The Use of Medical Grade Honey on Infected Chronic Diabetic Foot Ulcers—A Prospective Case-Control Study
Abstract
:1. Introduction
2. Results
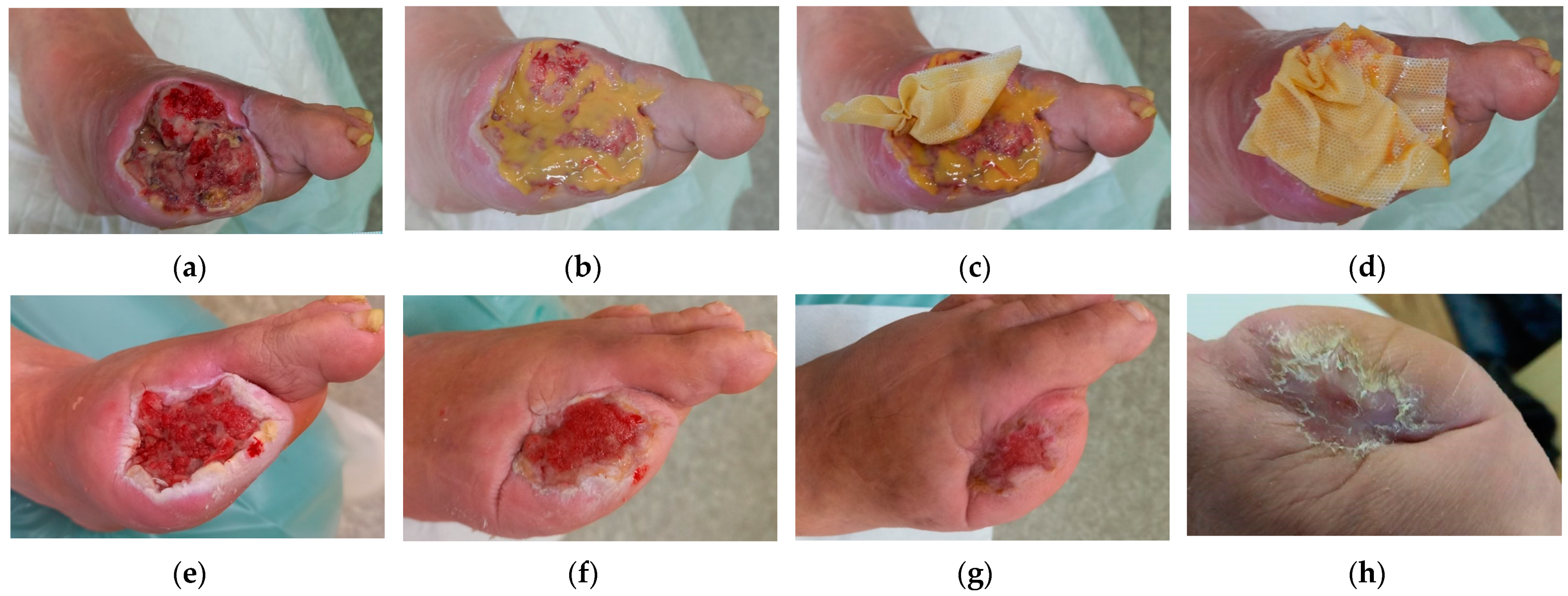
2.1. Case 1
2.2. Case 2
2.3. Case 3
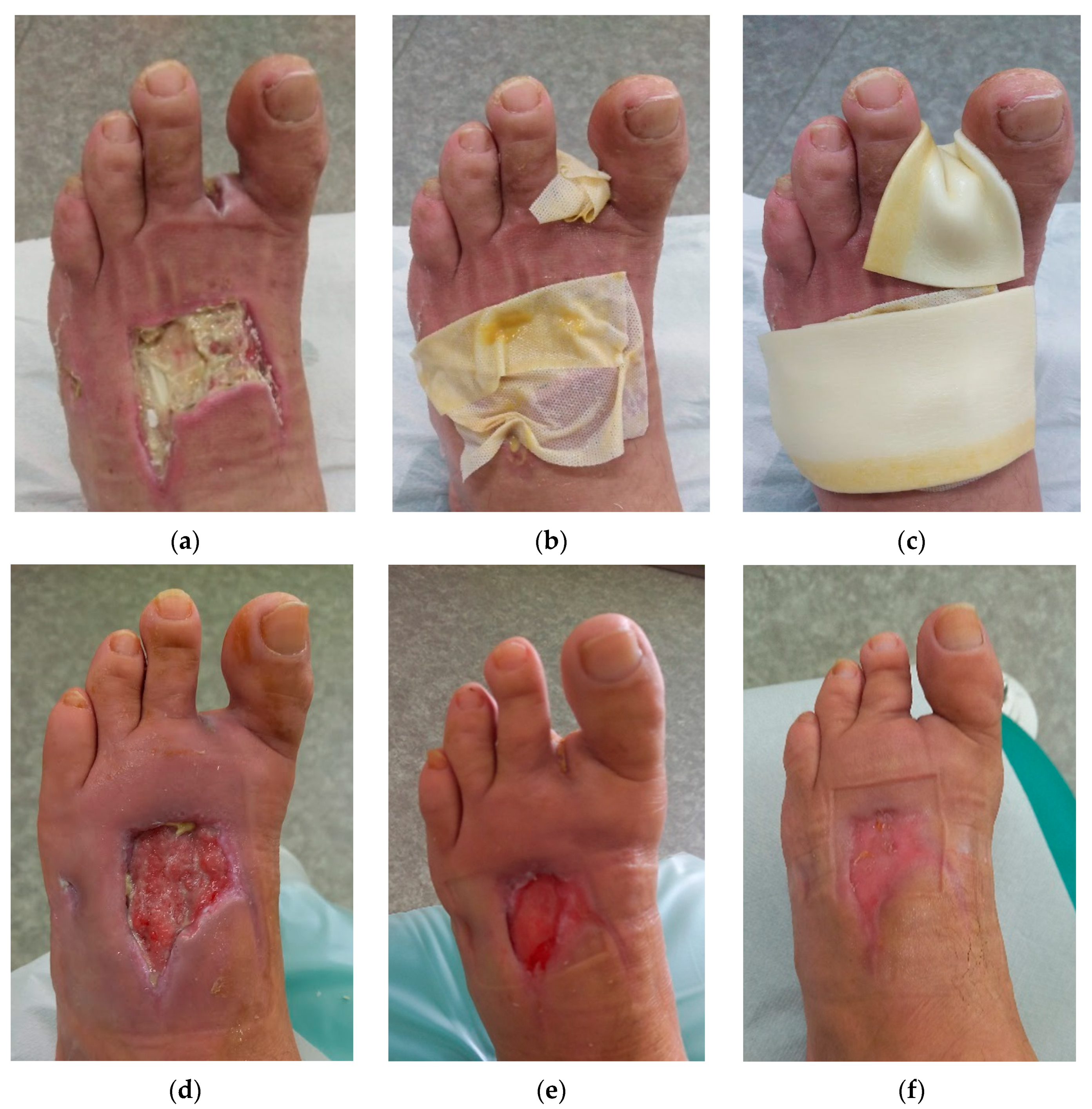
2.4. Case 4
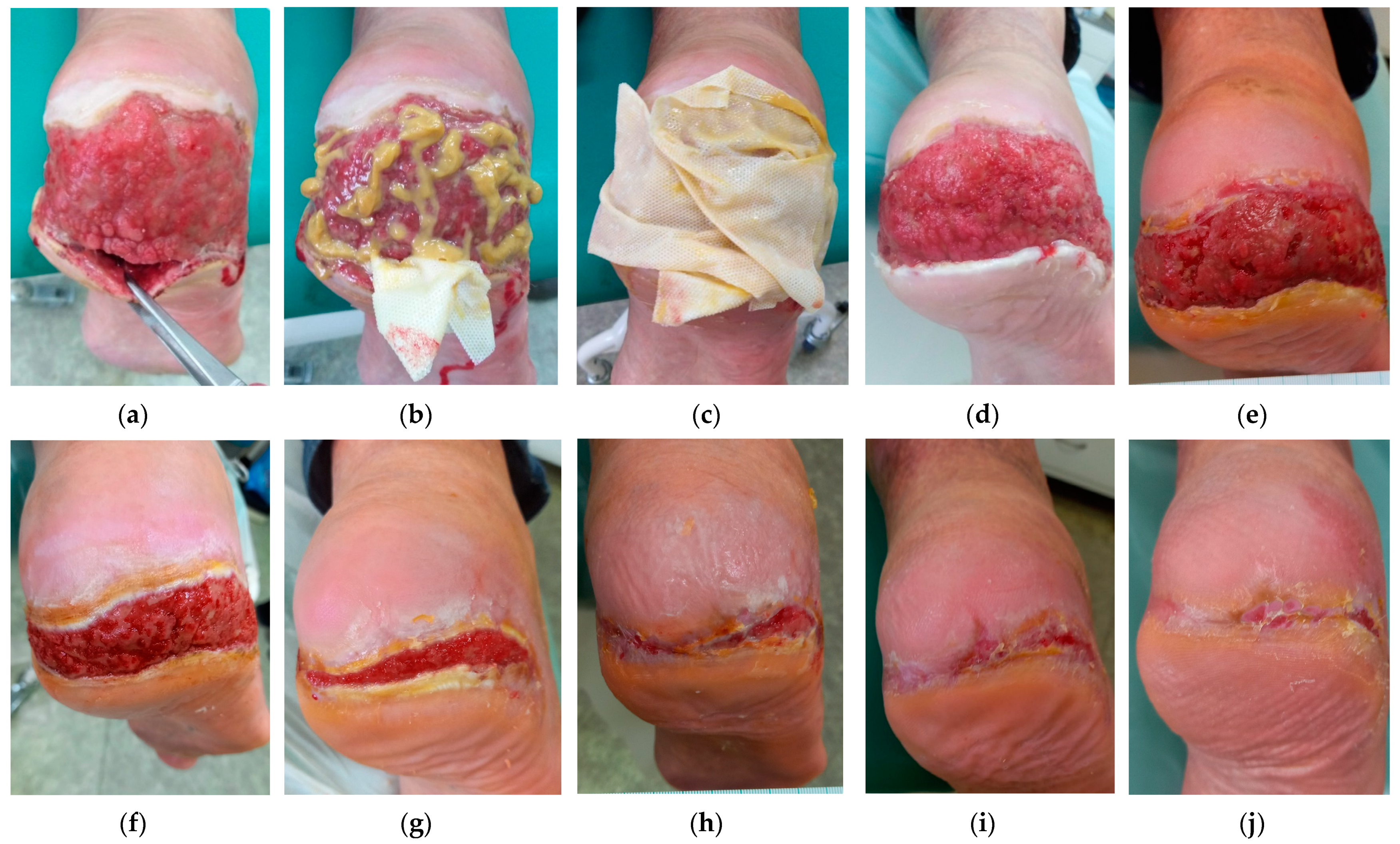
2.5. Case 5
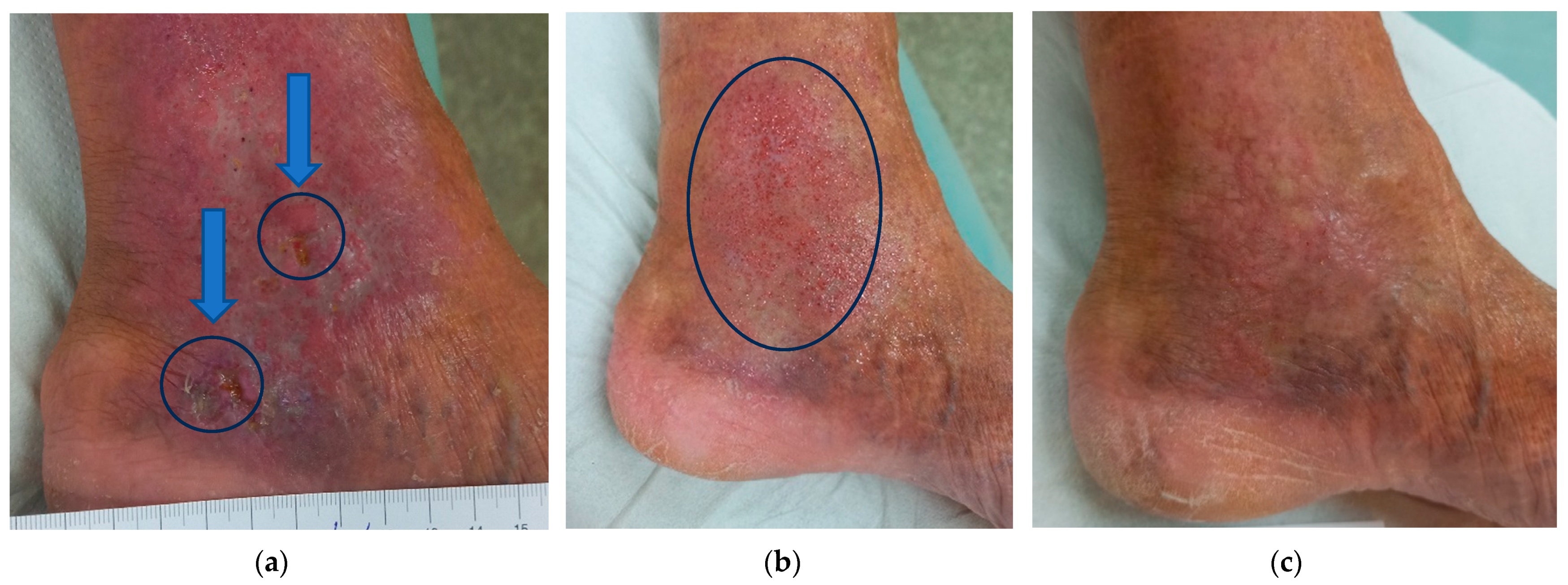
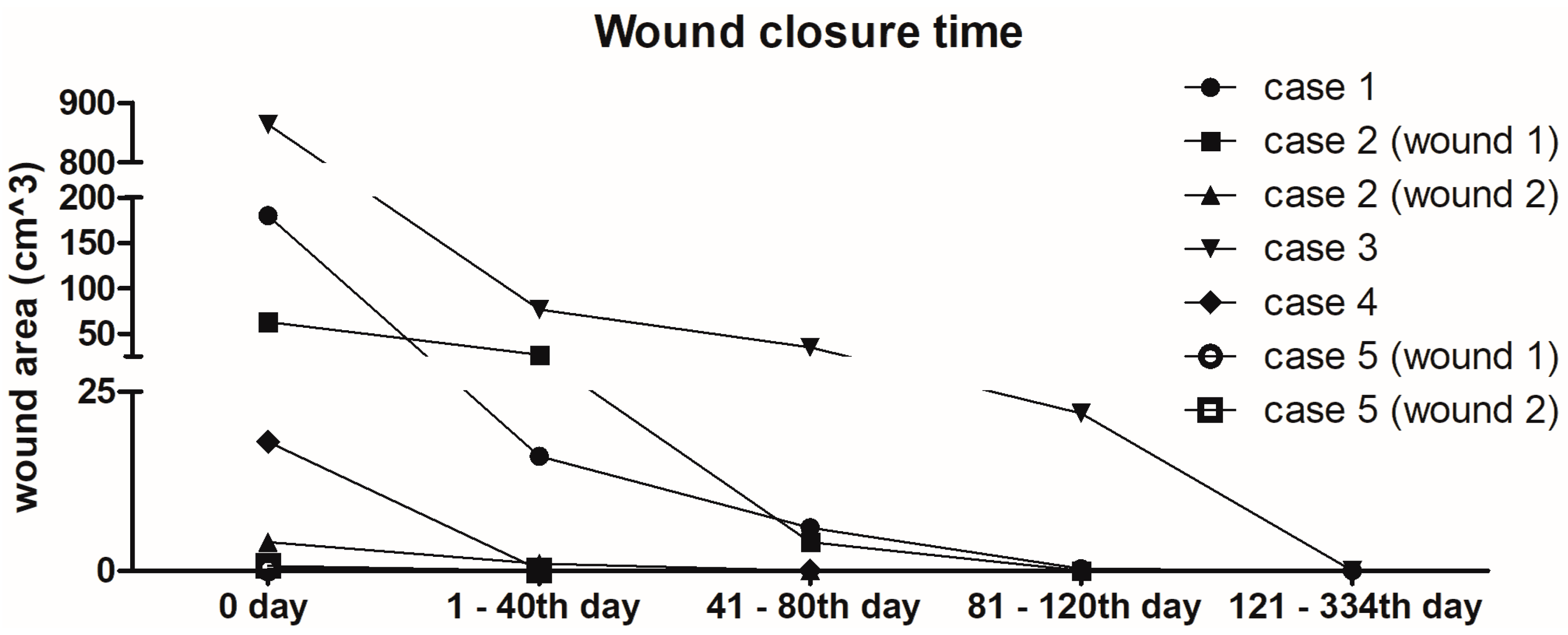
2.6. Overview of Wound Healing Time
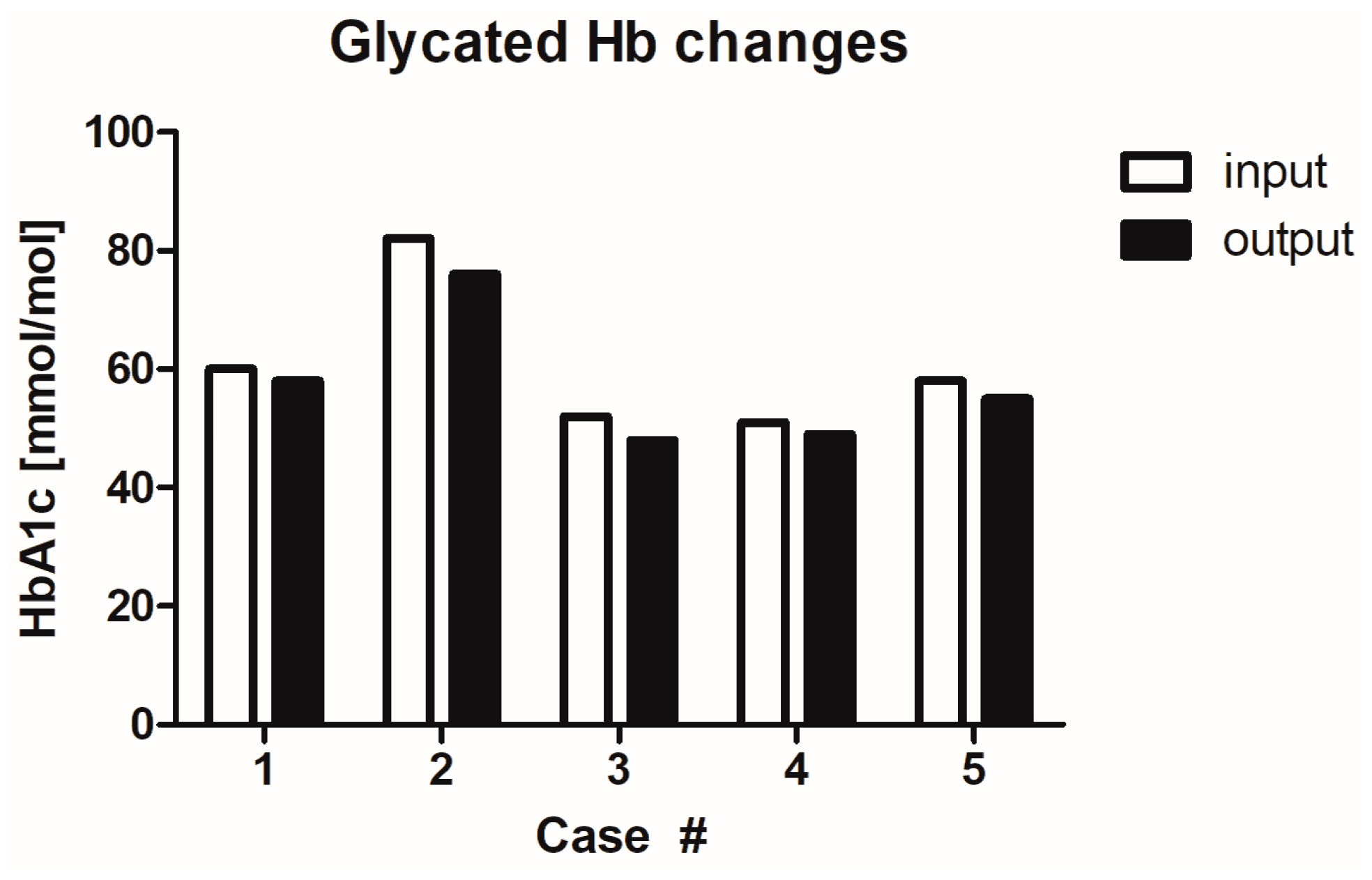
2.7. Influence of MGH on Glycaemia Levels
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Wound Care and Assessment Details
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Union of Wound Healing Societies (WUWHS). Florence Congress, Position Document. Advance in Wound Care: The Triangle of Wound Assessment; Wounds International: Hatfields, London, 2016. [Google Scholar]
- Pokorna, A.; Benesova, K.; Muzik, J.; Jarkovsky, J.; Dusek, L. Data Sources for Monitoring of Non-healing Wounds in a National Heal Information System—Epidemiology of Non-healing Wounds Analysis of the National Register of Hospitalized Patients in 2007–2015. Cesk. Slov. Neurol. 2017, 80, 8–17. [Google Scholar] [CrossRef]
- Deufert, D.; Graml, R. Disease-specific, health-related quality of life (HRQoL) of people with chronic wounds—A descriptive cross-sectional study using the Wound-QoL. Wound Med. 2017, 16, 29–33. [Google Scholar] [CrossRef]
- Krupova, L.; Pokorna, A. Quality of life in patient with non-healing wounds, with particular focus on assesment tools-a literature review. Cent. Eur. J. Nurs. Midwifery 2020, 11, 94–103. [Google Scholar] [CrossRef]
- International Best Practice Guidelines: Wound Management in Diabetic Foot Ulcers. Wounds International. 2013. Available online: www.woundsinternational.com (accessed on 15 June 2023).
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis (dagger). Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, K.; Garoufalis, M.; Wilson, P. POINT: Podiatry for international diabetic foot teams. J. Wound Care 2018, 27 (Suppl. S11), 1–32. [Google Scholar] [CrossRef] [PubMed]
- Lechleitner, M.; Abrahamian, H.; Francesconi, C.; Kofler, M.; Sturm, W.; Kohler, G. [Diabetic neuropathy and diabetic foot syndrome (Update 2019)]. Wien. Klin. Wochenschr. 2019, 131, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, C.; Hallern, B. The Triangle of Wound Assessment: A holistic framework from wound assessment to management goals and treatments. Wounds Int. 2017, 8, 34–39. [Google Scholar]
- Stryja, J. [Up-to-Date Approaches to Non-Healing Wound Treatment] Original Title: Moderní postupy v léčbě nehojících se ran. Remedia 2010, 20, 180–184. [Google Scholar]
- Fu, X.L.; Ding, H.; Miao, W.W.; Mao, C.X.; Zhan, M.Q.; Chen, H.L. Global recurrence rates in diabetic foot ulcers: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2019, 35, e3160. [Google Scholar] [CrossRef]
- Dayya, D.; O’Neill, O.; Habib, N.; Moore, J.; Iyer, K.; Huedo-Medina, T.B. Debridement of diabetic foot ulcers: Public health and clinical implications—A systematic review, meta-analysis, and meta-regression. BMJ Surg. Interv. Health Technol. 2022, 4, e000081. [Google Scholar] [CrossRef]
- Cremers, N.; Belas, A.; Santos Costa, S.; Couto, I.; de Rooster, H.; Pomba, C. In vitro antimicrobial efficacy of two medical grade honey formulations against common high-risk meticillin-resistant staphylococci and Pseudomonas spp. pathogens. Vet. Dermatol. 2020, 31, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Smaropoulos, E.; Cremers, N.A.J. Treating severe wounds in pediatrics with medical grade honey: A case series. Clin. Case Rep. 2020, 8, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, T.; Wolcott, R.D.; Peghetti, A.; Leaper, D.; Cutting, K.; Polignano, R.; Rosa Rita, Z.; Moscatelli, A.; Greco, A.; Romanelli, M.; et al. Recommendations for the management of biofilm: A consensus document. J. Wound Care 2016, 25, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.K.R.; Tatavilis, N.; Pospisilova, I.; Kucerova, J.; Cremers, N.A.J. Medical-Grade Honey Kills Antibiotic-Resistant Bacteria and Prevents Amputation in Diabetics with Infected Ulcers: A Prospective Case Series. Antibiotics 2020, 9, 529. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Mayer, A.; Slezak, V.; Takac, P.; Olejnik, J.; Majtan, J. Treatment of non-healing leg ulcers with honeydew honey. J. Tissue Viability 2014, 23, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.; Cremers, N.A. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Gethin, G.; Cowman, S. Bacteriological changes in sloughy venous leg ulcers treated with manuka honey or hydrogel: An RCT. J. Wound Care 2008, 17, 241–244. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martinez Florez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef]
- Pleeging, C.C.F.; Wagener, F.; de Rooster, H.; Cremers, N.A.J. Revolutionizing non-conventional wound healing using honey by simultaneously targeting multiple molecular mechanisms. Drug Resist. Updates 2022, 62, 100834. [Google Scholar] [CrossRef] [PubMed]
- Yildiz Karadeniz, E.; Kaplan Serin, E. Use of honey in diabetic foot ulcer: Systematic review and meta-analysis. J. Tissue Viability 2023, 32, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Shukrimi, A.; Sulaiman, A.R.; Halim, A.Y.; Azril, A. A comparative study between honey and povidone iodine as dressing solution for Wagner type II diabetic foot ulcers. Med. J. Malays. 2008, 63, 44–46. [Google Scholar]
- Labban, L. Honey as a promising treatment for diabetic foot ulcers (DFU). J. Med. Soc. 2014, 28, 64–68. [Google Scholar] [CrossRef]
- Molan, P.C. Re-introducing honey in the management of wounds and ulcers—Theory and practice. Ostomy Wound Manag. 2002, 48, 28–40. [Google Scholar]
- Moore, O.A.; Smith, L.A.; Campbell, F.; Seers, K.; McQuay, H.J.; Moore, R.A. Systematic review of the use of honey as a wound dressing. BMC Complement. Altern. Med. 2001, 1, 2. [Google Scholar] [CrossRef]
- Lusby, P.E.; Coombes, A.; Wilkinson, J.M. Honey: A potent agent for wound healing? J. Wound Ostomy Cont. Nurs. 2002, 29, 295–300. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- van Niekerk, G.; Davis, T.; Patterton, H.G.; Engelbrecht, A.M. How Does Inflammation-Induced Hyperglycemia Cause Mitochondrial Dysfunction in Immune Cells? Bioessays 2019, 41, e1800260. [Google Scholar] [CrossRef]
- Kirby, P.; Khan, N.; Dhillon, N.; Emmerson, E.; Fisher, A.; Thompson, J.; Burnside, J.; Chesterton, L.; Fernando, D. Do honey-impregnated dressings affect glycaemic control? Diabet. Foot J. 2009, 12, 177–180. [Google Scholar]
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair. Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cokcetin, N.N.; Burke, C.M.; Turnbull, L.; Liu, M.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 18160. [Google Scholar] [CrossRef] [PubMed]
- Kelechi, T.; Prentice, M.; Madisetti, M.; Brunette, G.; Mueller, M. Palliative Care in the Management of Pain, Odor, and Exudate in Chronic Wounds at the End of Life: A Cohort Study. J. Hosp. Palliat. Nurs. 2017, 19, 17–25. [Google Scholar] [CrossRef]
- Bates, M. The Future of Wound Care. IEEE Pulse 2020, 11, 22–25. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Al-Gharibi, K.A.; Sharstha, S.; Al-Faras, M.A. Cost-Effectiveness of Wound Care: A concept analysis. Sultan Qaboos Univ. Med. J. 2018, 18, e433–e439. [Google Scholar] [CrossRef]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13 (Suppl. S2), 5–15. [Google Scholar] [CrossRef]
- Biglari, B.; Moghaddam, A.; Santos, K.; Blaser, G.; Buchler, A.; Jansen, G.; Langler, A.; Graf, N.; Weiler, U.; Licht, V.; et al. Multicentre prospective observational study on professional wound care using honey (Medihoney). Int. Wound J. 2013, 10, 252–259. [Google Scholar] [CrossRef]
- Naik, P.P.; Chrysostomou, D.; Cinteza, M.; Pokorna, A.; Cremers, N.A. When time does not heal all wounds-the use of medical grade honey in wound healing: A case series. J. Wound Care 2022, 31, 548–558. [Google Scholar] [CrossRef]
- Papanikolaou, G.E.; Gousios, G.; Cremers, N.A.J. Use of Medical-Grade Honey to Treat Clinically Infected Heel Pressure Ulcers in High-Risk Patients: A Prospective Case Series. Antibiotics 2023, 12, 605. [Google Scholar] [CrossRef]
- Halstead, F.D.; Webber, M.A.; Oppenheim, B.A. Use of an engineered honey to eradicate preformed biofilms of important wound pathogens: An in vitro study. J. Wound Care 2017, 26, 442–450. [Google Scholar] [CrossRef]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S.; Mathew-Steiner, S.S.; Gordillo, G.M. Biofilm Management in Wound Care. Plast. Reconstr. Surg. 2021, 148, 275e–288e. [Google Scholar] [CrossRef] [PubMed]
- Burmolle, M.; Thomsen, T.R.; Fazli, M.; Dige, I.; Christensen, L.; Homoe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity—Monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef]
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int. Wound J. 2021, 18, 342–358. [Google Scholar] [CrossRef]
- Pleeging, C.C.F.; Coenye, T.; Mossialos, D.; de Rooster, H.; Chrysostomou, D.; Wagener, F.; Cremers, N.A.J. Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics 2020, 9, 866. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A.J. The pro-healing effects of medical grade honey supported by a pediatric case series. Complement. Ther. Med. 2019, 45, 14–18. [Google Scholar] [CrossRef]
- de Groot, T.; Janssen, T.; Faro, D.; Cremers, N.A.J.; Chowdhary, A.; Meis, J.F. Antifungal Activity of a Medical-Grade Honey Formulation against Candida auris. J. Fungi 2021, 7, 50. [Google Scholar] [CrossRef]
- Bocoum, A.; Riel, S.; Traore, S.O.; Ngo Oum Ii, E.F.; Traore, Y.; Thera, A.T.; Fane, S.; Dembele, B.T.; Cremers, N.A.J. Medical-Grade Honey Enhances the Healing of Caesarean Section Wounds and Is Similarly Effective to Antibiotics Combined with Povidone-Iodine in the Prevention of Infections-A Prospective Cohort Study. Antibiotics 2023, 12, 92. [Google Scholar] [CrossRef]
- Holubova, A.; Chlupacova, L.; Cetlova, L.; Cremers, N.A.J.; Pokorna, A. Medical-Grade Honey as an Alternative Treatment for Antibiotics in Non-Healing Wounds-A Prospective Case Series. Antibiotics 2021, 10, 918. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A. Medical grade honey for the treatment of paediatric abdominal wounds: A case series. J. Wound Care 2020, 29, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Sochor, M.; Slama, O. [Management of chronic and acute pain in patients with cancer diseases]. Klin. Onkol. 2015, 28, 94–98. [Google Scholar] [CrossRef]
- Juric Vukelic, D.; Juric, J. Hydrocolloid Dressing Application in the Treatment of Chronic Wounds and Relation to Quality of Life. Acta Clin. Croat. 2017, 56, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, C. An overview of integrative care options for patients with chronic wounds. Ostomy Wound Manag. 2012, 58, 44–51. [Google Scholar]
- Edwards, J. Dealing with wound-related pain at dressing change. J. Community Nurs. 2013, 27, 36–42. [Google Scholar]
- Upton, D.; Solowiej, K.; Hender, C.; Woodyatt, K.Y. Stress and pain associated with dressing change in patients with chronic wounds. J. Wound Care 2012, 21, 53–54. [Google Scholar] [CrossRef]
- Zelenikova, R.; Vyhlidalova, D. Applying honey dressings to non-healing wounds in elderly persons receiving home care. J. Tissue Viability 2019, 28, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ousey, K.; Roberts, D.; Gefen, A. Early identification of wound infection: Understanding wound odour. J. Wound Care 2017, 26, 577–582. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Gan, S.H.; Khalil, M.I. Honey: A potential therapeutic agent for managing diabetic wounds. Evid. Based Complement. Altern. Med. 2014, 2014, 169130. [Google Scholar] [CrossRef] [PubMed]
- Yapucu Gunes, U.; Eser, I. Effectiveness of a honey dressing for healing pressure ulcers. J. Wound Ostomy Cont. Nurs. 2007, 34, 184–190. [Google Scholar] [CrossRef] [PubMed]


| Case# | Gender/Age (years) | Patient Characteristics | Wound Type and Location | Wound Age | Previous Treatments | Signs of Infection | Pain VAS (Score 1–10) Daytime/Procedural | Analgesic/Antibiotic Treatment | Baseline/Final glycated Haemoglobin | Baseline/Final Glycaemia |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male, 63 | DM (oral antidiabetic drugs), diabetic gangrene, amputation of the big toe on the left foot, diabetic neuropathy, HT, obesity class III (BMI = 45), Charcot’s osteoarthropathy, phlegmon pedis, COVID-19 | DFU at the left foot | 2 months | Wound gel | Local warmth, exudate, delayed healing, malodour, pocketing | 1/1 (diabetic neuropathy) | No/No | 60/58 mmol/mol | 8/7.4 mmol/L |
| 2 | Male, 49 | DM (insulin therapy), diabetic gangrene, amputation of digit II on the left foot, diabetic neuropathy, HT, normal weight (BMI = 23.1), ischemic disease of the lower limbs second degree, chronic pancreatitis | DFUs at the left foot | 6 weeks | Iodinated povidone solution | Exudate, delayed healing, malodour | 1/1 (diabetic neuropathy) | No/No | 82/76 mmol/mol | 15.8/14.2 mmol/L |
| 3 | Male, 68 | DM (oral antidiabetic drugs), HT, hemochromatosis, CVI, dyslipidaemia, obesity class II (BMI = 35.6), Parkinson’s disease, polyneuropathy lower limbs | DFUs at the right foot on the heel | 6 weeks | Iodinated povidone solution | Increased pain, increased exudate, delayed healing, malodour, pocketing | 5/8 | No/No | 52/48 mmol/mol | 10.2/9 |
| 4 | Male, 63 | DM (insulin therapy), diabetic gangrene, diabetic neuropathy, overweight (BMI = 29.4) | DFUs at the left foot on the heel | 6 months | Rapeseed, wound gel, foam | Delayed healing, malodour, pocketing | 1/1 (diabetic neuropathy) | No/No | 51/49 mmol/mol | 8/7.6 mmol/L |
| 5 | Male, 65 | DM (oral antidiabetic drugs), HT, CVI, gouty disease, obesity class I (BMI = 30.9) | DFUs at the left foot under the media ankle | 6 weeks | Ointment, solution | Increased pain, delayed healing, malodour | 5/6 | No/No | 58/55 mmol/mol | 8.5/7.0 mmol/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holubová, A.; Chlupáčová, L.; Krocová, J.; Cetlová, L.; Peters, L.J.F.; Cremers, N.A.J.; Pokorná, A. The Use of Medical Grade Honey on Infected Chronic Diabetic Foot Ulcers—A Prospective Case-Control Study. Antibiotics 2023, 12, 1364. https://doi.org/10.3390/antibiotics12091364
Holubová A, Chlupáčová L, Krocová J, Cetlová L, Peters LJF, Cremers NAJ, Pokorná A. The Use of Medical Grade Honey on Infected Chronic Diabetic Foot Ulcers—A Prospective Case-Control Study. Antibiotics. 2023; 12(9):1364. https://doi.org/10.3390/antibiotics12091364
Chicago/Turabian StyleHolubová, Adéla, Lucie Chlupáčová, Jitka Krocová, Lada Cetlová, Linsey J. F. Peters, Niels A. J. Cremers, and Andrea Pokorná. 2023. "The Use of Medical Grade Honey on Infected Chronic Diabetic Foot Ulcers—A Prospective Case-Control Study" Antibiotics 12, no. 9: 1364. https://doi.org/10.3390/antibiotics12091364
APA StyleHolubová, A., Chlupáčová, L., Krocová, J., Cetlová, L., Peters, L. J. F., Cremers, N. A. J., & Pokorná, A. (2023). The Use of Medical Grade Honey on Infected Chronic Diabetic Foot Ulcers—A Prospective Case-Control Study. Antibiotics, 12(9), 1364. https://doi.org/10.3390/antibiotics12091364







