.; Kaeberlein, T.; Epstein, S.S. Short Peptide Induces an “Uncultivable” Microorganism to Grow In Vitro. Appl. Environ. Microb. 2008, 74, 4889–4897. [Google Scholar] [CrossRef]
- Kaeberlein, T.; Lewis, K.; Epstein, S.S. Isolating “Uncultivable” Microorganisms in Pure Culture in a Simulated Natural Environment. Science 2002, 296, 1127–1129. [Google Scholar] [CrossRef]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S.S. Use of Ichip for High-Throughput In Situ Cultivation of “Uncultivable” Microbial Species. Appl. Environ. Microb. 2010, 76, 2445–2450. [Google Scholar] [CrossRef]
- Zengler, K.; Toledo, G.; Rappé, M.; Elkins, J.; Mathur, E.J.; Short, J.M.; Keller, M. Cultivating the Uncultured. Proc. Natl. Acad. Sci. USA 2002, 99, 15681–15686. [Google Scholar] [CrossRef]
- Buerger, S.; Spoering, A.; Gavrish, E.; Leslin, C.; Ling, L.; Epstein, S.S. Microbial Scout Hypothesis and Microbial Discovery. Appl. Environ. Microb. 2012, 78, 3229–3233. [Google Scholar] [CrossRef]
- Buerger, S.; Spoering, A.; Gavrish, E.; Leslin, C.; Ling, L.; Epstein, S.S. Microbial Scout Hypothesis, Stochastic Exit from Dormancy, and the Nature of Slow Growers. Appl. Environ. Microb. 2012, 78, 3221–3228. [Google Scholar] [CrossRef]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A New Antibiotic Kills Pathogens without Detectable Resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Shukla, R.; Lavore, F.; Maity, S.; Derks, M.G.N.; Jones, C.R.; Vermeulen, B.J.A.; Melcrová, A.; Morris, M.A.; Becker, L.M.; Wang, X.; et al. Teixobactin Kills Bacteria by a Two-Pronged Attack on the Cell Envelope. Nature 2022, 608, 390–396. [Google Scholar] [CrossRef]
- Gavrish, E.; Sit, C.S.; Cao, S.; Kandror, O.; Spoering, A.; Peoples, A.; Ling, L.; Fetterman, A.; Hughes, D.; Bissell, A.; et al. Lassomycin, a Ribosomally Synthesized Cyclic Peptide, Kills Mycobacterium Tuberculosis by Targeting the ATP-Dependent Protease ClpC1P1P2. Chem. Biol. 2014, 21, 509–518. [Google Scholar] [CrossRef]
- Steen, A.D.; Crits-Christoph, A.; Carini, P.; DeAngelis, K.M.; Fierer, N.; Lloyd, K.G.; Thrash, J.C. High Proportions of Bacteria and Archaea across Most Biomes Remain Uncultured. ISME J. 2019, 13, 3126–3130. [Google Scholar] [CrossRef]
- Bodor, A.; Bounedjoum, N.; Vincze, G.E.; Kis, Á.E.; Laczi, K.; Bende, G.; Szilágyi, Á.; Kovács, T.; Perei, K.; Rákhely, G. Challenges of Unculturable Bacteria: Environmental Perspectives. Rev. Environ. Sci. Bio Technol. 2020, 19, 1–22. [Google Scholar] [CrossRef]
- Brady, S.F.; Chao, C.J.; Handelsman, J.; Clardy, J. Cloning and Heterologous Expression of a Natural Product Biosynthetic Gene Cluster from EDNA. Org. Lett. 2001, 3, 1981–1984. [Google Scholar] [CrossRef]
- Brady, S.F.; Clardy, J. Palmitoylputrescine, an Antibiotic Isolated from the Heterologous Expression of DNA Extracted from Bromeliad Tank Water. J. Nat. Prod. 2004, 67, 1283–1286. [Google Scholar] [CrossRef]
- Brady, S.F.; Chao, C.J.; Clardy, J. New Natural Product Families from an Environmental DNA (EDNA) Gene Cluster. J. Am. Chem. Soc. 2002, 124, 9968–9969. [Google Scholar] [CrossRef]
- Handelsman, J. Metagenomics: Application of Genomics to Uncultured Microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef]
- Garcia, J.A.L.; Fernández-Guerra, A.; Casamayor, E.O. A Close Relationship between Primary Nucleotides Sequence Structure and the Composition of Functional Genes in the Genome of Prokaryotes. Mol. Phylogenet Evol. 2011, 61, 650–658. [Google Scholar] [CrossRef]
- Owen, J.G.; Reddy, B.V.B.; Ternei, M.A.; Charlop-Powers, Z.; Calle, P.Y.; Kim, J.H.; Brady, S.F. Mapping Gene Clusters within Arrayed Metagenomic Libraries to Expand the Structural Diversity of Biomedically Relevant Natural Products. Proc. Natl. Acad. Sci. USA 2013, 110, 11797–11802. [Google Scholar] [CrossRef]
- Katz, M.; Hover, B.M.; Brady, S.F. Culture-Independent Discovery of Natural Products from Soil Metagenomes. J. Ind. Microbiol. Biot. 2016, 43, 129–141. [Google Scholar] [CrossRef]
- Li, L.; Maclntyre, L.W.; Brady, S.F. Refactoring Biosynthetic Gene Clusters for Heterologous Production of Microbial Natural Products. Curr. Opin. Biotech. 2021, 69, 145–152. [Google Scholar] [CrossRef]
- Tan, G.-Y.; Liu, T. Rational Synthetic Pathway Refactoring of Natural Products Biosynthesis in Actinobacteria. Metab. Eng. 2017, 39, 228–236. [Google Scholar] [CrossRef]
- Hover, B.M.; Kim, S.-H.; Katz, M.; Charlop-Powers, Z.; Owen, J.G.; Ternei, M.A.; Maniko, J.; Estrela, A.B.; Molina, H.; Park, S.; et al. Culture-Independent Discovery of the Malacidins as Calcium-Dependent Antibiotics with Activity against Multidrug-Resistant Gram-Positive Pathogens. Nat. Microbiol. 2018, 3, 415–422. [Google Scholar] [CrossRef]
- Owen, J.G.; Charlop-Powers, Z.; Smith, A.G.; Ternei, M.A.; Calle, P.Y.; Reddy, B.V.B.; Montiel, D.; Brady, S.F. Multiplexed Metagenome Mining Using Short DNA Sequence Tags Facilitates Targeted Discovery of Epoxyketone Proteasome Inhibitors. Proc. Natl. Acad. Sci. USA 2015, 112, 4221–4226. [Google Scholar] [CrossRef]
- Sun, Z.; Shang, Z.; Forelli, N.; Po, K.H.L.; Chen, S.; Brady, S.F.; Li, X. Total Synthesis of Malacidin A by Β-Hydroxyaspartic Acid Ligation-Mediated Cyclization and Absolute Structure Establishment. Angew. Chem. Int. Ed. 2020, 59, 19868–19872. [Google Scholar] [CrossRef]
- Kovalenko, N.; Howard, G.K.; Swain, J.A.; Hermant, Y.; Cameron, A.J.; Cook, G.M.; Ferguson, S.A.; Stubbing, L.A.; Harris, P.W.R.; Brimble, M.A. A Concise Synthetic Strategy Towards the Novel Calcium-Dependent Lipopeptide Antibiotic, Malacidin A and Analogues. Front. Chem. 2021, 9, 687875. [Google Scholar] [CrossRef]
- Li, L.; Koirala, B.; Hernandez, Y.; MacIntyre, L.W.; Ternei, M.A.; Russo, R.; Brady, S.F. Identification of Structurally Diverse Menaquinone-Binding Antibiotics with in Vivo Activity against Multidrug-Resistant Pathogens. Nat. Microbiol. 2022, 7, 120–131. [Google Scholar] [CrossRef]
- Chu, J.; Vila-Farres, X.; Inoyama, D.; Ternei, M.; Cohen, L.J.; Gordon, E.A.; Reddy, B.V.B.; Charlop-Powers, Z.; Zebroski, H.A.; Gallardo-Macias, R.; et al. Discovery of MRSA Active Antibiotics Using Primary Sequence from the Human Microbiome. Nat. Chem. Biol. 2016, 12, 1004–1006. [Google Scholar] [CrossRef]
- Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Park, S.; Perlin, D.S.; Brady, S.F. A Naturally Inspired Antibiotic to Target Multidrug-Resistant Pathogens. Nature 2022, 601, 606–611. [Google Scholar] [CrossRef]
- Miller, R.D.; Iinishi, A.; Modaresi, S.M.; Yoo, B.-K.; Curtis, T.D.; Lariviere, P.J.; Liang, L.; Son, S.; Nicolau, S.; Bargabos, R.; et al. Computational Identification of a Systemic Antibiotic for Gram-Negative Bacteria. Nat. Microbiol. 2022, 7, 1661–1672. [Google Scholar] [CrossRef]
- Kaur, N.; Chen, C.-C.; Luther, J.; Kao, J.Y. Intestinal Dysbiosis in Inflammatory Bowel Disease. Gut Microbes 2011, 2, 211–216. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Digest Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Interbacterial Mechanisms of Colonization Resistance and the Strategies Pathogens Use to Overcome Them. Mucosal Immunol. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Bohnhoff, M.; Drake, B.L.; Miller, C.P. Effect of Streptomycin on Susceptibility of Intestinal Tract to Experimental Salmonella Infection. Proc. Soc. Exp. Biol. Med. 1954, 86, 132–137. [Google Scholar] [CrossRef]
- RABIU, B.A.; GIBSON, G.R. Carbohydrates: A Limit on Bacterial Diversity within the Colon. Biol. Rev. 2002, 77, 443–453. [Google Scholar] [CrossRef]
- Bauer, M.A.; Kainz, K.; Carmona-Gutierrez, D.; Madeo, F. Microbial Wars: Competition in Ecological Niches and within the Microbiome. Microb. Cell 2018, 5, 215–219. [Google Scholar] [CrossRef]
- Celis, A.I.; Relman, D.A. Competitors versus Collaborators: Micronutrient Processing by Pathogenic and Commensal Human-Associated Gut Bacteria. Mol. Cell 2020, 78, 570–576. [Google Scholar] [CrossRef]
- Kamada, N.; Kim, Y.-G.; Sham, H.P.; Vallance, B.A.; Puente, J.L.; Martens, E.C.; Núñez, G. Regulated Virulence Controls the Ability of a Pathogen to Compete with the Gut Microbiota. Science 2012, 336, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Maltby, R.; Leatham-Jensen, M.P.; Gibson, T.; Cohen, P.S.; Conway, T. Nutritional Basis for Colonization Resistance by Human Commensal Escherichia Coli Strains HS and Nissle 1917 against E. coli O157:H7 in the Mouse Intestine. PLoS ONE 2013, 8, e53957. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Maurice, C.F.; Kinnebrew, M.A.; Abt, M.C.; Schenten, D.; Golovkina, T.V.; Bogatyrev, S.R.; Ismagilov, R.F.; Pamer, E.G.; Turnbaugh, P.J.; et al. Rapid Fucosylation of Intestinal Epithelium Sustains Host–Commensal Symbiosis in Sickness. Nature 2014, 514, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Rowley, C.A.; Anderson, C.J.; Kendall, M.M. Ethanolamine Influences Human Commensal Escherichia Coli Growth, Gene Expression, and Competition with Enterohemorrhagic E. coli O157:H7. mBio 2018, 9, e01429-18. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic Gut Microbial Modulation of Bile Acid Metabolism in Host Tissue Compartments. Proc. Natl. Acad. Sci. USA 2011, 108, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A Metabolic Pathway for Bile Acid Dehydroxylation by the Gut Microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Sorg, J.A.; Sonenshein, A.L. Bile Salts and Glycine as Cogerminants for Clostridium Difficile Spores. J. Bacteriol. 2008, 190, 2505–2512. [Google Scholar] [CrossRef]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision Microbiome Restoration of Bile Acid-Mediated Resistance to Clostridium Difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef]
- Sorg, J.A.; Sonenshein, A.L. Inhibiting the Initiation of Clostridium Difficile Spore Germination Using Analogs of Chenodeoxycholic Acid, a Bile Acid. J. Bacteriol. 2010, 192, 4983–4990. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Chen, C.; Zhang, N.; Graiziger, C.T.; Dosa, P.I.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Sadowsky, M.J.; Khoruts, A. Ursodeoxycholic Acid Inhibits Clostridium Difficile Spore Germination and Vegetative Growth, and Prevents the Recurrence of Ileal Pouchitis Associated With the Infection. J. Clin. Gastroenterol. 2016, 50, 624–630. [Google Scholar] [CrossRef]
- Coyne, M.J.; Comstock, L.E. Type VI Secretion Systems and the Gut Microbiota. Microbiol. Spectr. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Serapio-Palacios, A.; Woodward, S.E.; Vogt, S.L.; Deng, W.; Creus-Cuadros, A.; Huus, K.E.; Cirstea, M.; Gerrie, M.; Barcik, W.; Yu, H.; et al. Type VI Secretion Systems of Pathogenic and Commensal Bacteria Mediate Niche Occupancy in the Gut. Cell Rep. 2022, 39, 110731. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki-Livanis, M.; Geva-Zatorsky, N.; Comstock, L.E. Bacteroides Fragilis Type VI Secretion Systems Use Novel Effector and Immunity Proteins to Antagonize Human Gut Bacteroidales Species. Proc. Natl. Acad. Sci. USA 2016, 113, 3627–3632. [Google Scholar] [CrossRef]
- Jurėnas, D.; Journet, L. Activity, Delivery, and Diversity of Type VI Secretion Effectors. Mol. Microbiol. 2021, 115, 383–394. [Google Scholar] [CrossRef]
- Heilbronner, S.; Krismer, B.; Brötz-Oesterhelt, H.; Peschel, A. The Microbiome-Shaping Roles of Bacteriocins. Nat. Rev. Microbiol. 2021, 19, 726–739. [Google Scholar] [CrossRef]
- Lay, C.L.; Dridi, L.; Bergeron, M.G.; Ouellette, M.; Fliss, I. Nisin Is an Effective Inhibitor of Clostridium Difficile Vegetative Cells and Spore Germination. J. Med. Microbiol. 2016, 65, 169–175. [Google Scholar] [CrossRef]
- Rea, M.C.; Sit, C.S.; Clayton, E.; O’Connor, P.M.; Whittal, R.M.; Zheng, J.; Vederas, J.C.; Ross, R.P.; Hill, C. Thuricin CD, a Posttranslationally Modified Bacteriocin with a Narrow Spectrum of Activity against Clostridium Difficile. Proc. Natl. Acad. Sci. USA 2010, 107, 9352–9357. [Google Scholar] [CrossRef]
- Petersson, J.; Schreiber, O.; Hansson, G.C.; Gendler, S.J.; Velcich, A.; Lundberg, J.O.; Roos, S.; Holm, L.; Phillipson, M. Importance and Regulation of the Colonic Mucus Barrier in a Mouse Model of Colitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G327–G333. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kamada, N.; Jiao, Y.; Liu, M.Z.; Núñez, G.; Inohara, N. Protective Role of Commensals against Clostridium Difficile Infection via an IL-1β–Mediated Positive-Feedback Loop. J. Immunol. 2012, 189, 3085–3091. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Fu, J.; Xu, B.; Wang, Y.; Jin, M. Interplay between Gut Microbiota and Antimicrobial Peptides. Anim. Nutr. 2020, 6, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Lécuyer, E.; Rakotobe, S.; Lengliné-Garnier, H.; Lebreton, C.; Picard, M.; Juste, C.; Fritzen, R.; Eberl, G.; McCoy, K.D.; Macpherson, A.J.; et al. Segmented Filamentous Bacterium Uses Secondary and Tertiary Lymphoid Tissues to Induce Gut IgA and Specific T Helper 17 Cell Responses. Immunity 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Longo, D.L.; Leffler, D.A.; Lamont, J.T. Clostridium Difficile Infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Bien, J.; Palagani, V.; Bozko, P. The Intestinal Microbiota Dysbiosis and Clostridium Difficile Infection: Is There a Relationship with Inflammatory Bowel Disease? Ther. Adv. Gastroenter 2013, 6, 53–68. [Google Scholar] [CrossRef]
- Hopkins, R.J.; Wilson, R.B. Treatment of Recurrent Clostridium Difficile Colitis: A Narrative Review. Gastroenterol. Rep. 2018, 6, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.G.; Gardner, A. Mortality and Clostridium Difficile Infection: A Review. Antimicrob. Resist. Infect. Control 2012, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Hocquart, M.; Lagier, J.-C.; Cassir, N.; Saidani, N.; Eldin, C.; Kerbaj, J.; Delord, M.; Valles, C.; Brouqui, P.; Raoult, D.; et al. Early Fecal Microbiota Transplantation Improves Survival in Severe Clostridium Difficile Infections. Clin. Infect. Dis. 2017, 66, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Eiseman, B.; Silen, W.; Bascom, G.; Kauvar, A. Fecal Enema as an Adjunct in the Treatment of Pseudomembranous Enterocolitis. Surgery 1958, 5, 854–859. [Google Scholar]
- Schwan, A.; Sjolin, S.; Trottestam, U.; Aronsson, B. Relapsing Clostridium Difficile Enterocolitis Cured by Rectal Infusion of Normal Faeces. Scand. J. Infect. Dis. 1984, 16, 211–215. [Google Scholar] [CrossRef]
- Persky, S.E.; Brandt, L.J. Treatment of Recurrent Clostridium Difficile-Associated Diarrhea by Administration of Donated Stool Directly through a Colonoscope. Am. J. Gastroenterol. 2000, 95, 3283–3285. [Google Scholar] [CrossRef]
- Aas, J.; Gessert, C.E.; Bakken, J.S. Recurrent Clostridium Difficile Colitis: Case Series Involving 18 Patients Treated with Donor Stool Administered via a Nasogastric Tube. Clin. Infect. Dis. 2003, 36, 580–585. [Google Scholar] [CrossRef]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium Difficile Infection With Fecal Microbiota Transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium Difficile Infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef]
- Kassam, Z.; Lee, C.H.; Yuan, Y.; Hunt, R.H. Fecal Microbiota Transplantation for Clostridium Difficile Infection: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2013, 108, 500–508. [Google Scholar] [CrossRef]
- Khoruts, A.; Sadowsky, M.J. Understanding the Mechanisms of Faecal Microbiota Transplantation. Nat. Rev. Gastroentero 2016, 13, 508–516. [Google Scholar] [CrossRef]
- Seekatz, A.M.; Young, V.B. Clostridium Difficile and the Microbiota. J. Clin. Investig. 2014, 124, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Weingarden, A.; González, A.; Vázquez-Baeza, Y.; Weiss, S.; Humphry, G.; Berg-Lyons, D.; Knights, D.; Unno, T.; Bobr, A.; Kang, J.; et al. Dynamic Changes in Short- and Long-Term Bacterial Composition Following Fecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection. Microbiome 2015, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Broecker, F.; Klumpp, J.; Schuppler, M.; Russo, G.; Biedermann, L.; Hombach, M.; Rogler, G.; Moelling, K. Long-Term Changes of Bacterial and Viral Compositions in the Intestine of a Recovered Clostridium Difficile Patient after Fecal Microbiota Transplantation. Cold Spring Harb. Mol. Case Stud. 2016, 2, a000448. [Google Scholar] [CrossRef]
- Osman, M.; Stoltzner, Z.; O’Brien, K.; Ling, K.; Koelsch, E.; Dubois, N.; Amaratunga, K.; Smith, M.; Kassam, Z. Donor Efficacy in Fecal Microbiota Transplantation for Recurrent Clostridium Difficile: Evidence From a 1,999-Patient Cohort. Open Forum Infect. Dis. 2016, 3, 841. [Google Scholar] [CrossRef]
- Tariq, R.; Saha, S.; Solanky, D.; Pardi, D.S.; Khanna, S. Predictors and Management of Failed Fecal Microbiota Transplantation for Recurrent Clostridioides Difficile Infection. J. Clin. Gastroenterol. 2021, 55, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lebwohl, B.; Cuaresma, E.; Cadwell, K.; Green, P.H.R.; Freedberg, D.E. Gut Colonization with Vancomycin-Resistant Enterococcus and Risk for Subsequent Enteric Infection. Gut Pathog. 2018, 10, 28. [Google Scholar] [CrossRef]
- Kates, A.E.; Thapaliya, D.; Smith, T.C.; Chorazy, M.L. Prevalence and Molecular Characterization of Staphylococcus Aureus from Human Stool Samples. Antimicrob. Resist. Infect. Control 2018, 7, 42. [Google Scholar] [CrossRef]
- Niki, M.; Hirai, I.; Yoshinaga, A.; Ulzii-Orshikh, L.; Nakata, A.; Yamamoto, A.; Yamamoto, M.; Yamamoto, Y. Extended-Spectrum β-Lactamase-Producing Escherichia Coli Strains in the Feces of Carriers Contribute Substantially to Urinary Tract Infections in These Patients. Infection 2011, 39, 467. [Google Scholar] [CrossRef]
- Sakka, V.; Tsiodras, S.; Galani, L.; Antoniadou, A.; Souli, M.; Galani, I.; Pantelaki, M.; Siafakas, N.; Zerva, L.; Giamarellou, H. Risk-factors and Predictors of Mortality in Patients Colonised with Vancomycin-resistant Enterococci. Clin. Microbiol. Infect. 2008, 14, 14–21. [Google Scholar] [CrossRef]
- Davido, B.; Batista, R.; Fessi, H.; Michelon, H.; Escaut, L.; Lawrence, C.; Denis, M.; Perronne, C.; Salomon, J.; Dinh, A. Fecal Microbiota Transplantation to Eradicate Vancomycin-Resistant Enterococci Colonization in Case of an Outbreak. Méd. Mal. Infect. 2019, 49, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, L.; Allegretti, J.R.; Aroniadis, O.; Brandt, L.; Donovan, D.; Fischer, M.; Grinspan, A.; Kassam, Z.; Kelly, C.R.; Kim, C.; et al. Clearance of Vancomycin-Resistant Enterococcus Colonization With Fecal Microbiota Transplantation Among Patients With Recurrent Clostridium Difficile Infection. Open Forum Infect. Dis. 2016, 3, 2119. [Google Scholar] [CrossRef]
- Stripling, J.; Kumar, R.; Baddley, J.W.; Nellore, A.; Dixon, P.; Howard, D.; Ptacek, T.; Lefkowitz, E.J.; Tallaj, J.A.; Benjamin, W.H.; et al. Loss of Vancomycin-Resistant Enterococcus Fecal Dominance in an Organ Transplant Patient With Clostridium Difficile Colitis After Fecal Microbiota Transplant. Open Forum Infect. Dis. 2015, 2, ofv078. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Lee, S.K.; Cheon, J.H.; Yong, D.E.; Koh, H.; Kang, Y.K.; Jeong, W.Y.; Lee, W.J.; Sohn, Y.; Cho, Y.; et al. Fecal Microbiota Transplantation for Multidrug-Resistant Organism: Efficacy and Response Prediction. J. Infect. 2020, 81, 719–725. [Google Scholar] [CrossRef]
- Tavoukjian, V. Faecal Microbiota Transplantation for the Decolonisation of Antibiotic-Resistant Bacteria in the Gut: A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Manges, A.R.; Steiner, T.S.; Wright, A.J. Fecal Microbiota Transplantation for the Intestinal Decolonization of Extensively Antimicrobial-Resistant Opportunistic Pathogens: A Review. Infect. Dis. 2016, 48, 587–592. [Google Scholar] [CrossRef]
- Singh, R.; de Groot, P.F.; Geerlings, S.E.; Hodiamont, C.J.; Belzer, C.; ten Berge, I.J.M.; de Vos, W.M.; Bemelman, F.J.; Nieuwdorp, M. Fecal Microbiota Transplantation against Intestinal Colonization by Extended Spectrum Beta-Lactamase Producing Enterobacteriaceae: A Proof of Principle Study. BMC Res. Notes 2018, 11, 190. [Google Scholar] [CrossRef]
- Merrick, B.; Allen, L.; Zain, N.M.M.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, Risk and Safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Soto, M.T.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.-B.; Hohmann, E.L. Drug-Resistant E. Coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Tvede, M.; Rask-Madsen, J. Bacteriotherapy for Clostridium Difficile Diarrhoea. Lancet 1990, 335, 110. [Google Scholar] [CrossRef]
- Lawley, T.D.; Clare, S.; Walker, A.W.; Stares, M.D.; Connor, T.R.; Raisen, C.; Goulding, D.; Rad, R.; Schreiber, F.; Brandt, C.; et al. Targeted Restoration of the Intestinal Microbiota with a Simple, Defined Bacteriotherapy Resolves Relapsing Clostridium Difficile Disease in Mice. PLoS Pathog. 2012, 8, e1002995. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, M.; Menon, R.; Crossette, E.; Bhattarai, S.K.; Schneider, J.; Kim, Y.-G.; Reddy, S.; Caballero, S.; Felix, C.; Cornacchione, L.; et al. Colonization of the Live Biotherapeutic Product VE303 and Modulation of the Microbiota and Metabolites in Healthy Volunteers. Cell Host Microbe 2022, 30, 583–598.e8. [Google Scholar] [CrossRef] [PubMed]
- Biosciences, V. Phase 2 Study of VE303 for Prevention of Recurrent Clostridium Difficile Infection—Full Text View—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT03788434 (accessed on 19 May 2022).
- Singh, S.B.; Young, K.; Silver, L.L. What Is an “Ideal” Antibiotic? Discovery Challenges and Path Forward. Biochem. Pharmacol. 2017, 133, 63–73. [Google Scholar] [CrossRef]
- Outterson, K.; Rex, J.H.; Jinks, T.; Jackson, P.; Hallinan, J.; Karp, S.; Hung, D.T.; Franceschi, F.; Merkeley, T.; Houchens, C.; et al. Accelerating Global Innovation to Address Antibacterial Resistance: Introducing CARB-X. Nat. Rev. Drug Discov. 2016, 15, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Kostyanev, T.; Bonten, M.J.M.; O’Brien, S.; Steel, H.; Ross, S.; François, B.; Tacconelli, E.; Winterhalter, M.; Stavenger, R.A.; Karlén, A.; et al. The Innovative Medicines Initiative’s New Drugs for Bad Bugs Programme: European Public–Private Partnerships for the Development of New Strategies to Tackle Antibiotic Resistance. J. Antimicrob. Chemother. 2016, 71, 290–295. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Kohanski, M.A.; Collins, J.J. Role of Reactive Oxygen Species in Antibiotic Action and Resistance. Curr. Opin. Microbiol. 2009, 12, 482–489. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved Glycopeptide Antibacterial Drugs: Mechanism of Action and Resistance. CSH Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of Secondary Metabolites Using Tissue Culture-Based Biotechnological Applications. Front. Plant Sci. 2023, 14, 1132555. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacNair, C.R.; Tsai, C.N.; Rutherford, S.T.; Tan, M.-W. Returning to Nature for the Next Generation of Antimicrobial Therapeutics. Antibiotics 2023, 12, 1267. https://doi.org/10.3390/antibiotics12081267
MacNair CR, Tsai CN, Rutherford ST, Tan M-W. Returning to Nature for the Next Generation of Antimicrobial Therapeutics. Antibiotics. 2023; 12(8):1267. https://doi.org/10.3390/antibiotics12081267
Chicago/Turabian StyleMacNair, Craig R., Caressa N. Tsai, Steven T. Rutherford, and Man-Wah Tan. 2023. "Returning to Nature for the Next Generation of Antimicrobial Therapeutics" Antibiotics 12, no. 8: 1267. https://doi.org/10.3390/antibiotics12081267
APA StyleMacNair, C. R., Tsai, C. N., Rutherford, S. T., & Tan, M.-W. (2023). Returning to Nature for the Next Generation of Antimicrobial Therapeutics. Antibiotics, 12(8), 1267. https://doi.org/10.3390/antibiotics12081267





