Bacteriophage Therapy to Control Bovine Mastitis: A Review
Abstract
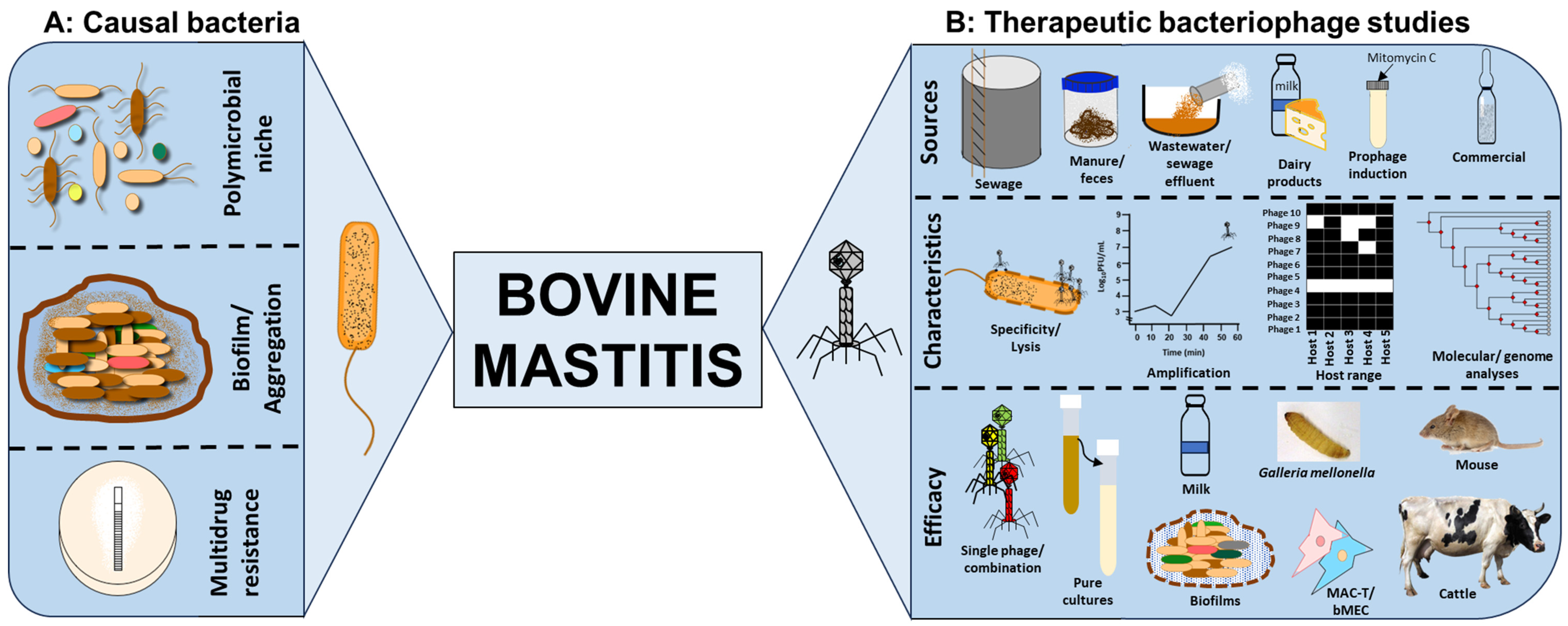
1. Impact of Bovine Mastitis
2. Microorganisms Causing or Associated with Bovine Mastitis
3. Treatment for Mastitis and Potential Problems with Current Control Methods
4. The Case for Phage Therapy to Control Bovine Mastitis
4.1. Phage Specificity, Lysis and Amplification
4.2. Isolation of Phages from a Wide Range of Sources
4.3. Cocktail Optimisation to Improve Therapeutic Activity
4.4. Characterisation of Phage Lysis and Stability in Pure Cultures
4.5. Phage Therapeutic Activity in Biofilms
4.6. Phage Therapeutic Assessments in Mastitis Ex Vivo and In Vivo Models
5. Barriers/Challenges to Therapeutic Phage Application to Control Bovine Mastitis
6. Thoughts on Phage Purification and Formulation for Safe and Optimal Delivery
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bond, D.M.; Morris, J.M.; Nassar, N. Study protocol: Evaluation of the probiotic Lactobacillus Fermentum CECT5716 for the prevention of mastitis in breastfeeding women: A randomised controlled trial. BMC Pregnancy Childbirth 2017, 17, 148. [Google Scholar] [CrossRef]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; Elsevier: St. Louis, MO, USA, 2007. [Google Scholar]
- Persson Waller, K.; Persson, Y.; Nyman, A.K.; Stengärde, L. Udder health in beef cows and its association with calf growth. Acta Vet. Scand. 2014, 56, 9. [Google Scholar] [CrossRef] [PubMed]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.Μ.; Wegener, H.C.; Rosdahl, V.T.; Jensen, Ν.E. Staphylococcal and other Bacterial Species Associated with Intramammary Infections in Danish Dairy Herds. Acta Vet. Scand. 1995, 36, 475–487. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Wegener, H.C.; Rosdahl, V.T. A comparative study of Staphylococcus aureus strains isolated from bovine subclinical mastitis during 1952–1956 and 1992. Acta Vet. Scand. 1995, 36, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Zhang, W.; Yang, Y.; Tang, F.; Nguyen, X.; Liu, G.; Lu, C. Characterization and genome sequencing of a novel bacteriophage infecting Streptococcus agalactiae with high similarity to a phage from Streptococcus pyogenes. Arch. Virol. 2013, 158, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.O.; Cortez, A.; Brandão, P.E.; da Silva, R.C.; Langoni, H. Molecular detection of Streptococcus agalactiae in bovine raw milk samples obtained directly from bulk tanks. Res. Vet. Sci. 2012, 93, 34–38. [Google Scholar] [CrossRef]
- Fahliyani, S.A.; Beheshti-Maal, K.; Ghandehari, F. Novel lytic bacteriophages of Klebsiella oxytoca ABG-IAUF-1 as the potential agents for mastitis phage therapy. Fems Microbiol. Lett. 2018, 365, fny223. [Google Scholar] [CrossRef]
- Farzaneh, M.; Derakhshandeh, A.; Al-Farha, A.A.A.; Petrovski, K.; Hemmatzadeh, F. A novel phage-displayed MilA ELISA for detection of antibodies against Myc. bovis in bovine milk. J. Appl. Microbiol. 2022, 133, 1496–1505. [Google Scholar] [CrossRef]
- Giannattasio-Ferraz, S.; Ene, A.; Gomes, V.J.; Queiroz, C.O.; Maskeri, L.; Oliveira, A.P.; Putonti, C.; Barbosa-Stancioli, E.F. Escherichia coli and Pseudomonas aeruginosa Isolated From Urine of Healthy Bovine Have Potential as Emerging Human and Bovine Pathogens. Front. Microbiol. 2022, 13, 764760. [Google Scholar] [CrossRef]
- Wipf, J.R.; Schwendener, S.; Perreten, V. The novel macrolide-Lincosamide-Streptogramin B resistance gene erm(44) is associated with a prophage in Staphylococcus xylosus. Antimicrob. Agents Chemother. 2014, 58, 6133–6138. [Google Scholar] [CrossRef]
- Richards, V.P.; Zadoks, R.N.; Pavinski Bitar, P.D.; Lefébure, T.; Lang, P.; Werner, B.; Tikofsky, L.; Moroni, P.; Stanhope, M.J. Genome characterization and population genetic structure of the zoonotic pathogen, Streptococcus canis. BMC Microbiol. 2012, 12, 293. [Google Scholar] [CrossRef]
- Larsen, H.D.; Sloth, K.H.; Elsberg, C.; Enevoldsen, C.; Pedersen, L.H.; Eriksen, N.H.; Aarestrup, F.M.; Jensen, N.E. The dynamics of Staphylococcus aureus intramammary infection in nine Danish dairy herds. Vet. Microbiol. 2000, 71, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.; Istiaq, A.; Clement, R.A.; Sultana, M.; Crandall, K.A.; Siddiki, A.Z.; Hossain, M.A. Metagenomic deep sequencing reveals association of microbiome signature with functional biases in bovine mastitis. Sci. Rep. 2019, 9, 13536. [Google Scholar]
- Aarestrup, F.M.; Wegener, H.C.; Rosdahl, V.T. Evaluation of phenotypic and genotypic methods for epidemiological typing of Staphylococcus aureus isolates from bovine mastitis in Denmark. Vet. Microbiol. 1995, 45, 139–150. [Google Scholar] [CrossRef]
- Bissong, M.E.A.; Ateba, C.N. Genotypic and Phenotypic Evaluation of Biofilm Production and Antimicrobial Resistance in Staphylococcus aureus Isolated from Milk, North West Province, South Africa. Antibiotics 2020, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.K.; Zadoks, R.N.; Gaskins, C.T. Biofilm production by Staphylococcus aureus associated with intramammary infection. Vet. Microbiol. 2005, 107, 295–299. [Google Scholar] [CrossRef]
- Li, X.; Xu, C.; Liang, B.; Kastelic, J.P.; Han, B.; Tong, X.; Gao, J. Alternatives to antibiotics for treatment of mastitis in dairy cows. Front. Vet. Sci. 2023, 10, 1160350. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M.; et al. Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 2016, 72, 957–968. [Google Scholar] [CrossRef]
- Saini, V.; McClure, J.T.; Léger, D.; Keefe, G.P.; Scholl, D.T.; Morck, D.W.; Barkema, H.W. Antimicrobial resistance profiles of common mastitis pathogens on Canadian dairy farms. J. Dairy Sci. 2012, 95, 4319–4332. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Bovine mastitis prevention and control in the post-antibiotic era. Trop. Anim. Health Prod. 2021, 53, 236. [Google Scholar] [CrossRef]
- Makumi, A.; Mhone, A.L.; Odaba, J.; Guantai, L.; Svitek, N. Phages for Africa: The Potential Benefit and Challenges of Phage Therapy for the Livestock Sector in Sub-Saharan Africa. Antibiotics 2021, 10, 1085. [Google Scholar] [PubMed]
- Han, J.E.; Kim, J.H.; Hwang, S.Y.; Choresca, C.H., Jr.; Shin, S.P.; Jun, J.W.; Chai, J.Y.; Park, Y.H.; Park, S.C. Isolation and characterization of a Myoviridae bacteriophage against Staphylococcus aureus isolated from dairy cows with mastitis. Res. Vet. Sci. 2013, 95, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Barasuol, B.M.; Cargnelutti, J.F.; Sangioni, L.A.; Pereira, D.I.B.; Varela, A.P.M.; Mayer, F.Q.; Pottker, E.S.; Goncalves, G.F.; Cibulski, S.; Botton, S.D. Characterization of novel of temperate phages of Staphylococcus aureus isolated from bovine milk. Arch. Microbiol. 2022, 204, 680. [Google Scholar] [CrossRef]
- Basdew, I.H.; Laing, M.D. Investigation of the lytic ability of South African bacteriophages specific for Staphylococcus aureus, associated with bovine mastitis. Biocontrol Sci. Technol. 2015, 25, 429–443. [Google Scholar] [CrossRef]
- Breyne, K.; Honaker, R.W.; Hobbs, Z.; Richter, M.; Żaczek, M.; Spangler, T.; Steenbrugge, J.; Lu, R.; Kinkhabwala, A.; Marchon, B. Efficacy and safety of a bovine-associated Staphylococcus aureus phage cocktail in a murine model of mastitis. Front. Microbiol. 2017, 8, 2348. [Google Scholar]
- Brouillette, E.; Millette, G.; Chamberland, S.; Roy, J.P.; Ster, C.; Kiros, T.; Hickey, S.; Hittle, L.; Woolston, J.; Malouin, F. Effective Treatment of Staphylococcus aureus Intramammary Infection in a Murine Model Using the Bacteriophage Cocktail StaphLyse™. Viruses 2023, 15, 887. [Google Scholar] [CrossRef]
- Dias, R.S.; Eller, M.R.; Duarte, V.S.; Pereira, Â.L.; Silva, C.C.; Mantovani, H.C.; Oliveira, L.L.; Silva Ede, A.; De Paula, S.O. Use of phages against antibiotic-resistant Staphylococcus aureus isolated from bovine mastitis. J. Anim. Sci. 2013, 91, 3930–3939. [Google Scholar] [CrossRef] [PubMed]
- Duarte, V.D.; Treu, L.; Sartori, C.; Dias, R.S.; Paes, I.D.; Vieira, M.S.; Santana, G.R.; Marcondes, M.I.; Giacomini, A.; Corich, V.; et al. Milk microbial composition of Brazilian dairy cows entering the dry period and genomic comparison between Staphylococcus aureus strains susceptible to the bacteriophage vB_SauM-UFV_DC4. Sci. Rep. 2020, 10, 5520. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Madera, C.; Martínez, B.; Rodríguez, A.; Evaristo Suárez, J. Prevalence of bacteriophages infecting Staphylococcus aureus in dairy samples and their potential as biocontrol agents. J. Dairy Sci. 2009, 92, 3019–3026. [Google Scholar] [CrossRef]
- Geng, H.; Zou, W.; Zhang, M.; Xu, L.; Liu, F.; Li, X.; Wang, L.; Xu, Y. Evaluation of phage therapy in the treatment of Staphylococcus aureus-induced mastitis in mice. Folia Microbiol 2020, 65, 339–351. [Google Scholar] [CrossRef]
- Hamza, A.; Perveen, S.; Abbas, Z.; Rehman, S.U. The Lytic SA Phage Demonstrate Bactericidal Activity against Mastitis Causing Staphylococcus aureus. Open Life Sci. 2016, 11, 39–45. [Google Scholar] [CrossRef]
- Iwano, H.; Inoue, Y.; Takasago, T.; Kobayashi, H.; Furusawa, T.; Taniguchi, K.; Fujiki, J.; Yokota, H.; Usui, M.; Tanji, Y.; et al. Bacteriophage Phi SA012 Has a Broad Host Range against Staphylococcus aureus and Effective Lytic Capacity in a Mouse Mastitis Model. Biology 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Synnott, A.J.; Kuang, Y.; Kurimoto, M.; Yamamichi, K.; Iwano, H.; Tanji, Y. Isolation from sewage influent and characterization of novel Staphylococcus aureus bacteriophages with wide host ranges and potent lytic capabilities. Appl. Environ. Microbiol. 2009, 75, 4483–4490. [Google Scholar] [CrossRef]
- Tanji, Y.; Tanaka, A.; Tani, K.; Kurimoto, M.; Miyanaga, K. IgG-dependent aggregation of Staphylococcus aureus inhibits bacteriophage attack. Biochem. Eng. J. 2015, 97, 17–24. [Google Scholar] [CrossRef]
- Jia, H.; Bai, Q.; Yang, Y.; Yao, H. Complete Genome Sequence of Staphylococcus aureus Siphovirus Phage JS01. Genome Announc. 2013, 1, e00797-13. [Google Scholar] [CrossRef]
- Jia, H.; Dong, W.; Yuan, L.; Ma, J.; Bai, Q.; Pan, Z.; Lu, C.; Yao, H. Characterization and complete genome sequence analysis of Staphylococcus aureus bacteriophage JS01. Virus Genes 2015, 50, 345–348. [Google Scholar] [CrossRef]
- Leite, J.A.; Pereira, H.P.; Borges, C.A.V.; Alves, B.R.C.; Ramos, A.; Martins, M.F.; Arcuri, E.F. Lytic bacteriophages as a potential alternative to control Staphylococcus aureus. Pesqui. Agropecu. Bras. 2019, 54. [Google Scholar] [CrossRef]
- Li, L.P.; Zhang, Z.Y. Isolation and characterization of a virulent bacteriophage SPW specific for Staphylococcus aureus isolated from bovine mastitis of lactating dairy cattle. Mol. Biol. Rep. 2014, 41, 5829–5838. [Google Scholar] [CrossRef]
- Ngassam-Tchamba, C.; Duprez, J.-N.; Fergestad, M.; De Visscher, A.; L’Abee-Lund, T.; De Vliegher, S.; Wasteson, Y.; Touzain, F.; Blanchard, Y.; Lavigne, R. In vitro and in vivo assessment of phage therapy against Staphylococcus aureus causing bovine mastitis. J. Glob. Antimicrob. Resist. 2020, 22, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Sangha, K.K.; Kumar, B.V.; Agrawal, R.K.; Deka, D.; Verma, R. Proteomic Characterization of Lytic Bacteriophages of Staphylococcus aureus Isolated from Sewage Affluent of India. Int. Sch. Res. Not. 2014, 2014, 265298. [Google Scholar] [CrossRef]
- Slanetz, L.W.; Jawetz, E. Isolation and Characteristics of Bacteriophages for Staphylococci of Bovine Mastitis. J. Bacteriol. 1941, 41, 447–455. [Google Scholar] [CrossRef]
- Song, J.; Ruan, H.; Chen, L.; Jin, Y.; Zheng, J.; Wu, R.; Sun, D. Potential of bacteriophages as disinfectants to control of Staphylococcus aureus biofilms. BMC Microbiol. 2021, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Srujana, A.S.; Sonalika, J.; Akhila, D.S.; Juliet, M.R.; Sheela, P. Isolation of Phages and Study of their In vitro Efficacy on Staphylococcus aureus Isolates Originating from Bovine Subclinical Mastitis. Indian. J. Anim. Res. 2022, 56, 754–758. [Google Scholar] [CrossRef]
- Teng, F.; Xiong, X.; Zhang, S.; Li, G.; Wang, R.; Zhang, L.; Wang, X.; Zhou, H.; Li, J.; Li, Y.; et al. Efficacy Assessment of Phage Therapy in Treating Staphylococcus aureus-Induced Mastitis in Mice. Viruses 2022, 14, 620. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zheng, P.P.; Ji, W.H.; Fu, Q.; Wang, H.G.; Yan, Y.X.; Sun, J.H. SLPW: A Virulent Bacteriophage Targeting Methicillin-Resistant Staphylococcus aureus In vitro and In vivo. Front. Microbiol. 2016, 7, 934. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, H.; Wei, C.; Zhang, H.; Zhou, Y.; Wang, R. Characterization and partial genomic analysis of a lytic Myoviridae bacteriophage against Staphylococcus aureus isolated from dairy cows with mastitis in Mid-east of China. Virus Genes 2015, 50, 111–117. [Google Scholar] [CrossRef]
- Zhang, L.L.; Sun, L.C.; Wei, R.C.; Gao, Q.; He, T.; Xu, C.F.; Liu, X.J.; Wang, R. Intracellular Staphylococcus aureus Control by Virulent Bacteriophages within MAC-T Bovine Mammary Epithelial Cells. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xing, S.Z.; Sun, Q.; Pei, G.Q.; Cheng, S.; Liu, Y.N.; An, X.P.; Zhang, X.L.L.; Qu, Y.G.; Tong, Y.G. Characterization and complete genome sequence analysis of a novel virulent Siphoviridae phage against Staphylococcus aureus isolated from bovine mastitis in Xinjiang, China. Virus Genes 2017, 53, 464–476. [Google Scholar] [CrossRef]
- Titze, I.; Krömker, V. Antimicrobial Activity of a Phage Mixture and a Lactic Acid Bacterium against Staphylococcus aureus from Bovine Mastitis. Vet. Sci. 2020, 7, 31. [Google Scholar] [CrossRef]
- Titze, I.; Lehnherr, T.; Lehnherr, H.; Krömker, V. Efficacy of Bacteriophages Against Staphylococcus aureus Isolates from Bovine Mastitis. Pharmaceuticals 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Yang, Y.; Lu, C. Isolation and characterization of siphovirus phages infecting bovine Streptococcus agalactiae. Wei Sheng Wu Xue Bao 2016, 56, 317–326. [Google Scholar] [PubMed]
- Gill, J.J.; Pacan, J.C.; Carson, M.E.; Leslie, K.E.; Griffiths, M.W.; Sabour, P.M. Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob. Agents Chemother. 2006, 50, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.J.; Sabour, P.M.; Leslie, K.E.; Griffiths, M.W. Bovine whey proteins inhibit the interaction of Staphylococcus aureus and bacteriophage K. J. Appl. Microbiol. 2006, 101, 377–386. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Coffey, A.; Meaney, W.J.; Fitzgerald, G.F.; Ross, R.P. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett. Appl. Microbiol. 2005, 41, 274–279. [Google Scholar] [CrossRef]
- Duarte, V.D.; Dias, R.S.; Kropinski, A.M.; Campanaro, S.; Treu, L.; Siqueira, C.; Vieira, M.S.; Paes, I.D.; Santana, G.R.; Martins, F.; et al. Genomic analysis and immune response in a murine mastitis model of vB_EcoM-UFV13, a potential biocontrol agent for use in dairy cows. Sci. Rep. 2018, 8, 6845. [Google Scholar] [CrossRef]
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Bacteriophage Cocktails Protect Dairy Cows Against Mastitis Caused By Drug Resistant Escherichia coli Infection. Front. Cell Infect. Microbiol. 2021, 11, 690377. [Google Scholar] [CrossRef]
- Porter, J.; Anderson, J.; Carter, L.; Donjacour, E.; Paros, M. In vitro evaluation of a novel bacteriophage cocktail as a preventative for bovine coliform mastitis. J. Dairy Sci. 2016, 99, 2053–2062. [Google Scholar] [CrossRef]
- Han, G.; Zhang, J.; Luo, Z.; Lu, B.; Zhang, P.; Yong, K.; Wang, Y.; Luo, Y.; Yang, Z.; Ren, M.; et al. Characteristics of a novel temperate bacteriophage against Staphylococcus arlettae (vB_SarS_BM31). Int. Microbiol. 2023, 26, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhao, W.; Han, B.; Barkema, H.W.; Niu, Y.D.; Liu, Y.; Kastelic, J.P.; Gao, J. Biological and genomic characteristics of two bacteriophages isolated from sewage, using one multidrug-resistant and one non-multidrug-resistant strain of Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 943279. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, W.; Liu, G.; Ali, T.; Chen, P.; Liu, Y.; Kastelic, J.P.; Han, B.; Gao, J. Bacteriophages isolated from dairy farm mitigated Klebsiella pneumoniae-induced inflammation in bovine mammary epithelial cells cultured in vitro. BMC Vet. Res. 2021, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shi, Y.; Liu, G.; Yang, J.; Yi, B.; Liu, Y.; Kastelic, J.P.; Han, B.; Gao, J. Bacteriophage has beneficial effects in a murine model of Klebsiella pneumoniae mastitis. J. Dairy Sci. 2021, 104, 3474–3484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, Y.; Gao, Y.; Guo, M.; Liu, Y.; Zou, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; et al. Phage vB_PaeS-PAJD-1 Rescues Murine Mastitis Infected With Multidrug-Resistant Pseudomonas aeruginosa. Front. Cell Infect. Microbiol. 2021, 11, 689770. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.Y.; He, D.L.; Li, D.; Liu, S.S.; Wang, G.; Ji, Y.L.; Wang, X.W.; Wang, Z.J.; Bi, L.T.; Zhao, R.H.; et al. Bacteriophage Protects Against Aerococcus viridans Infection in a Murine Mastitis Model. Front. Vet. Sci. 2020, 7, 588. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, H.; Sun, Y.; Zhangxiang, L.; Zhang, P.; Liu, G.; Qu, Y.; Tong, Y.; Li, Y. Isolation and characterization of a lytic phage infecting Enterococcus faecium of bovine mastitis. Acta Vet. Zootech. Sin. 2017, 48, 706–713. [Google Scholar]
- Wu, S.; Fang, Z.; Tan, J.; Li, M.; Wang, C.; Guo, Q.; Xu, C.; Jiang, X.; Zhu, H. DeePhage: Distinguishing virulent and temperate phage-derived sequences in metavirome data with a deep learning approach. Gigascience 2021, 10, giab056. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phage Therapy in Livestock and Companion Animals. Antibiotics 2021, 10, 559. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Taslem Mourosi, J.; Awe, A.; Guo, W.; Batra, H.; Ganesh, H.; Wu, X.; Zhu, J. Understanding Bacteriophage Tail Fiber Interaction with Host Surface Receptor: The Key “Blueprint” for Reprogramming Phage Host Range. Int. J. Mol. Sci. 2022, 23, 12146. [Google Scholar] [CrossRef]
- Kizziah, J.L.; Manning, K.A.; Dearborn, A.D.; Dokland, T. Structure of the host cell recognition and penetration machinery of a Staphylococcus aureus bacteriophage. PLoS Pathog. 2020, 16, e1008314. [Google Scholar] [CrossRef]
- Wang, I.N.; Smith, D.L.; Young, R. Holins: The protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 2000, 54, 799–825. [Google Scholar] [CrossRef]
- Hendrix, R.W.; Smith, M.C.M.; Burns, R.N.; Ford, M.E.; Hatfull, G.F. Evolutionary relationships among diverse bacteriophages and prophages: All the world’s a phage. Proc. Natl. Acad. Sci. USA 1999, 96, 2192–2197. [Google Scholar] [CrossRef]
- Mushegian, A.R. Are There 1031 Virus Particles on Earth, or More, or Fewer? J. Bacteriol. 2020, 202, 10–1128. [Google Scholar] [CrossRef]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Nale, J.Y.; Clokie, M.R.J. Preclinical data and safety assessment of phage therapy in humans. Curr. Opin. Biotechnol. 2021, 68, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef]
- Nale, J.Y.; Chutia, M.; Carr, P.; Hickenbotham, P.T.; Clokie, M.R.J. ‘Get in Early’; Biofilm and Wax Moth (Galleria mellonella) Models Reveal New Insights into the Therapeutic Potential of Clostridium difficile Bacteriophages. Front. Microbiol. 2016, 7, 1383. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid. Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.R.; Krömker, V.; Bjarnsholt, T.; Dahl-Pedersen, K.; Buhl, R.; Jørgensen, E. Biofilm Research in Bovine Mastitis. Front. Vet. Sci. 2021, 8, 656810. [Google Scholar] [CrossRef]
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic Engineering of Bacteriophages Against Infectious Diseases. Front. Microbiol. 2019, 10, 954. [Google Scholar] [CrossRef]
- Gibb, B.; Hyman, P.; Schneider, C.L. The Many Applications of Engineered Bacteriophages—An Overview. Pharmaceuticals 2021, 14, 634. [Google Scholar] [CrossRef]
- Guo, D.; Chen, J.; Zhao, X.; Luo, Y.; Jin, M.; Fan, F.; Park, C.; Yang, X.; Sun, C.; Yan, J.; et al. Genetic and Chemical Engineering of Phages for Controlling Multidrug-Resistant Bacteria. Antibiotics 2021, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]
- Vagenende, V.; Yap, M.G.; Trout, B.L. Mechanisms of protein stabilization and prevention of protein aggregation by glycerol. Biochemistry 2009, 48, 11084–11096. [Google Scholar] [CrossRef]
- Rosner, D.; Clark, J. Formulations for Bacteriophage Therapy and the Potential Uses of Immobilization. Pharmaceuticals 2021, 14, 359. [Google Scholar] [CrossRef]
| Bacteria | Phages | Therapeutic Activity | |||
|---|---|---|---|---|---|
| Species | Resistance | Name (Morphology/Classification *) | Source | Outcome | Reference(s) |
| Staphylococcus aureus | MDR | SAH-1 (M) | Enrichment of sewage effluent | Latent period of 20 min and burst of 100 PFU/cell, significantly reduced bacterial growth at MOIs of 1–100 | [27] |
| B_UFSM4L (S) B_UFSM5L (S) | Coagulase-positive Staphylococcus bovine milk | Broad host range (B_UFSM4, 45.8%; B_UFSM5, 4.16%; n = 24), intra-species infection on S. sciuri and Rothia terrae | [28] | ||
| MRSA | Six, only three fully characterised | Milk | Reduced S. aureus CFU counts by 64–95% | [29] | |
| ATCC 23361 (M) BP39 (R) | In-house directed evolution (ATCC 23361) Commercial (PhageLux, BP39) | Phage cocktail was effective in milk in vitro with/without supplementation with IgG. Reduced colonisation, high intramammary phage counts recorded, no phage systemic spread in mouse model | [30] | ||
| MSSA, MRSA VISA | SAML-4 (H) SAML-12 (H) SAML-150 (H) SAML-4229 (H) SATA-8505 (H) | Commercial (StaphLyseTM) | Wide host range (92.7% at 104 CFU/mL and 100% at 109 CFU/mL of 709 strains). Phages were stable at 4 °C and 37 °C and activity was dose dependent in milk. Reduced colonisation in mouse mammary gland 8 h after treatment and prophylactically 4 h before challenge was most effective | [31] | |
| Penicillin Ampicillin | Ufv-aur2 (M) Ufv-aur3 (M) Ufv-aur4 (M) Ufv-aur5 (M) Ufv-aur6 (M) Ufv-aur7 (M) Ufv-aur8 (M) Ufv-aur9 (M) Ufv-aur10 (M) Ufv-aur11 (M) | Sewage water | Reduced bacterial growth after 8 h, thermostable between 70 °C and 100 °C, lysed 80–100% of 20 isolates examined | [32] | |
| Ampicillin | vB_SauM-UFV_DC4 (M) | Wastewater of dairy farm | UFV_DC4 lysed two of the strains examined | [33] | |
| C1L ** P1L ** L7L ** L13L ** A8L ** H5L (S) A72L (S) | Enrichment of Cabrales cheese, Peñamellera cheese and raw milk | H5 and A72 were characterised, stable at 0–4 °C but reduced by 20–30% at 22–37 °C, respectively. Also stable at 72 °C for 15 s but inactivated after 1 min. Challenge assay in vitro showed bacterial inhibition in UHT and pasteurised milk but reduced activity in semi-skimmed and whole raw milk | [34] | ||
| vBSM-A1 (M) vBSP-A2 (P) | Mixed sewage samples from cattle farms | A cocktail of two phages was superior to single-phage treatments and comparable to ceftiofur sodium in mice; it improved mastitis pathology and reduced colonisation. High intramammary phage recovery was observed without systemic spread | [35] | ||
| Cefoxitin, Oxacillin, Vancomycin | SA (M) | Wastewater | Stable at pH 4–11 and temperatures 28–37 °C but significantly reduced at 50–105 °C. Host range of 50% (n = 12). Significantly reduced bacterial growth 8 h a phage treatment | [36] | |
| MRSA | PhiSA012 (M) PhiSA039 (M) | Previously isolated from sewage influent | PhiSA012 and 039 showed variable host range. SA012 activity was delayed by bovine IgG dependent aggregation. Intravenous and intra-peritoneal administration of SA012 reduced bacterial colonisation in and inflammation of mammary gland | [37,38,39] | |
| JS01L (S) | Milk of mastitis cows | 43,458 bp genome of 66 ORFs, 33.32%, G/C content and no tRNAs. Encodes two virulence factors, staphylokinase and Staphylococcal complement inhibitor | [40,41] | ||
| Phage 1 ** Phage 2 ** Phage 3 ** Phage 4 ** Phage 5 ** | Barn flush water from four dairy herds | Phage 2 and 4 showed wide host range lysing 69–100% susceptibility (n = 100). Highly conserved endolysin with 99% similarity to other Staphylococcal phages. Three domains for phage involved in phage recognition and bacterial lysis were identified | [42] | ||
| PSW (M) | Wastewater from dairy farm | 65–69 kb genome, small burst of 44 ± 3 PFU/mL/bacteria, attachment not influenced by calcium, stable at 40–60 °C and pH 2–9, resistant to chloroform, optimal lysis MOI is 0.01. Inhibited growth of four S. aureus strains and showed interspecies infection on E. coli | [43] | ||
| MRSA MSSA | Romulus (T) Remus (T) ISP (T) DSM105264 (Phage K, K) | Sewage water (Romulus and Remus), Unknown sources (ISP, Phage K) | Romulus, Remus, ISP showed lysis activity. A 50% survival in Galleria mellonella 4 days after phage treatment and incomplete recovery in mice 48 h after phage treatment with ISP phage | [44] | |
| ΦMSP (S) | Sewage | Possessed hydrolase of 70 kDA and induced twenty-six S. aureus proteins during infection | [45] | ||
| MDR | Phage 3 ** Phage 7 ** Phage 8 ** Phage 15 ** Phage 17 ** Phage 18 ** Phage 19 ** | Milk from mastitis cows shedding Staphylococci | Phages lysed both bovine and human bacterial isolates; they have similar plaque morphology to phages from human sources, not stable beyond 67 °C. No significant difference in susceptibility to mercuric chloride, hydrogen ion concentrations, sterile water or saline. Sterile water was toxic to low-concentrated phages | [46] | |
| MRSA | vB_SauM_SDQ (M) | Sewage | Lysed 20 of 24 strains, reduced established biofilms on polystyrene, milk, and mammary gland tissue after treatment | [47] | |
| MRSA MSSA | Phage 24 A2 ** | Cowshed wastewater | Lysed 19 of 30 strains examined. Phage cleared bacterial cultures on agar at MOI of 10, supporting topical application for therapeutic use | [48] | |
| MDR | 4086-1 (P) 4086-2 (P) 4086-3 (P) 4086-4 (P) 4086-6 (P) | Milk samples from mastitis cows | Phages 4086-1, 4086-2 and 4086-3 lysed four, while 4086-4 and -6 lysed two of the six S. aureus strains tested. Significantly reduced bacterial load at MOI 0.1, 2–4 h after phage treatment in vitro but resistance was observed 2–5 h afterwards. Significantly reduced biofilm mass and colonisation in the mammary gland, decreased expression of TNF-α and IL-6, reduction in mammary infiltration of S. aureus in mouse model | [49] | |
| MRSA | SLPW (P) | Faecal sewage in a pig farm | Lysed 36 of 40 isolates examined. Stable at up to 45 °C, chloroform and ultraviolet light but deactivated at 65 °C. Short latent (10 min), long lytic period (120 min), intraperitoneal phage administration remedially reduced colonisation and inflammation of cytokines in mice, effective in intra-abdominal infection for different MLST types | [50] | |
| vB_SauM_JS25 (M) | Sewage effluent in a dairy farm | Lysed 51 of 56 strains tested, stable at pH 6–9, deactivated at 70–80 °C for 10 min, significantly reduced bacterial load at MOI 1 in vitro. Ex vivo assays using MAC-T showed phage reached nucleus 3 h after infection and reduced colonisation in a time-dependent manner intracellularly; endocytotic activity was at 12% | [51,52] | ||
| vB_SauS_IMEP5 (S) | Manure from dairy farms | Stable at pH 3–10, inactivated at 70 °C for 20 min, reduced bacterial growth at MOI 0.001 | [53] | ||
| TA1.ST29 (M) EB1.ST11 (P) EB1.ST27 (P) | Sewage water (TA1.ST29) Pig manure (EB1.ST11 and EB1.ST27) | Two of three of bacterial isolates were lysed by at least a single phage, cocktail of the three phages along with and in combination with L. planetarium significantly reduced colonisation in pasteurised and raw milk | [54,55] | ||
| Streptococcus agalactiae | LYGO9L (S) HZ04L (S) pA11L (S) | Induction with mitomycin C | Specificity to S. galactiae; lysed 12, 13, 20 of 42 strains examined | [56] | |
| Bacteriophage K (K) | Unknown | Whey protein in milk could inhibit phage adhesion and proliferation in milk. Intramammary infusion of phages reduced colonisation in 16.7% of treated lactating cows. Large increases in somatic cells were observed in phage-treated healthy cows | [57,58,59] | ||
| JX01L (S) | Milk of mastitis cows | ~90% of phage adsorbed after 2.5 min, burst of 20/cell, latent period of 30 min. Deactivated at 60 °C at 30 min, with ~70% reduction at 50 °C. | [7] | ||
| Escherichia coli | MPEC | vB_EcoM_UFV13 (T) | Sewage | Stable at pH 4–12, temperature 37–62 °C, activity optimal at 22–37 °C and not affected by osmotic shock and organic solvents Sarksoyl and CTAB. A 10-fold reduction in bacterial load was observed at MOI of 10 in mice. From seven pro-inflammatory cytokines (IL-6, TNF-α, IL-2, IFN-γ, IL-4, IL-10 and IL-17A,) only IL-10, IL-6 and TNF-α expressions were statistically significant | [60] |
| MDR | vB_EcoM_SYGD1 (M) vB_EcoP_SYGE1 (A) SYGMH1 (M) | Sewage of dairy farms | Stable at 25–37 °C, deactivated at 60 °C. Optimal pH range is 5–9 and sensitive to ultraviolet light. Cocktail of the phages reduced colonisation, somatic cells, and inflammatory factors, alleviated symptoms of mastitis in cattle. Results were comparable to ceftiofur sodium-treated group | [61] | |
| Ampicillin | Four-phage cocktail | Sewage wastewater | Significant reduction in bacterial counts in raw milk and adherence to bovine mammary alveolar epithelial cell line, MAC-T | [62] | |
| Klebsiella oxytoca | P1 (M) P2 (M) P3 (P) P4 (M) | Wastewater | Stable at 37–50 °C, inactivated at pH 2, 5 and 11. Treatment caused 97% reduction in bacterial growth in pure cultures. P2 showed interspecies lysis clearing Enterobacter aerogenes as well. | [9] | |
| Staphylococcus arlettae | BM31L (S) | Milk of bovine mastitis cows | Stable at pH 6–9, temperatures 40–50 °C but significantly reduced at 60 °C, and in chloroform and ether. Optimal MOI was 0.001 and 1. First phage to be isolated for this bacterium | [63] | |
| Klebsiella pneumoniae | MDR and non-MDR | M_Kpn_HB132952 (S) CM_Kpn_HB143742 (P) | Sewage samples | Optimal MOI is 0.01 for M_Kpn_HB132952 and 1 for CM_Kpn_HB143742, pH 4–11, and 30–60 °C. Both phages had similar host range (30/31 strains), TNF-α and IL-1β expression not significantly different between treated and untreated mice | [64] |
| CM8-1 *** SJT-2 *** | Dairy farm wastewater | Phage treatment reduced bacteria adhesion, invasion and cytotoxicity. Phage treatment suppressed morphological changes in bMECs 4–8 h after treatment. Phage treatment mitigated expression of TLR4, NF-κB, TNF-α, IL-1β, IL-6, IL-8, caspase-3, caspase-9 and cyt-c in bMECs and increased apoptosis of bMECs | [65] | ||
| CM8-1 *** | Dairy farm wastewater | Stable at 30–50 °C, pH 6–10, reduced colonisation 2 h after phage treatment in mammary glands, reduced expression of TNF-α, IL-1β, IL-6, and IL-8 in murine model | [66] | ||
| Pseudomonas aeruginosa | MDR | vB_PaeS_PAJD-1 (S) | Sewage from dairy farm | Short latent period of 20 min, stable at 25–55 °C and pH 5–9. In murine model, phage treatment significantly reduced colonisation and repaired mammary glands | [67] |
| Aerococcus viridans | vB_AviM_AVP ** | Sewage | Optimal MOI was 0.001. Stable at pH 3–11, 25–50 °C. Reduced colonisation in damaged breast of mice with no bacteria detection with 107 PFU of phage treatment for 24 h. No significant difference in CFU load was recorded for 105 PFU treatment compared to control treatment with PBS. Reduced TNF-α, IL-1β, and IL-6 expression, and myeloperoxidase activity | [68] | |
| Enterococcus faecium | vB_EfaM_XJ3 (M) | Dairy cattle faecal sample | Optimal MOI was 0.001, latent period was 15 min, burst 84 and burst time was 175 min, stable at 50 °C and pH 5–11 | [69] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nale, J.Y.; McEwan, N.R. Bacteriophage Therapy to Control Bovine Mastitis: A Review. Antibiotics 2023, 12, 1307. https://doi.org/10.3390/antibiotics12081307
Nale JY, McEwan NR. Bacteriophage Therapy to Control Bovine Mastitis: A Review. Antibiotics. 2023; 12(8):1307. https://doi.org/10.3390/antibiotics12081307
Chicago/Turabian StyleNale, Janet Y., and Neil R. McEwan. 2023. "Bacteriophage Therapy to Control Bovine Mastitis: A Review" Antibiotics 12, no. 8: 1307. https://doi.org/10.3390/antibiotics12081307
APA StyleNale, J. Y., & McEwan, N. R. (2023). Bacteriophage Therapy to Control Bovine Mastitis: A Review. Antibiotics, 12(8), 1307. https://doi.org/10.3390/antibiotics12081307









