Missed Opportunities for Antifungal Stewardship during the COVID-19 Era
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Livorsi, D.; Comer, A.; Matthias, M.S.; Perencevich, E.N.; Bair, M.J. Factors influencing antibiotic-prescribing decisions among inpatient physicians: A qualitative investigation. Infect. Control Hosp. Epidemiol. 2015, 36, 1065–1072. [Google Scholar] [CrossRef]
- Baddley, J.W.; Thompson, G.R.; Chen, S.C.-A.; White, P.L.; Johnson, M.D.; Nguyen, M.H.; Schwartz, I.S.; Spec, A.; Ostrosky-Zeichner, L.; Jackson, B.R.; et al. Coronavirus disease 2019–associated invasive fungal infection. Open Forum Infect. Dis. 2021, 8, ofab510. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Cornely, O.A.; Böttiger, B.W.; Dusse, F.; Eichenauer, D.A.; Fuchs, F.; Hallek, M.; Jung, N.; Klein, F.; Persigehl, T.; et al. COVID-19 associated pulmonary aspergillosis. Mycoses 2020, 63, 528–534. [Google Scholar] [CrossRef]
- Verweij, P.E.; Gangneux, J.P.; Bassetti, M.; Brüggemann, R.J.M.; Cornely, O.A.; Koehler, P.; Lass-Flörl, C.; van de Veerdonk, F.L.; Chakrabarti, A.; Hoenigl, M. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e53–e55. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Rech, R.; Galimberti, L.; Castelli, A.; Angeli, E.; Fossali, T.; Bernasconi, D.; Covizzi, A.; Bonazzetti, C.; Torre, A.; et al. Invasive pulmonary aspergillosis complicating SARS-CoV-2 pneumonia: A diagnostic challenge. Travel Med. Infect. Dis. 2020, 38, 101752. [Google Scholar] [CrossRef] [PubMed]
- Blaize, M.; Mayaux, J.; Nabet, C.; Lampros, A.; Marcelin, A.-G.; Thellier, M.; Piarroux, R.; Demoule, A.; Fekkar, A. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg. Infect. Dis. 2020, 26, 1636–1637. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van De Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Ku, Y.H.; Chan, K.S.; Yang, C.C.; Tan, C.K.; Chuang, Y.C.; Yu, W.L. Higher mortality of severe influenza patients with probable aspergillosis than those with and without other coinfections. J. Formos. Med. Assoc. 2017, 116, 660–670. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Friedman, D.; Zapernick, L.; Dingle, T.C.; Lee, N.; Sligl, W.; Zelyas, N.; Smith, S.W. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: A retrospective cohort study from Alberta, Canada. Clin. Infect. Dis. 2020, 71, 1760–1763. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Chappell, L.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; et al. Dexamethasone in hospitalized patients with COVID-19. New Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (Recovery): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.E.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Alatorre-Alexander, J.; Pellegrini, R.d.C.; Estrada, V.; et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418. [Google Scholar] [CrossRef]
- Tocilizumab [Package Insert]; Genentech, Inc.: South San Francisco, CA, USA,, 2022; Available online: https://www.gene.com/download/pdf/actemra_prescribing.pdf (accessed on 22 May 2023).
- Baricitinib [Package Insert]; Lilly USA, LLC.: Indianapolis, IN, USA, 2022; Available online: https://uspl.lilly.com/olumiant/olumiant.html#pi (accessed on 22 May 2023).
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Hernández, S.; Echeverría-Esnal, D.; Almendral, A.; Ferrer, R.; Limón, E.; Horcajada, J.P.; Catalan Infection Control Antimicrobial Stewardship Program (VINCat-PROA). Antimicrobial consumption among 66 acute care hospitals in Catalonia: Impact of the COVID-19 pandemic. Antibiotics 2021, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, A.-L.; Bestion, A.; Pradat, P.; Richard, J.-C.; Argaud, L.; Guichon, C.; Roux, S.; Piriou, V.; Paillet, C.; Leboucher, G.; et al. Impact of COVID-19 pandemic on antifungal consumption: A multicenter retrospective analysis. Crit. Care 2022, 26, 384. [Google Scholar] [CrossRef]
- Nestler, M.; Godbout, E.; Lee, K.; Kim, J.; Noda, A.J.; Taylor, P.; Pryor, R.; Markley, J.D.; Doll, M.; Bearman, G.; et al. Stevens. Fungal superinfection in patients with COVID-19: Role of antifungal stewardship? Am. J. Infect. Control 2021, 49, 279–280. [Google Scholar] [CrossRef]
- Kariyawasam, R.M.; Dingle, T.C.; Kula, B.E.; Vandermeer, B.; Sligl, W.I.; Schwartz, I.S. Defining COVID-19-associated pulmonary aspergillosis: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 920–927. [Google Scholar] [CrossRef]
- Egger, M.; Bussini, L.; Hoenigl, M.; Bartoletti, M. Prevalence of COVID-19-associated pulmonary aspergillosis: Critical review and conclusions. J. Fungi 2022, 8, 390. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of america. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Balushi, A.A.; Ajmi, A.A.; Sinani, Q.A.; Menon, V.; Al Berieki, Z.; Al Shezawi, A.; Al Azri, S.; Al Rashdi, A.; Al Jardani, A.; Al Baluki, T.; et al. COVID-19-associated mucormycosis: An opportunistic fungal infection. A case series and review. Int. J. Infect. Dis. 2022, 121, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.-P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The emergence of COVID-19 associated mucormycosis: A review of cases from 18 countries. Lancet Microbe 2022, 3, e543–e552. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Lamoth, F.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. Navigating the uncertainties of COVID-19-associated aspergillosis: A comparison with influenza-associated aspergillosis. J. Infect. Dis. 2021, 224, 1631–1640. [Google Scholar] [CrossRef]
- Hashim, Z.; Neyaz, Z.; Marak, R.S.; Nath, A.; Nityanand, S.; Tripathy, N.K. Practice guidelines for the diagnosis of COVID-19-associated pulmonary aspergillosis in an intensive care setting. J. Intensive Care Med. 2021, 37, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, G.; Lupia, T.; Lombardo, D.; Stroffolini, G.; Corcione, S.; De Rosa, F.G.; Brazzi, L. Risk factors for invasive aspergillosis in ICU patients with COVID-19: Current insights and new key elements. Ann. Intensive Care 2021, 11, 136. [Google Scholar] [CrossRef]
- Calderón-Parra, J.; Mills-Sanchez, P.; Moreno-Torres, V.; Tejado-Bravo, S.; Romero-Sánchez, I.; Balandin-Moreno, B.; Calvo-Salvador, M.; Portero-Azorín, F.; García-Masedo, S.; Muñez-Rubio, E.; et al. COVID-19-associated pulmonary aspergillosis (CAPA): Risk factors and development of a predictive score for critically ill COVID-19 patients. Mycoses 2022, 65, 541–550. [Google Scholar] [CrossRef]
- Permpalung, N.; Chiang, T.P.-Y.; Avery, R.K.; Ostrander, D.; Datta, K.; Segev, D.L.; Durand, C.M.; Zhang, S.X.; Massie, A.B.; Marr, K.A. Coronavirus disease 2019–associated pulmonary aspergillosis: A noninvasive screening model for additional diagnostics. Open Forum Infect. Dis. 2023, 10, ofad155. [Google Scholar] [CrossRef]
- Zonios, D.; Yamazaki, H.; Murayama, N.; Natarajan, V.; Palmore, T.; Childs, R.; Skinner, J.; Bennett, J.E. Voriconazole metabolism, toxicity, and the effect of cytochrome P450 2C19 genotype. J. Infect. Dis. 2014, 209, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Inc. VFEND (Voriconazole) Prescribing Information; Pfizer: New York, NY, USA, 2022. [Google Scholar]
- Alkan, Y.; Haefeli, W.E.; Burhenne, J.; Stein, J.; Yaniv, I.; Shalit, I. Voriconazole-induced QT interval prolongation and ventricular tachycardia: A non-concentration-dependent adverse effect. Clin. Infect. Dis. 2004, 39, e49–e52. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Jain, R.; Xie, H.; Pottinger, P.; Fredricks, D.N. Voriconazole therapeutic drug monitoring: Retrospective cohort study of the relationship to clinical outcomes and adverse events. BMC Infect. Dis. 2013, 13, 105. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Yan, J.; Gao, L.; Zhou, L.; Wang, Y.; Li, Q.; Wang, J.; Chen, T.; Wang, T.; et al. Voriconazole therapeutic drug monitoring in critically ill patients improves efficacy and safety of antifungal therapy. Basic Clin. Pharmacol. Toxicol. 2020, 127, 495–504. [Google Scholar] [CrossRef]
- Bates, D.W.; Su, L.; Yu, D.T.; Chertow, G.M.; Seger, D.L.; Gomes, D.R.J.; Dasbach, E.J.; Platt, R. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin. Infect. Dis. 2001, 32, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Antosz, O.; Peyton-Thomas, B.; Selby, C.; Bozeman, A.; Leary, C.; Devarakonda, S.; Mansour, R.; Mills, G.; Koshy, N. Comparison of posaconazole versus voriconazole in the induction of acute myeloid leukemia: Impact on cost, safety, and efficacy. Biol. Blood Marrow Transplant. 2017, 23, S417. [Google Scholar] [CrossRef]
- Harrington, R.; Lee, E.; Yang, H.; Wei, J.; Messali, A.; Azie, N.; Wu, E.Q.; Spalding, J. Cost-Effectiveness Analysis of Isavuconazole vs. Voriconazole as First-Line Treatment for Invasive Aspergillosis. Adv. Ther. 2016, 34, 207–220. [Google Scholar] [CrossRef]
- Johnson, M.D.; Lewis, R.E.; Ashley, E.S.D.; Ostrosky-Zeichner, L.; Zaoutis, T.; Thompson, G.R.; Andes, D.R.; Walsh, T.J.; Pappas, P.G.; Cornely, O.A.; et al. Core recommendations for antifungal stewardship: A statement of the mycoses study group education and research consortium. J. Infect. Dis. 2020, 222 (Suppl. 3), S175–S198. [Google Scholar] [CrossRef]
- Hart, E.; Nguyen, M.; Allen, M.; Clark, C.M.; Jacobs, D.M. A systematic review of the impact of antifungal stewardship interventions in the United States. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 24. [Google Scholar] [CrossRef]
- Markogiannakis, A.; Korantanis, K.; Gamaletsou, M.N.; Samarkos, M.; Psichogiou, M.; Daikos, G.; Sipsas, N.V. Impact of a non-compulsory antifungal stewardship program on overuse and misuse of antifungal agents in a tertiary care hospital. Int. J. Antimicrob. Agents 2021, 57, 106255. [Google Scholar] [CrossRef]
- Muñoz, P.; Valerio, M.; Vena, A.; Bouza, E. Antifungal stewardship in daily practice and health economic implications. Mycoses 2015, 58 (Suppl. 2), 14–25. [Google Scholar] [CrossRef]
- Machado, M.; Chamorro de Vega, E.; Martínez-Jiménez, M.D.C.; Rodríguez-González, C.G.; Vena, A.; Navarro, R.; Zamora-Cintas, M.I.; Agnelli, C.; Olmedo, M.; Galar, A.; et al. Utility of 1,3 β-d-glucan assay for guidance in antifungal stewardship programs for oncologic patients and solid organ transplant recipients. J. Fungi 2021, 7, 59. [Google Scholar] [CrossRef]
- Murri, R.; Lardo, S.; De Luca, A.; Posteraro, B.; Torelli, R.; De Angelis, G.; Giovannenze, F.; Taccari, F.; Pavan, L.; Parroni, L.; et al. Post-prescription audit plus beta-d-glucan assessment decrease echinocandin use in people with suspected invasive candidiasis. Medicina 2021, 57, 656. [Google Scholar] [CrossRef]
- National Healthcare Safety Network. Chapter 14: Antimicrobial Use and Resistance (AUR) Module—January 2023. Centers for Disease Control and Prevention. 2023. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/11pscaurcurrent.pdf (accessed on 1 May 2023).
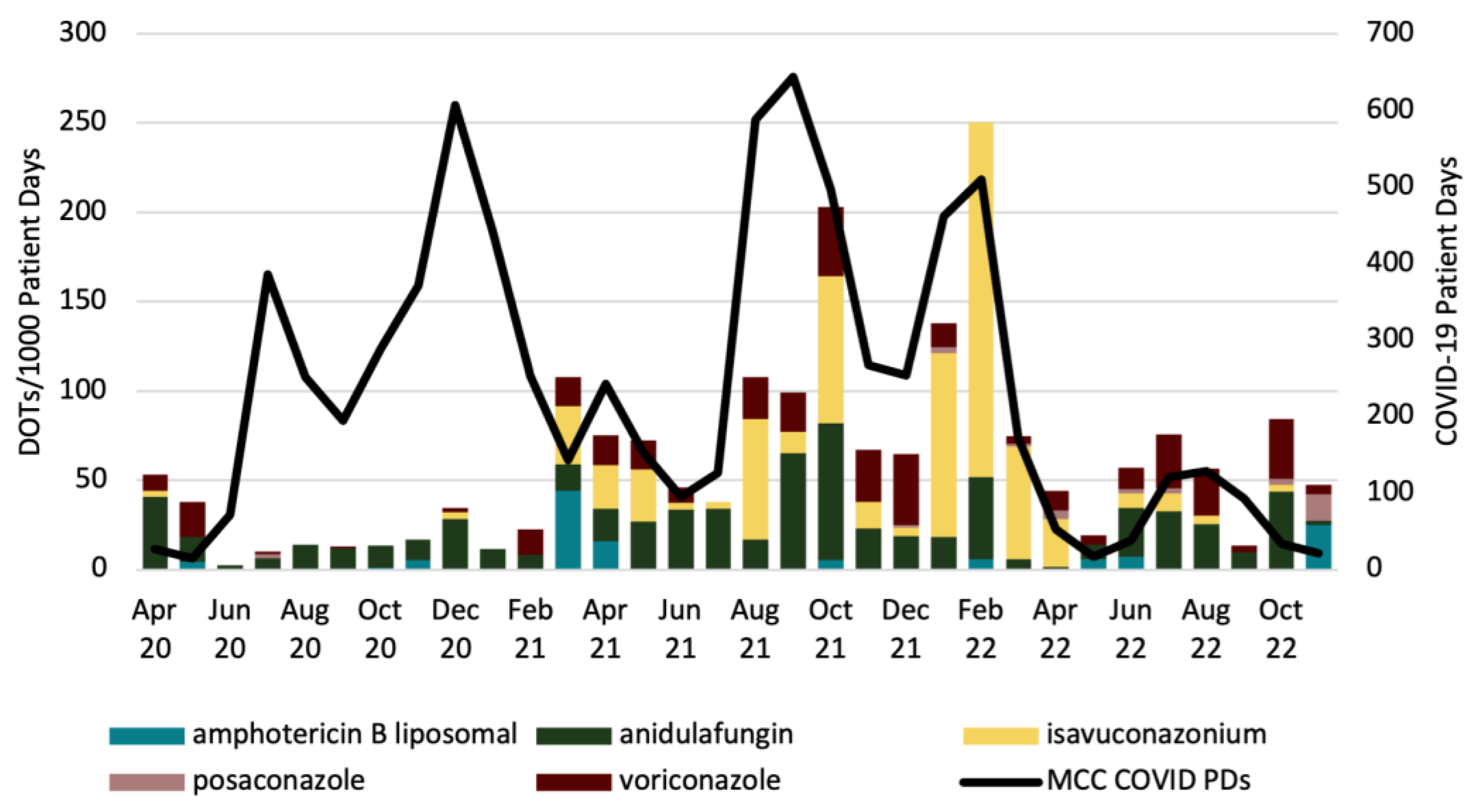
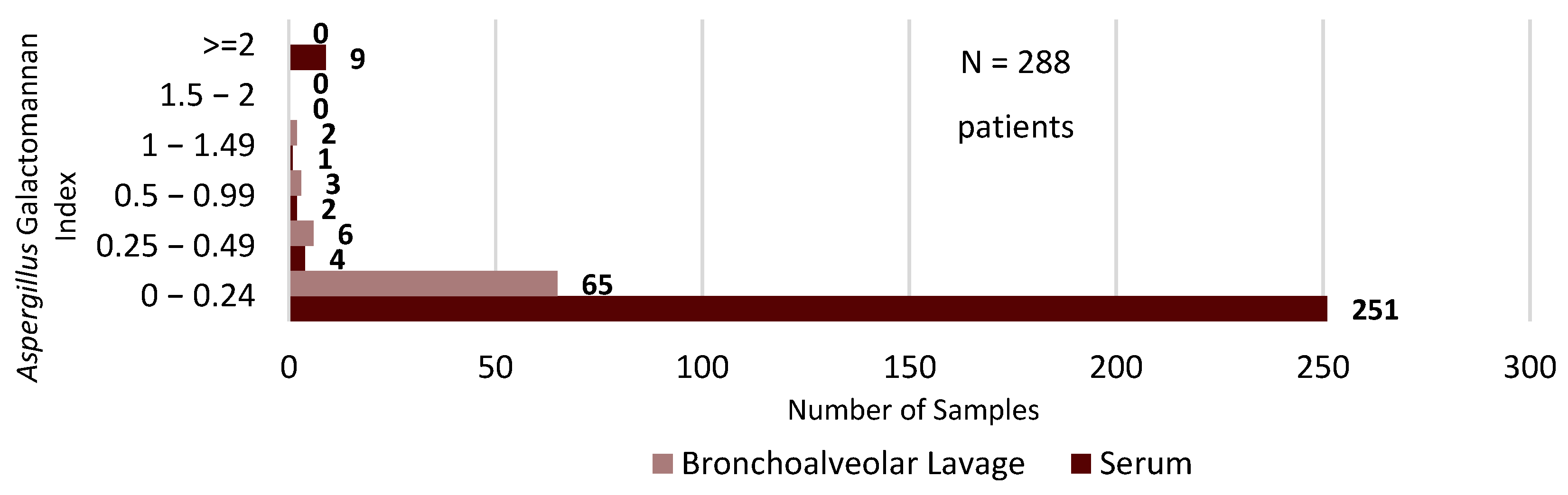
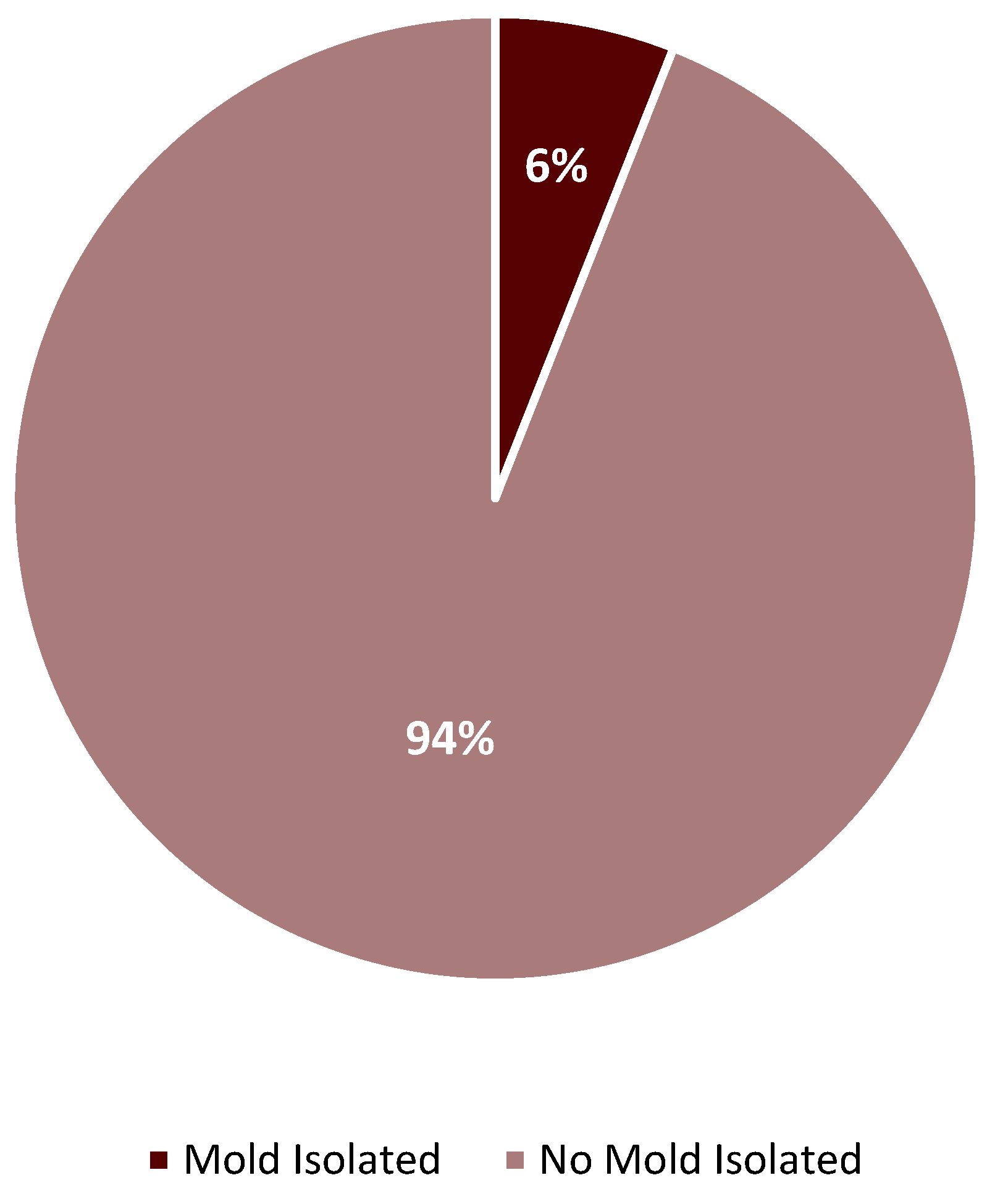
| Month | L-AMB | AFG | ISA | POS | VRC |
|---|---|---|---|---|---|
| Apr 2019–Mar 2020 Monthly Average (Baseline) | 5.82 | 23.15 | 1.05 | 0.79 | 6.66 |
| Apr 2020–Nov 2022 Monthly Average | 3.70 | 22.12 | 21.87 | 1.17 | 12.71 |
| p-value | 0.357 | 0.815 | 0.008 | 0.597 | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawkins, B.K.; Walker, S.D.; Shorman, M.A. Missed Opportunities for Antifungal Stewardship during the COVID-19 Era. Antibiotics 2023, 12, 1352. https://doi.org/10.3390/antibiotics12091352
Hawkins BK, Walker SD, Shorman MA. Missed Opportunities for Antifungal Stewardship during the COVID-19 Era. Antibiotics. 2023; 12(9):1352. https://doi.org/10.3390/antibiotics12091352
Chicago/Turabian StyleHawkins, Brandon K., Samantha D. Walker, and Mahmoud A. Shorman. 2023. "Missed Opportunities for Antifungal Stewardship during the COVID-19 Era" Antibiotics 12, no. 9: 1352. https://doi.org/10.3390/antibiotics12091352






