1. Introduction
Periprosthetic joint infection (PJI) is a feared complication in total hip arthroplasty (THA), with an infection rate of approximately 1–2% in patients who have undergone THA surgery [
1,
2,
3,
4]. Infection can damage bone stock and affect underlying bone integrity, thus limiting the potential success of future revision procedures [
5,
6,
7,
8]. The gold standard treatment for THA PJI is two-stage revision arthroplasty with implantation of an antibiotic spacer [
9,
10,
11,
12,
13]. More recently, implants have been utilized in an attempt to provide a functional spacer that may be able to last for a longer period of time or potentially be permanent, without the need for a second surgery [
14]. This approach uses cemented hip implants to allow for immediate weight-bearing and function [
5,
9,
15,
16]. However, functional spacers are subject to their own subset of complications, such as prosthetic dislocation and fracture [
9,
11,
17,
18,
19,
20].
Dislocation rates as high as 41% have been reported for articulating spacer constructs [
21]. Factors such as poor soft tissue quality and trochanteric insufficiency may exist in patients undergoing surgery that increase the instability of the construct and risk of dislocation. One alternative to reduce dislocation rates in such patient populations is the use of a constrained acetabular liner [
22]. Constrained liners may put more stress on the fixation of the acetabular component. Articulating spacers are placed using the cement technique and controversy exists over the methodology of cementation. Better cementation may provide a longer-lasting implant but makes removal of cement more difficult if a second-stage surgery is required.
The purpose of our study was to describe our results utilizing a cemented metal hip stem with an all-polyethylene constrained tripolar liner, secured with surgeon-mixed, high-dose antibiotic cement in the staged treatment of both PJI and native hip infections. Comparisons between PJI and native hip infection groups were made as patients with both types of infections are candidates for antibiotic spacer placement. We intended to examine rates of second surgery and dislocation rates of the construct. In addition, we examined the role of acetabular cement mantles and acetabular bone loss to predict the longer survival of the spacer. We hypothesized that a higher-grade acetabular cement mantle and a lesser degree of acetabular bone loss would be predictors of longer-term spacer survival. Our rationale was that due to improved fixation of components with high-grade cementation and superior scaffolding provided by healthier acetabular bone stock, a more robust implant interface would be created to prevent complications such as dislocation and fracture [
7,
8,
14].
2. Methods
After institutional review board approval, a retrospective review was conducted to identify patients who underwent placement of a functional, articulating hip spacer with a cemented, constrained, all-polyethylene acetabular liner and a metal femoral stem. These components were used in the treatment of PJI or native hip infection at a single tertiary care academic medical center from January 2016 through April 2020. Inclusion criteria were the placement of the articulating spacer and age greater than 18 years. Patients were excluded if no follow-up information was available in the health record (6) or if additional hardware such as a cage was implemented to provide stability to the implant (4). Our final cohort consisted of 103 articulating hip spacers placed from January 2016 to April 2020. Seventy-nine spacers were placed for PJI while 24 were placed for native hip chronic septic arthritis.
All infected hips underwent prosthesis removal and/or resection of infected bone and thorough irrigation and debridement of the hip. All hips were irrigated with at least 6 L of saline and a betadine solution using pulse lavage. A cemented, constrained all-polyethylene acetabular liner was used (Trident constrained liner, Stryker, Mahwah, NJ, USA). A cemented femoral implant and metal head were used (OmniFit, Stryker, Mahwah, NJ, USA). Both components were secured with 2–3 batches of plain Cobalt® bone cement (DJO Surgical, Austin, TX, USA) with surgeon-directed manual addition of 2–3 g of vancomycin and 2.4–3.6 g of tobramycin per batch of cement. On the acetabular side, acetabular reamers were used and the component was undersized by either 4 or 6 mm to gain a proper cement mantle. Femoral components were cemented only in the metaphyseal region to aid in future removal at the time of second-stage reimplantation. Cement restrictors were not used and a hand-packing technique was performed. Broaches were utilized to determine stem size and a size one under the broach was selected. Cobalt chrome femoral heads were used in all cases and the size was used in concordance with the acetabular liner. Following spacer placement, all patients were treated with 6 weeks of culture-directed intravenous antibiotics based on recommendations from our Musculoskeletal Infectious Disease Service. Post-operative weight-bearing status was determined per the surgeon’s discretion. Second-stage surgery was at the discretion of the operating surgeon. Surgeries were performed by 4 arthroplasty surgeons who have different philosophies of treatment. One surgeon was more likely to recommend early revision and the others performed a second surgery only if patients were symptomatic.
Demographic information is recorded in
Table 1. Microbiology cultures were positive in 79 of 103 patients and organisms are shown in
Table 2. Spacer components, spacer-related complications such as component failure, prosthetic dislocation, and periprosthetic fracture, rates of recurrent infection, and spacer longevity were recorded. Grading of acetabular bone loss was performed according to Paprosky classifications [
23] and cement mantle quality was measured using postoperative anteroposterior X-rays according to the criteria proposed by Hodgkinson, Shelley, and Wroblewski [
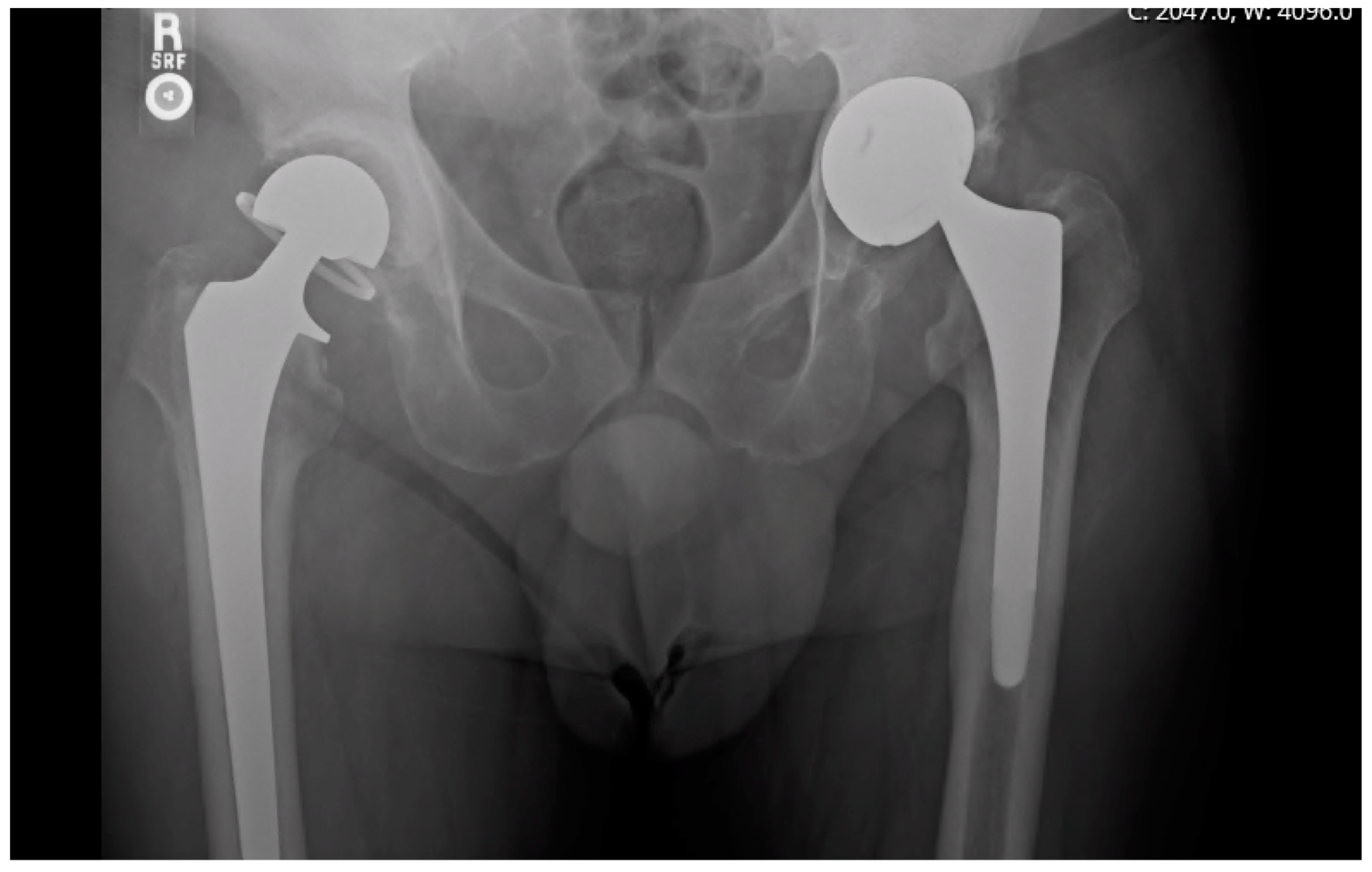
24]. A radiographic example of a prosthesis with a high-grade cement mantle and no acetabular bone loss can be seen in
Figure 1, contrasted with a low-grade cement mantle in the setting of moderate acetabular bone loss in
Figure 2. Spacer longevity was measured for patients who underwent second-stage reimplantation and for those with a retained spacer. Comparisons were also made between spacers placed for PJI and native hip septic arthritis. Within the patient subset who received second-stage reimplantation, factors leading to reimplantation such as component loosening and pain were analyzed. Rates of reinfection and spacer-related complications were compared between patients who retained their initial spacer and those who underwent a second-stage reimplantation, in addition to grading of bone loss and cement mantle quality between the two groups.
SAS v.9.4 software (SAS Institute Inc., Cary, NC, USA) was deployed for statistical analysis. Age and BMI were compared for group differences with the Kruskal–Wallis test, while all other variables were compared for group differences with Fisher’s exact test. p < 0.05 was considered statistically significant.
3. Results
At an average follow-up of 466.9 days, 70/103 (68.0%) of hips had undergone second-stage reimplantation (mean 151.9 days), while 33/103 (32.0%) had retained the spacer (mean duration 257.0 days). The majority of hips (94/103, 91.3%) were free from reinfection at one year with no statistically significant difference between the PJI (92.4%) and native groups (87.5%, p = 0.43).
There were 10 (9.7%) spacer-related complications, with no significant difference in spacer-related complication rate between PJI and native infections (7/79 PJI vs. 3/24 native,
p = 1.0). Spacer-related complications included acetabular component dislodgement (3), spacer dislocation (3), prosthetic dislocation (1), periprosthetic fracture (1), hematoma requiring reoperation (1), and femoral component loosening (1) (
Table 3). Spacer-related complications were observed in 9/72 (13%) of patients who received second-stage reimplantation, as opposed to 1/31 (3%) in those who retained their spacer (
p = 0.275).
A slightly greater number of patients who chose to retain their articulating constrained spacer had a cement mantle grade of 0 or 1 (15/31, 48%) as opposed to patients who underwent second-stage reimplantation (29/72, 40%) (
Table 4). Most patients with retained spacers had a cement mantle grade of 2 or greater (16/31, 52%) as well as patients who underwent second-stage reimplantation (43/72, 60%,
Table 4).
No acetabular bone loss (20) or mild loss (8) was observed in 28/31 (90%) of patients with retained spacers, compared to 59/72 (82%) of patients who underwent second-stage reimplantation (
Table 5). In the retained spacer group, 2/31 (6%) of patients had moderate or severe acetabular bone loss, compared to 10/72 (14%) of patients who underwent second-stage reimplantation (
Table 5).
4. Discussion
Periprosthetic hip joint infection represents a significant health burden to patients who have undergone total hip arthroplasty. In treating PJI, articulating antibiotic spacers have become popular for their numerous advantages for patient functionality. However, articulating spacers are subject to their own subset of complications, with fracture and periprosthetic dislocation being especially problematic. Earlier studies have shown fracture rates of 13.6% [
19] and rates of dislocation as high as 41% [
21]. By utilizing an articulating construct with a cemented constrained polyethylene acetabular liner and cemented femoral stem, we were able to minimize the risk of dislocation. The rate in our series was 5.8%. This is compared to 26% in the literature [
25]. The use of a constrained liner is thought to put additional stress on the bone–implant interface [
26]. Rates of acetabular implant loosening were 3.9%.
Recurrent infection was seen in both PJI (7.6%, 6/79) and native (12.5%, 3/24) infection groups. The literature has shown a reinfection rate of approximately 10% with two-stage treatment of PJI [
27]. Rates after native infections with arthritic change are less understood, but in a study by Fleck et al. were shown to be 7.2% [
28]. Persistent infection of the hip joint after surgical treatment is associated with significant morbidity [
29,
30], often leading to patients undergoing repeat two-stage procedures which have been associated with low success rates [
31].
Our implant retention at one year was only 32.0%. This may be somewhat due to differences in surgeon techniques or surgical decision-making. One of the treating surgeons plans reimplantation at 3 months while the other three surgeons are more likely to allow patients to keep their spacers if they are functioning well clinically. Tsung et al. described the 1.5-stage revision wherein 44.7% of patients kept a spacer that was functional [
32]. There may be a higher degradation level of retention as time goes on with additional loosening of implants. A case series by Choi et al. described long-term outcomes of retained articulating spacers fixated with antibiotic-laden cement, with 83.3% (15/18) of patients maintaining articulating spacers for up to 6 years with adequate functionality [
33]. Additionally, a case report by Luk et al. presented a patient who has retained an articulating spacer for 6 years with no evidence of infection, loosening of the spacer construct, or periprosthetic fracture [
34]. Another possible solution is a one-stage revision using uncemented revision implants or a better cement technique.
The quality of the cement technique did not seem to relate to reimplantation rates. There was no significant difference in cement mantle grade between those who kept their prosthesis and those who underwent reimplantation surgery. Improvements in technique may help this; however, at the expense of more difficulty later if a revision is required. Bone loss in the acetabulum was not found to be a risk factor for revision but may make revision surgery more difficult.
In addition to advantages in patient functionality, articulating spacers may provide an effective longer-term solution for patients who are unfit to undergo reimplantation surgery or who elect to retain their spacer, allowing patients a satisfactory quality of life while preventing the physical and psychological burden of repeat operation [
35]. This is an important consideration, especially in frail patients [
36]. The articulating spacer construct appears to be a feasible long-term solution as a significant number of patients elected to retain their initial spacer. Within the patient subset who retained their spacers, cement mantles were of a slightly higher grade, with 48% of patients having either a grade 0 or grade 1 cement mantle and a lesser degree of acetabular bone loss was observed when compared with patients who underwent eventual second-stage reimplantation, with 90% of patients having only mild bone loss or none at all. This suggests that the spacer construct is reliable as a long-term solution given cement mantles and bone stock are adequate at the time of implantation.
The burden of a second-stage revision procedure on patients is an important consideration. In attempting to solve this problem, a 1.5-stage revision surgery has been adopted by some centers for the treatment of infected total knee arthroplasty, allowing for patients to have a functional articulating spacer construct placed permanently with comparable infection eradication rates to those seen in retained articulating spacers [
37,
38]. Additionally, this method offers greater ease of resection and preservation of bone should a patient need a second surgery due to reinfection, construct loosening, or functional decline [
38]. In the future, having a similar construct available for the treatment of hip PJI would be advantageous if rates of infection eradication and construct longevity were comparable to articulating constructs in current use.
Our study has some limitations. The use of our articulating spacer is off-label and requires intraoperative surgeon adjustments depending on bone loss and cement supplementation needs. Because our surgeons intentionally do not cement the entire femur, we were unable to completely assess cement mantle quality and the subsequent need for revision surgery. Our study is a retrospective review of data. Surgeons had different philosophies regarding long-term spacer usage. The decision to reimplant versus leave in situ is often a complex, shared-decision process that considers many patient and implant factors.
5. Conclusions
Articulating antibiotic spacers with cemented constrained acetabular liners and metal femoral components represent a potential option for some patients in the treatment of PJI and native hip joint infections. The articulating spacer construct is able to eradicate hip joint infection while preserving good patient functional status. Articulating antibiotic spacers may be a feasible long-term solution, especially in frail patients. This spacer construct appears to be reliable with regard to component dislocation. The quality of acetabular cement mantles and acetabular bone loss were not related to the need for a second surgery.
Author Contributions
G.T.G.: study design, analysis, data acquisition, data interpretation, and manuscript composition. A.E.A.: data acquisition, analysis, and data interpretation. S.C.M.: Surgical decision-making, study design, analysis, and manuscript composition. C.L.B.: Surgical decision making. B.M.S.: Manuscript composition. E.R.S.: Data analysis. J.B.S.: Surgical decision-making, study design, analysis, data interpretation, and manuscript composition. All authors have read and agreed to the published version of the manuscript.
Funding
The research reported in this publication was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number P20GM125503. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, the Bill and Betty Petty Research Fund at the University of Arkansas for Medical Sciences supported this work.
Institutional Review Board Statement
IRB approval was obtained from our institution prior to the start of data acquisition and analysis.
Informed Consent Statement
Informed consent was not necessary due to the retrospective nature of the study as approved by the IRB.
Data Availability Statement
The data is stored securely on our encrypted BOX platform per our IRB designation.
Conflicts of Interest
Glenn declares no conflict of interest. Apple declares no conflict of interest. Mears—Stock or stock options from Delta Ortho LLC. Barnes—Royalties from DJO and Zimmer; Paid consultant for MicroPort Orthopaedics; Stock or stock options from Avant-garde Health; BEKHealth; Clozex Medical; Excelerate Health Ventures; Green OR; Hayle Surgical; In2Bones SAS; MiCare Path; Plakous; Ride Health; ROM3 Rehab, LLC; Sleep Partners, LLC; Sniffle. Stronach—Royalties from MiCare Path, Sawbones/Pacific Research Laboratories, Tightline Development LLC; Speakers bureau/paid presentations from DJ Orthopaedics; Paid consultant from DJ Orthopaedics, Johnson & Johnson; Stock or stock options from Joint Development LLC. Siegel declares no conflicts of interest. Stambough—Royalties from Signature Orthopaedics; Paid consultant from Smith & Nephew.
References
- Anagnostakos, K. Therapeutic use of antibiotic-loaded bone cement in the treatment of hip and knee joint infections. J. Bone Jt. Infect. 2017, 2, 29–37. [Google Scholar] [CrossRef]
- Citak, M.; Masri, B.A.; Springer, B.; Argenson, J.N.; Kendoff, D.O. Are preformed articulating spacers superior to surgeon-made articulating spacers in the treatment of PJI in THA? A literature review. Open Orthop. J. 2015, 9, 255–261. [Google Scholar] [CrossRef]
- Kang, J.-S.; Shin, E.-H.; Roh, T.-H.; Na, Y.; Moon, K.H.; Park, J.-H. Long-term clinical outcome of two-stage revision surgery for infected hip arthroplasty using cement spacer: Culture negative versus culture positive. J. Orthop. Surg. 2018, 26, 2309499017754095. [Google Scholar] [CrossRef]
- Çağlar, Ö.; Tokgözoğlu, M.; Akgün, R.C.; Atilla, B. Low-dose vancomycin-loaded cement spacer for two-stage revision of infected total hip arthroplasty. Jt. Dis. Relat. Surg. 2020, 31, 449–455. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Jung, J.; Schmid, N.V.; Schmitt, E.; Kelm, J. Mechanical complications and reconstruction strategies at the site of hip spacer implantation. Int. J. Med. Sci. 2009, 6, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Takahira, N.; Fukushima, K.; Moriya, M.; Yamamoto, T.; Minegishi, Y.; Sakai, R.; Itoman, M.; Takaso, M. Two-stage revision total hip arthroplasty for periprosthetic infections using antibiotic-impregnated cement spacers of various types and materials. Sci. World J. 2013, 2013, 147248. [Google Scholar] [CrossRef] [PubMed]
- Białecki, J.; Kogut, M.; Chaberek, S.; Bartosz, P.; Obrębski, M.; Marczyński, W.; Para, M. Two-stage revision arthroplasty in the treatment of periprosthetic hip infections with severe bone loss: Results from 182 cases. Orthop. Rev. 2020, 12, 8545. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Shih, C.H.; Chang, Y.H.; Lee, M.S.; Yang, W.E.; Shih, H.N. Treatment of deep infection of the hip associated with massive bone loss: Two-stage revision with an antibiotic-loaded interim cement prosthesis followed by reconstruction with allograft. J. Bone Jt. Surg. Br. 2005, 87, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, B.P.; Mabry, T.M.; Abdel, M.P.; Berry, D.J.; Hanssen, A.D.; Perry, K.I. Two-stage revision total hip arthroplasty with a specific articulating antibiotic spacer design: Reliable periprosthetic joint infection eradication and functional improvement. J. Arthroplast. 2018, 33, 3746–3753. [Google Scholar] [CrossRef]
- Charette, R.S.; Melnic, C.M. Two-stage revision arthroplasty for the treatment of prosthetic joint infection. Curr. Rev. Musculoskelet. Med. 2018, 11, 332–340. [Google Scholar] [CrossRef]
- Yang, F.S.; Lu, Y.D.; Wu, C.T.; Blevins, K.; Lee, M.S.; Kuo, F.C. Mechanical failure of articulating polymethylmethacrylate (PMMA) spacers in two-stage revision hip arthroplasty: The risk factors and the impact on interim function. BMC Musculoskelet. Disord. 2019, 20, 372. [Google Scholar] [CrossRef]
- Lee, H.D.; Prashant, K.; Shon, W.Y. Management of periprosthetic hip joint infection. Hip Pelvis 2015, 27, 63–71. [Google Scholar] [CrossRef]
- Slullitel, P.A.; Oñativia, J.I.; Buttaro, M.A.; Sánchez, M.L.; Comba, F.; Zanotti, G.; Piccaluga, F. State-of-the-art diagnosis and surgical treatment of acute peri-prosthetic joint infection following primary total hip arthroplasty. EFORT Open Rev. 2018, 3, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Kozaily, E.; Chisari, E.; Parvizi, J. Is there a role for spacer exchange in two-stage exchange arthroplasty for periprosthetic joint infection? J. Clin. Med. 2020, 9, 2901. [Google Scholar] [CrossRef] [PubMed]
- Kini, S.G.; Gabr, A.; Das, R.; Sukeik, M.; Haddad, F.S. Two-stage revision for periprosthetic hip and knee joint infections. Open Orthop. J. 2016, 10, 579–588. [Google Scholar] [CrossRef]
- Sukeik, M.; Haddad, F.S. Two-stage procedure in the treatment of late chronic hip infections--spacer implantation. Int. J. Med. Sci. 2009, 6, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Barreira, P.; Leite, P.; Neves, P.; Soares, D.; Sousa, R. Preventing mechanical complications of hip spacer implantation: Technical tips and pearls. Acta Orthop. Belg. 2015, 81, 344–348. [Google Scholar]
- Erivan, R.; Lecointe, T.; Villatte, G.; Mulliez, A.; Descamps, S.; Boisgard, S. Complications with cement spacers in 2-stage treatment of periprosthetic joint infection on total hip replacement. Orthop. Traumatol. Surg. Res. 2017, 104, 333–339. [Google Scholar] [CrossRef]
- Jung, J.; Schmid, N.V.; Kelm, J.; Schmitt, E.; Anagnostakos, K. Complications after spacer implantation in the treatment of hip joint infections. Int. J. Med. Sci. 2009, 6, 265–273. [Google Scholar] [CrossRef]
- Patel, J.N.; Pizzo, R.A.; Yoon, R.S.; Liporace, F.A. Addressing antibiotic hip spacer instability via hybrid screw-cement fixation of a constrained liner and cement-rebar interface techniques: A technical narrative. J. Am. Acad. Orthop. Surg. 2020, 28, 166–170. [Google Scholar] [CrossRef]
- Pizzo, R.A.; Patel, J.N.; Viola, A.; Keller, D.M.; Yoon, R.S.; Liporace, F.A. Reducing Dislocations of Antibiotic Hip Spacers via Hybrid Cement-screw Constrained Liner Fixation: A Case Series. Hip Pelvis 2020, 32, 207–213. [Google Scholar] [CrossRef]
- Harrison, S.J.; Leeder, D.J.; McWilliams, T.G.; Metcalf, R.W.; Sidhom, S.A. Outcome of the Stryker® Trident ‘all-poly’ constraint acetabular insert: A district general hospital experience. HIP Int. 2015, 25, 557–562. [Google Scholar] [CrossRef]
- Telleria, J.J.; Gee, A.O. Classifications in brief: Paprosky classification of acetabular bone loss. Clin. Orthop. Relat. Res. 2013, 471, 3725–3730. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, J.P.; Shelley, P.; Wroblewski, B.M. The correlation between the roentgenographic appearance and operative findings at the bone-cement junction of the socket in Charnley low friction arthroplasties. Clin. Orthop. Relat. Res. 1988, 228, 105–109. [Google Scholar] [CrossRef]
- Vargas-Vila, M.A.; Siljander, M.P.; Scudday, T.S.; Patel, J.J.; Barnett, S.L.; Nassif, N.A. Retained functional antibiotic hip spacers have high rates of stem loosening, subsidence, and reoperation. J. Arthroplast. 2023, 38, S405–S411. [Google Scholar] [CrossRef] [PubMed]
- Su, E.P.; Pellicci, P.M. The role of constrained liners in total hip arthroplasty. Clin. Orthop. Relat. Res. 2004, 420, 122–129. [Google Scholar] [CrossRef]
- Craig, A.; King, S.W.; van Duren, B.H.; Veysi, V.T.; Jain, S.; Palan, J. Articular spacers in two-stage revision arthroplasty for prosthetic joint infection of the hip and the knee. EFORT Open Rev. 2022, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Fleck, E.E.; Spangehl, M.J.; Rapuri, V.R.; Beauchamp, C.P. An articulating antibiotic spacer controls infection and improves pain and function in a degenerative septic hip. Clin. Orthop. Relat. Res. 2011, 469, 3055–3064. [Google Scholar] [CrossRef]
- Sigmund, I.K.; Winkler, T.; Önder, N.; Perka, C.; Renz, N.; Trampuz, A. Complications of resection arthroplasty in two-stage revision for the treatment of periprosthetic hip joint infection. J. Clin. Med. 2019, 8, 2224. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, T.; Arts, J.J.; Geurts, J. Antibiotic-loaded polymethylmethacrylate beads and spacers in treatment of orthopedic infections and the role of biofilm formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef]
- Steinicke, A.C.; Schwarze, J.; Gosheger, G.; Moellenbeck, B.; Ackmann, T.; Theil, C. Repeat two-stage exchange arthroplasty for recurrent periprosthetic hip or knee infection: What are the chances for success? Arch. Orthop. Trauma. Surg. 2023, 143, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Tsung, J.D.; Rohrsheim, J.A.; Whitehouse, S.L.; Wilson, M.J.; Howell, J.R. Management of periprosthetic joint infection after total hip arthroplasty using a custom made articulating spacer (CUMARS); the Exeter experience. J. Arthroplast. 2014, 29, 1813–1818. [Google Scholar] [CrossRef]
- Choi, H.R.; Freiberg, A.A.; Malchau, H.; Rubash, H.E.; Kwon, Y.M. The fate of unplanned retention of prosthetic articulating spacers for infected total hip and total knee arthroplasty. J. Arthroplast. 2014, 29, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Luk, M.H.; Ng, F.Y.; Fu, H.; Chan, P.K.; Yan, C.H.; Chiu, K.Y. Retention of prosthetic articulating spacer after infected hip arthroplasty as a semipermanent implant: A case report. J. Orthop. Trauma Rehabil. 2019, 26, 105–107. [Google Scholar] [CrossRef]
- Pignatti, G.; Nitta, S.; Rani, N.; Dallari, D.; Sabbioni, G.; Stagni, C.; Giunti, A. Two stage hip revision in periprosthetic infection: Results of 41 cases. Open Orthop. J. 2010, 4, 193–200. [Google Scholar] [CrossRef]
- Palmer, C.K.; Gooberman-Hill, R.; Blom, A.W.; Whitehouse, M.R.; Moore, A.J. Post-surgery and recovery experiences following one- and two-stage revision for prosthetic joint infection-A qualitative study of patients’ experiences. PLoS ONE 2020, 15, e0237047. [Google Scholar] [CrossRef]
- Zamora, T.; Garbuz, D.S.; Greidanus, N.V.; Masri, B.A. One and a half stage exchange arthroplasty for infected total knee arthroplasty. Orthop. Proc. 2020, 102, 5. [Google Scholar]
- Siddiqi, A.; Mahmoud, Y.; Forte, S.A.; Novack, T.A.; Nace, J. One and a half-stage revision with prosthetic articulating spacer for definitive management of knee periprosthetic joint infection. Arthroplast. Today 2022, 19, 100993. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).