Antimicrobial Resistance, Biofilm Formation, and Virulence Determinants in Enterococcus faecalis Isolated from Cultured and Wild Fish
Abstract
1. Introduction
2. Results
2.1. Occurrence of E. faecalis
2.2. Biofilm Formation in E. faecalis
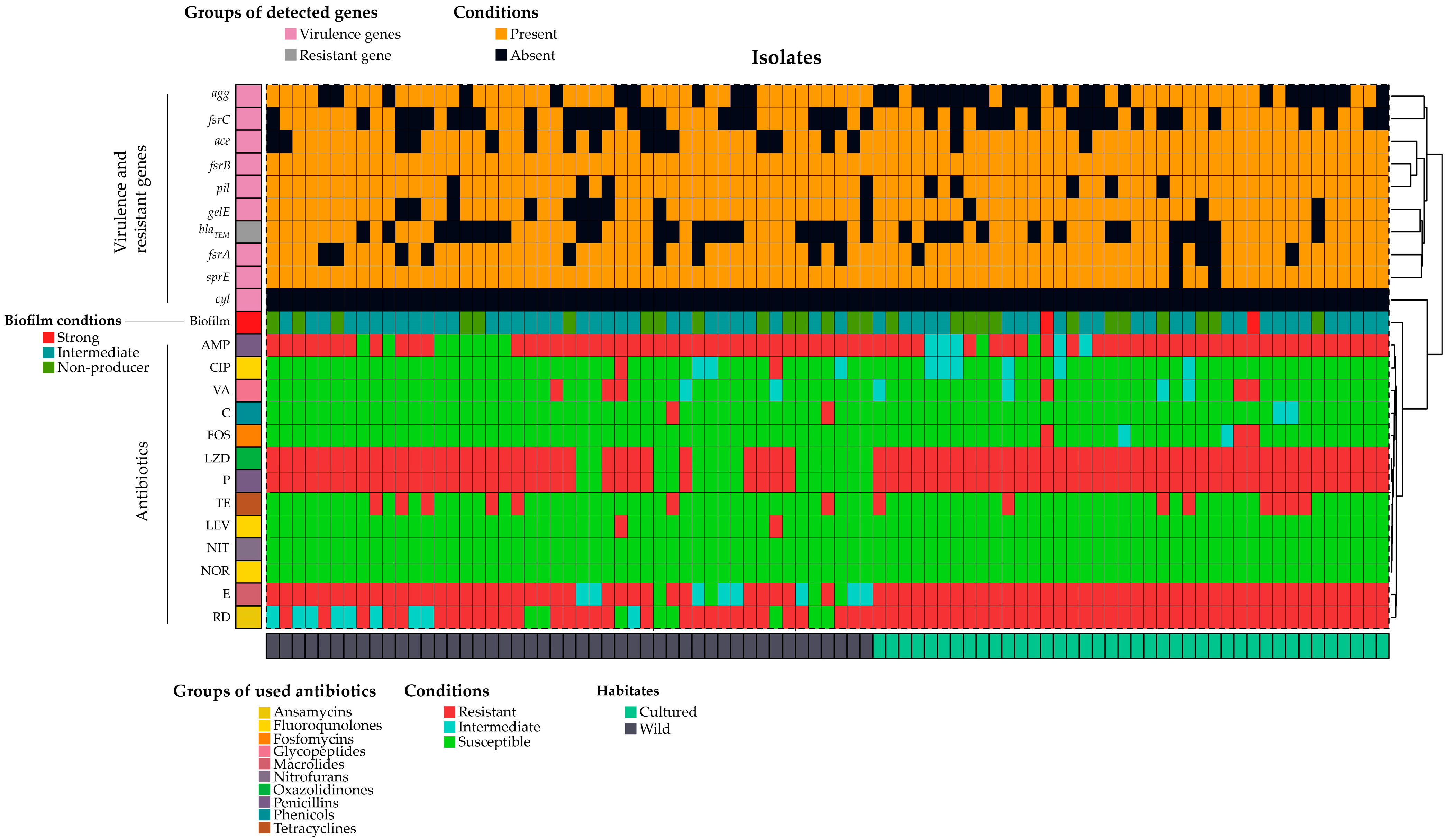
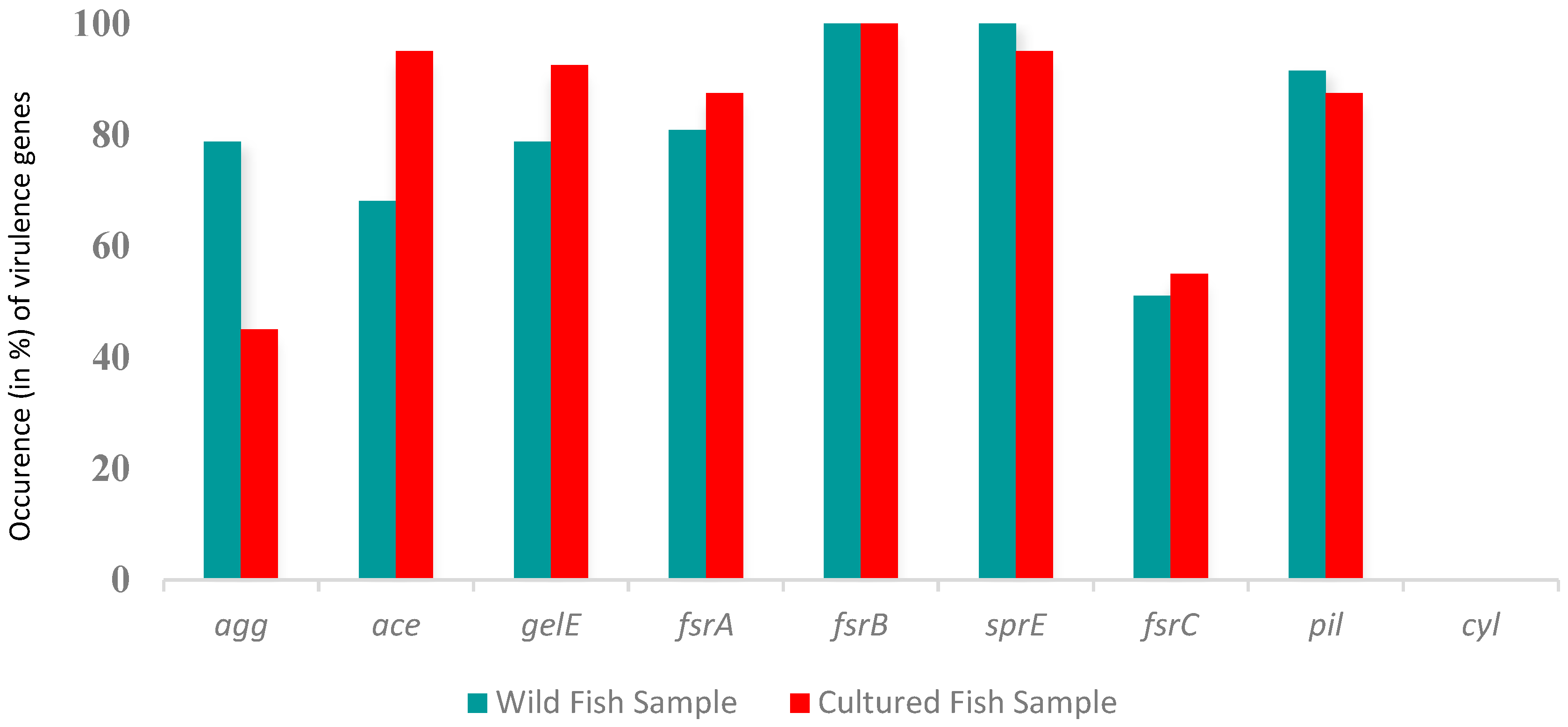
2.3. Presence of Virulence Genes in E. faecalis
2.4. Antibiogram Profile of E. faecalis
3. Discussion
3.1. Occurrence of Enterococcus faecalis
3.2. Antibiotic Resistance Pattern
3.3. Biofilm Formation and Virulence Genes
4. Materials and Methods
4.1. Ethical Approval
4.2. Sample Collection
4.3. Isolation and Molecular Identification of E. faecalis
4.4. Antibiotic Resistance, Biofilm, and Virulence Factors
4.5. Phenotypic Antimicrobial Resistance
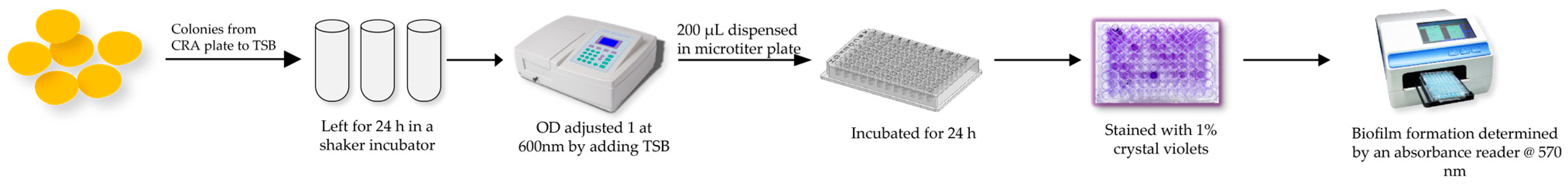
4.6. Biofilm Formation Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franz, C.M.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef]
- Kau, A.L.; Martin, S.M.; Lyon, W.; Hayes, E.; Caparon, M.G.; Hultgren, S.J. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 2005, 73, 2461–2468. [Google Scholar] [CrossRef]
- Shah, L.; Mulla, S.; Patel, K.G.; Rewadiwala, S. Prevalence of enterococci with higher resistance level in a tertiary care hospital: A matter of concern. Nat. J. Med. Res. 2012, 2, 25–27. [Google Scholar]
- Moro, M.; Gandin, C.; Bella, A.; Siepi, G.; Petrosillo, N. A National Survey on the Surveillance and Control of Nosocomial Infections in Public Hospitals in Italy (Rapporti ISTISAN 01/4); Istituto Superiore di Sanità: Rome, Italy, 2001. [Google Scholar]
- Duggan, J.M.; Sedgley, C.M. Biofilm formation of oral and endodontic Enterococcus faecalis. J. Endod. 2007, 33, 815–818. [Google Scholar] [CrossRef]
- Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic resistance and biofilm formation in Enterococcus spp. isolated from urinary tract infections. Pathogens 2023, 12, 34. [Google Scholar] [CrossRef]
- Janssens, J.C.; Steenackers, H.; Robijns, S.; Gellens, E.; Levin, J.; Zhao, H.; De Keersmaecker, S.C. Brominated furanones inhibit biofilm formation by Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2008, 74, 6639–6648. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 15 January 2023).
- de Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Threlfall, E.J.; Ward, L.R.; Frost, J.A.; Willshaw, G.A. The emergence and spread of antibiotic resistance in food-borne bacteria. Int. J. Food Microbiol. 2000, 62, 1–5. [Google Scholar] [CrossRef]
- Fisheries Resources Survey System (FRSS). Fisheries Statistical Report of Bangladesh 2017, 34, 129. Available online: https://fisheries.portal.gov.bd/sites/default/files/files/fisheries.portal.gov.bd/page/4cfbb3cc_c0c4_4f25_be21_b91f84bdc45c/Fisheries%20Statistical%20Yearboook%202017-18.pdf (accessed on 27 August 2023).
- Annual Report 2017; Dhaka Department of Fisheries, Ministry of Fisheries and Livestock, Government of Bangladesh: Dhaka, Bangladesh, 2017.
- Samani, R.J.; Tajbakhsh, E.; Momtaz, H.; Samani, M.K. Prevalence of virulence genes and antibiotic resistance pattern in Enterococcus faecalis isolated from urinary tract infection in Shahrekord, Iran. Rep. Biochem. Mol. Biol. 2021, 10, 50. [Google Scholar]
- About Department of Fisheries. Available online: http://www.fisheries.gov.bd/site/page/43ce3767-3981-4248-99bd-d321b6e3a7e5/%E0%A6%AA%E0%A6%9F%E0%A6%AD%E0%A7%82%E0%A6%AE%E0%A6%BF (accessed on 27 August 2023).
- Cattoir, V. The multifaceted lifestyle of enterococci: Genetic diversity, ecology and risks for public health. Curr. Opin. Microbiol. 2022, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Jose, M.M.; Cesar, A.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti-Infective Therapy 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Anderson, A.C.; Jonas, D.; Huber, I.; Karygianni, L.; Wölber, J.; Hellwig, E.; Arweiler, N.; Vach, K.; Wittmer, A.; Al-Ahmad, A. Enterococcus faecalis from food, clinical specimens, and oral sites: Prevalence of virulence factors in association with biofilm formation. Front Microbiol. 2016, 6, 1534. [Google Scholar] [CrossRef] [PubMed]
- Elgohary, I.; Eissa, A.E.; Fadel, N.G.; Ibrahim Abd, E.J.; Mahmoud, M.A. Bacteriological, molecular, and pathological studies on the Gram-positive bacteria Aerococcus viridans and Enterococcus faecalis and their effects on Oreochromis niloticus in Egyptian fish farms. Aquac. Res. 2021, 52, 2220–2232. [Google Scholar] [CrossRef]
- Petersen, A.; Dalsgaard, A. Species composition and antimicrobial resistance genes of Enterococcus spp., isolated from integrated and traditional fish farms in Thailand. Environ. Microbiol. 2003, 5, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, N.; Momtaz, H.; Tajbakhsh, E. Molecular characterization and antimicrobial resistance of Enterococcus faecalis isolated from seafood samples. Vet. Med. Sci. 2022, 8, 1104–1112. [Google Scholar] [CrossRef]
- Hassan, M.A.; Abdel-Naeim, N.S.; Mabrok, M.; Dessouki, A.A.; Hassan, A.M. Isolation and identification of Enterococcus faecalis from cultured Oreochromis niloticus and Mugil cephalus with a special emphasis on a possible integrated control strategy. Aquac. Res. 2022, 53, 5521–5535. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, M.M.; Deb, S.C.; Alam, M.S.; Alam, M.J.; Islam, M.T. Molecular identification of multiple antibiotic resistant fish pathogenic Enterococcus faecalis and their control by medicinal herbs. Sci. Rep. 2017, 7, 3747. [Google Scholar] [CrossRef]
- Agoba, E.E.; Adu, F.; Agyare, C.; Boamah, V.E.; Boakye, Y.D. Antibiotic resistance patterns of bacterial isolates from hatcheries and selected fish farms in the Ashanti region of Ghana. J. Microbiol. Antimicrob. 2017, 9, 35–46. [Google Scholar]
- Noroozi, N.; Momtaz, H.; Tajbakhsh, E. Occurrence and antibiotic resistance of Enterococcus faecalis strains isolated from fish and shrimp caught from the Persian Gulf. Int. J. Food Microbiol. 2022, 9, 16–23. [Google Scholar]
- Chowdhury, S.; Rheman, S.; Debnath, N.; Delamare-Deboutteville, J.; Akhtar, Z.; Ghosh, S.; Parveen, S.; Islam, K.; Islam, M.A.; Rashid, M.M.; et al. Antibiotics usage practices in aquaculture in Bangladesh and their associated factors. One Health 2022, 15, 100445. [Google Scholar] [CrossRef]
- Finisterra, L.; Duarte, B.; Peixe, L.; Novais, C.; Freitas, A.R. Industrial dog food is a vehicle of multidrug-resistant enterococci carrying virulence genes often linked to human infections. Int. J. Food Microbiol. 2021, 358, 109284. [Google Scholar] [CrossRef]
- Arumugam, U.; Nattan, S.; Gnanadesika, P.R. Isolation, molecular identification and antibiotic resistance of Enterococcus faecalis from diseased tilapia. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 136–146. [Google Scholar] [CrossRef][Green Version]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial resistance in aquaculture: A crisis for concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Datta, S. Management of water quality in intensive aquaculture. Respiration 2012, 6, 602. [Google Scholar]
- Sørum, H. Antimicrobial drug resistance in fish pathogens. In Antimicrobial Resistance in Bacteria of Animal Origin; Wiley: Hoboken, NJ, USA, 2005; pp. 213–238. [Google Scholar]
- Hughes, P.; Heritage, J. Antibiotic Growth-Promoters in Food Animals. FAO Animal Production and Health Paper; FAO: Rome, Italy, 2004; p. 160. [Google Scholar]
- Cui, P.; Feng, L.; Zhang, L.; He, J.; An, T.; Fu, X.; Yang, X. Antimicrobial resistance, virulence genes, and biofilm formation capacity among Enterococcus species from Yaks in Aba Tibetan autonomous prefecture, China. Front. Microbiol. 2020, 11, 1250. [Google Scholar] [CrossRef]
- Toledo-Arana, A.; Valle, J.; Solano, C.; Arrizubieta, M.J.; Cucarella, C.; Lamata, M.; Lasa, I. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 2001, 67, 4538–4545. [Google Scholar] [CrossRef]
- Rohde, H.; Knobloch, J.K.; Horstkotte, M.A.; Mack, D. Correlation of Staphylococcus aureus icaADBC genotype and biofilm expression phenotype. J. Clin. Microbiol 2001, 39, 4595. [Google Scholar] [CrossRef]
- Suchitra, U.; Kundabala, M. Enterococcus faecalis: An endodontic pathogen. Endodontology 2006, 18, 11–13. [Google Scholar]
- Rathnayake, I.U.; Hargreaves, M.; Huygens, F. Antibiotic resistance and virulence traits in clinical and environmental Enterococcus faecalis and Enterococcus faecium isolates. Syst. Appl. Microbiol. 2012, 35, 326–333. [Google Scholar] [CrossRef]
- Akter, T.; Haque, M.N.; Ehsan, R.; Paul, S.I.; Foysal, M.J.; Tay, A.C.Y.; Rahman, M.M. Virulence and antibiotic-resistance genes in Enterococcus faecalis associated with streptococcosis disease in fish. Sci. Rep. 2023, 13, 1551. [Google Scholar] [CrossRef] [PubMed]
- Del Papa, M.F.; Perego, M. Enterococcus faecalis virulence regulator FsrA binding to target promoters. J. Bacteriol. 2011, 193, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Didem, K.; Kuştimur, A.S. Investigation of gelatinase gene expression and growth of Enterococcus faecalis clinical isolates in biofilm models. Turk. J. Pharm. Sci. 2019, 16, 356. [Google Scholar]
- Lay, J.; Jackson, O. MALDI-TOF mass spectrometry of bacteria. Mass Spectrom. Rev. 2001, 20, 172–194. [Google Scholar] [CrossRef]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995, 33, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Ballah, F.M.; Islam, M.S.; Rana, M.L.; Ullah, M.A.; Ferdous, F.B.; Neloy, F.H.; Ievy, S.; Sobur, M.A.; Rahman, A.T.; Khatun, M.M.; et al. Virulence determinants and methicillin resistance in biofilm-forming Staphylococcus aureus from various food sources in Bangladesh. Antibiotics 2022, 11, 1666. [Google Scholar] [CrossRef] [PubMed]
- Hashem, Y.A.; Abdelrahman, K.A.; Aziz, R.K. Phenotype–genotype correlations and distribution of key virulence factors in Enterococcus faecalis isolated from patients with urinary tract infections. Infect. Drug Resist. 2021, 14, 1713–1723. [Google Scholar] [CrossRef]
- Randall, L.P.; Cooles, S.W.; Osborn, M.K.; Piddock, L.J.V.; Woodward, M.J. Antibiotic resistance genes, integrons and multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. J. Antimicrob. Chemother. 2004, 53, 208–216. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, M100-S32; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Bauer, A.T.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, B.; Zmantar, T.; Hentati, H.; Bakhrouf, A. Cell surface hydrophobicity, biofilm formation, adhesives properties and molecular detection of adhesins genes in Staphylococcus aureus associated to dental caries. Microb. Pathog. 2010, 49, 14–22. [Google Scholar] [CrossRef] [PubMed]


| Factors | Target Genes | The Nucleotide Sequence (5′-3′) | Annealing Temperature | Amplicon Size (bp) | References |
|---|---|---|---|---|---|
| Species identification | ddl | F′-ATCAAGTACAGTTAGTCTT R′-ACGATTCAAAGCTAACTG | 50 | 942 | [42] |
| Biofilm | agg | F′-TCTTGGACACGACCCATGAT R′-AGAAAGAACATCACCACGAGC | 58 | 413 | [44] |
| Virulence | gelE | F′-GGTGAAGAAGTTACTCTGAC R′-GGTATTGAGTTATGAGGGGC | 52 | 704 | |
| ace | F′-GAATGACCGAGAACGATGGC R′-CTTGATGTTGGCCTGCTTCC | 58 | 615 | ||
| pil | F′-GAAGAAACCAAAGCACCTAC R′-CTACCTAAGAAAAGAAACGCG | 53 | 620 | ||
| fsrA | F′-CGTTCCGTCTCTCATAGTTA R′-GCAGGATTTGAGGTTGCTAA | 53 | 474 | ||
| fsrB | F′-TAATCTAGGCTTAGTTCCCAC R′-CTAAATGGCTCTGTCGTCTAG | 55 | 428 | ||
| fsrC | F′-GTGTTTTTGATTTCGCCAGAGA R′-TATAACAATCCCCAACCGTG | 54 | 716 | ||
| sprE | F′-CTGAGGACAGAAGACAAGAAG R′-GGTTTTTCTCACCTGGATAG | 53 | 432 | ||
| Antibiotic-Resistant gene | BlaTEM | F: CATTTCCGTGTCGCCCTTAT R: TCCATAGTTGCCTGACTCCC | 56 | 793 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, M.L.; Firdous, Z.; Ferdous, F.B.; Ullah, M.A.; Siddique, M.P.; Rahman, M.T. Antimicrobial Resistance, Biofilm Formation, and Virulence Determinants in Enterococcus faecalis Isolated from Cultured and Wild Fish. Antibiotics 2023, 12, 1375. https://doi.org/10.3390/antibiotics12091375
Rana ML, Firdous Z, Ferdous FB, Ullah MA, Siddique MP, Rahman MT. Antimicrobial Resistance, Biofilm Formation, and Virulence Determinants in Enterococcus faecalis Isolated from Cultured and Wild Fish. Antibiotics. 2023; 12(9):1375. https://doi.org/10.3390/antibiotics12091375
Chicago/Turabian StyleRana, Md. Liton, Zannatul Firdous, Farhana Binte Ferdous, Md. Ashek Ullah, Mahbubul Pratik Siddique, and Md. Tanvir Rahman. 2023. "Antimicrobial Resistance, Biofilm Formation, and Virulence Determinants in Enterococcus faecalis Isolated from Cultured and Wild Fish" Antibiotics 12, no. 9: 1375. https://doi.org/10.3390/antibiotics12091375
APA StyleRana, M. L., Firdous, Z., Ferdous, F. B., Ullah, M. A., Siddique, M. P., & Rahman, M. T. (2023). Antimicrobial Resistance, Biofilm Formation, and Virulence Determinants in Enterococcus faecalis Isolated from Cultured and Wild Fish. Antibiotics, 12(9), 1375. https://doi.org/10.3390/antibiotics12091375










