Sequential or Combination Treatments as Rescue Therapies in Immunocompromised Patients with Persistent SARS-CoV-2 Infection in the Omicron Era: A Case Series
Abstract
:1. Introduction
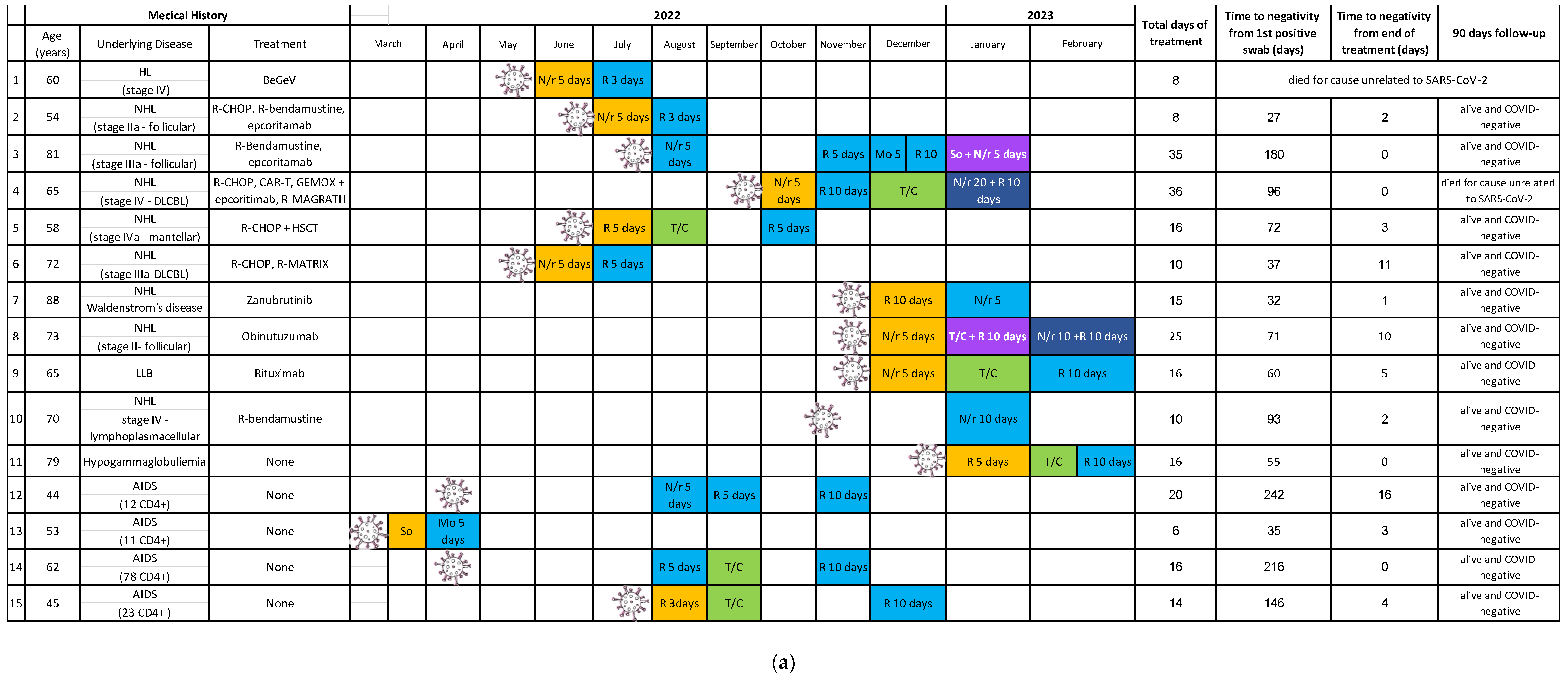
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aydillo, T.; Gonzalez-Reiche, A.S.; Aslam, S.; van de Guchte, A.; Khan, Z.; Obla, A.; Dutta, J.; van Bakel, H.; Aberg, J.; García-Sastre, A.; et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N. Engl. J. Med. 2020, 383, 2586–2588. [Google Scholar] [CrossRef]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.H.; Boucau, J.; Bowman, K.; et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N. Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Shah, M.K.; Hoyos, D.; Solovyov, A.; Douglas, M.; Taur, Y.; Maslak, P.; Babady, N.E.; Greenbaum, B.; Kamboj, M.; et al. Prolonged SARS-CoV-2 infection in patients with lymphoid malignancies. Cancer Discov. 2022, 12, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Sepulcri, C.; Dentone, C.; Mikulska, M.; Bruzzone, B.; Lai, A.; Fenoglio, D.; Bozzano, F.; Bergna, A.; Parodi, A.; Altosole, T.; et al. The longest persistence of viable SARS-CoV-2 with recurrence of viremia and relapsing symptomatic COVID-19 in an immunocompromised patient—A case study. Open Forum Infect. Dis. 2021, 8, ofab217. [Google Scholar] [CrossRef]
- Westblade, L.F.; Brar, G.; Pinheiro, L.C.; Paidoussis, D.; Rajan, M.; Martin, P.; Goyal, P.; Sepulveda, J.L.; Zhang, L.; George, G.; et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell 2020, 38, 661–671.e2. [Google Scholar] [CrossRef] [PubMed]
- Belkin, A.; Leibowitz, A.; Shargian, L.; Yahav, D. The unique presentation of SARS-CoV-2 infection in patients with B-cell depletion: Definition of “persistent inflammatory sero-negative COVID”. Clin. Microbiol. Infect. 2023, 29, 1–3. [Google Scholar] [CrossRef]
- Mikulska, M.; Sepulcri, C.; Dentone, C.; Magne, F.; Balletto, E.; Baldi, F.; Labate, L.; Russo, C.; Mirabella, M.; Magnasco, L.; et al. Triple combination therapy with two antivirals and monoclonal antibodies for persistent or relapsed SARS-CoV-2 infection in immunocompromised patients. Clin. Infect. Dis. 2023, 77, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Duléry, R.; Lamure, S.; Delord, M.; Di Blasi, R.; Chauchet, A.; Hueso, T.; Rossi, C.; Drenou, B.; Deau Fischer, B.; Soussain, C.; et al. Prolonged in-hospital stay and higher mortality after COVID-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am. J. Hematol. 2021, 96, 934–944. [Google Scholar] [CrossRef]
- Gandhi, S.; Klein, J.; Robertson, A.; Peña-Hernández, M.A.; Lin, M.J.; Roychoudhury, P.; Lu, P.; Fournier, J.; Ferguson, D.; Mohamed Bakhash, S.A.; et al. De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: A case report. Nat. Commun. 2022, 13, 1547. [Google Scholar] [CrossRef]
- Cele, S.; Karim, F.; Lustig, G.; San, J.E.; Hermanus, T.; Tegally, H.; Snyman, J.; Moyo-Gwete, T.; Wilkinson, E.; Bernstein, M.; et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe 2022, 30, 154–162.e5. [Google Scholar] [CrossRef]
- Hogan, J.I.; Duerr, R.; Dimartino, D.; Marier, C.; Hochman, S.E.; Mehta, S.; Wang, G.; Heguy, A. Remdesivir Resistance in Transplant Recipients with Persistent Coronavirus Disease 2019. Clin. Infect. Dis. 2023, 76, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Owusu, D.; Pomeroy, M.A.; Lewis, N.M.; Wadhwa, A.; Yousaf, A.R.; Whitaker, B.; Dietrich, E.; Hall, A.J.; Chu, V.; Thornburg, N.; et al. Persistent SARS-CoV-2 RNA Shedding without Evidence of Infectiousness: A Cohort Study of Individuals with COVID-19. J. Infect. Dis. 2021, 224, 1362–1371. [Google Scholar] [CrossRef]
- Corey, L.; Beyrer, C.; Cohen, M.S.; Michael, N.L.; Bedford, T.; Rolland, M. SARS-CoV-2 Variants in Patients with Immunosuppression. N. Engl. J. Med. 2021, 385, 562–566. [Google Scholar] [CrossRef]
- Kareff, S.A.; Khan, A.; Barreto-Coelho, P.; Iyer, S.G.; Pico, B.; Stanchina, M.; Dutcher, G.; Monteiro de Oliveira Novaes, J.; Nallagangula, A.; Lopes, G. Prevalence and Outcomes of COVID-19 among Hematology/Oncology Patients and Providers of a Community-Facing Health System during the B1.1.529 (“Omicron”) SARS-CoV-2 Variant Wave. Cancers 2022, 14, 4629. [Google Scholar] [CrossRef] [PubMed]
- Cattel, L.; Giordano, S.; Traina, S.; Lupia, T.; Corcione, S.; Angelone, L.; La Valle, G.; De Rosa, F.G.; Cattel, F. Vaccine development and technology for SARS-CoV-2: Current insight. J. Med. Virol. 2022, 94, 878–896. [Google Scholar] [CrossRef]
- Mornese Pinna, S.; Lupia, T.; Scabini, S.; Vita, D.; De Benedetto, I.; Gaviraghi, A.; Colasanto, I.; Varese, A.; Cattel, F.; De Rosa, F.G.; et al. Monoclonal antibodies for the treatment of COVID-19 patients: An umbrella to overcome the storm? Int. Immunopharmacol. 2021, 101 Pt A, 108200. [Google Scholar] [CrossRef]
- Bassetti, M.; Corcione, S.; Dettori, S.; Lombardi, A.; Lupia, T.; Vena, A.; De Rosa, F.G.; Gori, A.; Giacobbe, D.R. Antiviral treatment selection for SARS-CoV-2 pneumonia. Expert Rev. Respir. Med. 2021, 15, 985–992. [Google Scholar] [CrossRef]
- Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-dashboard (accessed on 24 May 2023).
- Yanez, N.D.; Weiss, N.S.; Romand, J.A.; Treggiari, M.M. COVID-19 mortality risk for older men and women. BMC Public Health 2020, 20, 1742. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Barranco, P.; García-Roa, M.; Trelles-Martínez, R.; Arribalzaga, K.; Velasco, M.; Guijarro, C.; Marcos, J.; Campelo, C.; Acedo-Sanz, J.M.; Villalón, L.; et al. Management of Persistent SARS-CoV-2 Infection in Patients with Follicular Lymphoma. Acta Haematol. 2022, 145, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Hettle, D.; Hutchings, S.; Muir, P.; Moran, E. COVID-19 Genomics UK (COG-UK) consortium. Persistent SARS-CoV-2 infection in immunocompromised patients facilitates rapid viral evolution: Retrospective cohort study and literature review. Clin. Infect. Pract. 2022, 16, 100210. [Google Scholar] [CrossRef] [PubMed]
- Blennow, O.; Vesterbacka, J.; Tovatt, T.; Nowak, P. Successful combination treatment for persistent SARS-CoV-2 infection. Clin. Infect. Dis. 2023, 76, 1864–1865. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, D.; Antonello, R.M.; Coppi, M.; Palazzo, M.; Nassi, L.; Streva, N.; Povolo, L.; Malentacchi, F.; Zammarchi, L.; Rossolini, G.M.; et al. Combination regimen of nirmatrelvir/ritonavir and molnupiravir for the treatment of persistent SARS-CoV-2 infection: A case report and a scoping review of the literature. Int. J. Infect. Dis. IJID 2023, 133, 53–56. [Google Scholar] [CrossRef]
- Maponga, T.G.; Jeffries, M.; Tegally, H.; Sutherland, A.; Wilkinson, E.; Lessells, R.J.; Msomi, N.; van Zyl, G.; de Oliveira, T.; Preiser, W. Persistent Severe Acute Respiratory Syndrome Coronavirus 2 Infection with accumulation of mutations in a patient with poorly controlled Human Immunodeficiency Virus infection. Clin. Infect. Dis. 2023, 76, e522–e525. [Google Scholar] [CrossRef]
- Riemersma, K.K.; Haddock, L.A., 3rd; Wilson, N.A.; Minor, N.; Eickhoff, J.; Grogan, B.E.; Kita-Yarbro, A.; Halfmann, P.J.; Segaloff, H.E.; Kocharian, A.; et al. Shedding of infectious SARS-CoV-2 despite vaccination. PLoS Pathog. 2022, 18, e1010876. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.K.; Moran, E.; Goodman, A.; Baxendale, H.; Bermingham, W.; Buckland, M.; AbdulKhaliq, I.; Jarvis, H.; Hunter, M.; Karanam, S.; et al. Treatment of chronic or relapsing COVID-19 in immunodeficiency. J. Allergy Clin. Immunol. 2022, 149, 557–561.e1. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Wada, D.; Nakamori, Y.; Maruyama, S.; Shimazu, H.; Saito, F.; Yoshiya, K.; Kuwagata, Y. Novel treatment combining antiviral and neutralizing antibody-based therapies with monitoring of spike-specific antibody and viral load for immunocompromised patients with persistent COVID-19 infection. Exp. Hematol. Oncol. 2022, 11, 53. [Google Scholar] [CrossRef]
- Ford, E.S.; Simmons, W.; Karmarkar, E.N.; Yoke, L.H.; Braimah, A.B.; Orozco, J.J.; Ghiuzeli, C.M.; Barnhill, S.; Sack, C.L.; Benditt, J.O.; et al. Successful Treatment of Prolonged, Severe Coronavirus Disease 2019 Lower Respiratory Tract Disease in a B cell Acute Lymphoblastic Leukemia Patient with an Extended Course of Remdesivir and Nirmatrelvir/Ritonavir. Clin. Infect. Dis. 2023, 76, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Trottier, C.A.; Wong, B.; Kohli, R.; Boomsma, C.; Magro, F.; Kher, S.; Anderlind, C.; Golan, Y. Dual Antiviral Therapy for Persistent Coronavirus Disease 2019 and Associated Organizing Pneumonia in an Immunocompromised Host. Clin. Infect. Dis. 2023, 76, 923–925. [Google Scholar] [CrossRef]
- Schultz, D.C.; Johnson, R.M.; Ayyanathan, K.; Miller, J.; Whig, K.; Kamalia, B.; Dittmar, M.; Weston, S.; Hammond, H.L.; Dillen, C.; et al. Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature 2022, 604, 134–140. [Google Scholar] [CrossRef]
- Carlin, A.F.; Clark, A.E.; Chaillon, A.; Garretson, A.F.; Bray, W.; Porrachia, M.; Santos, A.T.; Rana, T.M.; Smith, D.M. Virologic and immunologic characterization of Coronavirus Disease 2019 recrudescence after nirmatrelvir/ritonavir treatment. Clin. Infect. Dis. 2023, 76, e530–e532. [Google Scholar] [CrossRef] [PubMed]
- Boucau, J.; Uddin, R.; Marino, C.; Regan, J.; Flynn, J.P.; Choudhary, M.C.; Chen, G.; Stuckwisch, A.M.; Mathews, J.; Liew, M.Y.; et al. Characterization of virologic rebound following nirmatrelvir-ritonavir treatment for Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2023, 76, e526–e529. [Google Scholar] [CrossRef] [PubMed]
- Carey, T.S.; Boden, S.D. A critical guide to case series reports. Spine 2003, 28, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Data on SARS-CoV-2 variants in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/data-virus-variants-covid-19-eueea (accessed on 13 July 2023).

| Characteristics | N (%) | IQR |
|---|---|---|
| Male sex | 9 (60) | |
| Age (median) | 65 | (58–73) |
| Underlying diseases | N (%) | |
| Onco-hematological disease | 11 (73) | |
| Non-Hodgkin lymphoma | 8 (72) | |
| Hodgkin lymphoma | 1 (9) | |
| Chronic lymphocytic leukemia | 1 (9) | |
| Hypogammaglobulinemia | 1 (9) | |
| HSCT | 1 (9) | |
| Other causes of immunocompromise | ||
| Ongoing immunomodulant therapies (other than anti-CD20 biologics) | 8 (73) | |
| Anti-CD20 treatment within 6 months | 7 (64) | |
| HIV/AIDS patients and characteristics | 4 (27) | |
| nadir CD4+T-cell count, median | 23 | (12–78) |
| Treatment-naïve at COVID-19 onset | 3 (75) | |
| Concomitant opportunistic infections | 4 (100) | |
| Comorbidities of immunocompromised population | N (%) | |
| Cardiovascular diseases | 6 (40) | |
| Type 2 diabetes mellitus | 4 (27) | |
| BMI > 30 | 2 (13) | |
| Chronic respiratory disease | 2 (13) | |
| SARS-CoV-2 prophylaxis | N (%) | |
| Vaccinated for SARS-CoV-2 | 15 (100) | |
| No. of doses of vaccine, median | 3 | (1–5) |
| Time to diagnosis, days, from last dose of vaccine, median | 408 | (337–447) |
| Prophylactic monoclonal antibodies | 4 (27) | |
| Clinical parameters | N (%) | |
| Symptomatic for SARS-CoV-2 | 15 (100) | |
| Mild symptoms | 8 (53) | |
| Moderate symptoms | 5 (33) | |
| Severe symptoms | 2 (13) | |
| Pneumonia | 9 (60) | |
| Hospitalization | 11 (73) | |
| Oxygen therapy | 7 (47) | |
| Nasal cannula | 4 (57) | |
| NIV | 3 (29) | |
| ICU admission | 1 (33) | |
| Adjuvant treatment with steroids | 7 (47) | |
| Previous SARS-CoV-2 therapies | N (%) | |
| Previous early therapy for the same SARS-CoV-2 infection episode | 11 (73) | |
| Remdesivir for 3 days | 1 (9) | |
| Remdesivir for 5 days | 2 (18) | |
| Remdesivir for 10 days | 1 (9) | |
| Molnupiravir 5 days | 0 (0) | |
| Nirmatrelvir/ritonavir for 5 days | 6 (55) | |
| Tixagevimab/cilgavimab | 0 (0) | |
| Sotrovimab | 1 (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, B.M.; Venuti, F.; Gaviraghi, A.; Lupia, T.; Ranzani, F.A.; Pepe, A.; Ponzetta, L.; Vita, D.; Allice, T.; Gregorc, V.; et al. Sequential or Combination Treatments as Rescue Therapies in Immunocompromised Patients with Persistent SARS-CoV-2 Infection in the Omicron Era: A Case Series. Antibiotics 2023, 12, 1460. https://doi.org/10.3390/antibiotics12091460
Longo BM, Venuti F, Gaviraghi A, Lupia T, Ranzani FA, Pepe A, Ponzetta L, Vita D, Allice T, Gregorc V, et al. Sequential or Combination Treatments as Rescue Therapies in Immunocompromised Patients with Persistent SARS-CoV-2 Infection in the Omicron Era: A Case Series. Antibiotics. 2023; 12(9):1460. https://doi.org/10.3390/antibiotics12091460
Chicago/Turabian StyleLongo, Bianca Maria, Francesco Venuti, Alberto Gaviraghi, Tommaso Lupia, Fabio Antonino Ranzani, Andrea Pepe, Laura Ponzetta, Davide Vita, Tiziano Allice, Vanesa Gregorc, and et al. 2023. "Sequential or Combination Treatments as Rescue Therapies in Immunocompromised Patients with Persistent SARS-CoV-2 Infection in the Omicron Era: A Case Series" Antibiotics 12, no. 9: 1460. https://doi.org/10.3390/antibiotics12091460
APA StyleLongo, B. M., Venuti, F., Gaviraghi, A., Lupia, T., Ranzani, F. A., Pepe, A., Ponzetta, L., Vita, D., Allice, T., Gregorc, V., Frascione, P. M. M., De Rosa, F. G., Calcagno, A., & Bonora, S. (2023). Sequential or Combination Treatments as Rescue Therapies in Immunocompromised Patients with Persistent SARS-CoV-2 Infection in the Omicron Era: A Case Series. Antibiotics, 12(9), 1460. https://doi.org/10.3390/antibiotics12091460







