Enhancing Chemical Stability through Structural Modification of Antimicrobial Peptides with Non-Proteinogenic Amino Acids
Abstract
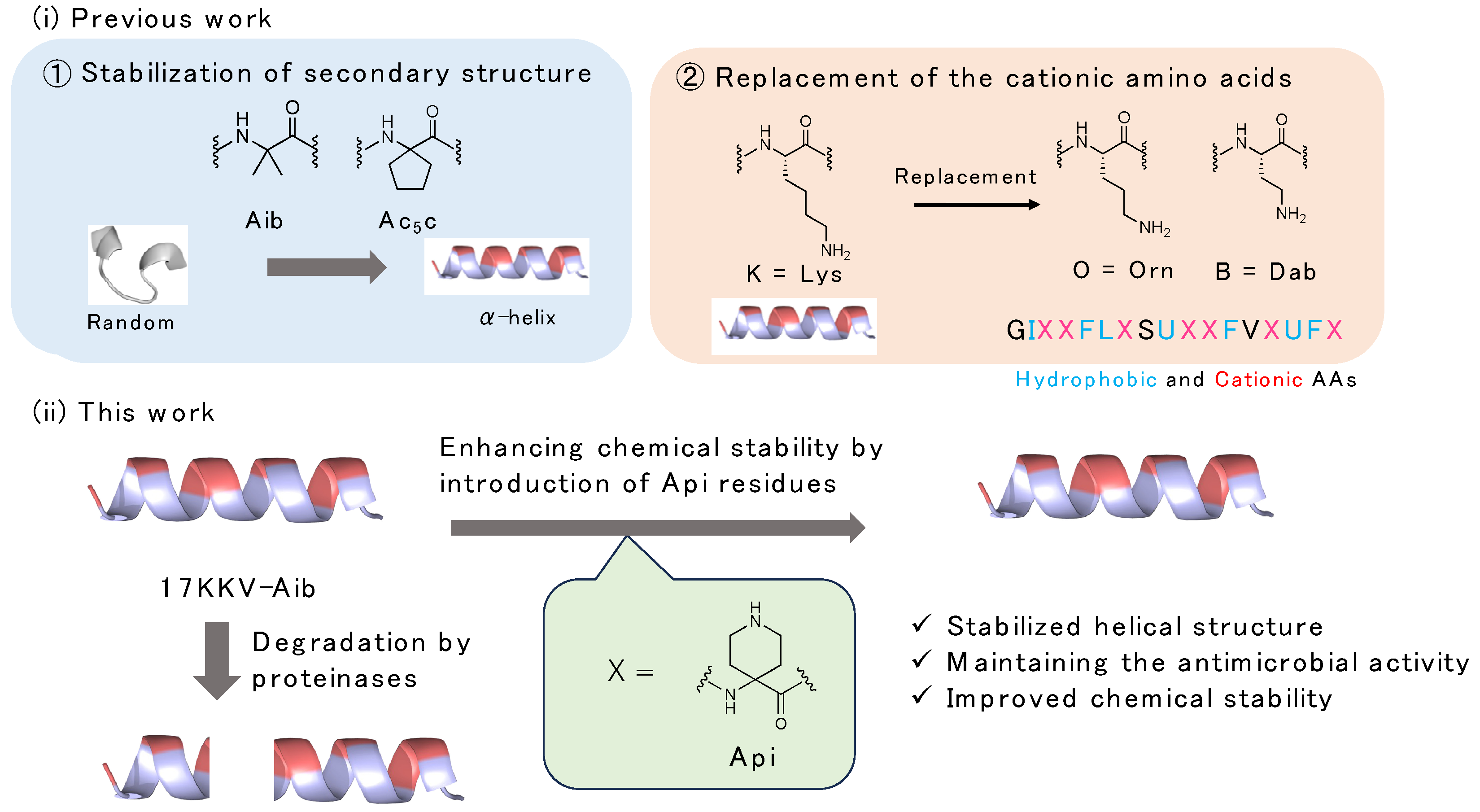
:1. Introduction
2. Results
2.1. Synthesis of Fmoc-Protected Api
2.2. Design and Synthesis of AMPs Containing Api Residue
2.3. Preferred Secondary Structural Analysis by CD Spectra
2.4. Antimicrobial Activity of the Synthesized Peptides
2.5. Hemolysis Activity of the Synthesized Peptides against Red Blood Cells
2.6. Chemical Stability of the Synthesized Peptides against Digestive Enzymes
2.7. Calculation Analysis of Charge Surface with or without Api Residue
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Synthesis of Api
4.3. Synthesis of Fmoc-Protected Api
4.4. Peptide Synthesis
4.5. CD Spectrometry
4.6. Antimicrobial Activity
4.7. Hemolysis Activity
4.8. Digestion Assay
4.9. Calculation Analysis of Charge Surface
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, J.L.C.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global buden of bacterial antimicrobial resistance in 2019: A systematic. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Rajeev, A.; Chattopadhyay, I. Application of antimicrobial peptides as next-generation therapeutics in the biomedical world. Biotechnol. Genet. Eng. Rev. 2023, 10, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial peptides: A new hope in biomedical and pharmaceutical fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial machanisms and clinical application prospects of antimicrobial peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Juárez-López, D.; Morales-Ruiz, E.; Herrera-Zúñiga, L.D.; González-Carrera, Z.; Cuevas-Reyes, E.; Corzo, G.; Schcolnik-Cabrera, A.; Villegas, E. The resilience of Pseudomonas aeruginosa to antibiotics and the designing of antimicrobial peptides to overcome microbial resistance. Curr. Med. Chem. 2022, 30, 72–103. [Google Scholar]
- Yu, L.; Li, K.; Zhang, J.; Jin, H.; Saleem, A.; Song, Q.; Jia, Q.; Li, P. Antimicrobial peptides and macromolecules for combating microbial infections: From agents to interfaces. ACS Appl. Bio Mater. 2022, 5, 366–393. [Google Scholar] [CrossRef]
- Zhu, Y.; Hao, W.; Wang, X.; Ouyang, J.; Deng, X.; Yu, H.; Wang, Y. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infection. Med. Res. Rev. 2022, 42, 1377–1422. [Google Scholar] [CrossRef]
- Bowers, S.R.; Klimov, D.K.; Lockhart, C. Mechanisms of binding of antimicrobial peptide PGLa to DMPC/DMPG membrane. J. Chem. Inf. Model. 2022, 62, 1525–1537. [Google Scholar] [CrossRef]
- Goto, C.; Hirano, M.; Hayashi, K.; Kikuchi, Y.; Hara-Kudo, Y.; Misawa, T.; Demizu, Y. Development of amphipathic antimicrobial peptide foldamers based on Magainin 2 sequence. ChemMedChem 2019, 14, 1911–1916. [Google Scholar] [CrossRef]
- Hirano, M.; Saito, C.; Yokoo, H.; Goto, C.; Kawano, R.; Misawa, T.; Demizu, Y. Development of antimicrobial stapled peptides based on Magainin 2 sequence. Molecules 2021, 26, 444. [Google Scholar] [CrossRef]
- Takada, M.; Ito, T.; Kurashima, M.; Matsunaga, N.; Demizu, Y.; Misawa, T. Structure-activity relationship studies of substitutions of cationic amino acid residues on antimicrobial peptides. Antibiotics 2023, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Wysong, C.L.; Yokum, T.S.; Morales, G.A.; Gundry, R.L.; McLaughlin, M.L.; Hammer, R.P. 4-Aminopiperidine-4-carboxylic acid: A cyclic alpha,alpha-disubstituted amino acid for preparation of water-soluble highly helical peptides. J. Org. Chem. 1996, 61, 7650–7651. [Google Scholar] [CrossRef] [PubMed]
- Zanella, S.; Bocchinfuso, G.; De Zotti, M.; Arosio, D.; Marino, F.; Raniolo, S.; Pignataro, L.; Sacco, G.; Palleschi, A.; Siano, A.S.; et al. Rational design of antiangiogenic helical oligopeptides targeting the vascular endothelial growth factor receptors. Front. Chem. 2019, 7, 170. [Google Scholar] [CrossRef]
- Hammarström, L.G.; Gauthier, T.J.; Hammer, R.P.; McLaughlin, M.L. Amphipathic control of the 3(10)-/alpha-helix equilibrium in synthetic peptides. J. Pept. Res. 2001, 58, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, L.G.J.; Fu, Y.; Vail, S.; Hammer, R.P.; McLaughlin, M.L. A convenient prepareation of an orthogonally protected alpha, alpha disubstituted amino acid analogue of Lysine: 1-tert-butyloxycarbonyl-4-((9-fluorenylmethyloxycarbonyl)amino)-piperidine-4-carboxylic acid. Org. Synth. 2005, 81, 213–224. [Google Scholar]
- Yamashita, H.; Oba, M.; Misawa, T.; Tanaka, M.; Hattori, T.; Naito, M.; Kurihara, M.; Demizu, Y. A helix-stabilized cell-penetrating peptides as an intracellular delivery tool. ChemBioChem 2016, 17, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Horne, W.S.; Johnson, L.M.; Ketas, T.J.; Klasse, P.J.; Lu, M.; Moore, J.P.; Gellman, S.H. Structural and biological mimicry of protein surface recognition by alpha/beta-peptide foldamers. Proc. Natl. Acad. Sci. USA 2009, 106, 14751–14756. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kato, T.; Oba, M.; Misawa, T.; Hattori, T.; Ohoka, N.; Tanaka, M.; Naito, M.; Kurihara, M.; Demizu, Y. Development of a cell-penetrating peptide that exhibits responsive changes in its secondary structure in the cellular environment. Sci. Rep. 2016, 6, 33003. [Google Scholar] [CrossRef]
- Toniolo, C.; Polese, A.; Formaggio, F.; Crisma, M.; Kamphuis, J. Circular dichroism spectrum of a peptide 310-helix. J. Am. Chem. Soc. 1996, 118, 2744–2745. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Akishiba, M.; Takeuchi, T.; Kawaguchi, Y.; Sakamoto, K.; Yu, H.H.; Nakase, I.; Takatani-Nakase, T.; Madani, F.; Gräslund, A.; Futaki, S. Cytosolic antibody delivery by lipid-sensitive endosomolytic peptides. Nat. Chem. 2017, 9, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Oddo, A.; Hansen, P.R. Hemolytic activity of antimicrobial peptides. Methods Mol. Biol. 2017, 1548, 427–435. [Google Scholar] [PubMed]
- Misawa, T.; Goto, C.; Shibata, N.; Hirano, M.; Kikuchi, Y.; Naito, M.; Demizu, Y. Rational design of novel amphipathic antimicrobial peptides focused on the distribution of cationic amino acid residues. MedChemComm 2019, 10, 896–900. [Google Scholar] [CrossRef]





| Peptide | Sequence | |
|---|---|---|
| 1 | GIKKFLKSUKKFVKUFK |   |
| 2 | GIXKFLKSUKKFVKUFK | |
| 3 | GIKXFLKSUKKFVKUFK | |
| 4 | GIKKFLXSUKKFVKUFK | |
| 5 | GIKKFLKSUXKFVKUFK | |
| 6 | GIKKFLKSUKXFVKUFK | |
| 7 | GIKKFLKSUKKFVXUFK | |
| 8 | GIKKFLKSUKKFVKUFX |
| Peptide | Sequence | MIC (μM) | Hemolysis (μM) | |||
|---|---|---|---|---|---|---|
| Gram(−) | Gram(+) | |||||
| E. Coli DH5α | P. aeruginosa | MDRP | S. aureus | |||
| 1 | GIKKFLKSUKKFVKUFK | 1.56 | 3.125 | 6.25 | 12.5 | 100 |
| 2 | GIXKFLKSUKKFVKUFK | 3.125 | 3.125 | 3.125 | 12.5 | 12.5 |
| 3 | GIKXFLKSUKKFVKUFK | 3.125 | 3.125 | 3.125 | 25 | 25 |
| 4 | GIKKFLXSUKKFVKUFK | 1.56 | 3.125 | 6.25 | 12.5 | 12.5 |
| 5 | GIKKFLKSUXKFVKUFK | 1.56 | 3.125 | 6.25 | 25 | 50 |
| 6 | GIKKFLKSUKXFVKUFK | 1.56 | 3.125 | 6.25 | 12.5 | 50 |
| 7 | GIKKFLKSUKKFVXUFK | 1.56 | 3.125 | 6.25 | 12.5 | 12.5 |
| 8 | GIKKFLKSUKKFVKUFX | 3.125 | 3.125 | 12.5 | 12.5 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, T.; Matsunaga, N.; Kurashima, M.; Demizu, Y.; Misawa, T. Enhancing Chemical Stability through Structural Modification of Antimicrobial Peptides with Non-Proteinogenic Amino Acids. Antibiotics 2023, 12, 1326. https://doi.org/10.3390/antibiotics12081326
Ito T, Matsunaga N, Kurashima M, Demizu Y, Misawa T. Enhancing Chemical Stability through Structural Modification of Antimicrobial Peptides with Non-Proteinogenic Amino Acids. Antibiotics. 2023; 12(8):1326. https://doi.org/10.3390/antibiotics12081326
Chicago/Turabian StyleIto, Takahito, Natsumi Matsunaga, Megumi Kurashima, Yosuke Demizu, and Takashi Misawa. 2023. "Enhancing Chemical Stability through Structural Modification of Antimicrobial Peptides with Non-Proteinogenic Amino Acids" Antibiotics 12, no. 8: 1326. https://doi.org/10.3390/antibiotics12081326
APA StyleIto, T., Matsunaga, N., Kurashima, M., Demizu, Y., & Misawa, T. (2023). Enhancing Chemical Stability through Structural Modification of Antimicrobial Peptides with Non-Proteinogenic Amino Acids. Antibiotics, 12(8), 1326. https://doi.org/10.3390/antibiotics12081326







