Antibiotic Resistance Mediated by Escherichia coli in Kuwait Marine Environment as Revealed through Genomic Analysis
Abstract
:1. Introduction
2. Results
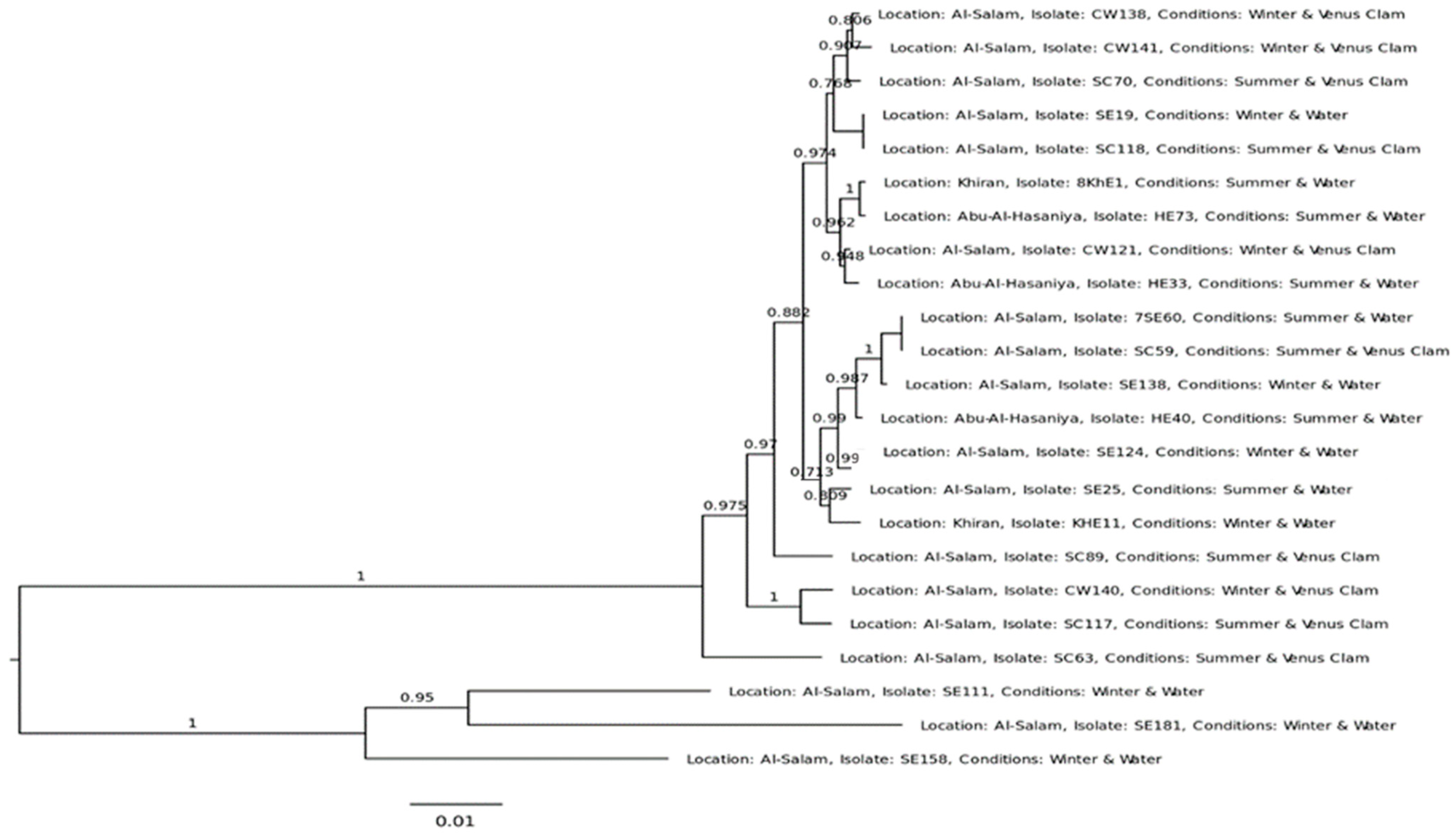
2.1. MLST and Phylogenetic Analysis
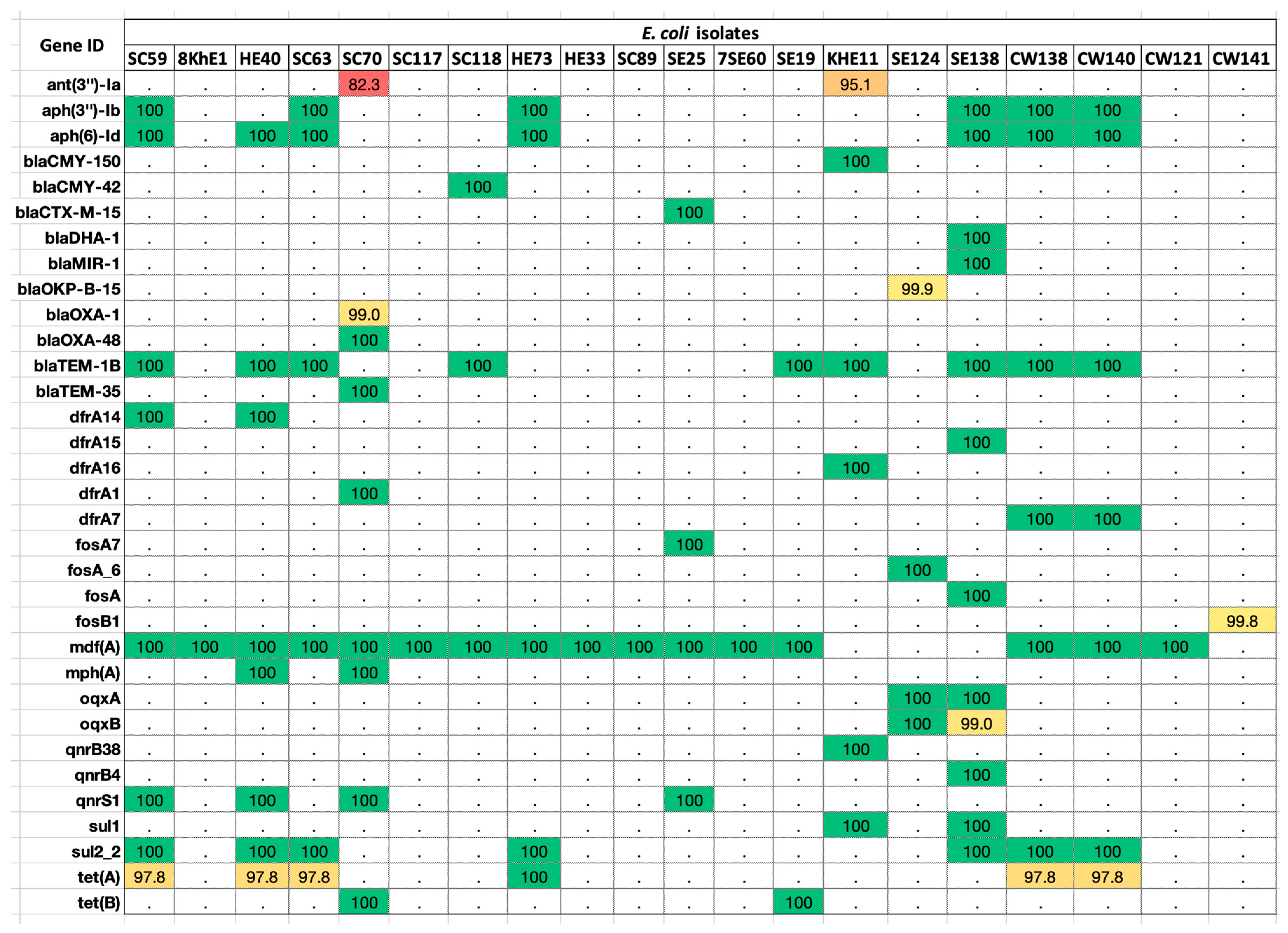
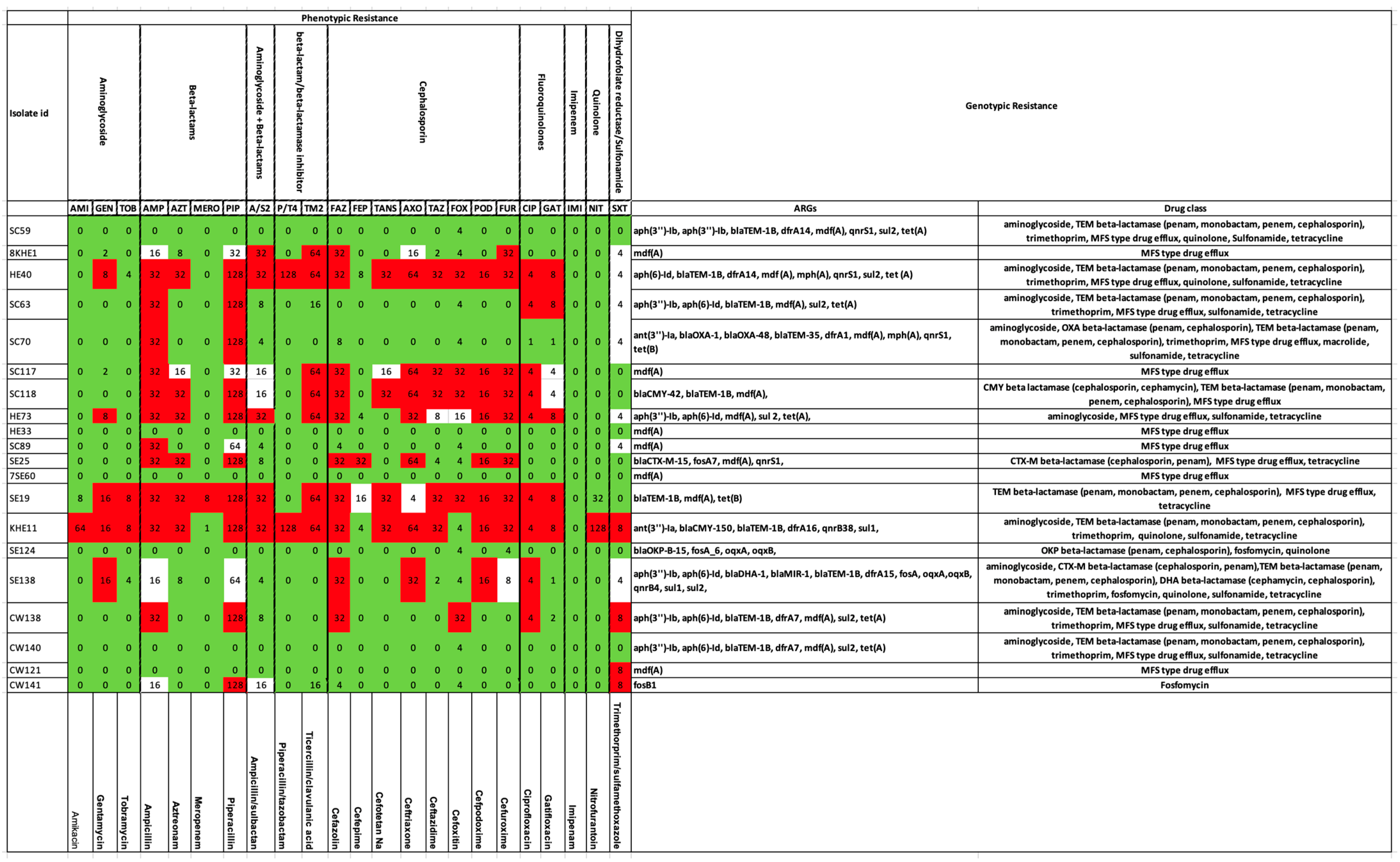
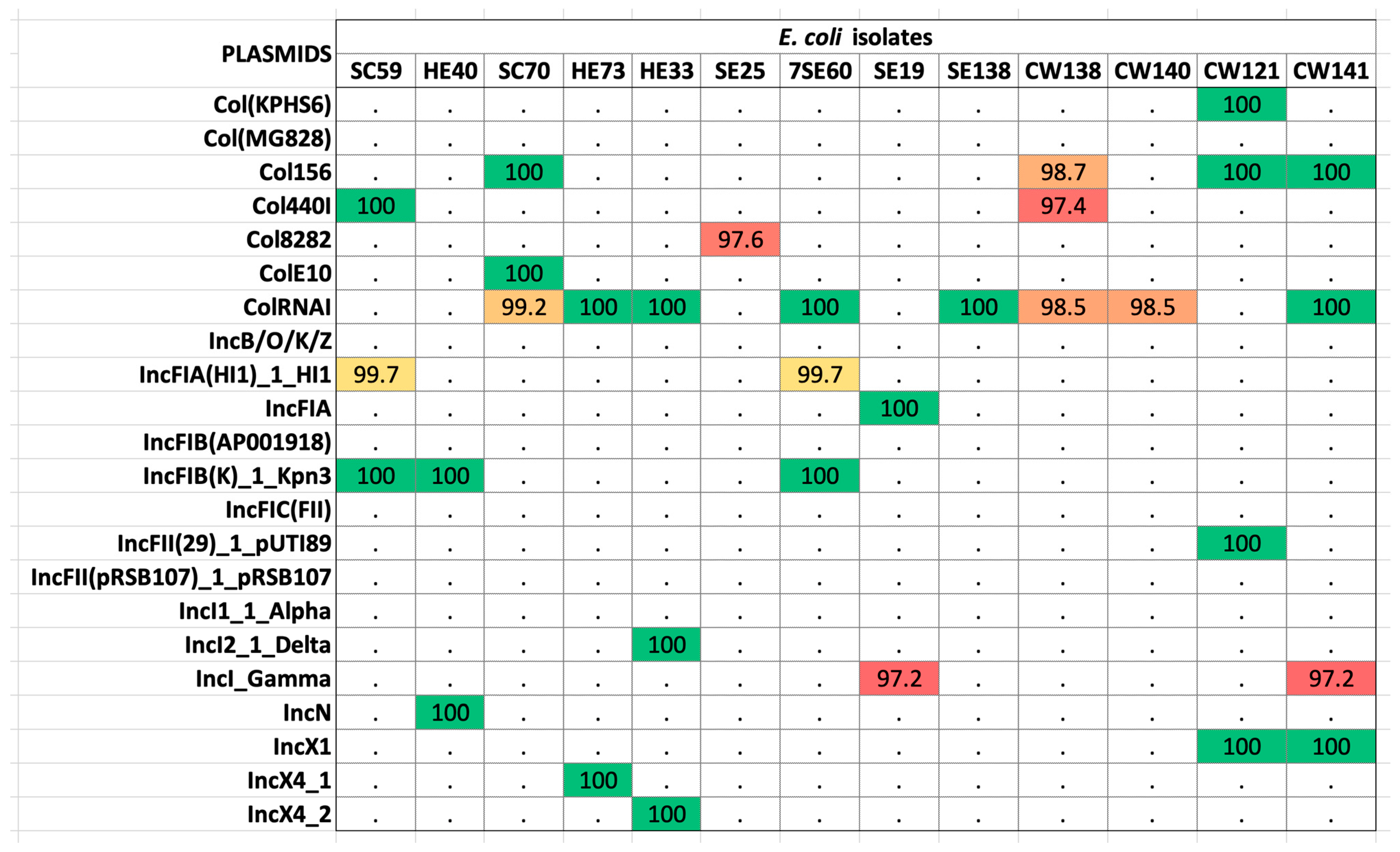
2.2. Antibiotic-Resistance Gene Elements
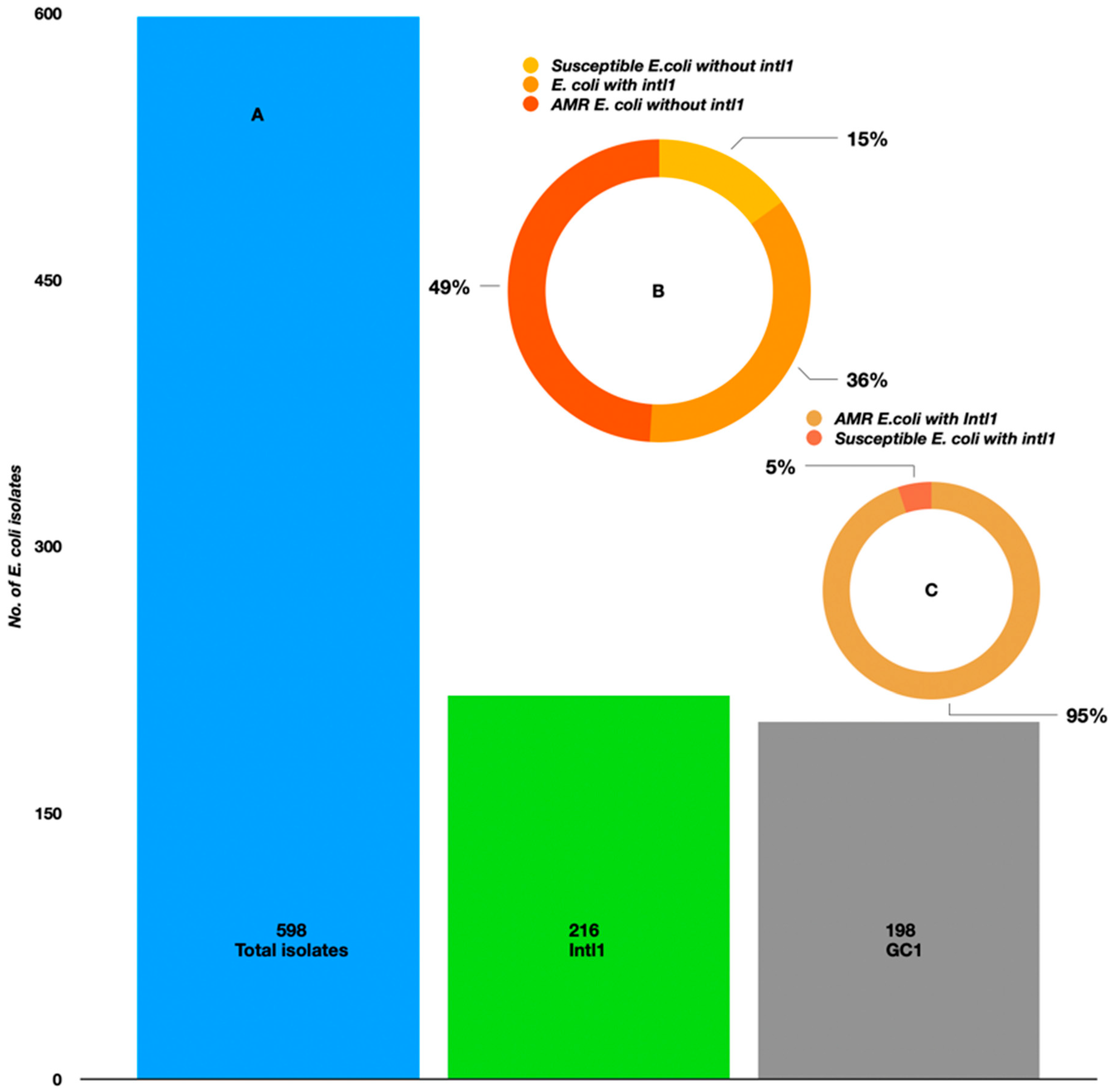
2.3. PCR-Based Identification of Intl1 and GCs
3. Discussion
4. Materials and Methods
4.1. Sampling Locations, Collection and Isolation of Strains
4.1.1. Marine Waters
4.1.2. Mollusk Samples
4.1.3. Antimicrobial Susceptibility Testing
4.2. Whole-Genome Sequencing (WGS) and Filtering of ARGEs
4.3. MLST (Muti-Locus Sequence Typing) and Phylogenetic Analysis
4.4. Screening for Class 1 Integrons and Associated Gene Cassettes
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bungau, S.; Tit, D.M.; Behl, T.; Aleya, L.; Zaha, D.C. Aspects of excessive antibiotic consumption and environmental influences correlated with the occurrence of resistance to antimicrobial agents. Curr. Opin. Environ. Sci. Health 2021, 19, 100224. [Google Scholar]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar]
- Thompson, T. The staggering death toll of drug-resistant bacteria. Nature 2022. Online ahead of print. [Google Scholar]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef]
- Baquero, F.; Alvarez-Ortega, C.; Martinez, J.L. Ecology and evolution of antibiotic resistance. Environ. Microbiol. Rep. 2009, 1, 469–476. [Google Scholar]
- Al-Ghadban, A.; Uddin, S.; Aba, A.; Ali, L.N.; Al-Sharmoukh, D.; Al-Khabbaz, A.; Al-Mutairi, A. Measurement and Assessment of Radionuclide Concentrations in the Coastal Marine Environment; Kuwait Institute for Scientific Research: Kuwait City, Kuwait, 2011; p. 128. [Google Scholar]
- Gevao, B.; Jaward, F.M.; Uddin, S.; Al-Ghadban, A.N. Occurrence and concentrations of polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) in coastal marine sediments in Kuwait. Mar. Pollut. Bull. 2009, 58, 452–455. [Google Scholar]
- Gevao, B.; Uddin, S.; Dupont, S. Baseline concentrations of pharmaceuticals in Kuwait’s coastal marine environment. Mar. Pollut. Bull. 2021, 173, 113040. [Google Scholar]
- Uddin, S.; Aba, A.; Fowler, S.; Behbehani, M.; Ismaeel, A.; Al-Shammari, H.; Alboloushi, A.; Mietelski, J.; Al-Ghadban, A.; Al-Ghunaim, A. Radioactivity in the Kuwait marine environment—Baseline measurements and review. Mar. Pollut. Bull. 2015, 100, 651–661. [Google Scholar]
- Uddin, S.; Fowler, S.W.; Saeed, T. Microplastic particles in the Persian/Arabian Gulf–a review on sampling and identification. Mar. Pollut. Bull. 2020, 154, 111100. [Google Scholar]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Behbehani, M. Micro-Nano Plastic in the Aquatic Environment: Methodological Problems and Challenges. Animals 2022, 12, 297. [Google Scholar]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar]
- Chen, J.; McIlroy, S.E.; Archana, A.; Baker, D.M.; Panagiotou, G. A pollution gradient contributes to the taxonomic, functional, and resistome diversity of microbial communities in marine sediments. Microbiome 2019, 7, 1–12. [Google Scholar]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A review of microplastic distribution in sediment profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar]
- Habibi, N.; Uddin, S.; Bottein, M.Y.D.; Faizuddin, M. Ciguatera in the Indian Ocean with Special Insights on the Arabian Sea and Adjacent Gulf and Seas: A Review. Toxins 2021, 13, 525. [Google Scholar]
- Uddin, S.; Al-Ghadban, A.; Gevao, B.; Al-Shamroukh, D.; Al-Khabbaz, A. Estimation of suspended particulate matter in Gulf using MODIS data. Aquat. Ecosyst. Health Manag. 2012, 15, 41–44. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Al-Sarawi, H.; Aldhameer, A.; Shajan, A.; Zakir, F.; Abdul Razzack, N.; Alam, F. Metagenomes from Coastal Sediments of Kuwait: Insights into the Microbiome, Metabolic Functions and Resistome. Microorganisms 2023, 11, 531. [Google Scholar]
- Wright, G.D. Antibiotic resistance in the environment: A link to the clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar]
- Perry, J.A.; Wright, G.D. The antibiotic resistance “mobilome”: Searching for the link between environment and clinic. Front. Microbiol. 2013, 4, 138. [Google Scholar]
- Von Wintersdorff, C.J.; Penders, J.; Van Niekerk, J.M.; Mills, N.D.; Majumder, S.; Van Alphen, L.B.; Savelkoul, P.H.; Wolffs, P.F. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar]
- Perry, J.A.; Wright, G.D. Forces shaping the antibiotic resistome. BioEssays 2014, 36, 1179–1184. [Google Scholar]
- Anonymous. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Devlin, M.; Le Quesne, W.J.; Lyons, B.P. The marine environment of Kuwait—Emerging issues in a rapidly changing environment. Mar. Pollut. Bull. 2015, 100, 593–596. [Google Scholar]
- Le Quesne, W.J.; Baker-Austin, C.; Verner-Jeffreys, D.W.; Al-Sarawi, H.A.; Balkhy, H.H.; Lyons, B.P. Antimicrobial resistance in the Gulf Cooperation Council region: A proposed framework to assess threats, impacts and mitigation measures associated with AMR in the marine and aquatic environment. Environ. Int. 2018, 121, 1003–1010. [Google Scholar]
- Habibi, N.; Uddin, S.; Lyons, B.; Al-Sarawi, H.A.; Behbehani, M.; Shajan, A.; Razzack, N.A.; Zakir, F.; Alam, F. Antibiotic Resistance Genes Associated with Marine Surface Sediments: A Baseline from the Shores of Kuwait. Sustainability 2022, 14, 8029. [Google Scholar]
- Al-Sarawi, H.A.; Jha, A.N.; Al-Sarawi, M.A.; Lyons, B.P. Historic and contemporary contamination in the marine environment of Kuwait: An overview. Mar. Pollut. Bull. 2015, 100, 621–628. [Google Scholar]
- Al-Sarawi, H.A.; Jha, A.N.; Baker-Austin, C.; Al-Sarawi, M.A.; Lyons, B.P. Baseline screening for the presence of antimicrobial resistance in E. coli isolated from Kuwait’s marine environment. Mar. Pollut. Bull. 2018, 129, 893–898. [Google Scholar]
- Al-Sarawi, H.A.; Najem, A.B.; Lyons, B.P.; Uddin, S.; Al-Sarawi, M.A. Antimicrobial Resistance in Escherichia coli Isolated from Marine Sediment Samples from Kuwait Bay. Sustainability 2022, 14, 11325. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Zhang, Z.-W.; Liang, D.; Jiang, D.; Wang, C.; Du, N.; Zhang, Q.; Mitchelson, K.; Cheng, J. Multiplex asymmetric PCR-based oligonucleotide microarray for detection of drug resistance genes containing single mutations in Enterobacteriaceae. Antimicrob. Agents Chemother. 2007, 51, 3707–3713. [Google Scholar]
- Papan, C.; Meyer-Buehn, M.; Laniado, G.; Nicolai, T.; Griese, M.; Huebner, J. Assessment of the multiplex PCR-based assay Unyvero pneumonia application for detection of bacterial pathogens and antibiotic resistance genes in children and neonates. Infection 2018, 46, 189–196. [Google Scholar]
- Burakoff, A.; Brown, K.; Knutsen, J.; Hopewell, C.; Rowe, S.; Bennett, C.; Cronquist, A. Outbreak of fluoroquinolone-resistant Campylobacter jejuni infections associated with raw milk consumption from a herdshare dairy—Colorado, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 146. [Google Scholar]
- Nemergut, D.; Martin, A.; Schmidt, S. Integron diversity in heavy-metal-contaminated mine tailings and inferences about integron evolution. Appl. Environ. Microbiol. 2004, 70, 1160–1168. [Google Scholar]
- Pournajaf, A.; Ardebili, A.; Goudarzi, L.; Khodabandeh, M.; Narimani, T.; Abbaszadeh, H. PCR-based identification of methicillin–resistant Staphylococcus aureus strains and their antibiotic resistance profiles. Asian Pac. J. Trop. Biomed. 2014, 4, S293–S297. [Google Scholar]
- Khan, M.W.; Habibi, N.; Shaheed, F.; Mustafa, A.S. Draft genome sequences of five clinical strains of Brucella melitensis isolated from patients residing in Kuwait. Genome Announc. 2016, 4, e01144-16. [Google Scholar] [CrossRef]
- Light, E.; Baker-Austin, C.; Card, R.M.; Ryder, D.; Alves, M.T.; Al-Sarawi, H.A.; Abdulla, K.H.; Stahl, H.; Aliya, A.-G.; Al Ghoribi, M. Establishing a marine monitoring programme to assess antibiotic resistance: A case study from the Gulf Cooperation Council (GCC) region. medRxiv 2022, 9, 100268. [Google Scholar]
- Cabello-Yeves, P.J.; Callieri, C.; Picazo, A.; Mehrshad, M.; Haro-Moreno, J.M.; Roda-Garcia, J.J.; Dzhembekova, N.; Slabakova, V.; Slabakova, N.; Moncheva, S. The microbiome of the Black Sea water column analyzed by shotgun and genome centric metagenomics. Environ. Microbiome 2021, 16, 1–15. [Google Scholar]
- Ruppé, E.; Cherkaoui, A.; Charretier, Y.; Girard, M.; Schicklin, S.; Lazarevic, V.; Schrenzel, J. From genotype to antibiotic susceptibility phenotype in the order Enterobacterales: A clinical perspective. Clin. Microbiol. Infect. 2020, 26, 643.e641–643.e647. [Google Scholar]
- Zhang, X.-X.; Zhang, T.; Zhang, M.; Fang, H.H.; Cheng, S.-P. Characterization and quantification of class 1 integrons and associated gene cassettes in sewage treatment plants. Appl. Microbiol. Biotechnol. 2009, 82, 1169–1177. [Google Scholar]
- Grevskott, D.H.; Svanevik, C.S.; Sunde, M.; Wester, A.L.; Lunestad, B.T. Marine bivalve mollusks as possible indicators of multidrug-resistant Escherichia coli and other species of the Enterobacteriaceae family. Front. Microbiol. 2017, 8, 24. [Google Scholar] [CrossRef]
- Beaz-Hidalgo, R.; Balboa, S.; Romalde, J.L.; Figueras, M.J. Diversity and pathogenecity of Vibrio species in cultured bivalve molluscs. Environ. Microbiol. Rep. 2010, 2, 34–43. [Google Scholar]
- Giacometti, F.; Pezzi, A.; Galletti, G.; Tamba, M.; Merialdi, G.; Piva, S.; Serraino, A.; Rubini, S. Antimicrobial resistance patterns in Salmonella enterica subsp. enterica and Escherichia coli isolated from bivalve molluscs and marine environment. Food Control 2021, 121, 107590. [Google Scholar]
- Gevao, B.; Uddin, S.; Krishnan, D.; Rajagopalan, S.; Habibi, N. Antibiotics in Wastewater: Baseline of the Influent and Effluent Streams in Kuwait. Toxics 2022, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007, 5, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.S.; Baker-Austin, C.; Lindell, A.H.; Stepanauskas, R.; Stokes, H.W.; McArthur, J.V. Influence of industrial contamination on mobile genetic elements: Class 1 integron abundance and gene cassette structure in aquatic bacterial communities. ISME J. 2008, 2, 417–428. [Google Scholar] [CrossRef]
- Waśko, I.; Kozińska, A.; Kotlarska, E.; Baraniak, A. Clinically Relevant β-Lactam Resistance Genes in Wastewater Treatment Plants. Int. J. Environ. Res. Public Health 2022, 19, 13829. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Pongchaikul, P.; Mongkolsuk, P. Comprehensive Analysis of Imipenemase (IMP)-Type Metallo-β-Lactamase: A Global Distribution Threatening Asia. Antibiotics 2022, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Uddin, S.; Behbehani, M.; Kishk, M.; Abdul Razzack, N.; Zakir, F.; Shajan, A. Antibiotic Resistance Genes in Aerosols: Baseline from Kuwait. Int. J. Mol. Sci. 2023, 24, 6756. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-H.; Park, S.-G.; Choi, S.-M.; Hwang, Y.-O.; Ham, H.-J.; Kim, S.-U.; Lee, Y.-K.; Kim, M.-S.; Park, G.-Y.; Kim, K.-S. Antimicrobial resistance and resistance genes in Escherichia coli strains isolated from commercial fish and seafood. Int. J. Food Microbiol. 2012, 152, 14–18. [Google Scholar] [CrossRef]
- Koczura, R.; Mokracka, J.; Jabłońska, L.; Gozdecka, E.; Kubek, M.; Kaznowski, A. Antimicrobial resistance of integron-harboring Escherichia coli isolates from clinical samples, wastewater treatment plant and river water. Sci. Total Environ. 2012, 414, 680–685. [Google Scholar] [CrossRef]
- Lyons, B.; Devlin, M.; Hamid, S.A.; Al-Otiabi, A.; Al-Enezi, M.; Massoud, M.; Al-Zaidan, A.; Smith, A.; Morris, S.; Bersuder, P. Microbial water quality and sedimentary faecal sterols as markers of sewage contamination in Kuwait. Mar. Pollut. Bull. 2015, 100, 689–698. [Google Scholar] [CrossRef]
- Lyons, B.; Barber, J.; Rumney, H.; Bolam, T.; Bersuder, P.; Law, R.; Mason, C.; Smith, A.; Morris, S.; Devlin, M. Baseline survey of marine sediments collected from the State of Kuwait: PAHs, PCBs, brominated flame retardants and metal contamination. Mar. Pollut. Bull. 2015, 100, 629–636. [Google Scholar] [CrossRef]
- Gaze, W.H.; Zhang, L.; Abdouslam, N.A.; Hawkey, P.M.; Calvo-Bado, L.; Royle, J.; Brown, H.; Davis, S.; Kay, P.; Boxall, A. Impacts of anthropogenic activity on the ecology of class 1 integrons and integron-associated genes in the environment. ISME J. 2011, 5, 1253–1261. [Google Scholar] [CrossRef]
- Stalder, T.; Barraud, O.; Casellas, M.; Dagot, C.; Ploy, M.-C. Integron involvement in environmental spread of antibiotic resistance. Front. Microbiol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Liu, P.; Sun, Y.; Dong, X.; Hu, X. Unveiling the occurrence, hosts and mobility potential of antibiotic resistance genes in the deep ocean. Sci. Total Environ. 2022, 816, 151539. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, R.; Liu, H.; Wang, C.; Yin, X.; Zhang, M.; Fang, J.; Zhang, T.; Ma, L. Evidence for Long-Term Anthropogenic Pollution: The Hadal Trench as a Depository and Indicator for Dissemination of Antibiotic Resistance Genes. Environ. Sci. Technol. 2021, 55, 15136–15148. [Google Scholar] [CrossRef] [PubMed]
- Domingues, S.; Da Silva, G.J.; Nielsen, K.M. Global dissemination patterns of common gene cassette arrays in class 1 integrons. Microbiology 2015, 161, 1313–1337. [Google Scholar] [CrossRef] [PubMed]
- Hastey, C.J.; Boyd, H.; Schuetz, A.N.; Anderson, K.; Citron, D.M.; Dzink-Fox, J.; Hackel, M.; Hecht, D.W.; Jacobus, N.V.; Jenkins, S.G. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007–2009 to 2010–2012 based on the CLSI methodology. Anaerobe 2016, 42, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Al Salameen, F.; Habibi, N.; Uddin, S.; Al Mataqi, K.; Kumar, V.; Al Doaij, B.; Al Amad, S.; Al Ali, E.; Shirshikhar, F. Spatio-temporal variations in bacterial and fungal community associated with dust aerosol in Kuwait. PLoS ONE 2020, 15, e0241283. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A quality control tool for high throughput sequence data. Retrieved May 2010, 17, 2018. [Google Scholar]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Seemann, T. ABRicate: Mass Screening of Contigs for Antibiotic Resistance Genes; GitHub: San Francisco, CA, USA, 2016. [Google Scholar]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Carattoli, A.; Hasman, H. PlasmidFinder and In Silico pMLST: Identification and Typing of Plasmid Replicons in Whole-Genome Sequencing (WGS). In Horizontal Gene Transfer. Methods in Molecular Biology; de la Cruz, F., Ed.; Humana: New York, NY, USA, 2020; Volume 2075, pp. 285–294. [Google Scholar] [CrossRef]
- Néron, B.; Littner, E.; Haudiquet, M.; Perrin, A.; Cury, J.; Rocha, E.P. IntegronFinder 2.0: Identification and analysis of integrons across bacteria, with a focus on antibiotic resistance in Klebsiella. Microorganisms 2022, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Tate, J.; Mistry, J.; Coggill, P.C.; Sammut, S.J.; Hotz, H.-R.; Ceric, G.; Forslund, K.; Eddy, S.R.; Sonnhammer, E.L. The Pfam protein families database. Nucleic Acids Res. 2007, 36, D281–D288. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.i.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Team, U. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- FigTree. Molecular, Evolution, Phylogenetic and Epidemiology v1.4.2. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 17 May 2017).
- Alsarawi, H.A. Developing an Integrated Strategy for the Assessment of Hazardous Substances in Kuwait’s Marine Environment; University of Plymouth: Plymouth, UK, 2017. [Google Scholar]
- Mustafa, A.S.; Habibi, N.; Osman, A.; Shaheed, F.; Khan, M.W. Species identification and molecular typing of human Brucella isolates from Kuwait. PLoS ONE 2017, 12, e0182111. [Google Scholar] [CrossRef]
- Mazel, D.; Dychinco, B.; Webb, V.A.; Davies, J. Antibiotic resistance in the ECOR collection: Integrons and identification of a novel aad gene. Antimicrob. Agents Chemother. 2000, 44, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
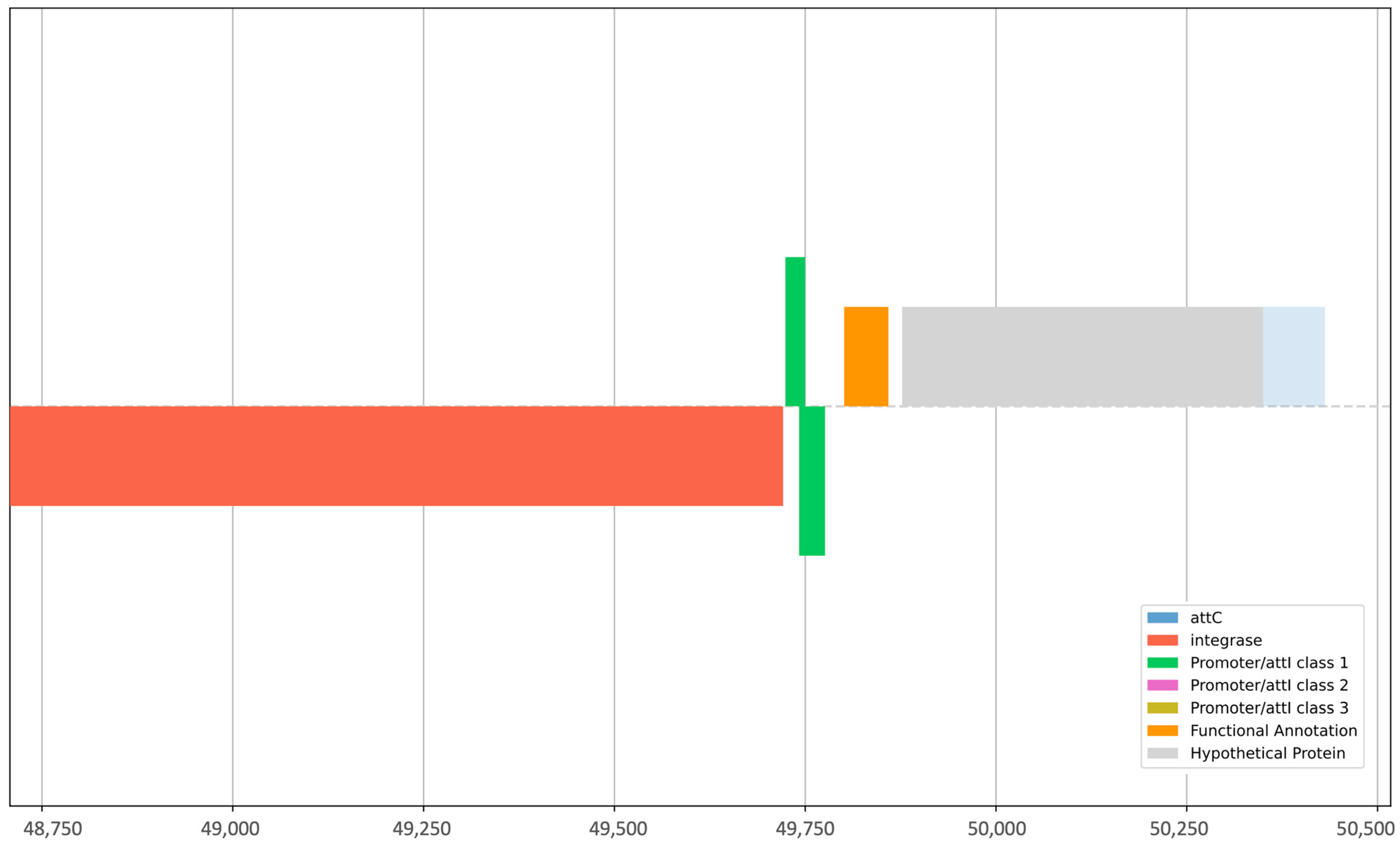
| Sample | Elements Identified by Integron Finder | Integron Class | |||||
|---|---|---|---|---|---|---|---|
| attC | intI | Pc_1 | Pint_1 | attI_1 | intl1 | GC | |
| HE40 | - | Yes | Yes | - | Yes | - | - |
| CW138 | Yes | Yes | - | Yes | - | Yes | Yes |
| CW121 | Yes | Yes | - | Yes | - | Yes | Yes |
| CW141 | Yes | Yes | Yes | Yes | Yes | Yes | - |
| SC70 | Yes | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sarawi, H.A.; Habibi, N.; Uddin, S.; Jha, A.N.; Al-Sarawi, M.A.; Lyons, B.P. Antibiotic Resistance Mediated by Escherichia coli in Kuwait Marine Environment as Revealed through Genomic Analysis. Antibiotics 2023, 12, 1366. https://doi.org/10.3390/antibiotics12091366
Al-Sarawi HA, Habibi N, Uddin S, Jha AN, Al-Sarawi MA, Lyons BP. Antibiotic Resistance Mediated by Escherichia coli in Kuwait Marine Environment as Revealed through Genomic Analysis. Antibiotics. 2023; 12(9):1366. https://doi.org/10.3390/antibiotics12091366
Chicago/Turabian StyleAl-Sarawi, Hanan A., Nazima Habibi, Saif Uddin, Awadhesh N. Jha, Mohammed A. Al-Sarawi, and Brett P. Lyons. 2023. "Antibiotic Resistance Mediated by Escherichia coli in Kuwait Marine Environment as Revealed through Genomic Analysis" Antibiotics 12, no. 9: 1366. https://doi.org/10.3390/antibiotics12091366








