Antimicrobial Resistance: Is There a ‘Light’ at the End of the Tunnel?
Abstract
:1. Antimicrobial Resistance: How Do Microbes Evade Antimicrobial Agents?
1.1. Intrinsic Resistance
1.2. Phenotypic Strategies That Induce Antimicrobial Resistance
1.3. Acquired Resistance
1.3.1. Single Nucleotide Polymorphisms Drives of Resistance through Selective Pressure
1.3.2. Expression of Enzymes That Modify or Hydrolyze Antibiotics
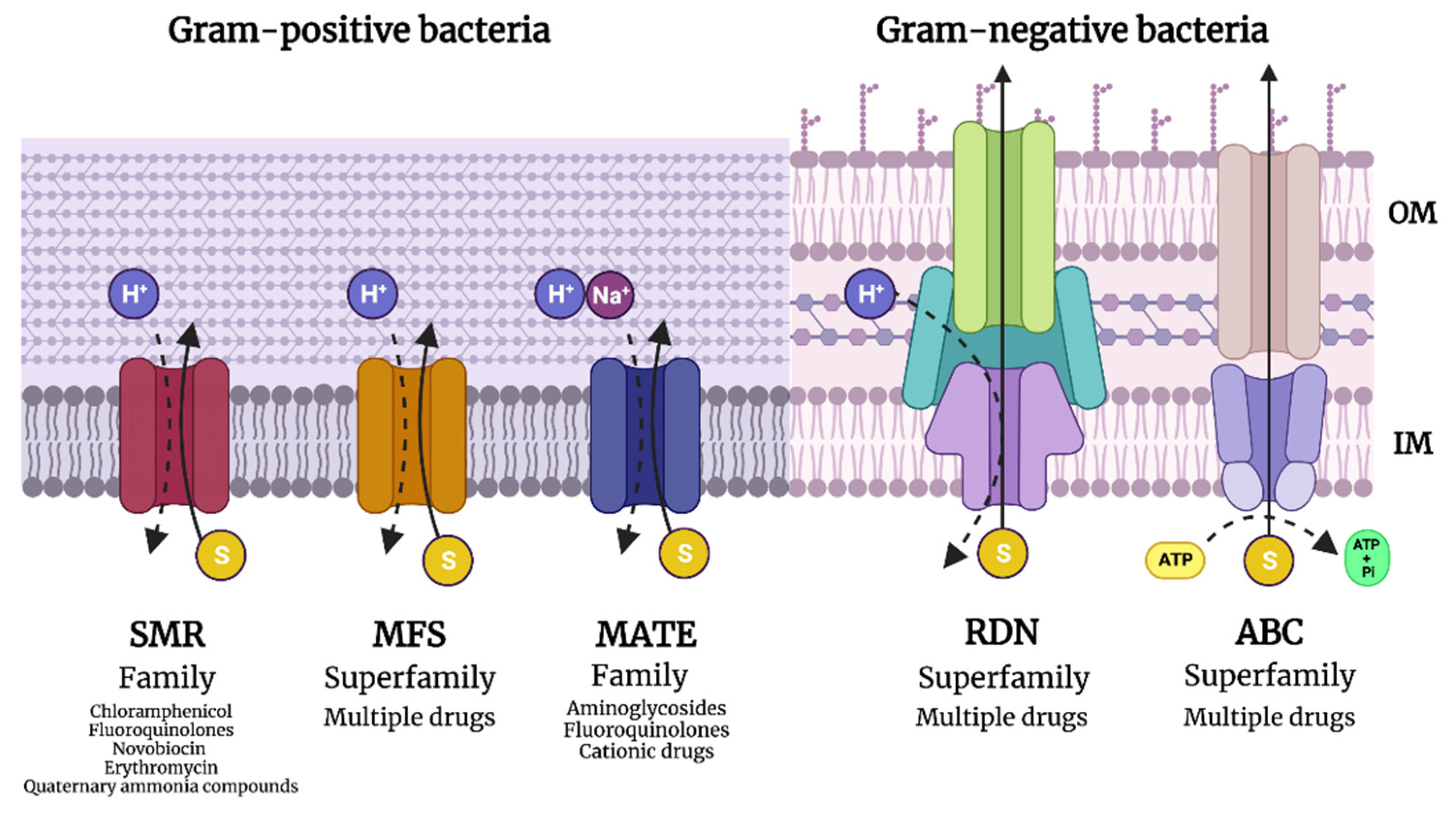
1.3.3. Efflux Pump Expression to Eject Antibiotics
2. Light-Based Anti-Infective Agents: Can They Overcome Antimicrobial Resistance?
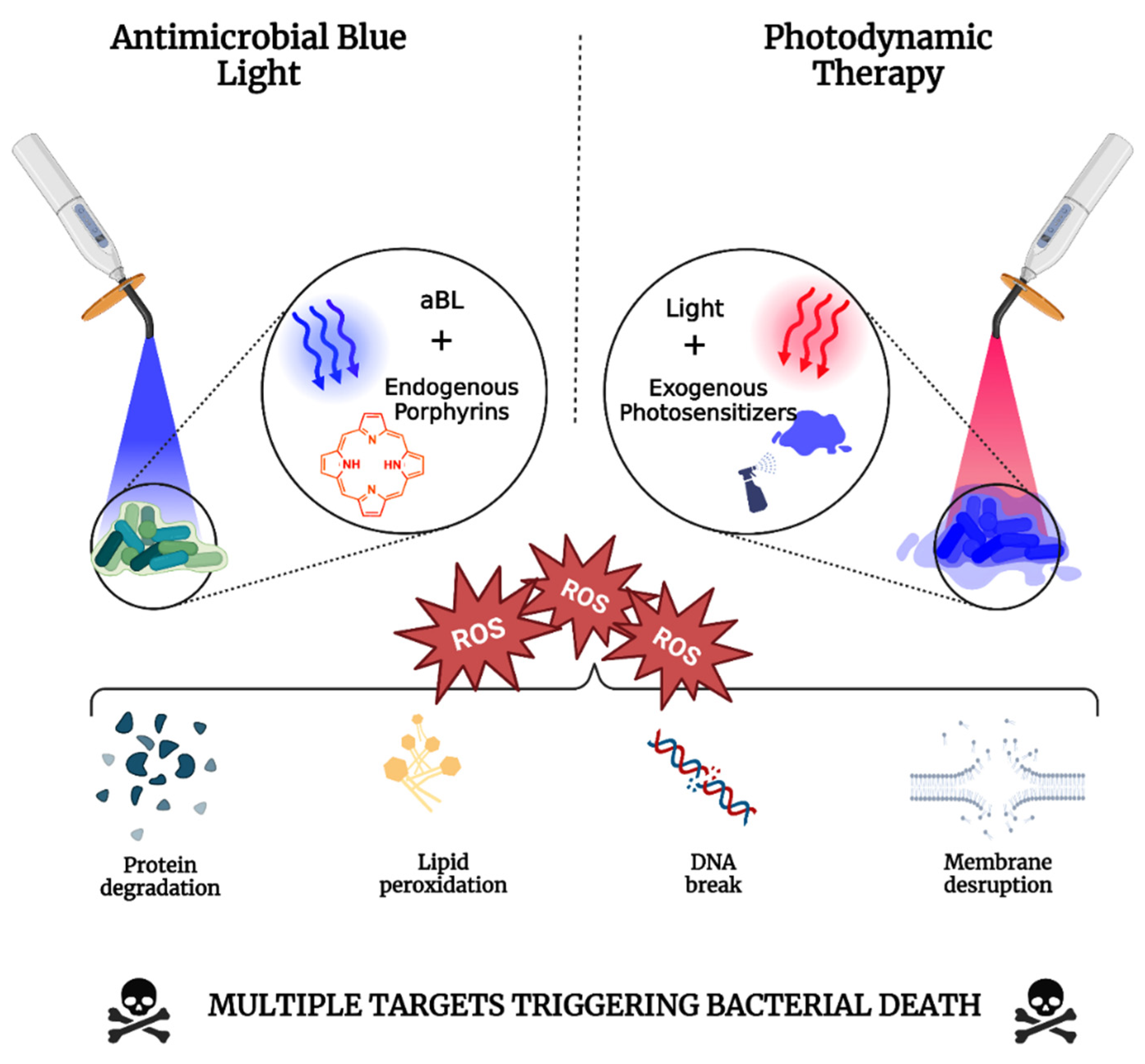
2.1. Antimicrobial Photodynamic Therapy
2.1.1. Mechanisms of Photodynamic Therapy
2.1.2. Photodynamic Inactivation of Microbes: A Function of Wavelength and Photosensitizer
Blue Light-Mediated Antimicrobial Photodynamic Therapy
Green Light-Mediated Antimicrobial Photodynamic Therapy
Red Light-Mediated Antimicrobial Photodynamic Therapy
Near-Infrared-Mediated Antimicrobial Photodynamic Therapy
2.2. Antimicrobial Blue Light
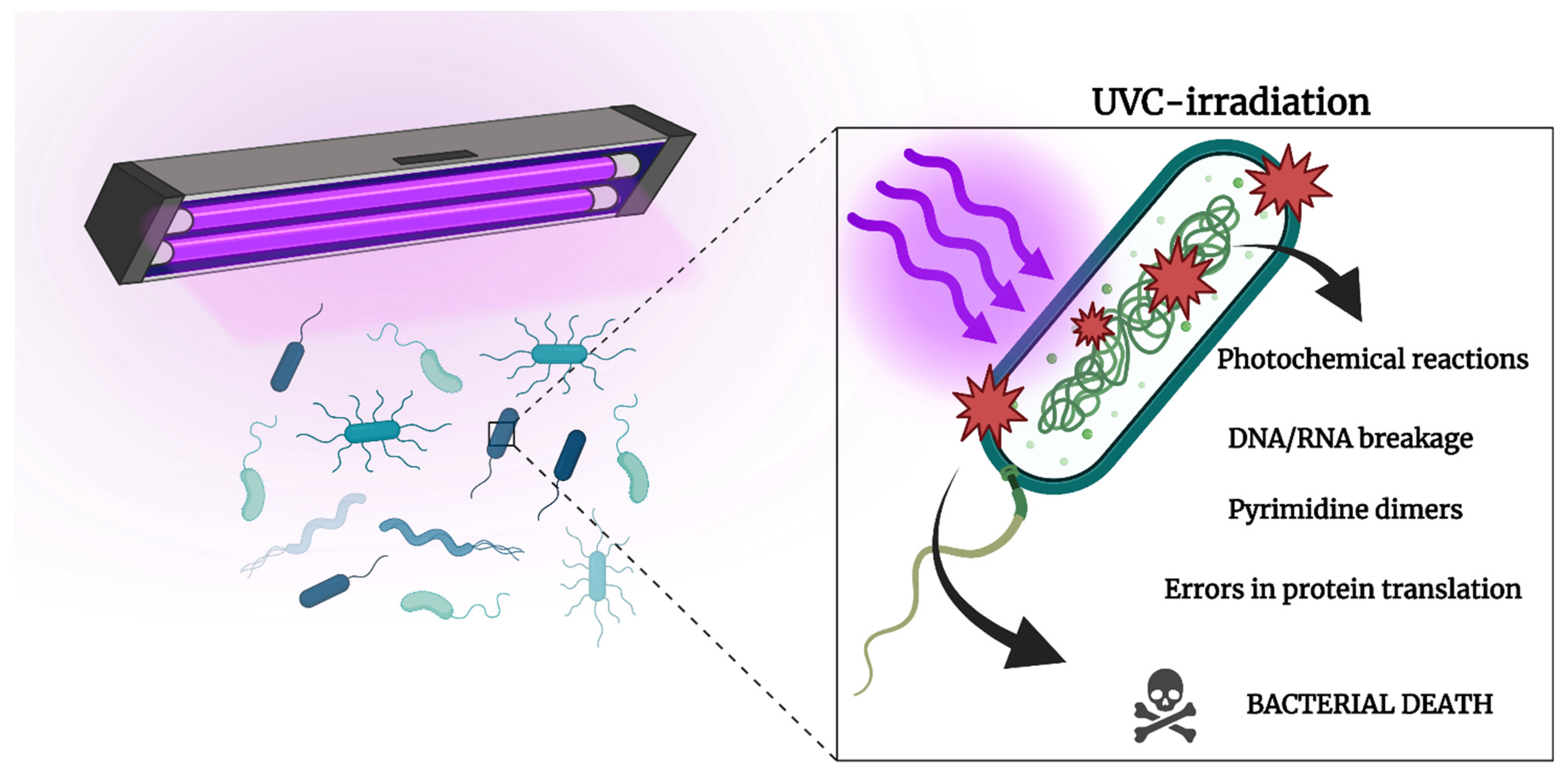
2.3. Ultraviolet Light
2.4. Can Light Fight against Antimicrobial Resistance?
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Langford, B.J.; Soucy, J.R.; Leung, V.; So, M.; Kwan, A.T.H.; Portnoff, J.S.; Bertagnolio, S.; Raybardhan, S.; MacFadden, D.R.; Daneman, N. Antibiotic resistance associated with the COVID-19 pandemic: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Nauta, K.M.; Ho, T.D.; Ellermeier, C.D. The Penicillin-Binding Protein PbpP Is a Sensor of β-Lactams and Is Required for Activation of the Extracytoplasmic Function σ Factor σP in Bacillus thuringiensis. mBio 2021, 12, e00179-21. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Molano, J.; Bowers, B.; Cabib, E. Distribution of chitin in the yeast cell wall. An ultrastructural and chemical study. J. Cell Biol. 1980, 85, 199–212. [Google Scholar] [CrossRef]
- Walker, S.S.; Black, T.A. Are outer-membrane targets the solution for MDR Gram-negative bacteria? Drug Discov. Today 2021, 26, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Coico, R. Gram staining. Curr. Protoc. Microbiol. 2005, 00, A.3C.1–A.3C.2. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better tests, better care: Improved diagnostics for infectious diseases. Clin. Infect. Dis. 2013, 3 (Suppl. S3), S139–S170. [Google Scholar] [CrossRef]
- Love, A.C.; Wagner, G.P. Co-option of stress mechanisms in the origin of evolutionary novelties. Evolution 2022, 76, 394–413. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Gilbert, P.; McBain, A.J. Biofilms: Their impact on health and their recalcitrance toward biocides. Am. J. Infect. Control. 2001, 29, 252–255. [Google Scholar] [CrossRef]
- Potera, C. Antibiotic Resistance: Biofilm Dispersing Agent Rejuvenates Older Antibiotics. Environ. Health Perspect. 2010, 118, A288. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004, 230, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Cloeckaert, A.; Zygmunt, M.S.; Doublet, B. Editorial: Genetics of Acquired Antimicrobial Resistance in Animal and Zoonotic Pathogens. Front. Microbiol. 2017, 8, 2428. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.J.; Munck, C.; Ellabaan, M.M.H.; Sommer, M.O.A. Adaptive Laboratory Evolution of Antibiotic Resistance Using Different Selection Regimes Lead to Similar Phenotypes and Genotypes. Front. Microbiol. 2017, 8, 816. [Google Scholar] [CrossRef]
- Revitt-Mills, S.A.; Robinson, A. Antibiotic-Induced Mutagenesis: Under the Microscope. Front. Microbiol. 2020, 11, 585175. [Google Scholar] [CrossRef]
- Martinez, J.L.; Baquero, F. Mutation frequencies and antibiotic resistance. Antimicrob. Agents Chemother. 2000, 44, 1771–1777. [Google Scholar] [CrossRef]
- Kolár, M.; Urbánek, K.; Látal, T. Antibiotic selective pressure and development of bacterial resistance. Int. J. Antimicrob. Agents 2001, 17, 357–363. [Google Scholar] [CrossRef]
- Egorov, A.M.; Ulyashova, M.M.; Rubtsova, M.Y. Bacterial Enzymes and Antibiotic Resistance. Acta Nat. 2018, 10, 33–48. [Google Scholar] [CrossRef]
- Eiamphungporn, W.; Schaduangrat, N.; Malik, A.A.; Nantasenamat, C. Tackling the Antibiotic Resistance Caused by Class A β-Lactamases through the Use of β-Lactamase Inhibitory Protein. Int. J. Mol. Sci. 2018, 19, 2222. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol. Med. 2020, 26, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Beheshti, M.; Ardebili, A.; Beheshti, F.; Lari, A.R.; Siyadatpanah, A.; Pournajaf, A.; Gautam, D.; Dolma, K.G.; Nissapatorn, V. Tetracycline resistance mediated by tet efflux pumps in clinical isolates of Acinetobacter baumannii. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e88. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, C.; Gao, H.; Xu, C.; Dai, M.; Huang, L.; Hao, H.; Wang, X.; Cheng, G. Bacterial Multidrug Efflux Pumps at the Frontline of Antimicrobial Resistance: An Overview. Antibiotics 2022, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Martins, A.; Spengler, G.; Molnar, J. Efflux pumps of Gram-negative bacteria: What they do, how they do it, with what and how to deal with them. Front. Pharmacol. 2014, 4, 168. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, G.; Hryc, C.F.; Blaza, J.N.; Serysheva, I.I.; Schmid, M.F.; Chiu, W.; Luisi, B.F.; Du, D. An allosteric transport mechanism for the AcrAB-TolC multidrug efflux pump. Elife 2017, 6, e24905. [Google Scholar] [CrossRef]
- Pan, Y.P.; Xu, Y.H.; Wang, Z.X.; Fang, Y.P.; Shen, J.L. Overexpression of MexAB-OprM efflux pump in carbapenem-resistant Pseudomonas aeruginosa. Arch. Microbiol. 2016, 198, 565–571. [Google Scholar] [CrossRef]
- Roesler, H. Niels Ryberg Finsen’s Disease and His Self-instituted Treatment. Ann. Med. Hist. 1936, 8, 353–356. [Google Scholar]
- Göring, H.D. Zum 100. Todestag von Niels Ryberg Finsen [In memoriam: Niels Ryberg Finsen]. Hautarzt 2004, 55, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; Dos Anjos, C.; Mushtaq, S.; Dai, T. Antimicrobial blue light: A ‘Magic Bullet’ for the 21st century and beyond? Adv. Drug Deliv. Rev. 2022, 180, 114057. [Google Scholar] [CrossRef]
- Hamzavi, I.; Lui, H. Using light in dermatology: An update on lasers, ultraviolet phototherapy, and photodynamic therapy. Dermatol. Clin. 2005, 23, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent advances in photodynamic therapy for cancer and infectious diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1560. [Google Scholar] [CrossRef]
- Kolarikova, M.; Hosikova, B.; Dilenko, H.; Barton-Tomankova, K.; Valkova, L.; Bajgar, R.; Malina, L.; Kolarova, H. Photodynamic therapy: Innovative approaches for antibacterial and anticancer treatments. Med. Res. Rev. 2023, 43, 717–774. [Google Scholar] [CrossRef]
- Daniell, M.D.; Hill, J.S. A history of photodynamic therapy. Aust. N. Z. J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef]
- Shen, J.J.; Jemec, G.B.E.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic therapy treatment of superficial fungal infections: A systematic review. Photodiagn. Photodyn. Ther. 2020, 31, 101774. [Google Scholar] [CrossRef]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef]
- Warrier, A.; Mazumder, N.; Prabhu, S.; Satyamoorthy, K.; Murali, T.S. Photodynamic therapy to control microbial biofilms. Photodiagn. Photodyn. Ther. 2021, 33, 102090. [Google Scholar] [CrossRef]
- Marasini, S.; Leanse, L.G.; Dai, T. Can microorganisms develop resistance against light based anti-infective agents? Adv. Drug Deliv. Rev. 2021, 175, 113822. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front. Med. 2021, 8, 642609. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer-A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Oxygen-Independent Antimicrobial Photoinactivation: Type III Photochemical Mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Połaska, M.; Marszałek, K.; Skąpska, S. Photosensitizing Furocoumarins: Content in Plant Matrices and Kinetics of Supercritical Carbon Dioxide Extraction. Molecules 2020, 25, 3805. [Google Scholar] [CrossRef] [PubMed]
- Oginsky, E.L.; Green, G.S.; Griffith, D.G.; Fowlks, W.L. Lethal photosensitization of bacteria with 8-methoxypsoralen to long wavelength ultraviolet radiation. J. Bacteriol. 1959, 78, 821–833. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, C.; Xiong, H.; Shi, D. Photodynamic therapy, a promising treatment approach for cutaneous infectious granulomas. Photodiagn. Photodyn. Ther. 2022, 39, 102952. [Google Scholar] [CrossRef]
- Reinhard, A.; Sandborn, W.J.; Melhem, H.; Bolotine, L.; Chamaillard, M.; Peyrin-Biroulet, L. Photodynamic therapy as a new treatment modality for inflammatory and infectious conditions. Expert. Rev. Clin. Immunol. 2015, 11, 637–657. [Google Scholar] [CrossRef]
- de Paiva, A.C.M.; Ferreira, M.D.C.; da Fonseca, A.S. Photodynamic therapy for treatment of bacterial keratitis. Photodiagn. Photodyn. Ther. 2022, 37, 102717. [Google Scholar] [CrossRef] [PubMed]
- de Souza da Fonseca, A.; de Paoli, F.; Mencalha, A.L. Photodynamic therapy for treatment of infected burns. Photodiagn. Photodyn. Ther. 2022, 38, 102831. [Google Scholar] [CrossRef] [PubMed]
- Vital-Fujii, D.G.; Baptista, M.S. Progress in the photodynamic therapy treatment of Leishmaniasis. Braz. J. Med. Biol. Res. 2021, 54, e11570. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Fabbrocini, G.; Sanchez-Blanco, B.; López-Barcenas, A.; El-Samahy, M.; Juárez-Durán, E.R.; González-Cespón, J.L. New Applications of Photodynamic Therapy in the Management of Candidiasis. J. Fungi 2021, 7, 1025. [Google Scholar] [CrossRef]
- Baptista, M.S.; Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT) for the treatment of malaria, leishmaniasis and trypanosomiasis. Braz. J. Med. Biol. Res. 2011, 44, 1–10. [Google Scholar] [CrossRef]
- Yoo, J.O.; Ha, K.S. New insights into the mechanisms for photodynamic therapy-induced cancer cell death. Int. Rev. Cell Mol. Biol. 2012, 295, 139–174. [Google Scholar] [CrossRef]
- Raiskup, F.; Terai, N.; Veliká, V.; Spörl, E. Hornhautvernetzung mit Riboflavin und UV-A-Licht bei Keratokonus [Corneal Cross-Linking with Riboflavin and UVA in Keratoconus]. Klin. Monbl Augenheilkd. 2016, 233, 938–944. (In German) [Google Scholar] [CrossRef]
- O’Brart, D.P. Riboflavin for corneal cross-linking. Drugs Today 2016, 52, 331–346. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef]
- Etemadi, A.; Hamidain, M.; Parker, S.; Chiniforush, N. Blue Light Photodynamic Therapy With Curcumin and Riboflavin in the Management of Periodontitis: A Systematic Review. J. Lasers Med. Sci. 2021, 12, e15. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Shao, C.; Xie, J. Chlorophyllin-Based 405 nm Light Photodynamic Improved Fresh-Cut Pakchoi Quality at Postharvest and Inhibited the Formation of Biofilm. Foods 2022, 11, 2541. [Google Scholar] [CrossRef]
- Luke-Marshall, N.R.; Hansen, L.A.; Shafirstein, G.; Campagnari, A.A. Antimicrobial Photodynamic Therapy with Chlorin e6 Is Bactericidal against Biofilms of the Primary Human Otopathogens. mSphere 2020, 5, e00492-20. [Google Scholar] [CrossRef]
- Bumah, V.V.; Morrow, B.N.; Cortez, P.M.; Bowman, C.R.; Rojas, P.; Masson-Meyers, D.S.; Suprapto, J.; Tong, W.G.; Enwemeka, C.S. The importance of porphyrins in blue light suppression of Streptococcus agalactiae. J. Photochem. Photobiol. B 2020, 212, 111996. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.X. Visualization and elimination of polymicrobial biofilms by a combination of ALA-carvacrol-blue light. J. Photochem. Photobiol. B 2022, 234, 112525. [Google Scholar] [CrossRef]
- Barbaric, J.; Abbott, R.; Posadzki, P.; Car, M.; Gunn, L.H.; Layton, A.M.; Majeed, A.; Car, J. Light therapies for acne. Cochrane Database Syst. Rev. 2016, 9, CD007917. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, J.; Qi, Y.; Yu, W.; Yang, S.; Zhang, J.; Zhao, J. Curcumin: A review of experimental studies and mechanisms related to periodontitis treatment. J. Periodontal Res. 2021, 56, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.C.; Fontana, C.R.; Gerbi, M.E.; Bagnato, V.S. Overall-mouth disinfection by photodynamic therapy using curcumin. Photomed. Laser Surg. 2012, 30, 96–101. [Google Scholar] [CrossRef]
- Leite, D.P.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: A randomized controlled trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef] [PubMed]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Soares, A.B.; Mima, E.G.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Carrera, E.T.; Dias, H.B.; Corbi, S.C.T.; Marcantonio, R.A.C.; Bernardi, A.C.A.; Bagnato, V.S.; Hamblin, M.R.; Rastelli, A.N.S. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: A critical review. Laser Phys. 2016, 26, 123001. [Google Scholar] [CrossRef]
- Hung, J.-H.; Lee, C.-N.; Hsu, H.-W.; Ng, I.-S.; Wu, C.-J.; Yu, C.-K.; Lee, N.-Y.; Chang, Y.; Wong, T.-W. Recent Advances in Photodynamic Therapy against Fungal Keratitis. Pharmaceutics 2021, 13, 2011. [Google Scholar] [CrossRef]
- Naranjo, A.; Arboleda, A.; Martinez, J.D.; Durkee, H.; Aguilar, M.C.; Relhan, N.; Nikpoor, N.; Galor, A.; Dubovy, S.R.; Leblanc, R.; et al. Rose Bengal Photodynamic Antimicrobial Therapy for Patients with Progressive Infectious Keratitis: A Pilot Clinical Study. Am. J. Ophthalmol. 2019, 208, 387–396. [Google Scholar] [CrossRef]
- Kamegaya, Y.; Farinelli, W.A.; Vila Echague, A.V.; Akita, H.; Gallagher, J.; Flotte, T.J.; Anderson, R.R.; Redmond, R.W.; Kochevar, I.E. Evaluation of photochemical tissue bonding for closure of skin incisions and excisions. Lasers Surg. Med. 2005, 37, 264–270. [Google Scholar] [CrossRef]
- Halili, F.; Arboleda, A.; Durkee, H.; Taneja, M.; Miller, D.; Alawa, K.A.; Aguilar, M.C.; Amescua, G.; Flynn, H.W., Jr.; Parel, J.-M. Rose bengal–and riboflavin-mediated photodynamic therapy to inhibit methicillin-resistant Staphylococcus aureus keratitis isolates. Am. J. Ophthalmol. 2016, 166, 194–202. [Google Scholar] [CrossRef]
- Germann, J.A.; Martínez-Enríquez, E.; Martínez-García, M.C.; Kochevar, I.E.; Marcos, S. Corneal Collagen Ordering After In Vivo Rose Bengal and Riboflavin Cross-Linking. Investig. Ophthalmol. Vis. Sci. 2020, 61, 28. [Google Scholar] [CrossRef]
- Fila, G.; Kasimova, K.; Arenas, Y.; Nakonieczna, J.; Grinholc, M.; Bielawski, K.P.; Lilge, L. Murine Model Imitating Chronic Wound Infections for Evaluation of Antimicrobial Photodynamic Therapy Efficacy. Front. Microbiol. 2016, 7, 1258. [Google Scholar] [CrossRef]
- Maytin, E.V.; Kaw, U.; Ilyas, M.; Mack, J.A.; Hu, B. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: A bilaterally controlled comparison study. Photodiagn. Photodyn. Ther. 2018, 22, 7–13. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Salomatina, E.V.; Yaroslavsky, A.N.; Herman, I.M.; Hamblin, M.R. Low-level light stimulates excisional wound healing in mice. Lasers Surg. Med. 2007, 39, 706–715. [Google Scholar] [CrossRef]
- Monteiro, F.; Carvalho, Ó.; Sousa, N.; Silva, F.S.; Sotiropoulos, I. Photobiomodulation and visual stimulation against cognitive decline and Alzheimer’s disease pathology: A systematic review. Alzheimers Dement 2022, 8, e12249. [Google Scholar] [CrossRef]
- Hennessy, M.; Hamblin, M.R. Photobiomodulation and the brain: A new paradigm. J. Opt. 2017, 19, 013003. [Google Scholar] [CrossRef] [PubMed]
- Songsantiphap, C.; Vanichanan, J.; Chatsuwan, T.; Asawanonda, P.; Boontaveeyuwat, E. Methylene Blue-Mediated Antimicrobial Photodynamic Therapy Against Clinical Isolates of Extensively Drug Resistant Gram-Negative Bacteria Causing Nosocomial Infections in Thailand, An In Vitro Study. Front. Cell Infect. Microbiol. 2022, 12, 929242. [Google Scholar] [CrossRef]
- Cefalu, J.N.; Joshi, T.V.; Spalitta, M.J.; Kadi, C.J.; Diaz, J.H.; Eskander, J.P.; Cornett, E.M.; Kaye, A.D. Methemoglobinemia in the Operating Room and Intensive Care Unit: Early Recognition, Pathophysiology, and Management. Adv. Ther. 2020, 37, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Shentu, Y.; Zhang, L.; Gu, H.; Mao, F.; Cai, M.; Ding, Z.; Wang, Z. A new technique combining virtual simulation and methylene blue staining for the localization of small peripheral pulmonary lesions. BMC Cancer 2014, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.; Lu, G.; Jahn, A.; Mockenhaupt, F.P. How worthwhile is methylene blue as a treatment of malaria? Expert. Rev. Anti Infect. Ther. 2019, 17, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Chen, C.J.; Ding, S.J.; Chen, C.C. Antimicrobial efficacy of methylene blue-mediated photodynamic therapy on titanium alloy surfaces in vitro. Photodiagn. Photodyn. Ther. 2019, 25, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Sabino, C.P.; Wainwright, M.; Dos Anjos, C.; Sellera, F.P.; Baptista, M.S.; Lincopan, N.; Ribeiro, M.S. Inactivation kinetics and lethal dose analysis of antimicrobial blue light and photodynamic therapy. Photodiagn. Photodyn. Ther. 2019, 28, 186–191. [Google Scholar] [CrossRef]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Rezusta, A.; Gilaberte, Y. Photodynamic therapy using methylene blue, combined or not with gentamicin, against Staphylococcus aureus and Pseudomonas aeruginosa. Photodiagn. Photodyn. Ther. 2020, 31, 101810. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent. Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Dai, T.; Bil de Arce, V.J.; Tegos, G.P.; Hamblin, M.R. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob. Agents Chemother. 2011, 55, 5710–5717. [Google Scholar] [CrossRef]
- Freire, F.; Ferraresi, C.; Jorge, A.O.; Hamblin, M.R. Photodynamic therapy of oral Candida infection in a mouse model. J. Photochem. Photobiol. B 2016, 159, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ragàs, X.; Dai, T.; Tegos, G.P.; Agut, M.; Nonell, S.; Hamblin, M.R. Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: In vitro and in vivo studies. Lasers Surg. Med. 2010, 42, 384–390. [Google Scholar] [CrossRef]
- Soundarajan, S.; Rajasekar, A. Comparative evaluation of combined efficacy of methylene blue mediated antimicrobial photodynamic therapy (a-PDT) using 660 nm diode laser versus Erbium-chromium-yttrium-scandium-gallium-garnet (Er, Cr: YSGG) laser as an adjunct to scaling and root planing on clinical parameters in supportive periodontal therapy: A randomized split-mouth trial. Photodiagn. Photodyn. Ther. 2022, 39, 102971. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Adami, F.; Correa, J.A.; Pinhal, M.A.; Baptista, M.S. A clinical trial testing the efficacy of PDT in preventing amputation in diabetic patients. Photodiagn. Photodyn. Ther. 2014, 11, 342–350. [Google Scholar] [CrossRef]
- Noro Filho, G.A.; Casarin, R.C.; Casati, M.Z.; Giovani, E.M. PDT in non-surgical treatment of periodontitis in HIV patients: A split-mouth, randomized clinical trial. Lasers Surg. Med. 2012, 44, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.P.; Carvalho, C.R.; Rezende, R.R.; Lima, C.J.; Santos, F.V.; Moreira, L.M. Synergism between fluconazole and methylene blue-photodynamic therapy against fluconazole-resistant Candida strains. Indian J. Med. Microbiol. 2016, 34, 506–508. [Google Scholar] [CrossRef]
- Entezari, S.; Moezzimoghadam, N.; Lawaf, S.; Azizi, A. In vitro Effect of Photodynamic Therapy with Curcumin and Methylene Blue Photosensitizers on Staphylococcus Aureus. J. Dent. 2022, 23 (Suppl. S2), 387–392. [Google Scholar] [CrossRef]
- Aspiroz, C.; Sevil, M.; Toyas, C.; Gilaberte, Y. Photodynamic Therapy With Methylene Blue for Skin Ulcers Infected With Pseudomonas aeruginosa and Fusarium spp. Actas Dermosifiliogr. 2017, 108, e45–e48, English, Spanish. [Google Scholar] [CrossRef]
- Sbeghen, M.R.; Voltarelli, E.M.; Campois, T.G.; Kimura, E.; Aristides, S.M.; Hernandes, L.; Caetano, W.; Hioka, N.; Lonardoni, M.V.; Silveira, T.G. Topical and Intradermal Efficacy of Photodynamic Therapy with Methylene Blue and Light-Emitting Diode in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis. J. Lasers Med. Sci. 2015, 6, 106–111. [Google Scholar] [CrossRef]
- Figueiredo-Godoi, L.M.A.; Garcia, M.T.; Pinto, J.G.; Ferreira-Strixino, J.; Faustino, E.G.; Pedroso, L.L.C.; Junqueira, J.C. Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii. Antibiotics 2022, 11, 619. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Baptista, M.S. Treatment of osteomyelitis in the feet of diabetic patients by photodynamic antimicrobial chemotherapy. Photomed. Laser Surg. 2009, 27, 145–150. [Google Scholar] [CrossRef]
- Boltes Cecatto, R.; Siqueira de Magalhães, L.; Fernanda Setúbal Destro Rodrigues, M.; Pavani, C.; Lino-Dos-Santos-Franco, A.; Teixeira Gomes, M.; Fátima Teixeira Silva, D. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: The state of the art. Photodiagn. Photodyn. Ther. 2020, 31, 101828. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.G.; Nicholls, P.; Cooper, C.E. Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: Implications for non invasive in vivo monitoring of tissues. Biochim. Biophys. Acta 2014, 1837, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, P.A.; Neustein, T.M.; Fang, R.C.; Payne, D.E. Indocyanine Green Angiography: A Helpful Tool for Intraoperative Assessment of Upper Extremity Perfusion. Tech. Hand Up. Extrem. Surg. 2017, 21, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Shirata, C.; Kaneko, J.; Inagaki, Y.; Kokudo, T.; Sato, M.; Kiritani, S.; Akamatsu, N.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; et al. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci. Rep. 2017, 7, 13958. [Google Scholar] [CrossRef]
- Topaloglu, N.; Güney, M.; Yuksel, S.; Gülsoy, M. Antibacterial photodynamic therapy with 808-nm laser and indocyanine green on abrasion wound models. J. Biomed. Opt. 2015, 20, 28003. [Google Scholar] [CrossRef]
- Wong, T.W.; Liao, S.Z.; Ko, W.C.; Wu, C.J.; Wu, S.B.; Chuang, Y.C.; Huang, I.H. Indocyanine Green-Mediated Photodynamic Therapy Reduces Methicillin-Resistant Staphylococcus aureus Drug Resistance. J. Clin. Med. 2019, 8, 411. [Google Scholar] [CrossRef]
- Kassab, G.; Cheburkanov, V.; Willis, J.; Moule, M.G.; Kurachi, C.; Yakovlev, V.; Cirillo, J.D.; Bagnato, V.S. Safety and delivery efficiency of a photodynamic treatment of the lungs using indocyanine green and extracorporeal near infrared illumination. J. Biophotonics 2020, 13, e202000176. [Google Scholar] [CrossRef]
- Geralde, M.C.; Leite, I.S.; Inada, N.M.; Salina, A.C.; Medeiros, A.I.; Kuebler, W.M.; Kurachi, C.; Bagnato, V.S. Pneumonia treatment by photodynamic therapy with extracorporeal illumination—An experimental model. Physiol. Rep. 2017, 5, e13190. [Google Scholar] [CrossRef]
- Liu, X.; Chang, Q.; Ferrer-Espada, R.; Leanse, L.G.; Goh, X.S.; Wang, X.; Gelfand, J.A.; Dai, T. Photoinactivation of Moraxella catarrhalis Using 405-nm Blue Light: Implications for the Treatment of Otitis Media. Photochem. Photobiol. 2020, 96, 611–617. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Bumah, V.V.; Castel, J.C.; Suess, S.L. Pulsed blue light, saliva and curcumin significantly inactivate human coronavirus. J. Photochem. Photobiol. B 2022, 227, 112378. [Google Scholar] [CrossRef]
- Haag, R.; Sieber, N.; Heßling, M. Cataract Development by Exposure to Ultraviolet and Blue Visible Light in Porcine Lenses. Medicina 2021, 57, 535. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Wang, X.; Wan, M.; Zhang, L.; Dai, Y.; Hai, Y.; Yue, C.; Xu, J.; Ding, Y.; Wang, M.; Xie, J.; et al. ALA_PDT Promotes Ferroptosis-Like Death of Mycobacterium abscessus and Antibiotic Sterilization via Oxidative Stress. Antioxidants 2022, 11, 546. [Google Scholar] [CrossRef]
- Oliver, I.T.; Rawlinson, W.A. The absorption spectra of porphyrin alpha and derivatives. Biochem. J. 1955, 61, 641–646. [Google Scholar] [CrossRef]
- Wang, Y.; Ferrer-Espada, R.; Baglo, Y.; Gu, Y.; Dai, T. Antimicrobial Blue Light Inactivation of Neisseria gonorrhoeae: Roles of Wavelength, Endogenous Photosensitizer, Oxygen, and Reactive Oxygen Species. Lasers Surg. Med. 2019, 51, 815–823. [Google Scholar] [CrossRef]
- Fila, G.; Krychowiak, M.; Rychlowski, M.; Bielawski, K.P.; Grinholc, M. Antimicrobial blue light photoinactivation of Pseudomonas aeruginosa: Quorum sensing signaling molecules, biofilm formation and pathogenicity. J. Biophotonics. 2018, 11, e201800079. [Google Scholar] [CrossRef]
- Dos Anjos, C.; Leanse, L.G.; Ribeiro, M.S.; Sellera, F.P.; Dropa, M.; Arana-Chavez, V.E.; Lincopan, N.; Baptista, M.S.; Pogliani, F.C.; Dai, T.; et al. New Insights into the Bacterial Targets of Antimicrobial Blue Light. Microbiol. Spectr. 2023, 11, e0283322. [Google Scholar] [CrossRef]
- Tsutsumi-Arai, C.; Arai, Y.; Terada-Ito, C.; Imamura, T.; Tatehara, S.; Ide, S.; Shirakawa, J.; Wakabayashi, N.; Satomura, K. Inhibitory effect of 405-nm blue LED light on the growth of Candida albicans and Streptococcus mutans dual-species biofilms on denture base resin. Lasers Med. Sci. 2022, 37, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Espada, R.; Liu, X.; Goh, X.S.; Dai, T. Antimicrobial Blue Light Inactivation of Polymicrobial Biofilms. Front. Microbiol. 2019, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Huang, Y.Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: Efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, C.; Leanse, L.G.; Liu, X.; Miranda, H.V.; Anderson, R.R.; Dai, T. Antimicrobial Blue Light for Prevention and Treatment of Highly Invasive Vibrio vulnificus Burn Infection in Mice. Front. Microbiol. 2022, 13, 932466. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; Goh, X.S.; Dai, T. Quinine Improves the Fungicidal Effects of Antimicrobial Blue Light: Implications for the Treatment of Cutaneous Candidiasis. Lasers Surg. Med. 2020, 52, 569–575. [Google Scholar] [CrossRef]
- Leanse, L.G.; Zeng, X.; Dai, T. Potentiated antimicrobial blue light killing of methicillin resistant Staphylococcus aureus by pyocyanin. J. Photochem. Photobiol. B 2021, 215, 112109. [Google Scholar] [CrossRef]
- Jusuf, S.; Cheng, J.X. Blue Light Improves Antimicrobial Efficiency of Silver Sulfadiazine Via Catalase Inactivation. Photobiomodul Photomed. Laser Surg. 2023, 41, 80–87. [Google Scholar] [CrossRef]
- Fila, G.; Kawiak, A.; Grinholc, M.S. Blue light treatment of Pseudomonas aeruginosa: Strong bactericidal activity, synergism with antibiotics and inactivation of virulence factors. Virulence 2017, 8, 938–958. [Google Scholar] [CrossRef]
- Leanse, L.G.; Harrington, O.D.; Fang, Y.; Ahmed, I.; Goh, X.S.; Dai, T. Evaluating the Potential for Resistance Development to Antimicrobial Blue Light (at 405 nm) in Gram-Negative Bacteria: In vitro and in vivo Studies. Front. Microbiol. 2018, 9, 2403. [Google Scholar] [CrossRef]
- Dong, P.T.; Mohammad, H.; Hui, J.; Leanse, L.G.; Li, J.; Liang, L.; Dai, T.; Seleem, M.N.; Cheng, J.X. Photolysis of Staphyloxanthin in Methicillin-Resistant Staphylococcus aureus Potentiates Killing by Reactive Oxygen Species. Adv. Sci. 2019, 6, 1900030. [Google Scholar] [CrossRef]
- Leanse, L.G.; Goh, X.S.; Cheng, J.X.; Hooper, D.C.; Dai, T. Dual-wavelength photo-killing of methicillin-resistant Staphylococcus aureus. JCI Insight 2020, 5, e134343. [Google Scholar] [CrossRef]
- Dong, P.T.; Zhan, Y.; Jusuf, S.; Hui, J.; Dagher, Z.; Mansour, M.K.; Cheng, J.X. Photoinactivation of Catalase Sensitizes Candida albicans and Candida auris to ROS-Producing Agents and Immune Cells. Adv. Sci. 2022, 9, e2104384. [Google Scholar] [CrossRef]
- Ceburkov, O.; Gollnick, H. Photodynamic therapy in dermatology. Eur. J. Dermatol. 2000, 10, 568–575. [Google Scholar]
- Thai, T.P.; Houghton, P.E.; Keast, D.H.; Campbell, K.E.; Woodbury, M.G. Ultraviolet light C in the treatment of chronic wounds with MRSA: A case study. Ostomy/Wound Manag. 2002, 48, 52–60. [Google Scholar]
- Marasini, S.; Zhang, A.C.; Dean, S.J.; Swift, S.; Craig, J.P. Safety and efficacy of UV application for superficial infections in humans: A systematic review and meta-analysis. Ocul. Surf. 2021, 21, 331–344. [Google Scholar] [CrossRef]
- Thai, T.P.; Keast, D.H.; Campbell, K.E.; Woodbury, M.G.; Houghton, P.E. Effect of ultraviolet light C on bacterial colonization in chronic wounds. Ostomy/Wound Manag. 2005, 51, 32–45. [Google Scholar]
- Hetty, R.; Nagaraja, H.; Jayadev, C.; Shivanna, Y.; Kugar, T. Collagen crosslinking in the management of advanced non-resolving microbial keratitis. Br. J. Ophthalmol. 2014, 98, 1033–1035. [Google Scholar]
- Dai, T.; Kharkwal, G.B.; Zhao, J.; St. Denis, T.G.; Wu, Q.; Xia, Y.; Huang, L.; Sharma, S.K.; d’Enfert, C.; Hamblin, M.R. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem. Photobiol. 2011, 87, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Garcia, B.; Murray, C.K.; Vrahas, M.S.; Hamblin, M.R. UVC light prophylaxis for cutaneous wound infections in mice. Antimicrob. Agents Chemother. 2012, 56, 3841–3848. [Google Scholar] [CrossRef] [PubMed]
- Marasini, S.; Mugisho, O.O.; Swift, S.; Read, H.; Rupenthal, I.D.; Dean, S.J.; Craig, J.P. Effect of therapeutic UVC on corneal DNA: Safety assessment for potential keratitis treatment. Ocul. Surf. 2021, 20, 130–138. [Google Scholar] [CrossRef]
- Marasini, S.; Dean, S.J.; Swift, S.; Perera, J.; Rupenthal, I.D.; Wang, T.; Read, H.; Craig, J.P. Preclinical confirmation of UVC efficacy in treating infectious keratitis. Ocul. Surf. 2022, 25, 76–86. [Google Scholar] [CrossRef]
- Onigbinde, A.T.; Adedoyin, R.A.; Ojoawo, O.A.; Johnson, O.E.; Obembe, A.O.; Olafimihan, F.K.; Omiyale, O.M.; Oniyangi, S. Effects of ultraviolet radiation (type B) on wound exudates, appearance and depth description. Technol. Health Care. 2010, 18, 297–302. [Google Scholar] [CrossRef]
- Leanse, L.G.; dos Anjos, C.; Anderson, R.R.; Hooper, D.C.; Dai, T. Blue light enhances antibiotic activity in multidrug-resistant bacteria. In Photonic Diagnosis, Monitoring, Prevention, and Treatment of Infections and Inflammatory Diseases 2023; PC123580A; SPIE: Bellingham, WA, USA, 2023; Volume PC12358. [Google Scholar]
- Yu, Y.; Zhao, Y.; He, Y.; Pang, J.; Yang, Z.; Zheng, M.; Yin, R. Inhibition of efflux pump encoding genes and biofilm formation by sub-lethal photodynamic therapy in methicillin susceptible and resistant Staphylococcus aureus. Photodiagn. Photodyn. Ther. 2022, 39, 102900. [Google Scholar] [CrossRef]
- Dos Anjos, C.; Sellera, F.P.; Ribeiro, M.S.; Baptista, M.S.; Pogliani, F.C.; Lincopan, N.; Sabino, C.P. Antimicrobial blue light and photodynamic therapy inhibit clinically relevant β-lactamases with extended-spectrum (ESBL) and carbapenemase activity. Photodiagn. Photodyn. Ther. 2020, 32, 102086. [Google Scholar] [CrossRef]
- Feng, Y.; Palanisami, A.; Ashraf, S.; Bhayana, B.; Hasan, T. Photodynamic inactivation of bacterial carbapenemases restores bacterial carbapenem susceptibility and enhances carbapenem antibiotic effectiveness. Photodiagn. Photodyn. Ther. 2020, 30, 101693. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leanse, L.G.; Marasini, S.; dos Anjos, C.; Dai, T. Antimicrobial Resistance: Is There a ‘Light’ at the End of the Tunnel? Antibiotics 2023, 12, 1437. https://doi.org/10.3390/antibiotics12091437
Leanse LG, Marasini S, dos Anjos C, Dai T. Antimicrobial Resistance: Is There a ‘Light’ at the End of the Tunnel? Antibiotics. 2023; 12(9):1437. https://doi.org/10.3390/antibiotics12091437
Chicago/Turabian StyleLeanse, Leon G., Sanjay Marasini, Carolina dos Anjos, and Tianhong Dai. 2023. "Antimicrobial Resistance: Is There a ‘Light’ at the End of the Tunnel?" Antibiotics 12, no. 9: 1437. https://doi.org/10.3390/antibiotics12091437
APA StyleLeanse, L. G., Marasini, S., dos Anjos, C., & Dai, T. (2023). Antimicrobial Resistance: Is There a ‘Light’ at the End of the Tunnel? Antibiotics, 12(9), 1437. https://doi.org/10.3390/antibiotics12091437





