Palladium(II) Metal Complex Fabricated Titanium Implant Mitigates Dual-Species Biofilms in Artificial Synovial Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Culture Condition and Metal Complex
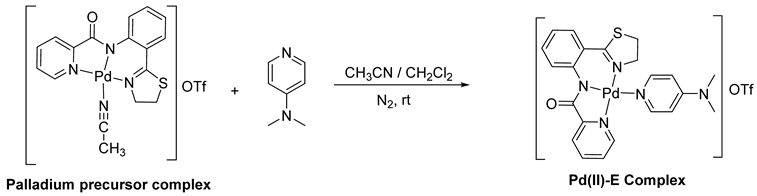
2.2. Evaluation of the Minimum Inhibitory Concentration (MIC)
2.3. Assessment of the Biofilm Inhibition Capacity of Pd(II)-E in Artificial Synovial Fluid
2.4. Stability of Pd(II)-E-Coated Titanium Plates in Artificial Synovial Fluid
2.5. Biofilm Inhibitory Potency of Pd(II)-E-Coated Titanium Plates
2.6. Evaluation of Viable Cells Residing in Biofilms
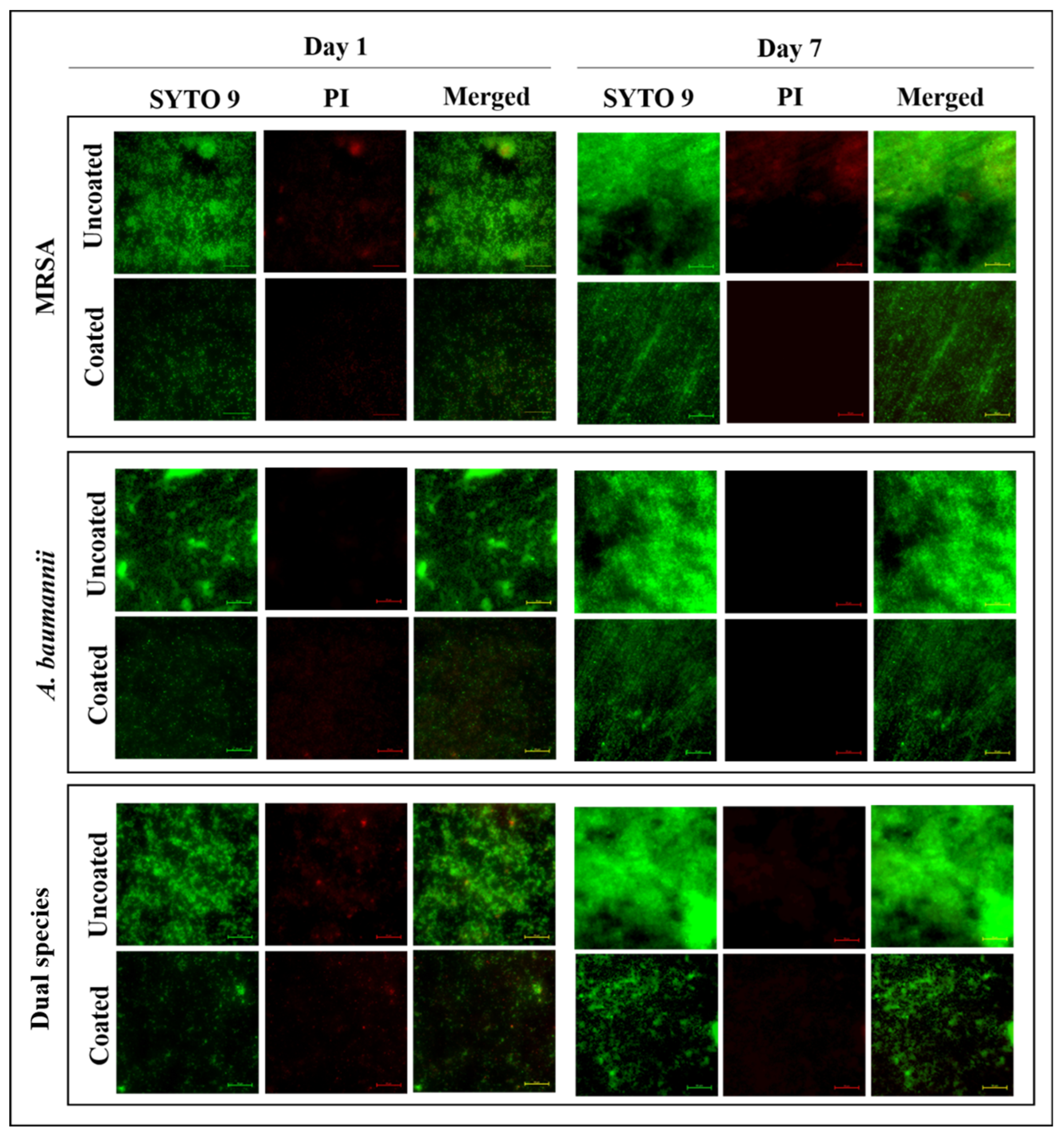
2.7. Live/Dead Fluorescent Staining of Biofilms
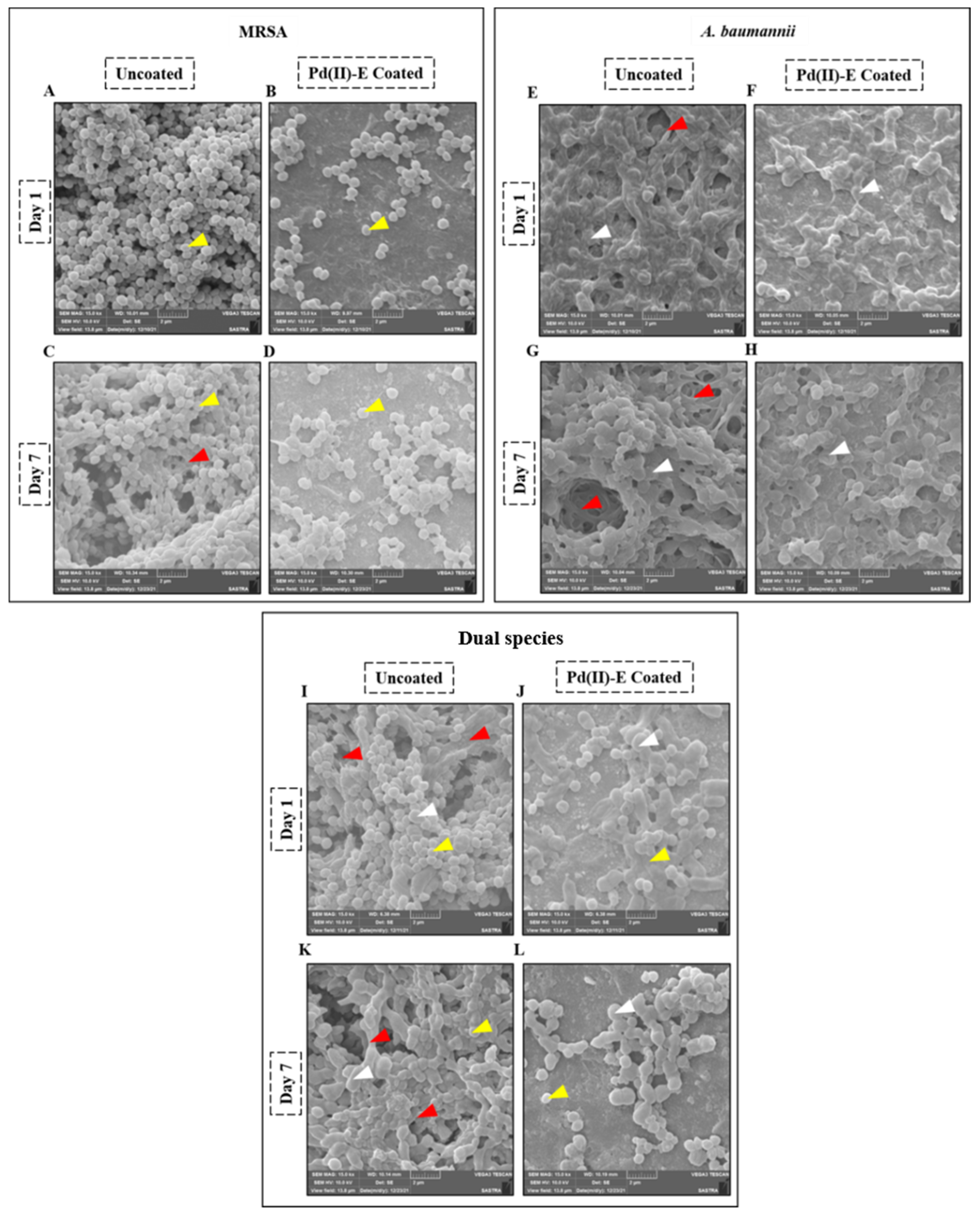
2.8. Scanning Electron Microscopy (SEM) Examination of Biofilms
2.9. Assessment of Polysaccharides in the ECM Matrix in ASF
2.10. Comparison of Virulence Gene Expression between Mono- and Dual-Species Biofilms Formed in ASF
2.11. Downregulation of Virulence Gene Expression in Mono- and Dual-Species Biofilms in Pd(II)-E Impregnated Titanium Plates
2.12. Indirect Cytotoxicity Assessment of Pd(II)-E-Coated Titanium Plates Using Osteoblastic Cells
2.13. Quantification of Calcium Deposition by Alizarin Red Staining
2.14. Real-Time PCR Analysis of Osteoblast Differentiation Marker Genes
2.15. Statistics
3. Results
3.1. Minimum Inhibitory Concentration of Pd(II)-E against Dual-Species Planktonic Cells
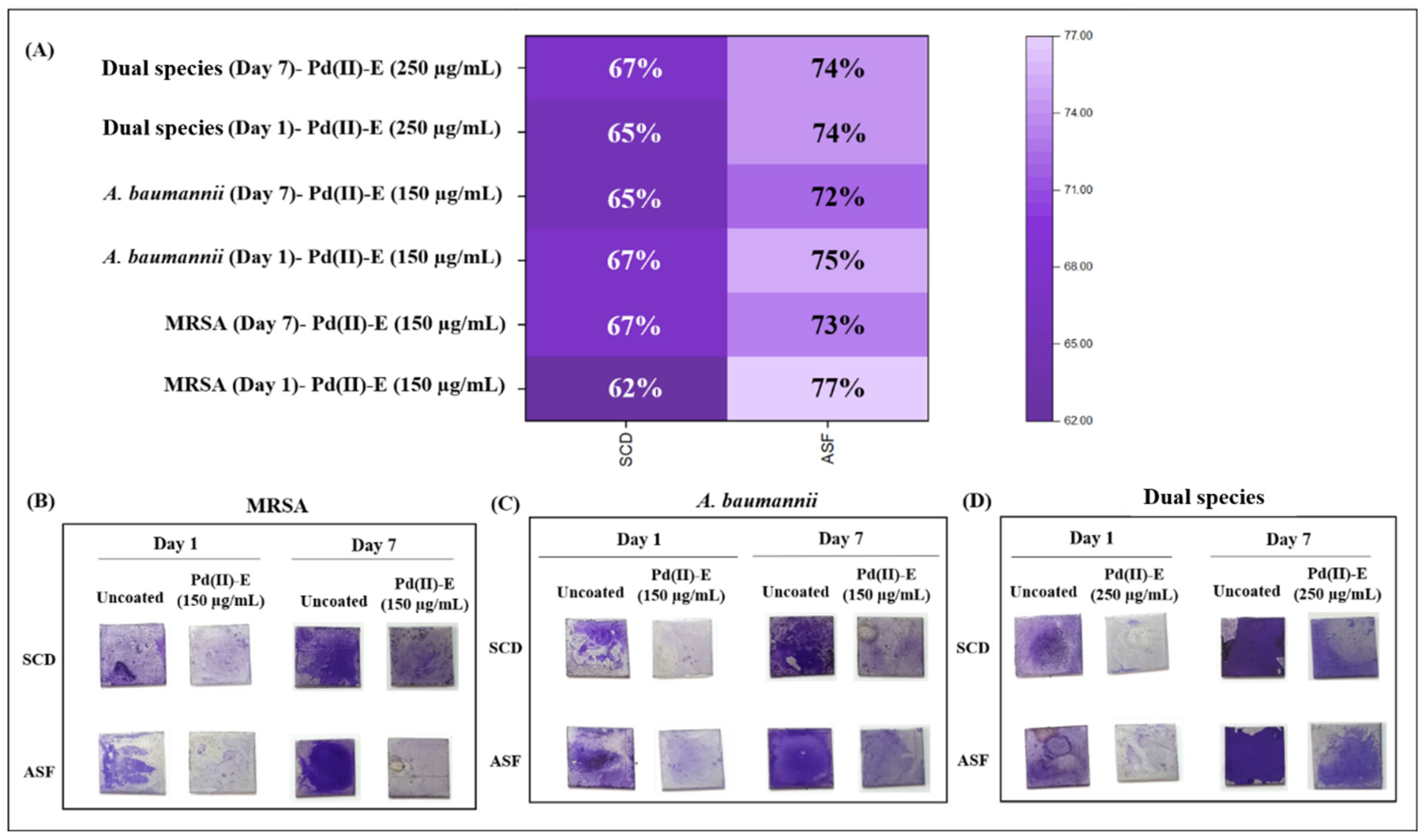
3.2. Pd(II)-E Inhibited Mono- and Dual-Species Biofilms in ASF
3.3. Pd(II)-E Coating on Titanium Plates Remains Stable in ASF
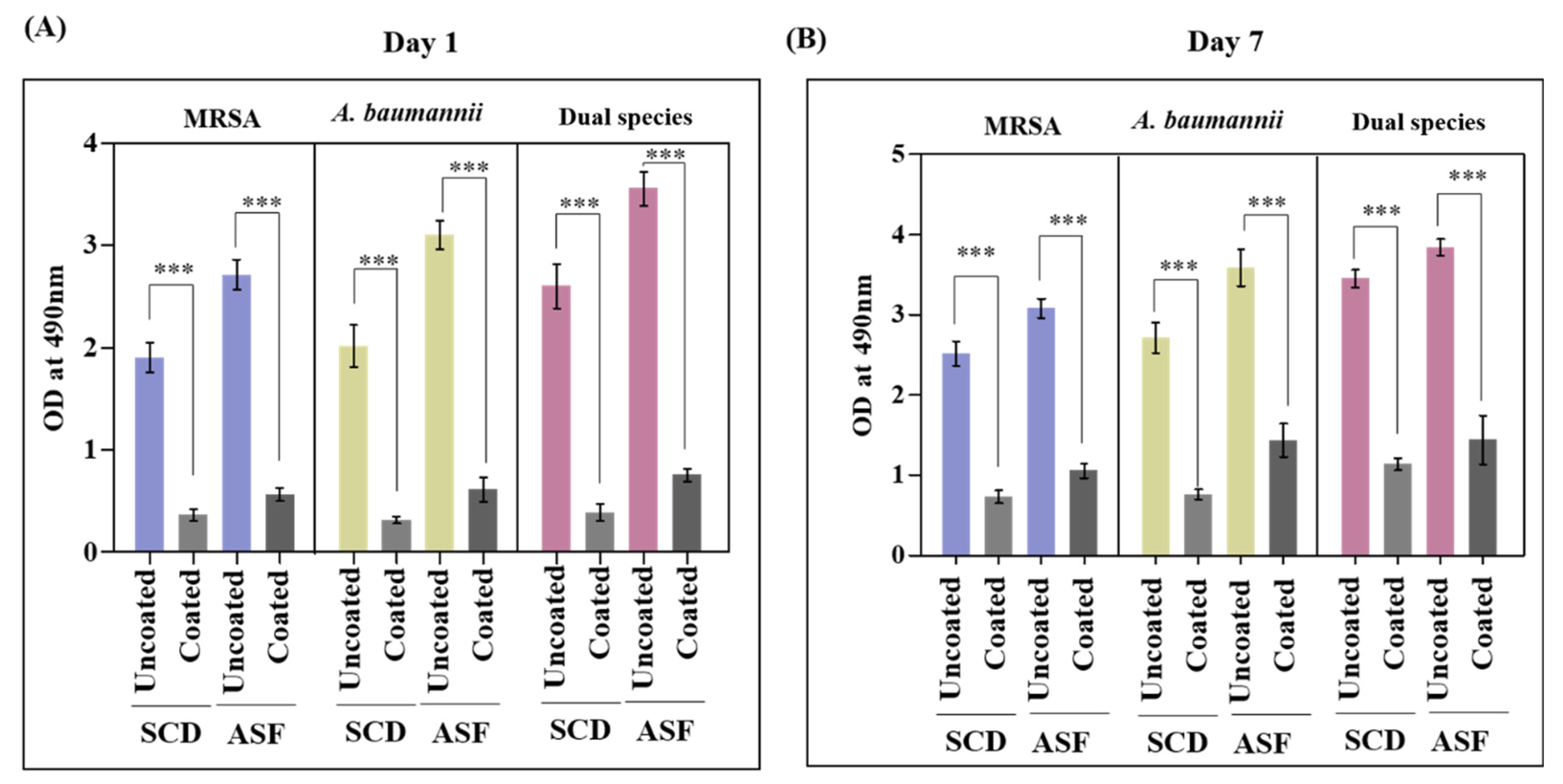
3.4. Pd(II)-E Fabricated Titanium Plates Impede Biofilm Adherence
3.5. Viable Biofilm Cells Were Lowered in Pd(II)-E-Coated Titanium Plates
3.6. Coated Titanium Plates Disrupt the Extracellular Matrix of Biofilms in ASF
3.7. ASF-Induced Virulence Gene Expression by Pathogens
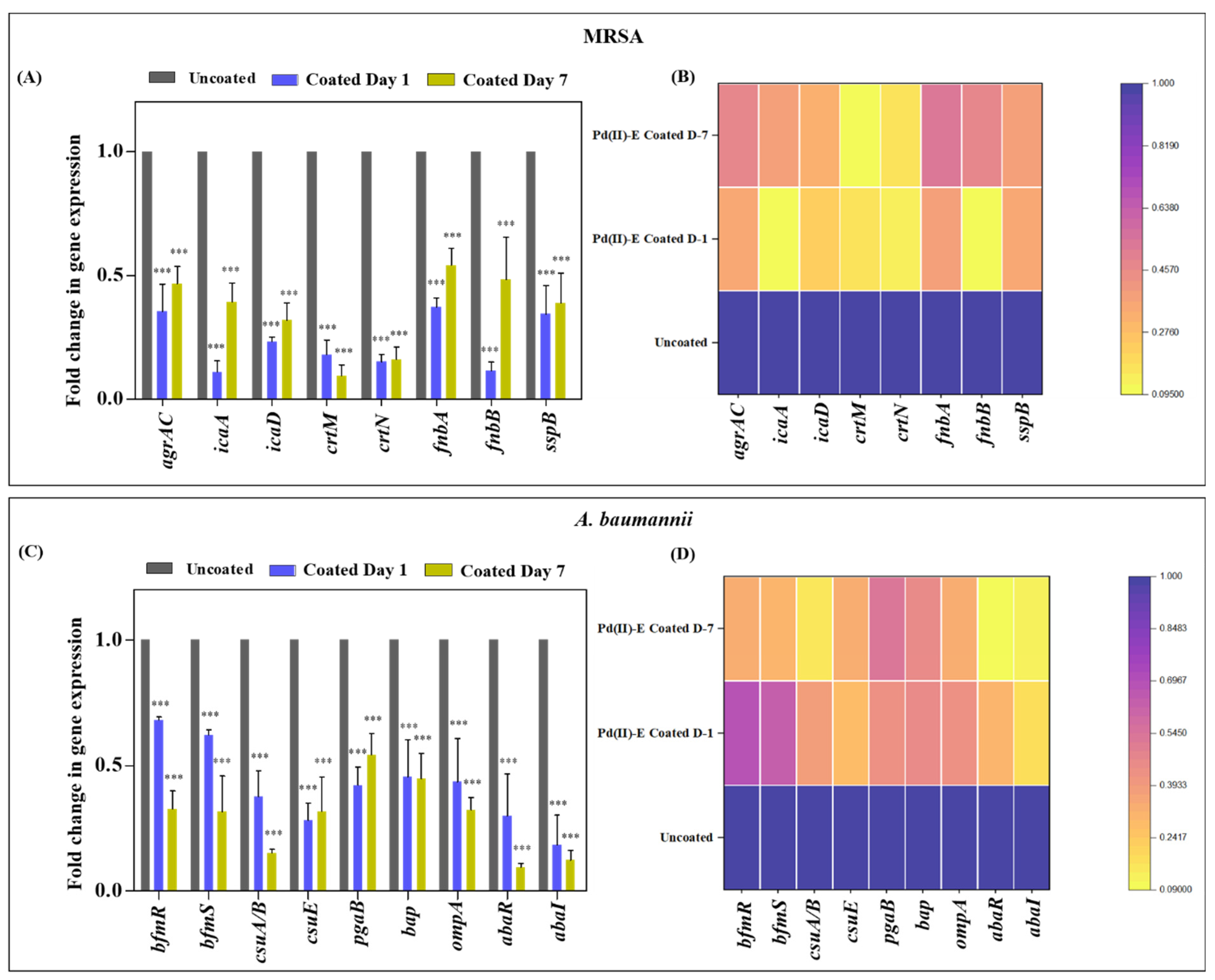
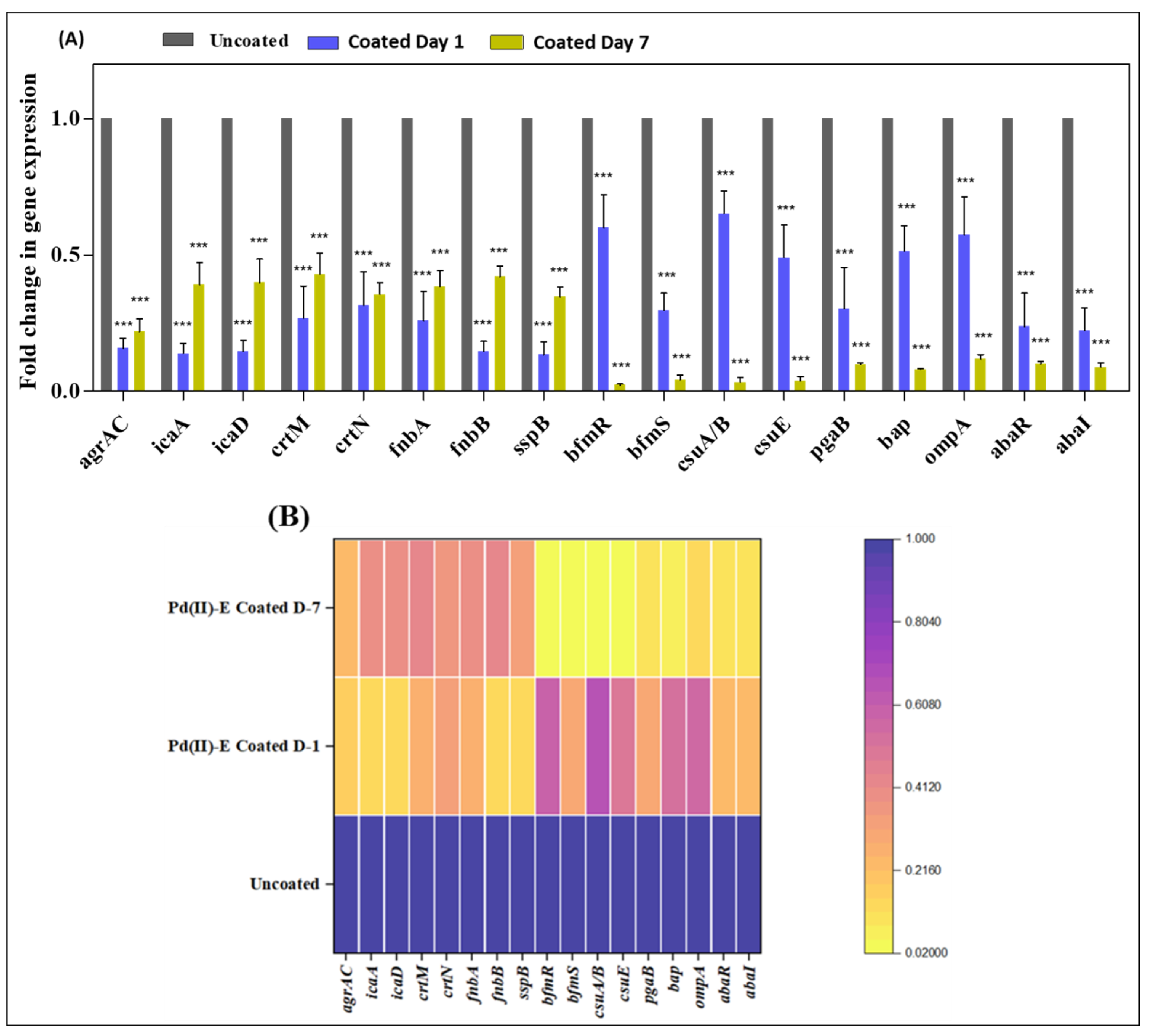
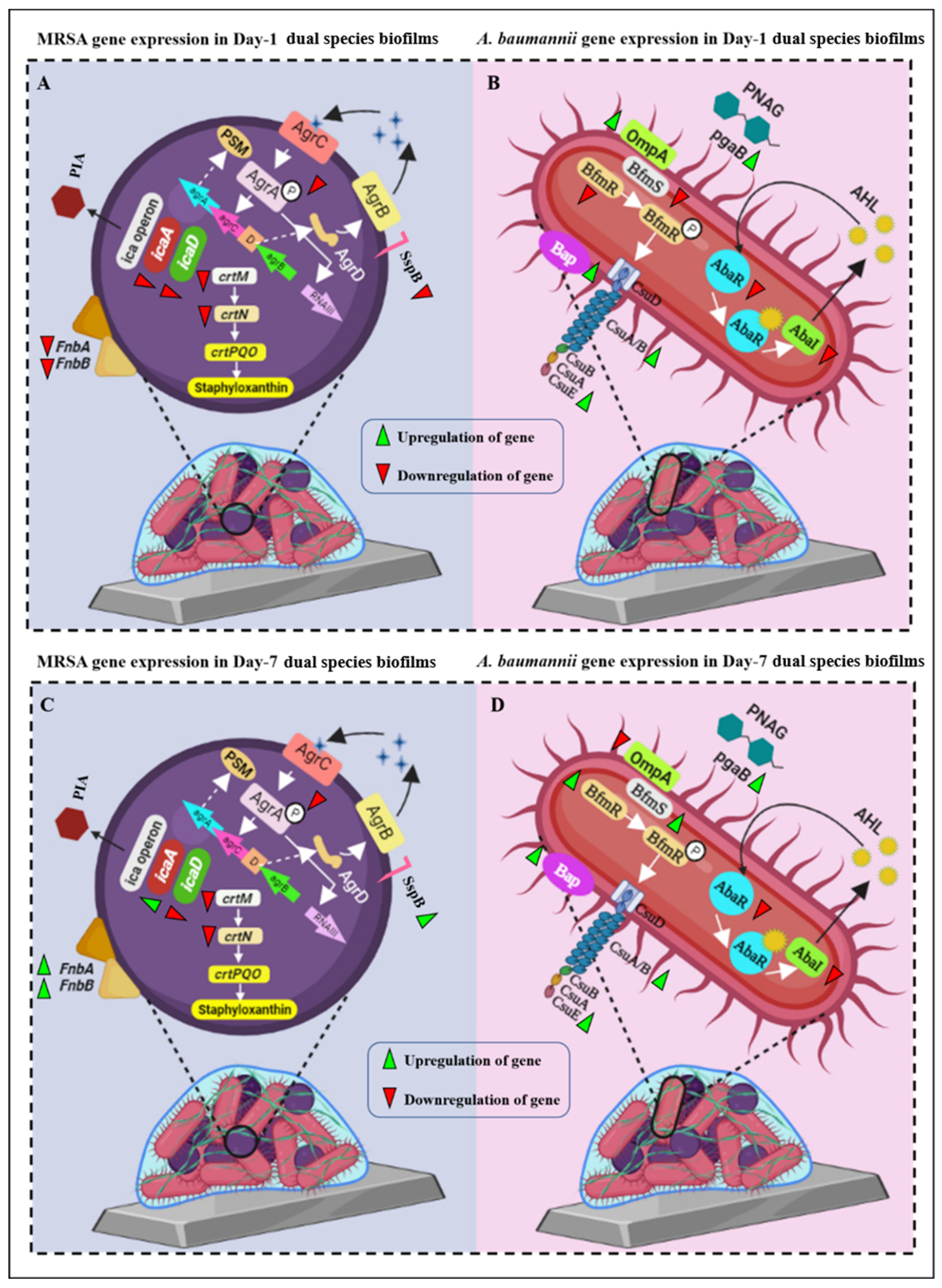
3.8. Coated Titanium Plates Downregulate Virulence Gene
3.9. Pd(II)-E-Coated Titanium Plate Is Biocompatible with Human Osteoblasts
3.10. Pd(II)-E-Coated Plates Enhanced Calcium Deposition
3.11. Pd(II)-E-Coated Plates Promote Osteogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahyussalim, A.J.; Marsetio, A.F.; Saleh, I.; Kurniawati, T.; Whulanza, Y. The Needs of Current Implant Technology in Orthopaedic Prosthesis Biomaterials Application to Reduce Prosthesis Failure Rate. J. Nanomater. 2016, 2016, 5386924. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Chu, P.K. Orthopedic Implants. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–439. [Google Scholar]
- Souza, J.G.S.; Bertolini, M.M.; Costa, R.C.; Nagay, B.E.; Dongari-Bagtzoglou, A.; Barão, V.A.R. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. iScience 2020, 24, 102008. [Google Scholar] [CrossRef]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria. Medicines 2018, 5, 125. [Google Scholar] [CrossRef] [Green Version]
- Seebach, E.; Kubatzky, K.F. Chronic Implant-Related Bone Infections-Can Immune Modulation be a Therapeutic Strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, L.; Yang, Y.; Yang, S.; Fan, Q.; Yu, Z.; Hu, X.L.; James, T.D.; He, X.P.; Tang, T. Preferential Colonization of Osteoblasts Over Co-cultured Bacteria on a Bifunctional Biomaterial Surface. Front. Microbiol. 2018, 9, 2219. [Google Scholar] [CrossRef] [Green Version]
- Macias-Valcayo, A.; Staats, A.; Aguilera-Correa, J.J.; Brooks, J.; Gupta, T.; Dusane, D.; Stoodley, P.; Esteban, J. Synovial Fluid Mediated Aggregation of Clinical Strains of Four Enterobacterial Species. Adv. Exp. Med. Biol. 2021, 1323, 81–90. [Google Scholar] [PubMed]
- Junka, A.; Szymczyk, P.; Ziółkowski, G.; Karuga-Kuzniewska, E.; Smutnicka, D.; Bil-Lula, I.; Bartoszewicz, M.; Mahabady, S.; Sedghizadeh, P.P. Bad to the Bone: On In Vitro and Ex Vivo Microbial Biofilm Ability to Directly Destroy Colonized Bone Surfaces without Participation of Host Immunity or Osteoclastogenesis. PLoS ONE 2017, 12, e0169565. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert. Rev. Anti. Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Shi, M.; Xu, F.; Qiu, Y.; Zhang, P.; Shen, K.; Zhao, Q.; Yu, J.; Zhang, Y. Graphdiyne-modified TiO2 nanofibers with osteoinductive and enhanced photocatalytic antibacterial activities to prevent implant infection. Nat. Commun. 2020, 11, 4465. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Dias, M. The Microbiological Profiles of Infected Prosthetic Implants with an Emphasis on the Organisms which Form Biofilms. J. Clin. Diagn. Res. 2019, 7, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. 2018, 36, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Assad, M.; Downey, A.M.; Cluzel, C.; Trudel, Y.; Doyle, N.; Authier, S. Characterization of an Acute Rodent Osteomyelitis Infectious Model Using a Tibial Intramedullary Implant Inoculation. Front. Bioeng. Biotechnol. 2020, 8, 567647. [Google Scholar] [CrossRef]
- Howlin, R.P.; Winnard, C.; Frapwell, C.J.; Webb, J.S.; Cooper, J.J.; Aiken, S.S.; Stoodley, P. Biofilm prevention of gram-negative bacterial pathogens involved in periprosthetic infection by antibiotic-loaded calcium sulfate beads in vitro. Biomed. Mater. 2016, 12, 015002. [Google Scholar] [CrossRef]
- Vasiliadis, A.V.; Poutoglidou, F.; Chatziravdeli, V.; Metaxiotis, D.; Beletsiotis, A. Acute Periprosthetic Hip Joint Infection Caused by Multidrug-Resistant Acinetobacter baumannii: Is Debridement, Antibiotics, Irrigation, and Implant Retention a Viable Treatment Option? Cureus 2021, 13, e13090. [Google Scholar] [CrossRef]
- Stacy, D.M.; Welsh, M.A.; Rather, P.N.; Blackwell, H.E. Attenuation of quorum sensing in the pathogen Acinetobacter baumannii using non-native N-Acyl homoserine lactones. ACS Chem. Biol. 2012, 7, 1719–1728. [Google Scholar] [CrossRef] [Green Version]
- Urish, K.L.; Cassat, J.E. Staphylococcus aureus Osteomyelitis: Bone, Bugs, and Surgery. Infect. Immun. 2020, 88, e00932-19. [Google Scholar] [CrossRef]
- Connaughton, A.; Childs, A.; Dylewski, S.; Sabesan, V.J. Biofilm Disrupting Technology for Orthopedic Implants: What’s on the Horizon? Front. Med. 2014, 1, 22. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.R.; Camara, J.; Camara, R.; Duffy, L.; Waites, K.; Kim, H.; Zinn, K. Contaminated open fracture and crush injury: A murine model. Bone. Res. 2015, 3, 14050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellanos, N.; Nakanouchi, J.; Yüzen, D.I.; Fung, S.; Fernandez, J.S.; Barberis, C.; Tuchscherr, L.; Ramirez, M.S. A Study on Acinetobacter baumannii and Staphylococcus aureus Strains Recovered from the Same Infection Site of a Diabetic Patient. Curr. Microbiol. 2019, 76, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Zhai, Z.; Gao, C. Adaptive antibacterial biomaterial surfaces and their applications. Mater. Today Bio 2019, 2, 100017. [Google Scholar] [CrossRef]
- Sudharsan, M.; Nethaji, M.; Bhuvanesh, N.S.; Suresh, D. Heteroleptic Palladium(II) Complexes of Thiazolinyl-picolinamide Derived N∩N∩N Pincer Ligand: An Efficient Catalyst for Acylative Suzuki Coupling Reactions. Asian J. Org. Chem. 2021, 10, 2982–2992. [Google Scholar] [CrossRef]
- Sowndarya, J.; Suresh, D.; Sankaran, V.S.; Thamotharan, S.; Shanmugasundaram, K.; Preethi, V.; Saravanan, S.; Gowrishankar, S.; Karutha Pandian, S.; Nithyanand, P. Heteroleptic Pincer Palladium(II) Complex coated orthopedic implants impedes AbaI/AbaR quorum sensing system and biofilm development by Acinetobacter baumannii. Biofouling 2021, 38, 55–70. [Google Scholar]
- Sowndarya, J.; Suresh, D.; Saravanan, S.; Sudharsan, M.; Raghunandhakumar, S.; Nithyanand, P. Transition metal complex laminated bioactive implant alleviates Methicillin Resistant Staphylococcus aureus virulence. Biomater. Adv. 2022, 137, 212813. [Google Scholar]
- Senthilganesh, J.; Ravichandran, S.; Durairajan, R.; Siva, B.; Lakshmi, K.; Anbazhagan, V.; Nithyanand, P. Metal Sulfide Nanoparticles Based Phytolectin Scaffolds Inhibit Vulvovaginal Candidiasis Causing Candida albicans. J. Clust. Sci. 2021, 33, 1361–1372. [Google Scholar] [CrossRef]
- Pestrak, M.J.; Gupta, T.T.; Dusane, D.H.; Guzior, D.V.; Staats, A.; Harro, J.; Horswill, A.R.; Stoodley, P. Investigation of synovial fluid induced Staphylococcus aureus aggregate development and its impact on surface attachment and biofilm formation. PLoS ONE 2020, 15, e0231791. [Google Scholar]
- Solis-Velazquez, O.A.; Gutiérrez-Lomelí, M.; Guerreo-Medina, P.J.; Rosas-García, M.L.; Iñiguez-Moreno, M.; Avila-Novoa, M.G. Nosocomial pathogen biofilms on biomaterials: Different growth medium conditions and components of biofilms produced in vitro. J. Microbiol. Immunol. Infect. 2021, 54, 1038–1047. [Google Scholar] [CrossRef]
- Rubini, D.; Vedha Hari, B.N.; Nithyanand, P. Chitosan coated catheters alleviates mixed species biofilms of Staphylococcus epidermidis and Candida albicans. Carbohydr. Polym. 2021, 252, 117192. [Google Scholar] [CrossRef]
- Rossoni, R.D.; de Barros, P.P.; Lopes, L.A.D.C.; Ribeiro, F.C.; Nakatsuka, T.; Kasaba, H.; Junqueira, J.C. Effects of surface pre-reacted glass-ionomer (S-PRG) eluate on Candida spp.: Antifungal activity, anti-biofilm properties, and protective effects on Galleria mellonella against C. albicans infection. Biofouling 2019, 35, 997–1006. [Google Scholar] [CrossRef]
- Nithyanand, P.; Beema Shafreen, R.M.; Muthamil, S.; Karutha Pandian, S. Usnic acid inhibits biofilm formation and virulent morphological traits of Candida albicans. Microbiol. Res. 2015, 179, 20–28. [Google Scholar] [CrossRef]
- Drago, L.; Agrappi, S.; Bortolin, M.; Toscano, M.; Romanò, C.L.; De Vecchi, E. How to Study Biofilms after Microbial Colonization of Materials Used in Orthopaedic Implants. Int. J. Mol. Sci. 2016, 17, 293. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Ge, Y.; Wu, Y.; Chen, J.; Xu, H.H.K.; Yang, M.; Li, M.; Ren, B.; Feng, M.; Weir, M.D.; et al. Anti-Bacteria and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules 2017, 22, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowrishankar, S.; Kamaladevi, A.; Ayyanar, K.S.; Balamurugan, K.; Pandian, S.K. Bacillus amyloliquefaciens-secreted 1 cyclic dipeptide–cyclo (L-leucyl-L-prolyl) inhibits biofilm and virulence production in methicillin-resistant Staphylococcus aureus. RSC Adv. 2015, 5, 95788–95804. [Google Scholar] [CrossRef]
- Hetta, H.F.; Al-Kadmy, I.M.S.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Ellah, N.H.A.; Ahmed, E.A.; Abd-Ellatief, R.B.; El-Masry, E.A.; et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef] [PubMed]
- Balagangadharan, K.; Viji Chandran, S.; Arumugam, B.; Saravanan, S.; Devanand Venkatasubbu, G.; Selvamurugan, N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. Int. J. Biol. Macromol. 2018, 111, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Vimalraj, S.; Vairamani, M.; Selvamurugan, N. Role of Mesoporous Wollastonite (Calcium Silicate) in Mesenchymal Stem Cell Proliferation and Osteoblast Differentiation: A Cellular and Molecular Study. J. Biomed. Nanotechnol. 2015, 11, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.W.; Yang, J.; Guo, H.; Ji, Y. The AirSR two-component system contributes to Staphylococcus aureus survival in human blood and transcriptionally regulates sspABC operon. Front. Microbiol. 2015, 6, 682. [Google Scholar] [CrossRef]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus Virulence. Microbiol Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Knott, S.; Curry, D.; Zhao, N.; Metgud, P.; Dastgheyb, S.S.; Purtill, C.; Harwood, M.; Chen, A.F.; Schaer, T.P.; Otto, M.; et al. Staphylococcus aureus Floating Biofilm Formation and Phenotype in Synovial Fluid Depends on Albumin, Fibrinogen, and Hyaluronic Acid. Front. Microbiol. 2021, 12, 655873. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.K.; Rasouli, M.R.; Parvizi, J. Periprosthetic joint infection: Current concept. Indian. J. Orthop. 2013, 47, 10–17. [Google Scholar] [CrossRef]
- Dastgheyb, S.; Parvizi, J.; Shapiro, I.M.; Hickok, N.J.; Otto, M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J. Infect. Dis. 2015, 211, 641–650. [Google Scholar] [CrossRef]
- Beloin, C.; McDougald, D. Speciality Grand Challenge for “Biofilms”. Front. Cell. Infect. Microbiol. 2021, 11, 632429. [Google Scholar] [CrossRef]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface Modification Techniques of Titanium and its Alloys to Functionally Optimize Their Biomedical Properties: Thematic Review. Front. Bioeng. Biotechnol. 2020, 8, 603072. [Google Scholar] [CrossRef]
- Shiels, S.M.; Mangum, L.H.; Wenke, J.C. Revisiting the "race for the surface" in a pre-clinical model of implant infection. Eur. Cell. Mater. 2020, 39, 77–95. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Arora, S.; Liu, W.; Churion, K.; Wu, Y.; Höök, M. vhp Is a Fibrinogen-Binding Protein Related to vWbp in Staphylococcus aureus. mBio 2021, 12, e0116721. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1923–1931. [Google Scholar] [CrossRef]
- Smani, Y.; McConnell, M.J.; Pachón, J. Role of fibronectin in the adhesion of Acinetobacter baumannii to host cells. PLoS ONE 2012, 7, e33073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahma, U.; Kothari, R.; Sharma, P.; Bhandari, V. Antimicrobial and anti-biofilm activity of hexadentated macrocyclic complex of copper (II) derived from thiosemicarbazide against Staphylococcus aureus. Sci. Rep. 2018, 8, 8050. [Google Scholar] [CrossRef] [PubMed]
- Viganor, L.; Galdino, A.C.; Nunes, A.P.; Santos, K.R.; Branquinha, M.H.; Devereux, M.; Kellett, A.; McCann, M.; Santos, A.L. Anti-Pseudomonas aeruginosa activity of 1,10-phenanthroline-based drugs against both planktonic- and biofilm-growing cells. J. Antimicrob. Chemother. 2016, 71, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Amod, A.; Pandey, P.; Bose, P.; Pingali, M.S.; Shivalkar, S.; Varadwaj, P.K.; Sahoo, A.K.; Samanta, S.K. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed. Mater. 2022, 17, 022003. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends. Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Hofstee, M.I.; Muthukrishnan, G.; Atkins, G.J.; Riool, M.; Thompson, K.; Morgenstern, M.; Stoddart, M.J.; Richards, R.G.; Zaat, S.A.J.; Moriarty, T.F. Current Concepts of Osteomyelitis: From Pathologic Mechanisms to Advanced Research Methods. Am. J. Pathol. 2020, 190, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef] [Green Version]
- Hardy, B.L.; Bansal, G.; Hewlett, K.H.; Arora, A.; Schaffer, S.D.; Kamau, E.; Bennett, J.W.; Merrell, D.S. Antimicrobial Activity of Clinically Isolated Bacterial Species Against Staphylococcus aureus. Front. Microbiol. 2020, 10, 2977. [Google Scholar] [CrossRef]
- Dastgheyb, S.S.; Villaruz, A.E.; Le, K.Y.; Tan, V.Y.; Duong, A.C.; Chatterjee, S.S.; Cheung, G.Y.; Joo, H.S.; Hickok, N.J.; Otto, M. Role of Phenol-Soluble Modulins in Formation of Staphylococcus aureus Biofilms in Synovial Fluid. Infect. Immun. 2015, 83, 2966–2975. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jothipandiyan, S.; Suresh, D.; Sekaran, S.; Paramasivam, N. Palladium(II) Metal Complex Fabricated Titanium Implant Mitigates Dual-Species Biofilms in Artificial Synovial Fluid. Antibiotics 2023, 12, 1296. https://doi.org/10.3390/antibiotics12081296
Jothipandiyan S, Suresh D, Sekaran S, Paramasivam N. Palladium(II) Metal Complex Fabricated Titanium Implant Mitigates Dual-Species Biofilms in Artificial Synovial Fluid. Antibiotics. 2023; 12(8):1296. https://doi.org/10.3390/antibiotics12081296
Chicago/Turabian StyleJothipandiyan, Sowndarya, Devarajan Suresh, Saravanan Sekaran, and Nithyanand Paramasivam. 2023. "Palladium(II) Metal Complex Fabricated Titanium Implant Mitigates Dual-Species Biofilms in Artificial Synovial Fluid" Antibiotics 12, no. 8: 1296. https://doi.org/10.3390/antibiotics12081296
APA StyleJothipandiyan, S., Suresh, D., Sekaran, S., & Paramasivam, N. (2023). Palladium(II) Metal Complex Fabricated Titanium Implant Mitigates Dual-Species Biofilms in Artificial Synovial Fluid. Antibiotics, 12(8), 1296. https://doi.org/10.3390/antibiotics12081296





