In Vitro Impact of Fluconazole on Oral Microbial Communities, Bacterial Growth, and Biofilm Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. pH Assay
2.3. Viability Assay
2.4. Biomass
2.5. Real-Time PCR
2.6. Construction of Luciferase Reporters
2.7. Luciferase Reporter Assay
2.8. Planktonic Growth
| Strain or Primers | Description or Sequence a | Source or Reference b |
|---|---|---|
| Strains | ||
| CCUG31611 | Streptococcus mitis type strain | CCUG |
| UA159 | Streptococcus mutans wild-type reference strain | [32] |
| MI048 | CCUG31611, but pldh-luc; Spcr | [31] |
| SM120 | UA159, but but pldh-luc; Spcr | This study |
| Primer pairs | ||
| FP514 c forward | agctagcACCGCATCAATTGTTTCTCC | pFW5-luc |
| FP515 d reverse | aggatccCACCATCACCAACAAGGATG | |
| FP1067 forward | CCATGAAGTCGGAATCGCTAG | 16S rRNA |
| FP1068 reverse | GCTTGACGGGCGGTGT | |
| FP2387 forward | GATACATAGCCGACCTGAG | Streptococcus spp. New England Biolabs |
| FP2388 reverse | CCATTGCCGAAGATTCC | |
| FP2297 forward | GTACAGTTGCTTCAGGACGTATC | Streptococcus spp. [33] |
| FP2298 reverse | ACGTTCGATTTCATCACGTTG | |
| FP2341 forward | TCTCTTGTTGAAGAATTAGAACGC | Veillonella atypica [34] |
| FP2342 reverse | GTGTAACAAGGGAGTACGGACC | |
| FP2389 forward | CCGTGATGGGATGGAAACTGC | Veillonella dispar [34] |
| FP2390 reverse | CCTTCGCCACTGGTGTTCTTC | |
| FP2303 forward | CTCAAAACTAAACAAAGTTTC | Lactobacillus spp. [35] |
| FP2304 reverse | CTTGTACACACCGCCCGTCA | |
| FP2383 forward | CGGTCTGTTAAGCGTGTTGTG | Prevotella intermedia [36] |
| FP2384 reverse | CACCATGAATTCCGCATACG | |
| FP2381 forward | GGATTTATTGGGCGTAAAGC | Fusobacterium nucleatum [33] |
| FP2382 reverse | GGCATTCCTACAAATATCTACGAA |
2.9. S. mitis Biofilm Assay
2.10. Statistical Analyses
3. Results
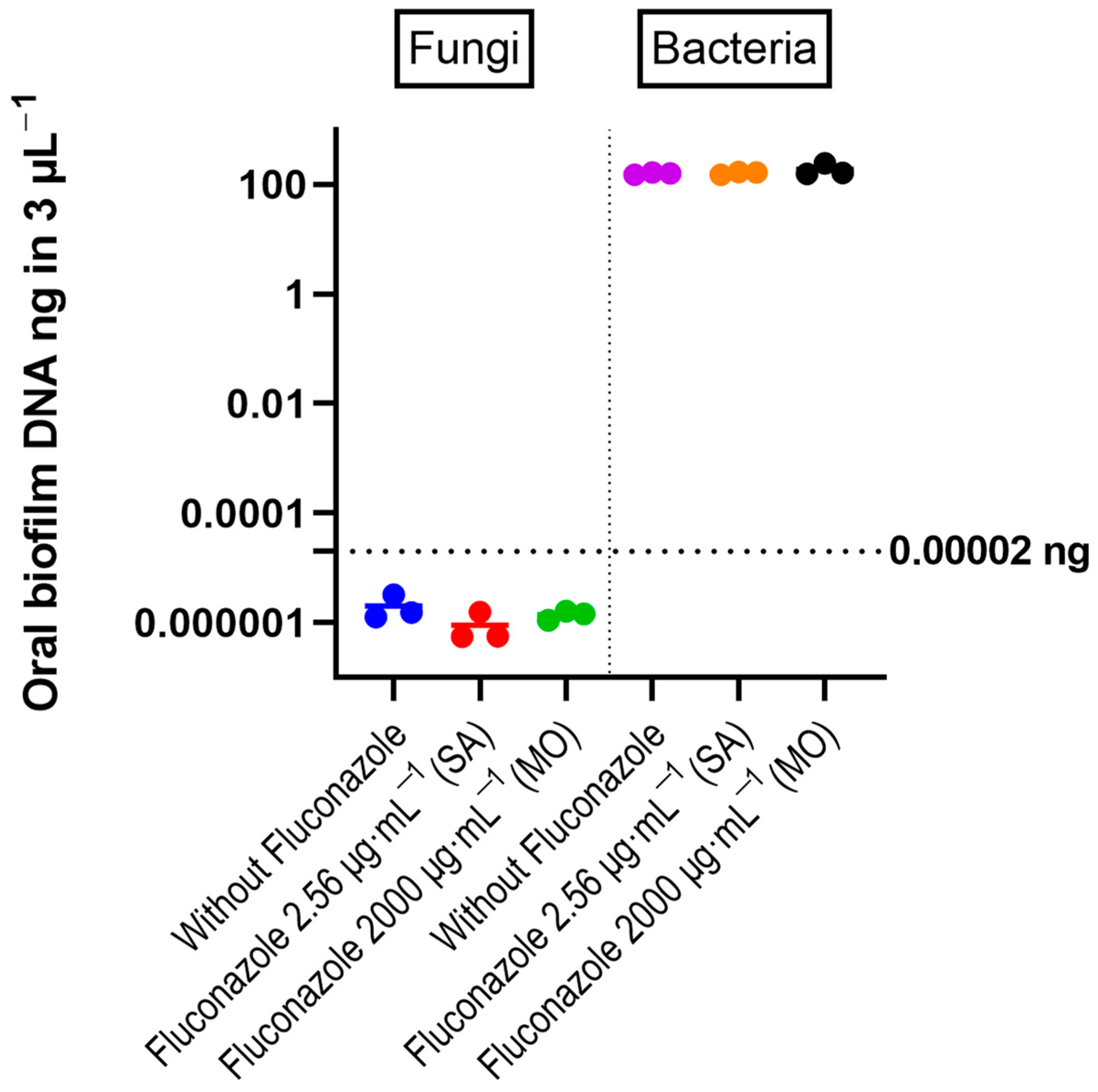
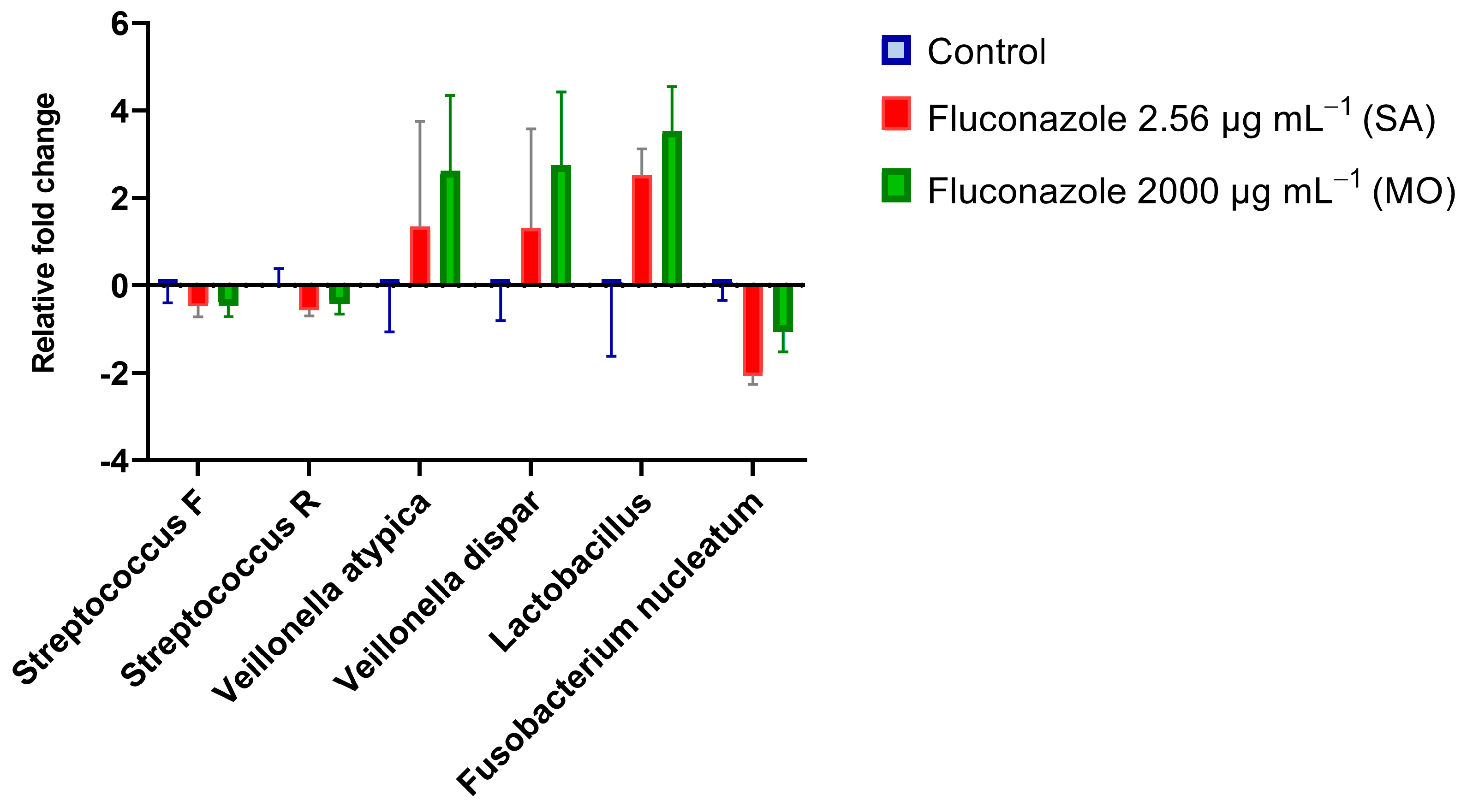
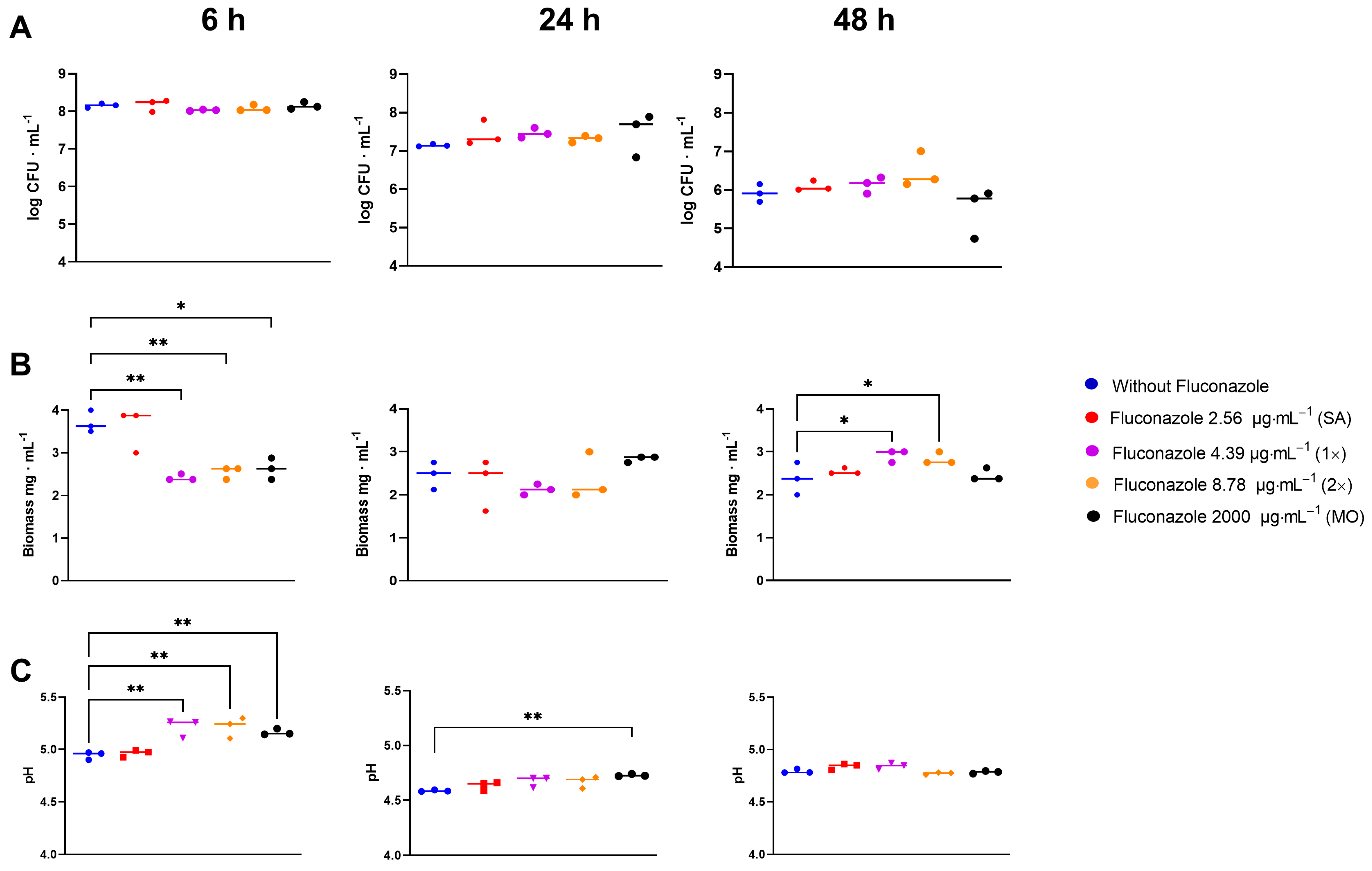
3.1. Oral Microbiota Biofilm
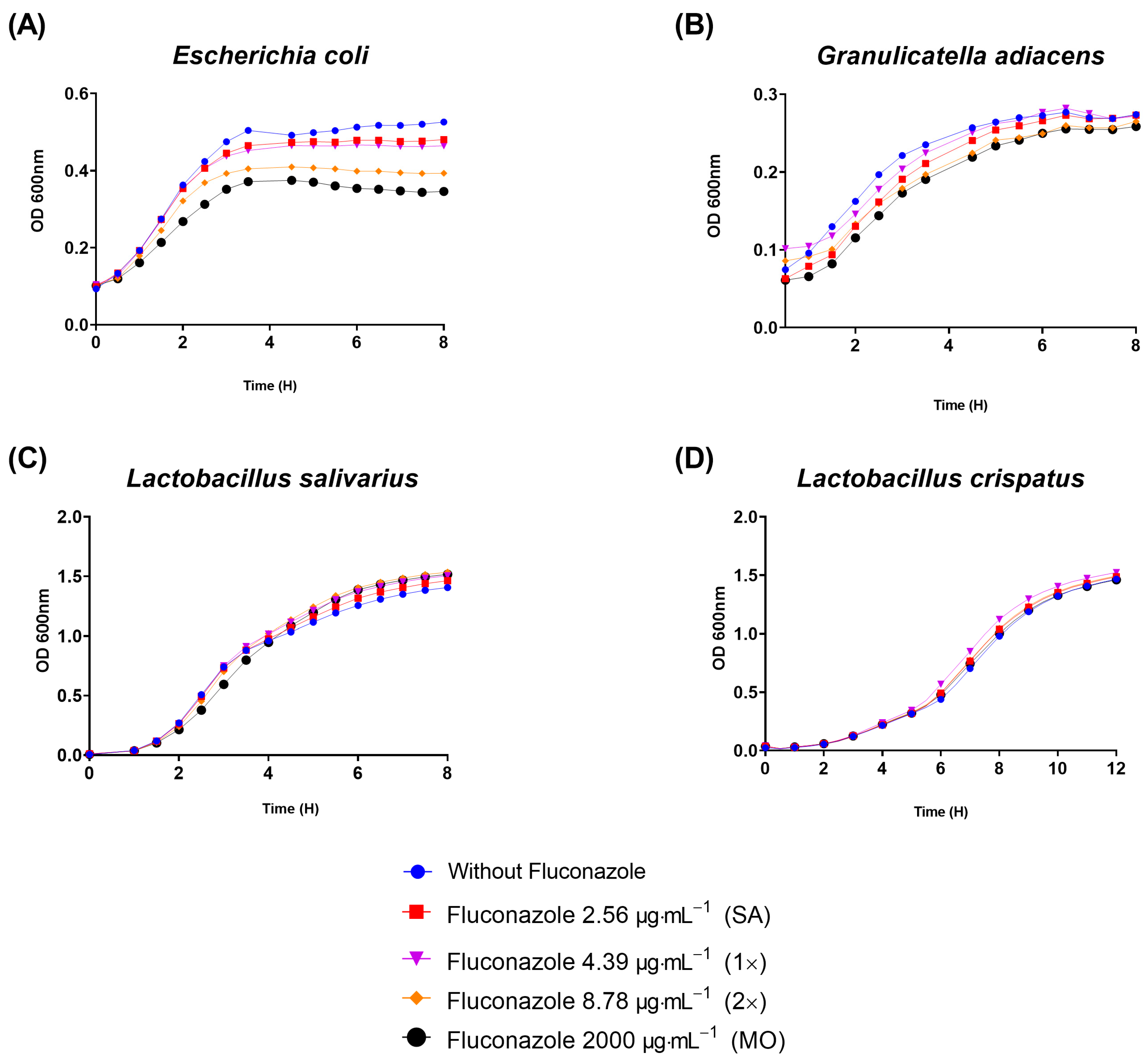
3.2. Fluconazole Effect on Bacterial Planktonic Growth
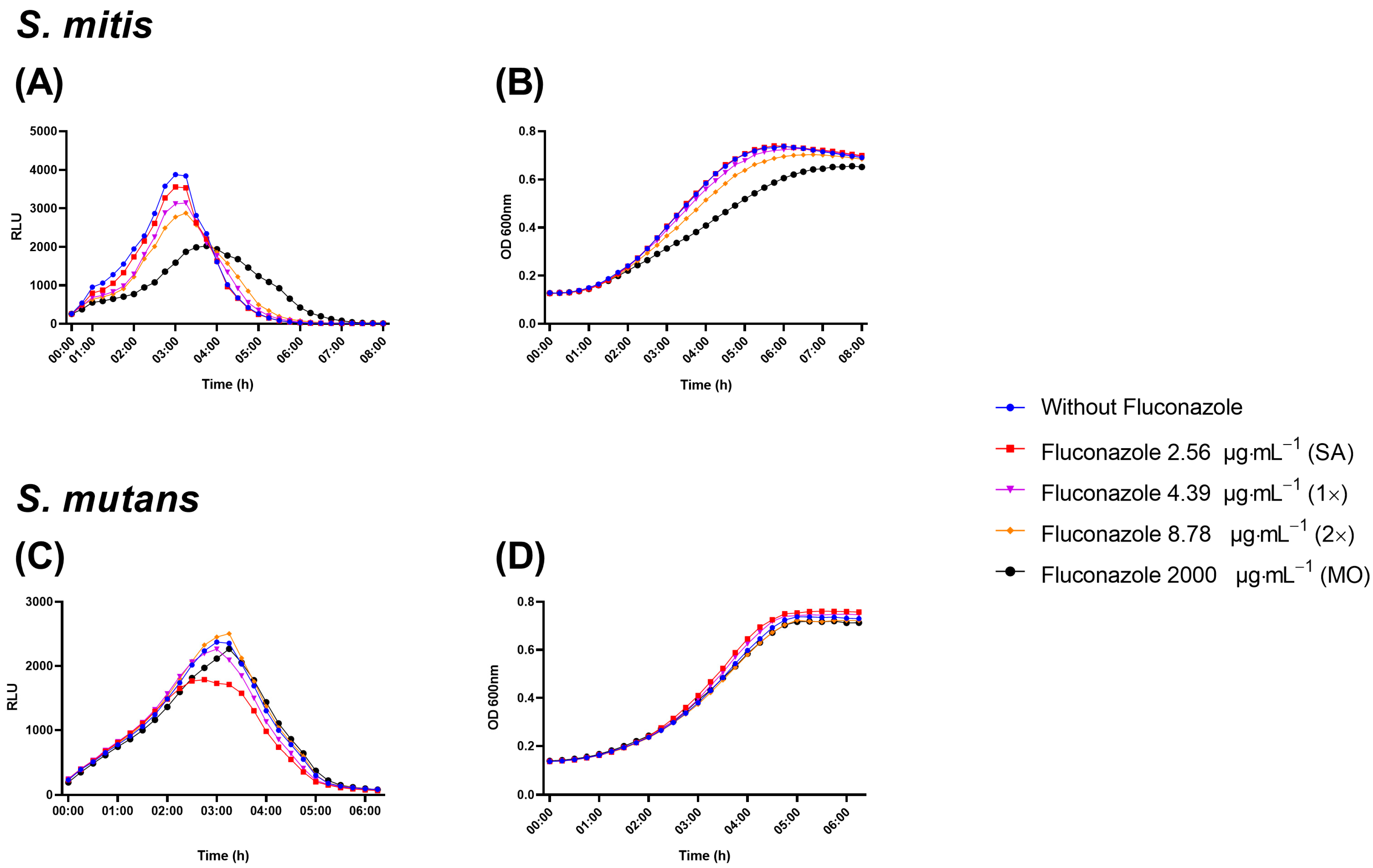
3.3. Effect of Fluconazole on S. mitis Biofilms
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parolo, C.C.F.; Arthur, R.A. Oral Microbial Biofilms. Monogr. Oral Sci. 2023, 31, 62–77. [Google Scholar] [PubMed]
- Cicchinelli, S.; Rosa, F.; Manca, F.; Zanza, C.; Ojetti, V.; Covino, M.; Candelli, M.; Gasbarrini, A.; Franceschi, F.; Piccioni, A. The Impact of Smoking on Microbiota: A Narrative Review. Biomedicines 2023, 11, 1144. [Google Scholar] [CrossRef]
- Kim, S.; Covington, A.; Pamer, E.G. The Intestinal Microbiota: Antibiotics, Colonization Resistance, and Enteric Pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef]
- Dawan, J.; Ahn, J. Bacterial Stress Responses as Potential Targets in Overcoming Antibiotic Resistance. Microorganisms 2022, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Berkow, E.L.; Lockhart, S.R. Infection and Drug Resistance Dovepress Fluconazole Resistance in Candida Species: A Current Perspective. Infect. Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Hamza, O.J.M.; Matee, M.I.N.; Brüggemann, R.J.M.; Moshi, M.J.; Simon, E.N.M.; Mugusi, F.; Mikx, F.H.M.; Van Der Lee, H.A.L.; Verweij, P.E.; Van Der Ven, A.J.A.M. Single-Dose Fluconazole versus Standard 2-Week Therapy for Oropharyngeal Candidiasis in HIV-Infected Patients: A Randomized, Double-Blind, Double-Dummy Trial. Clin. Infect. Dis. 2008, 47, 1270–1276. [Google Scholar] [CrossRef]
- Ng, T.B.; Cheung, R.C.F.; Ye, X.J.; Fang, E.F.; Chan, Y.S.; Pan, W.L.; Dan, X.L.; Yin, C.M.; Lam, S.K.; Lin, P.; et al. Pharmacotherapy Approaches to Antifungal Prophylaxis. Expert Opin. Pharmacother. 2012, 13, 1695–1705. [Google Scholar] [CrossRef]
- Ramírez-Carmona, W.; Fernandes, G.L.P.; Díaz-Fabregat, B.; Oliveira, E.C.; do Prado, R.L.; Pessan, J.P.; Monteiro, D.R. Effectiveness of Fluconazole as Antifungal Prophylaxis in Cancer Patients Undergoing Chemotherapy, Radiotherapy, or Immunotherapy: Systematic Review and Meta-Analysis. APMIS 2023, 1–17. [Google Scholar] [CrossRef]
- Hornik, C.D.; Bondi, D.S.; Greene, N.M.; Cober, M.P.; John, B. Review of Fluconazole Treatment and Prophylaxis for Invasive Candidiasis in Neonates. J. Pediatr. Pharmacol. Ther. 2021, 26, 115–122. [Google Scholar] [CrossRef]
- Rios, J.F.d.S.; Camargos, P.A.M.; Corrêa, L.P.; Romanelli, R.M.d.C. Fluconazole Prophylaxis in Preterm Infants: A Systematic Review. Braz. J. Infect. Dis. 2017, 21, 333–338. [Google Scholar] [CrossRef]
- Davis, M.R.; Nguyen, M.V.H.; Donnelley, M.A.; Thompson, G.R. Tolerability of Long-Term Fluconazole Therapy. J. Antimicrob. Chemother. 2019, 74, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Quindós, G.; Gil-Alonso, S.; Marcos-Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic Tools for Oral Candidiasis: Current and New Antifungal Drugs. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e172–e180. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Gorsky, M.; Caldwell, J. Fluconazole Mouthrinses for Oral Candidiasis in Postirradiation, Transplant, and Other Patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Koks, C.H.W.; Meenhorst, P.L.; Hillebrand, M.J.X.; Bult, A.; Beijnen, J.H. Pharmacokinetics of Fluconazole in Saliva and Plasma after Administration of an Oral Suspension and Capsules. Antimicrob. Agents Chemother. 1996, 40, 1935–1937. [Google Scholar] [CrossRef] [PubMed]
- Force, R.W.; Nahata, M.C. Salivary Concentrations of Ketoconazole and Fluconazole: Implications for Drug Efficacy in Oropharyngeal and Esophageal Candidiasis. Ann. Pharmacother. 1995, 29, 10–15. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 2016, 19, 865–873. [Google Scholar] [CrossRef]
- McLean, K.J.; Marshall, K.R.; Richmond, A.; Hunter, I.S.; Fowler, K.; Kieser, T.; Gurcha, S.S.; Besra, G.S.; Munro, A.W. Azole Antifungals Are Potent Inhibitors of Cytochrome P450 Mono-Oxygenases and Bacterial Growth in Mycobacteria and Streptomycetes. Microbiology 2002, 148, 2937–2949. [Google Scholar] [CrossRef]
- Dehner, C.; Fine, R.; Kriegel, M.A. The Microbiome in Systemic Autoimmune Disease: Mechanistic Insights from Recent Studies. Curr. Opin. Rheumatol. 2019, 31, 201–207. [Google Scholar] [CrossRef]
- Kleinstein, S.E.; Nelson, K.E.; Freire, M. Inflammatory Networks Linking Oral Microbiome with Systemic Health and Disease. J. Dent. Res. 2020, 99, 1131–1139. [Google Scholar] [CrossRef]
- Nikitakis, N.G.; Papaioannou, W.; Sakkas, L.I.; Kousvelari, E. The Autoimmunity-Oral Microbiome Connection. Oral Dis. 2017, 23, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Valdórsson, O.; Frimodt-Møller, N.; Hollingshead, S.; Kilian, M. Commensal Streptococci Serve as a Reservoir for β-Lactam Resistance Genes in Streptococcus Pneumoniae. Antimicrob. Agents Chemother. 2015, 59, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Morley, V.J.; Woods, R.J.; Read, A.F. Bystander Selection for Antimicrobial Resistance: Implications for Patient Health. Trends Microbiol. 2019, 27, 864–877. [Google Scholar] [CrossRef]
- Tedijanto, C.; Olesen, S.W.; Grad, Y.H.; Lipsitch, M. Estimating the Proportion of Bystander Selection for Antibiotic Resistance among Potentially Pathogenic Bacterial Flora. Proc. Natl. Acad. Sci. USA 2018, 115, E11988–E11995. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.; Yang, Y.; Hall, A.P.; Guo, L.; Lux, R.; He, X.; Nelson, K.E.; Nealson, K.H.; Yooseph, S.; Shi, W.; et al. An in Vitro Biofilm Model System Maintaining a Highly Reproducible Species and Metabolic Diversity Approaching That of the Human Oral Microbiome. Microbiome 2013, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.; Kreth, J.; Qi, F.; Sullivan, R.; Shi, W. Non-Disruptive, Real-Time Analyses of the Metabolic Status and Viability of Streptococcus Mutans Cells in Response to Antimicrobial Treatments. J. Microbiol. Methods 2005, 61, 161–170. [Google Scholar] [CrossRef]
- Podbielski, A.; Spellerberg, B.; Woischnik, M.; Pohl, B.; Lütticken, R. Novel Series of Plasmid Vectors for Gene Inactivation and Expression Analysis in Group A Streptococci (GAS). Gene 1996, 177, 137–147. [Google Scholar] [CrossRef]
- Khan, R.; Rukke, H.V.; Ricomini Filho, A.P.; Fimland, G.; Arntzen, M.Ø.; Thiede, B.; Petersen, F.C. Extracellular Identification of a Processed Type II ComR/ComS Pheromone of Streptococcus Mutans. J. Bacteriol. 2012, 194, 3781–3788. [Google Scholar] [CrossRef]
- Salvadori, G.; Junges, R.; Khan, R.; Åmdal, H.A.; Morrison, D.A.; Petersen, F.C. Natural Transformation of Oral Streptococci by Use of Synthetic Pheromones. Methods Mol. Biol. 2017, 1537, 219–232. [Google Scholar]
- Junges, R.; Sturød, K.; Salvadori, G.; Åmdal, H.A.; Chen, T.; Petersen, F.C. Characterization of a Signaling System in Streptococcus Mitis That Mediates Interspecies Communication with Streptococcus Pneumoniae. Appl. Environ. Microbiol. 2019, 85, e02297-18. [Google Scholar] [CrossRef] [PubMed]
- Ajdić, D.; McShan, W.M.; McLaughlin, R.E.; Savić, G.; Chang, J.; Carson, M.B.; Primeaux, C.; Tian, R.; Kenton, S.; Jia, H.; et al. Genome Sequence of Streptococcus Mutans UA159, a Cariogenic Dental Pathogen. Proc. Natl. Acad. Sci. USA 2002, 99, 14434–14439. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Millhouse, E.; Lappin, D.F.; Murray, C.; Culshaw, S.; Nile, C.J.; Ramage, G. Investigating the Biological Properties of Carbohydrate Derived Fulvic Acid (CHD-FA) as a Potential Novel Therapy for the Management of Oral Biofilm Infections. BMC Oral Health 2013, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Kolenbrander, P.E. Mutualistic Biofilm Communities Develop with Porphyromonas Gingivalis and Initial, Early, and Late Colonizers of Enamel. J. Bacteriol. 2009, 191, 6804–6811. [Google Scholar] [CrossRef]
- Dubernet, S.; Desmasures, N.; Guéguen, M. A PCR-Based Method for Identification of Lactobacilli at the Genus Level. FEMS Microbiol. Lett. 2002, 214, 271–275. [Google Scholar] [CrossRef]
- Loozen, G.; Boon, N.; Pauwels, M.; Quirynen, M.; Teughels, W. Live/Dead Real-Time Polymerase Chain Reaction to Assess New Therapies against Dental Plaque-Related Pathologies. Mol. Oral Microbiol. 2011, 26, 253–261. [Google Scholar] [CrossRef]
- Kraneveld, E.A.; Buijs, M.J.; Bonder, M.J.; Visser, M.; Keijser, B.J.F.; Crielaard, W.; Zaura, E. The Relation between Oral Candida Load and Bacterial Microbiome Profiles in Dutch Older Adults. PLoS ONE 2012, 7, e42770. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic Relationship between Streptococcus Mutans and Candida Albicans Synergizes Virulence of Plaque Biofilms In Vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Petersen, F.C.; Scheie, A.A. AI-2/LuxS Is Involved in Increased Biofilm Formation by Streptococcus Intermedius in the Presence of Antibiotics. Antimicrob. Agents Chemother. 2009, 53, 4258–4263. [Google Scholar] [CrossRef]
- Penesyan, A.; Paulsen, I.T.; Gillings, M.R.; Kjelleberg, S.; Manefield, M.J. Secondary Effects of Antibiotics on Microbial Biofilms. Front. Microbiol. 2020, 11, 2109. [Google Scholar] [CrossRef]
- Kean, R.; Delaney, C.; Rajendran, R.; Sherry, L.; Metcalfe, R.; Thomas, R.; McLean, W.; Williams, C.; Ramage, G. Gaining Insights from Candida Biofilm Heterogeneity: One Size Does Not Fit All. J. Fungi 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Khmelevtsova, L.E.; Sazykin, I.S.; Sazykina, M.A.; Seliverstova, E.Y. Prokaryotic Cytochromes P450 (Review). Appl. Biochem. Microbiol. 2017, 53, 401–409. [Google Scholar] [CrossRef]
- Padayachee, T.; Nzuza, N.; Chen, W.; Nelson, D.R.; Syed, K. Impact of Lifestyle on Cytochrome P450 Monooxygenase Repertoire Is Clearly Evident in the Bacterial Phylum Firmicutes. Sci. Rep. 2020, 10, 13982. [Google Scholar] [CrossRef] [PubMed]
- Garipov, M.R.; Pavelyev, R.S.; Lisovskaya, S.A.; Nikitina, E.V.; Kayumov, A.R.; Sabirova, A.E.; Bondar, O.V.; Malanyeva, A.G.; Aimaletdinov, A.M.; Iksanova, A.G.; et al. Fluconazole-Pyridoxine Bis-Triazolium Compounds with Potent Activity against Pathogenic Bacteria and Fungi Including Their Biofilm-Embedded Forms. J. Chem. 2017, 2017, 4761650. [Google Scholar] [CrossRef]
- Debruyne, D.; Ryckelynck, J.P. Clinical Pharmacokinetics of Fluconazole. Clin. Pharmacokinet. 1993, 24, 10–27. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dornelas-Figueira, L.M.; Ricomini Filho, A.P.; Junges, R.; Åmdal, H.A.; Cury, A.A.D.B.; Petersen, F.C. In Vitro Impact of Fluconazole on Oral Microbial Communities, Bacterial Growth, and Biofilm Formation. Antibiotics 2023, 12, 1433. https://doi.org/10.3390/antibiotics12091433
Dornelas-Figueira LM, Ricomini Filho AP, Junges R, Åmdal HA, Cury AADB, Petersen FC. In Vitro Impact of Fluconazole on Oral Microbial Communities, Bacterial Growth, and Biofilm Formation. Antibiotics. 2023; 12(9):1433. https://doi.org/10.3390/antibiotics12091433
Chicago/Turabian StyleDornelas-Figueira, Louise Morais, Antônio Pedro Ricomini Filho, Roger Junges, Heidi Aarø Åmdal, Altair Antoninha Del Bel Cury, and Fernanda Cristina Petersen. 2023. "In Vitro Impact of Fluconazole on Oral Microbial Communities, Bacterial Growth, and Biofilm Formation" Antibiotics 12, no. 9: 1433. https://doi.org/10.3390/antibiotics12091433





