Translational PK/PD for the Development of Novel Antibiotics—A Drug Developer’s Perspective
Abstract
:1. Introduction
2. Translational PK/PD for Antibacterial Development in Health Authority Guidelines
2.1. History and Current Approach of Antibacterial PK/PD
2.2. Antibacterial PK/PD in the Regulatory Context
3. Potential Limitations of the PK/PD Index Approach and Risk Mitigation Strategies
3.1. Risk Mitigation by Conducting Additional Studies/Enhanced Study Design
3.1.1. Incorporating Resistance in PK/PD Assessments
3.1.2. Considering the Drug Concentration-Time Profile
3.1.3. Use of In Vivo Models Mimicking Human Disease and Treatment
3.1.4. Considering the Local Free Tissue Concentration
3.2. Risk Mitigation by Applying an Advanced PK/PD Analysis Approach
4. Conclusions
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, C.L.J.I.; Ikuta, K.S.; Sharara, F.; The Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet Infect. Dis. 2016, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- CDC. Antibiotic Resistance Threats in the United States—2019; CDC: Atlanta, GA, USA, 2019.
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the eu and the european economic area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the clinical pipeline of treatments for drugresistant bacterial infections: Despite progress, more action is needed. Antimicrob. Agents Chemother. 2022, 66, e01921–e01991. [Google Scholar] [CrossRef]
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed; WHO: Geneva, Switzerland, 2017.
- Årdal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic development—Economic, regulatory and societal challenges. Nat. Rev. Microbiol. 2020, 18, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Shorr, A.F.; Wunderink, R.G.; Du, B.; Poirier, G.E.; Rana, K.; Miller, A.; Lewis, D.; O’Donnell, J.; Chen, L.; et al. Efficacy and safety of sulbactam-durlobactam versus colistin for the treatment of patients with serious infections caused by acinetobacter baumannii-calcoaceticus complex: A multicentre, randomised, active-controlled, phase 3, non-inferiority clinical trial (attack). Lancet Infect. Dis. 2023, 23, 1072–1084. [Google Scholar] [PubMed]
- Rex, J.H.; Eisenstein, B.I.; Alder, J.; Goldberger, M.; Meyer, R.; Dane, A.; Friedland, I.; Knirsch, C.; Sanhai, W.R.; Tomayko, J.; et al. A comprehensive regulatory framework to address the unmet need for new antibacterial treatments. Lancet Infect. Dis. 2013, 13, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Ambrose, P.G.; Chambers, H.F.; Ebright, R.H.; Jezek, A.; Murray, B.E.; Newland, J.G.; Ostrowsky, B.; Rex, J.H.; Infectious Diseases Society of America. White paper: Developing antimicrobial drugs for resistant pathogens, narrow-spectrum indications, and unmet needs. J. Infect. Dis. 2017, 216, 228–236. [Google Scholar] [CrossRef] [PubMed]
- FDA-CDER. Antibacterial Therapies for Patients with an Unmet Medical Need for the Treatment of Serious Bacterial Diseases—Questions and Answers (Revision 1) Guidance for Industry. 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/antibacterial-therapies-patients-unmet-medical-need-treatment-serious-bacterial-diseases-questions (accessed on 1 October 2023).
- EMA-CHMP. Guideline on the Evaluation of Medicinal Products Indicated for Treatment of Bacterial Infections (cpmp/ewp/558/95 rev 3). 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-3_en.pdf (accessed on 1 October 2023).
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 us hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef]
- Kaye, K.S.; Bhowmick, T.; Metallidis, S.; Bleasdale, S.C.; Sagan, O.S.; Stus, V.; Vazquez, J.; Zaitsev, V.; Bidair, M.; Chorvat, E.; et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: The tango i randomized clinical trial. JAMA 2018, 319, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Giamarellos-Bourboulis, E.J.; Rahav, G.; Mathers, A.J.; Bassetti, M.; Vazquez, J.; Cornely, O.A.; Solomkin, J.; Bhowmick, T.; Bishara, J.; et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant enterobacteriaceae infections: The tango ii randomized clinical trial. Infect. Dis. Ther. 2018, 7, 439–455. [Google Scholar] [CrossRef]
- Sabet, M.; Tarazi, Z.; Rubio-Aparicio, D.; Nolan, T.G.; Parkinson, J.; Lomovskaya, O.; Dudley, M.N.; Griffitha, D.C. Activity of simulated human dosage regimens of meropenem and vaborbactam against carbapenem-resistant enterobacteriaceae in an in vitro hollow-fiber model. Antimicrob. Agents Chemother. 2018, 62, e01917–e01969. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.M.; Asempa, T.E.; Nicolau, D.P. Efficacy of human-simulated exposures of meropenem/vaborbactam and meropenem against oxa-48 beta-lactamase-producing enterobacterales in the neutropenic murine thigh infection model. J. Antimicrob. Chemother. 2021, 76, 184–188. [Google Scholar] [CrossRef] [PubMed]
- FDA-CDER. Combined Cross-Discipline Team Leader, Division and Office Director Review. Nda 209766 Vabomere (Meropenem/Vaborbactam); Application Number: 209776orig1s000; CDER-FDA: Beltsville, MD, USA, 2017.
- Food and Drug Administration. FDA Approves New Treatment for Pneumonia Caused by Certain Difficult-to-Treat Bacteria; News Release May 2023; FDA: Beltsville, MD, USA, 2023.
- FDA-CDER. Integrated Review: Sulbactam/Durlobactam; Application Number: 216974orig1s000; CDER-FDA: Beltsville, MD, USA, 2023.
- Eagle, H.; Fleischman, R.; Musselman, A.D. Effect of schedule of administration on the therapeutic efficacy of penicillin; importance of the aggregate time penicillin remains at effectively bactericidal levels. Am. J. Med. 1950, 9, 280–299. [Google Scholar] [CrossRef]
- Eagle, H.; Fleischman, R.; Musselman, A.D. The effective concentrations of penicillin in vitro and in vivo for streptococci, pneumococci, and treponema pallidum. J. Bacteriol. 1950, 59, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Eagle, H.; Fleischman, R.; Levy, M. “Continuous” vs. “Discontinuous” therapy with penicillin; the effect of the interval between injections on therapeutic efficacy. N. Engl. J. Med. 1953, 248, 481–488. [Google Scholar] [CrossRef]
- Eagle, H.; Fleischman, R.; Levy, M. On the duration of penicillin action in relation to its concentration in the serum. J. Lab. Clin. Med. 1953, 41, 122–132. [Google Scholar] [PubMed]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef]
- Craig, W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 2003, 17, 479–501. [Google Scholar] [CrossRef]
- Odenholt, I.; Isaksson, B.; Nilsson, L.; Cars, O. Postantibiotic and bactericidal effect of imipenem against pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 136–141. [Google Scholar] [CrossRef]
- Tornqvist, I.O.; Holm, S.E.; Cars, O. Pharmacodynamic effects of subinhibitory antibiotic concentrations. Scand. J. Infect. Dis. Suppl. 1990, 74, 94–101. [Google Scholar]
- Loewdin, E.; Odenholt, I.; Bengtsson, S.; Cars, O. Pharmacodynamic effects of sub-mics of benzylpenicillin against streptococcus pyogenes in a newly developed in vitro kinetic model. Antimicrob. Agents Chemother. 1996, 40, 2478–2482. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, P.G.; Bhavnani, S.M.; Ellis-Grosse, E.J.; Drusano, G.L. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: Look before you leap! Clin. Infect. Dis. 2010, 51 (Suppl. S1), S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Role of pharmacokinetics in the outcome of infections. Antimicrob. Agents Chemother. 1998, 32, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Dalhoff, A.; Ambrose, P.G.; Mouton, J.W. A long journey from minimum inhibitory concentration testing to clinically predictive breakpoints: Deterministic and probabilistic approaches in deriving breakpoints. Infection 2009, 37, 296–305. [Google Scholar] [CrossRef]
- Mouton, J.W.; Brown, D.F.; Apfalter, P.; Canton, R.; Giske, C.G.; Ivanova, M.; MacGowan, A.P.; Rodloff, A.; Soussy, C.J.; Steinbakk, M.; et al. The role of pharmacokinetics/pharmacodynamics in setting clinical mic breakpoints: The eucast approach. Clin. Microbiol. Infect. 2012, 18, E37–E45. [Google Scholar] [CrossRef]
- Moore, J.N.; Poon, L.; Pahwa, S.; Bensman, T.; Wei, X.T.; Danielsen, Z.Y.; Jang, S. Animal pharmacokinetics/pharmacodynamics (pk/pd) infection models for clinical development of antibacterial drugs: Lessons from selected cases. J. Antimicrob. Chemother. 2023, 78, 1337–1343. [Google Scholar] [CrossRef]
- Rizk, M.L.; Bhavnani, S.M.; Drusano, G.; Dane, A.; Eakin, A.E.; Guina, T.; Jang, S.H.; Tomayko, J.F.; Wang, J.; Zhuang, L.; et al. Considerations for dose selection and clinical pharmacokinetics/pharmacodynamics for the development of antibacterial agents. Antimicrob. Agents Chemother. 2019, 63, e02309–e02318. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Hope, W.W.; Eakin, A.E.; Guina, T.; Tam, V.H.; Louie, A.; Drusano, G.L.; Hoover, J.L. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob. Agents Chemother. 2019, 63, e02307–e02318. [Google Scholar] [CrossRef]
- Arrazuria, R.; Kerscher, B.; Huber, K.E.; Hoover, J.L.; Lundberg, C.V.; Hansen, J.U.; Sordello, S.; Renard, S.; Aranzana-Climent, V.; Hughes, D.; et al. Variability of murine bacterial pneumonia models used to evaluate antimicrobial agents. Front. Microbiol. 2022, 13, 988728. [Google Scholar] [CrossRef]
- Andes, D.R.; Lepak, A.J. In vivo infection models in the pre-clinical pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Curr. Opin. Pharmacol. 2017, 36, 94–99. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef]
- Berry, A.V.; Kuti, J.L. Pharmacodynamic thresholds for beta-lactam antibiotics: A story of mouse versus man. Front. Pharmacol. 2022, 13, 833189. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- EMA-CHMP. Guideline on the Use of Pharmacokinetics and Pharmacodynamics in the Development of Antimicrobial Medicinal Products; EMA: London, UK, 2017.
- Craig, W.A.; Andes, D.R. In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against pseudomonas aeruginosa and enterobacteriaceae, including strains with extended-spectrum beta-lactamases, in the thighs of neutropenic mice. Antimicrob. Agents Chemother. 2013, 57, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Melchers, M.J.; Mavridou, E.; van Mil, A.C.; Lagarde, C.; Mouton, J.W. Pharmacodynamics of ceftolozane combined with tazobactam against enterobacteriaceae in a neutropenic mouse thigh model. Antimicrob. Agents Chemother. 2016, 60, 7272–7279. [Google Scholar] [CrossRef]
- Vanscoy, B.; Mendes, R.E.; McCauley, J.; Bhavnani, S.M.; Bulik, C.C.; Okusanya, O.O.; Forrest, A.; Jones, R.N.; Friedrich, L.V.; Steenbergen, J.N.; et al. ; et al. Pharmacological basis of beta-lactamase inhibitor therapeutics: Tazobactam in combination with ceftolozane. Antimicrob. Agents Chemother. 2013, 57, 5924–5930. [Google Scholar] [CrossRef]
- Xiao, A.J.; Miller, B.W.; Huntington, J.A.; Nicolau, D.P. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived dose justification for phase 3 studies in patients with nosocomial pneumonia. J. Clin. Pharmacol. 2016, 56, 56–66. [Google Scholar] [CrossRef]
- FDA-CDER. Summary Review: Ceftolozane/Tazobactam; Application Number 206829orig1s000; CDER-FDA: Beltsville, MD, USA, 2014.
- FDA-CDER. Clinical Pharmacology and Biopharmaceutics Review(s): Ceftolozane/Tazobactam; Application Number 206829orig1s000; CDER-FDA: Beltsville, MD, USA, 2014.
- FDA-CDER. Microbiology/Virology Review(s): Ceftolozane/Tazobactam; Application Number 206829orig1s000; CDER-FDA: Beltsville, MD, USA, 2014; ref 89.
- FDA-CDER. Approval Package for: Ceftolozane and Tazobactum; Application Number: 206829sorig1s008; CDER-FDA: Beltsville, MD, USA, 2019; ref 99.
- FDA-CDER. Clinical Pharmacology and Biopharmaceutics Review(s): Ceftazidime/Avibactam; Application Number 206494orig1s000; CDER-FDA: Beltsville, MD, USA, 2015.
- FDA-CDER. Microbiology/Virology Review(s): Ceftazidime/Avibactam; Application Number 206494orig1s000; CDER-FDA: Beltsville, MD, USA, 2015.
- FDA-CDER. Pharmacology Review(s) Ceftazidime/Avibactam; Application Number 206494orig1s000; CDER-FDA: Beltsville, MD, USA, 2015.
- Das, S.; Li, J.; Riccobene, T.; Carrothers, T.J.; Newell, P.; Melnick, D.; Critchley, I.A.; Stone, G.G.; Nichols, W.W. Dose selection and validation for ceftazidime-avibactam in adults with complicated intra-abdominal infections, complicated urinary tract infections, and nosocomial pneumonia. Antimicrob. Agents Chemother. 2019, 63, e02118–e02187, ref 109. [Google Scholar] [CrossRef]
- Griffith, D.C.; Sabet, M.; Tarazi, Z.; Lomovskaya, O.; Dudley, M.N. Pharmacokinetics/pharmacodynamics of vaborbactam, a novel beta-lactamase inhibitor, in combination with meropenem. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- FDA-CDER. Clinical Pharmacology and Biopharmaceutics Review(s): Meropenem/Vaborbactam; Approval Number 209776orig1s000; CDER-FDA: Beltsville, MD, USA, 2017.
- FDA-CDER. Clinical Microbiology/Virology Review(s): Meropenem/Vaborbactam; Application Number: 209776orig1s000; CDER-FDA: Beltsville, MD, USA, 2017; ref 96.
- FDA-CDER. Summary Review: Plazomicin; Application Number: 210303orig1s000; CDER-FDA: Beltsville, MD, USA, 2018.
- FDA-CDER. Clinical Pharmacology and Biopharmaceutics Review(s): Plazomicin; Application Number: 210303orig1s000; CDER-FDA: Beltsville, MD, USA, 2018.
- FDA-CDER. Clinical Microbiology/Virology Review(s): Plazomicin; Application Number: 210303orig1s000; CDER-FDA: Beltsville, MD, USA, 2018; ref 87.
- Ghazi, I.M.; Monogue, M.L.; Tsuji, M.; Nicolau, D.P. Humanized exposures of cefiderocol, a siderophore cephalosporin, display sustained in vivo activity against siderophore-resistant Pseudomonas aeruginosa. Pharmacology 2018, 101, 278–284. [Google Scholar] [CrossRef]
- FDA-CDER. Approval Package for: Cefiderocol; Application Number: 209445orig1s002; CDER-FDA: Beltsville, MD, USA, 2020; ref 98.
- FDA-CDER. Multi-Discipline review: Cefiderocol; Application Number: 209445orig1s000; CDER-FDA: Beltsville, MD, USA, 2019.
- FDA-CDER. Multi-Discipline Review: Imipenem-Cilastatin/Relebactam; Application Number: 212819orig1s000; CDER-FDA: Beltsville, MD, USA, 2018.
- Ambrose, P.G. Antibacterial drug development program successes and failures: A pharmacometric explanation. Curr. Opin. Pharmacol. 2017, 36, 1–7. [Google Scholar] [CrossRef]
- Nielsen, E.I.; Friberg, L.E. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharmacol. Rev. 2013, 65, 1053–1090. [Google Scholar] [CrossRef] [PubMed]
- Landersdorfer, C.B.; Nation, R.L. Limitations of antibiotic mic-based pk-pd metrics: Looking back to move forward. Front. Pharmacol. 2021, 12, 770518. [Google Scholar] [CrossRef] [PubMed]
- van Os, W.; Zeitlinger, M. Predicting antimicrobial activity at the target site: Pharmacokinetic/pharmacodynamic indices versus time-kill approaches. Antibiotics 2021, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Bulman, Z.P.; Wicha, S.G.; Nielsen, E.I.; Lenhard, J.R.; Nation, R.L.; Theuretzbacher, U.; Derendorf, H.; Tangden, T.; Zeitlinger, M.; Landersdorfer, C.B.; et al. Research priorities towards precision antibiotic therapy to improve patient care. Lancet Microbe 2022, 3, e795–e802. [Google Scholar] [CrossRef]
- Bhavnani, S.M.; Hammel, J.P.; Lakota, E.A.; Trang, M.; Bader, J.C.; Bulik, C.C.; VanScoy, B.D.; Rubino, C.M.; Huband, M.D.; Friedrich, L.; et al. Pharmacokinetic-pharmacodynamic target attainment analyses evaluating omadacycline dosing regimens for the treatment of patients with community-acquired bacterial pneumonia arising from streptococcus pneumoniae and haemophilus influenzae. Antimicrob. Agents Chemother. 2023, 67, e0221321. [Google Scholar] [CrossRef]
- Fernandez, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef]
- Toprak, E.; Veres, A.; Michel, J.B.; Chait, R.; Hartl, D.L.; Kishony, R. Evolutionary paths to antibiotic resistance under dynamically sustained drug selection. Nat. Genet. 2011, 44, 101–105. [Google Scholar] [CrossRef]
- Adam, M.; Murali, B.; Glenn, N.O.; Potter, S.S. Epigenetic inheritance based evolution of antibiotic resistance in bacteria. BMC Evol. Biol. 2008, 8, 52. [Google Scholar] [CrossRef]
- Mouton, J.W.; Vinks, A.A.T.M.M.; Punt, N.C. Pharmacokinetic-pharmacodynamic modeling of activity of ceftazidime during continuous and intermittent infusion. Antimicrob. Agents Chemother. 1997, 41, 733–738. [Google Scholar] [CrossRef]
- Meagher, A.K.; Forrest, A.; Dalhoff, A.; Stass, H.; Schentag, J.J. Novel pharmacokinetic-pharmacodynamic model for prediction of outcomes with an extended-release formulation of ciprofloxacin. Antimicrob. Agents Chemother. 2004, 48, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Schuck, E.L.; Dalhoff, A.; Stass, H.; Derendorf, H. Pharmacokinetic/pharmacodynamic (pk/pd) evaluation of a once-daily treatment using ciprofloxacin in an extended-release dosage form. Infection 2005, 33 (Suppl. S2), 22–28. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.F.; Nielsen, E.I.; Cars, O.; Friberg, L.E. Pharmacokinetic-pharmacodynamic model for gentamicin and its adaptive resistance with predictions of dosing schedules in newborn infants. Antimicrob. Agents Chemother. 2012, 56, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What antibiotic exposures are required to suppress the emergence of resistance for gram-negative bacteria? A systematic review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Hope, W.W.; Roberts, J.A. Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: Optimizing efficacy and reducing resistance development. Semin. Respir. Crit. Care Med. 2015, 36, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L.; Louie, A.; MacGowan, A.; Hope, W. Suppression of emergence of resistance in pathogenic bacteria: Keeping our powder dry, part 1. Antimicrob. Agents Chemother. 2015, 60, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramos, J.; Gras-Martin, L.; Ramirez, P. Antimicrobial pharmacokinetics and pharmacodynamics in critical care: Adjusting the dose in extracorporeal circulation and to prevent the genesis of multiresistant bacteria. Antibiotics 2023, 12, 475. [Google Scholar] [CrossRef]
- MacGowan, A.P.; Rogers, C.A.; Holt, H.A.; Bowker, K.E. Activities of moxifloxacin against, and emergence of resistance in, streptococcus pneumoniae and pseudomonas aeruginosa in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 2003, 47, 1088–1095. [Google Scholar] [CrossRef]
- Drlica, K.; Malik, M. Fluoroquinolones: Action and resistance. Curr. Top. Med. Chem. 2003, 3, 249–282. [Google Scholar] [CrossRef]
- Hansen, G.T.; Metzler, K.; Drlica, K.; Blondeau, J.M. Mutant prevention concentration of gemifloxacin for clinical isolates of streptococcus pneumoniae. Antimicrob. Agents Chemother. 2003, 47, 440–441. [Google Scholar] [CrossRef]
- Drlica, K.; Zhao, X. Mutant selection window hypothesis updated. Clin. Infect. Dis. 2007, 44, 681–688. [Google Scholar] [CrossRef]
- Safdar, N.; Andes, D.; Craig, W.A. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 2004, 48, 63–68. [Google Scholar] [CrossRef]
- Smith, H.J.; Nichol, K.A.; Hoban, D.J.; Zhanel, G.G. Stretching the mutant prevention concentration (mpc) beyond its limits. J. Antimicrob. Chemother. 2003, 51, 1323–1325. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Overstretching the mutant prevention concentration. J. Antimicrob. Chemother. 2003, 52, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Bacconi, A.; Richmond, G.S.; Baroldi, M.A.; Laffler, T.G.; Blyn, L.B.; Carolan, H.E.; Frinder, M.R.; Toleno, D.M.; Metzgar, D.; Gutierrez, J.R.; et al. Improved sensitivity for molecular detection of bacterial and candida infections in blood. J. Clin. Microbiol. 2014, 52, 3164–3174. [Google Scholar] [CrossRef]
- Adembri, C.; Novelli, A.; Nobili, S. Some suggestions from pk/pd principles to contain resistance in the clinical setting-focus on icu patients and gram-negative strains. Antibiotics 2020, 9, 676. [Google Scholar] [CrossRef]
- Zedtwitz-Liebenstein, K.; Schenk, P.; Apfalter, P.; Fuhrmann, V.; Stoiser, B.; Graninger, W.; Schuster, E.; Frass, M.; Burgmann, H. Ventilator-associated pneumonia: Increased bacterial counts in bronchoalveolar lavage by using urea as an endogenous marker of dilution. Crit. Care Med. 2005, 33, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L.; Corrado, M.L.; Girardi, G.; Ellis-Grosse, E.J.; Wunderink, R.G.; Donnelly, H.; Leeper, K.V.; Brown, M.; Malek, T.; Hite, R.D.; et al. Dilution factor of quantitative bacterial cultures obtained by bronchoalveolar lavage in patients with ventilator-associated bacterial pneumonia. Antimicrob. Agents Chemother. 2018, 62, e01317–e01323. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Liu, J.; O’Donnell, J.N.; Dulhunty, J.M.; Abdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged infusion piperacillin-tazobactam decreases mortality and improves outcomes in severely ill patients: Results of a systematic review and meta-analysis. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef]
- Kesteman, A.S.; Ferran, A.A.; Perrin-Guyomard, A.; Laurentie, M.; Sanders, P.; Toutain, P.L.; Bousquet-Melou, A. Influence of inoculum size and marbofloxacin plasma exposure on the amplification of resistant subpopulations of klebsiella pneumoniae in a rat lung infection model. Antimicrob. Agents Chemother. 2009, 53, 4740–4748. [Google Scholar] [CrossRef]
- Ferran, A.A.; Kesteman, A.S.; Toutain, P.L.; Bousquet-Melou, A. Pharmacokinetic/pharmacodynamic analysis of the influence of inoculum size on the selection of resistance in escherichia coli by a quinolone in a mouse thigh bacterial infection model. Antimicrob. Agents Chemother. 2009, 53, 3384–3390. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.M.; Santini, D.; Takemura, M.; Longshaw, C.; Yamano, Y.; Echols, R.; Nicolau, D.P. In vivo efficacy & resistance prevention of cefiderocol in combination with ceftazidime/avibactam, ampicillin/sulbactam or meropenem using human-simulated regimens versus acinetobacter baumannii. J. Antimicrob. Chemother. 2023, 78, 983–990. [Google Scholar] [PubMed]
- Huo, W.; Busch, L.M.; Hernandez-Bird, J.; Hamami, E.; Marshall, C.W.; Geisinger, E.; Cooper, V.S.; van Opijnen, T.; Rosch, J.W.; Isberg, R.R. Immunosuppression broadens evolutionary pathways to drug resistance and treatment failure during acinetobacter baumannii pneumonia in mice. Nat. Microbiol. 2022, 7, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.I.; Cars, O.; Friberg, L.E. Pharmacokinetic/pharmacodynamic (pk/pd) indices of antibiotics predicted by a semimechanistic pkpd model: A step toward model-based dose optimization. Antimicrob. Agents Chemother. 2011, 55, 4619–4630. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersson, A.N.; David-Pierson, P.; Parrott, N.J.; Kuhlmann, O.; Lave, T.; Friberg, L.E.; Nielsen, E.I. Simulation-based evaluation of pk/pd indices for meropenem across patient groups and experimental designs. Pharm. Res. 2016, 33, 1115–1125. [Google Scholar] [CrossRef]
- Gerber, A.U.; Brugger, H.P.; Feller, C.; Stritzko, T.; Stalder, B. Antibiotic therapy of infections due to pseudomonas aeruginosa in normal and granulocytopenic mice: Comparison of murine and human pharmacokinetics. J. Infect. Dis. 1986, 153, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Deziel, M.R.; Heine, H.; Louie, A.; Kao, M.; Byrne, W.R.; Basset, J.; Miller, L.; Bush, K.; Kelly, M.; Drusano, G.L. Effective antimicrobial regimens for use in humans for therapy of bacillus anthracis infections and postexposure prophylaxis. Antimicrob. Agents Chemother. 2005, 49, 5099–5106. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.W.; Goodwin, J.; O’Connor, L.; Sharp, A.; Gregson, L.; Livermore, J.; Howard, S.J.; Neely, M.N.; Hope, W.W. Impact of bolus dosing versus continuous infusion of piperacillin and tazobactam on the development of antimicrobial resistance in pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 5811–5819. [Google Scholar] [CrossRef]
- Dhaese, S.; Heffernan, A.; Liu, D.; Abdul-Aziz, M.H.; Stove, V.; Tam, V.H.; Lipman, J.; Roberts, J.A.; De Waele, J.J. Prolonged versus intermittent infusion of beta-lactam antibiotics: A systematic review and meta-regression of bacterial killing in preclinical infection models. Clin. Pharmacokinet. 2020, 59, 1237–1250. [Google Scholar] [CrossRef]
- Stasek, J.; Keller, F.; Koci, V.; Klucka, J.; Klabusayova, E.; Wiewiorka, O.; Strasilova, Z.; Benovska, M.; Skardova, M.; Malaska, J. Update on therapeutic drug monitoring of beta-lactam antibiotics in critically ill patients-a narrative review. Antibiotics 2023, 12, 568. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. Dali: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of area under the inhibitory curve (auic) and time above the minimum inhibitory concentration (t > mic) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, P.G.; Bhavnani, S.M.; Owens, R.C., Jr. Clinical pharmacodynamics of quinolones. Infect. Dis. Clin. N. Am. 2003, 17, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Mouton, J.W.; Mattina, R.; Fraschini, F. Pharmacodynamics of levofloxacin and ciprofloxacin in a murine pneumonia model: Peak concentration/mic versus area under the curve/mic ratios. Antimicrob. Agents Chemother. 2003, 47, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Bland, C.M.; Pai, M.P.; Lodise, T.P. Reappraisal of contemporary pharmacokinetic and pharmacodynamic principles for informing aminoglycoside dosing. Pharmacotherapy 2018, 38, 1229–1238. [Google Scholar] [CrossRef]
- Gill, C.M.; Asempa, T.E.; Nicolau, D.P. Human-simulated antimicrobial regimens in animal models: Transparency and validation are imperative. Antimicrob. Agents Chemother. 2020, 64, 00520–00594. [Google Scholar] [CrossRef]
- Monogue, M.L.; Giovagnoli, S.; Bissantz, C.; Zampaloni, C.; Nicolau, D.P. In vivo efficacy of meropenem with a novel non-β-lactam–β-lactamase inhibitor, nacubactam, against gram-negative organisms exhibiting various resistance mechanisms in a murine complicated urinary tract infection model. Antimicrob. Agents Chemother. 2018, 62, e02596-17. [Google Scholar] [CrossRef] [PubMed]
- Asempa, T.E.; Motos, A.; Abdelraouf, K.; Bissantz, C.; Zampaloni, C.; Nicolau, D.P. Efficacy of human-simulated epithelial lining fluid exposure of meropenem-nacubactam combination against class a serine beta-lactamase-producing enterobacteriaceae in the neutropenic murine lung infection model. Antimicrob. Agents Chemother. 2019, 63, e02382-18. [Google Scholar] [CrossRef]
- Asempa, T.E.; Motos, A.; Abdelraouf, K.; Bissantz, C.; Zampaloni, C.; Nicolau, D.P. Meropenem-nacubactam activity against ampc-overproducing and kpc-expressing pseudomonas aeruginosa in a neutropenic murine lung infection model. Int. J. Antimicrob. Agents 2020, 55, 105838. [Google Scholar] [CrossRef]
- Crandon, J.L.; Schuck, V.J.; Banevicius, M.A.; Beaudoin, M.E.; Nichols, W.W.; Tanudra, M.A.; Nicolau, D.P. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2012, 56, 6137–6146. [Google Scholar] [CrossRef]
- Wenzler, E.; Scoble, P.J. An appraisal of the pharmacokinetic and pharmacodynamic properties of meropenem-vaborbactam. Infect. Dis. Ther. 2020, 9, 769–784. [Google Scholar] [CrossRef]
- Monogue, M.L.; Tsuji, M.; Yamano, Y.; Echols, R.; Nicolau, D.P. Efficacy of humanized exposures of cefiderocol (s-649266) against a diverse population of gram-negative bacteria in a murine thigh infection model. Antimicrob. Agents Chemother. 2017, 61, e01017–e01022. [Google Scholar] [CrossRef] [PubMed]
- Luna, B.M.; Yan, J.; Reyna, Z.; Moon, E.; Nielsen, T.B.; Reza, H.; Lu, P.; Bonomo, R.; Louie, A.; Drusano, G.; et al. Natural history of acinetobacter baumannii infection in mice. PLoS ONE 2019, 14, e0219824. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.M.; Waack, U.; Weinstein, E.A.; Joshi, A.; Shurland, S.M.; Iarikov, D.; Bulitta, J.B.; Diep, B.A.; Guina, T.; Hope, W.W.; et al. FDA public workshop summary: Advancing animal models for antibacterial drug development. Antimicrob. Agents Chemother. 2020, 65. [Google Scholar] [CrossRef]
- Jacobs, A.C.; Thompson, M.G.; Black, C.C.; Kessler, J.L.; Clark, L.P.; McQueary, C.N.; Gancz, H.Y.; Corey, B.W.; Moon, J.K.; Si, Y.; et al. Ab5075, a highly virulent isolate of acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. mBio 2014, 5, e01014–e01076. [Google Scholar] [CrossRef]
- Lawrenz, M.B.; Biller, A.E.; Cramer, D.E.; Kraenzle, J.L.; Sotsky, J.B.; Vanover, C.D.; Yoder-Himes, D.R.; Pollard, A.; Warawa, J.M. Development and evaluation of murine lung-specific disease models for pseudomonas aeruginosa applicable to therapeutic testing. Pathog. Dis. 2015, 73, ftv025. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.M.; Sibila, O.; Agusti, C.; Torres, A. Animal models of ventilator-associated pneumonia. Eur. Respir. J. 2009, 33, 182–188. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Naing, E.; Aung, T.; Thi, W.P.; Kavaliauskas, P.; Win Maung, B.B.; Michel, A.O.; Ricart Arbona, R.J.; DeRyke, A.C.; et al. Ceftolozane-tazobactam in the treatment of experimental pseudomonas aeruginosa pneumonia in persistently neutropenic rabbits: Impact on strains with genetically defined mechanisms of resistance. Antimicrob. Agents Chemother. 2019, 63, e00344-19. [Google Scholar] [CrossRef]
- Rodvold, K.A.; Hope, W.W.; Boyd, S.E. Considerations for effect site pharmacokinetics to estimate drug exposure: Concentrations of antibiotics in the lung. Curr. Opin. Pharmacol. 2017, 36, 114–123. [Google Scholar] [CrossRef]
- Rodvold, K.A.; George, J.M.; Yoo, L. Penetration of anti-infective agents into pulmonary epithelial lining fluid. Clin. Pharmacokinet. 2011, 50, 637–664. [Google Scholar] [CrossRef]
- Enlo-Scott, Z. Understanding Local Respiratory Toxicity and Bioavailability of Inhaled Pesticides in an Occupational Exposure Setting; King’s College London: London, UK, 2021. [Google Scholar]
- Backstrom, E.; Erngren, T.; Fihn, B.M.; Sadiq, M.W.; Lindberg, W.; Friden, M.; Grime, K. Possible extraction of drugs from lung tissue during broncho-alveolar lavage suggest uncertainty in the procedure’s utility for quantitative assessment of airway drug exposure. J. Pharm. Sci. 2022, 111, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Keemink, J.; Cantrill, C.; Riboulet, W.; Jurmanovic, S.; Pavlovski, I.; Bissantz, C. Estimating unbound drug concentrations in simulated human lung fluid: Relevance to lung antibiotic pkpd. Mol. Pharm. 2023, 20, 3578–3588. [Google Scholar] [CrossRef]
- Khalid, K.; Rox, K. All roads lead to rome: Enhancing the probability of target attainment with different pharmacokinetic/pharmacodynamic modelling approaches. Antibiotics 2023, 12, 690. [Google Scholar] [CrossRef]
- Nguyen, D.; Shaik, J.S.; Tai, G.; Tiffany, C.; Perry, C.; Dumont, E.; Gardiner, D.; Barth, A.; Singh, R.; Hossain, M. Comparison between physiologically based pharmacokinetic and population pharmacokinetic modelling to select paediatric doses of gepotidacin in plague. Br. J. Clin. Pharmacol. 2022, 88, 416–428. [Google Scholar] [CrossRef]
- FDA-CDER. Multi-discipline review: Lefamulin (application number: 211672orig1s000 211673orig1s000); CDER-FDA: Beltsville, MD, USA, 2018.
- Friberg, L.E. Pivotal role of translation in anti-infective development. Clin. Pharmacol. Ther. 2021, 109, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Landersdorfer, C.B.; Forrest, A.; Brown, S.V.; Neely, M.N.; Brian T Tsuji, A.L. Relevance of pharmacokinetic and pharmacodynamic modeling to clinical care of critically ill patients. Curr. Pharm. Biotechnol. 2011, 12, 2044–2061. [Google Scholar] [CrossRef]
- Sou, T.; Hansen, J.; Liepinsh, E.; Backlund, M.; Ercan, O.; Grinberga, S.; Cao, S.; Giachou, P.; Petersson, A.; Tomczak, M.; et al. Model-informed drug development for antimicrobials: Translational pk and pk/pd modeling to predict an efficacious human dose for apramycin. Clin. Pharmacol. Ther. 2021, 109, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Aranzana-Climent, V.; Hughes, D.; Cao, S.; Tomczak, M.; Urbas, M.; Zabicka, D.; Vingsbo Lundberg, C.; Hansen, J.; Lindberg, J.; Hobbie, S.N.; et al. Translational in vitro and in vivo pkpd modelling for apramycin against gram-negative lung pathogens to facilitate prediction of human efficacious dose in pneumonia. Clin. Microbiol. Infect. 2022, 28, 1367–1374. [Google Scholar] [CrossRef]
- Jacobs, M.; Gregoire, N.; Couet, W.; Bulitta, J.B. Distinguishing antimicrobial models with different resistance mechanisms via population pharmacodynamic modeling. PLoS Comput. Biol. 2016, 12, e1004782. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Ly, N.S.; Xu, H.; Tsuji, B.T.; Bulitta, J.B. Quantifying subpopulation synergy for antibiotic combinations via mechanism-based modeling and a sequential dosing design. Antimicrob. Agents Chemother. 2013, 57, 2343–2351. [Google Scholar] [CrossRef]
- Brill, M.J.E.; Kristoffersson, A.N.; Zhao, C.; Nielsen, E.I.; Friberg, L.E. Semi-mechanistic pharmacokinetic-pharmacodynamic modelling of antibiotic drug combinations. Clin. Microbiol. Infect. 2018, 24, 697–706. [Google Scholar] [CrossRef]
- Aranzana-Climent, V.; Buyck, J.M.; Smani, Y.; Pachon-Diaz, J.; Marchand, S.; Couet, W.; Gregoire, N. Semi-mechanistic pk/pd modelling of combined polymyxin b and minocycline against a polymyxin-resistant strain of acinetobacter baumannii. Clin. Microbiol. Infect. 2020, 26, 1254.e9–1254.e15. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, B.V.; Diniz, A.; Palma, E.C.; Buffe, C.; Dalla Costa, T. Pk-pd modeling of beta-lactam antibiotics: In vitro or in vivo models? J. Antibiot. 2011, 64, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Nonejuie, P.; Munguia, J.; Hollands, A.; Olson, J.; Dam, Q.; Kumaraswamy, M.; Rivera, H., Jr.; Corriden, R.; Rohde, M.; et al. Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant gram-negative bacterial pathogens. EBioMedicine 2015, 2, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Cornforth, D.M.; Dees, J.L.; Ibberson, C.B.; Huse, H.K.; Mathiesen, I.H.; Kirketerp-Moller, K.; Wolcott, R.D.; Rumbaugh, K.P.; Bjarnsholt, T.; Whiteley, M. Pseudomonas aeruginosa transcriptome during human infection. Proc. Natl. Acad. Sci. USA 2018, 115, E5125–E5134. [Google Scholar] [CrossRef]
- Fahnoe, K.C.; Flanagan, M.E.; Gibson, G.; Shanmugasundaram, V.; Che, Y.; Tomaras, A.P. Non-traditional antibacterial screening approaches for the identification of novel inhibitors of the glyoxylate shunt in gram-negative pathogens. PLoS ONE 2012, 7, e51732. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, B.E.; Yang, R.; Clatworthy, A.E.; White, T.; Osmulski, S.J.; Li, L.; Penaranda, C.; Lander, E.S.; Shoresh, N.; Hung, D.T. Defining the core essential genome of pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2019, 116, 10072–10080. [Google Scholar] [CrossRef] [PubMed]
- Villenave, R.; Thavagnanam, S.; Sarlang, S.; Parker, J.; Douglas, I.; Skibinski, G.; Heaney, L.G.; McKaigue, J.P.; Coyle, P.V.; Shields, M.D.; et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 5040–5045. [Google Scholar] [CrossRef]
- Jonsdottir, H.R.; Dijkman, R. Coronaviruses and the human airway: A universal system for virus-host interaction studies. Virol. J. 2016, 13, 24. [Google Scholar] [CrossRef]
- Epithelix. Available online: https://www.epithelix.com/services/bacterial-infections (accessed on 12 November 2023).
- Wong, F.; de la Fuente-Nunez, C.; Collins, J.J. Leveraging artificial intelligence in the fight against infectious diseases. Science 2023, 381, 164–170. [Google Scholar] [CrossRef]
- Lin, S.; de la Fuente, C. The Emerging Field of Digital Antibiotic Discovery. Available online: https://revive.gardp.org/the-emerging-field-of-digital-antibiotic-discovery/ (accessed on 20 November 2023).
- Ali, O.; Abdelbaki, W.; Shrestha, A.; Elbasi, E.; Alryalat, M.A.A.; Dwivedi, Y.K. A systematic literature review of artificial intelligence in the healthcare sector: Benefits, challenges, methodologies, and functionalities. J. Innov. Knowl. 2023, 8, 100333. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Alhumaid, S.; Mutair, A.A.; Garout, M.; Abulhamayel, Y.; Halwani, M.A.; Alestad, J.H.; Bshabshe, A.A.; Sulaiman, T.; AlFonaisan, M.K.; et al. Application of artificial intelligence in combating high antimicrobial resistance rates. Antibiotics 2022, 11, 784. [Google Scholar] [CrossRef]
- Rayner, C.R.; Smith, P.F.; Andes, D.; Andrews, K.; Derendorf, H.; Friberg, L.E.; Hanna, D.; Lepak, A.; Mills, E.; Polasek, T.M.; et al. Model-informed drug development for anti-infectives: State of the art and future. Clin. Pharmacol. Ther. 2021, 109, 867–891. [Google Scholar] [CrossRef]
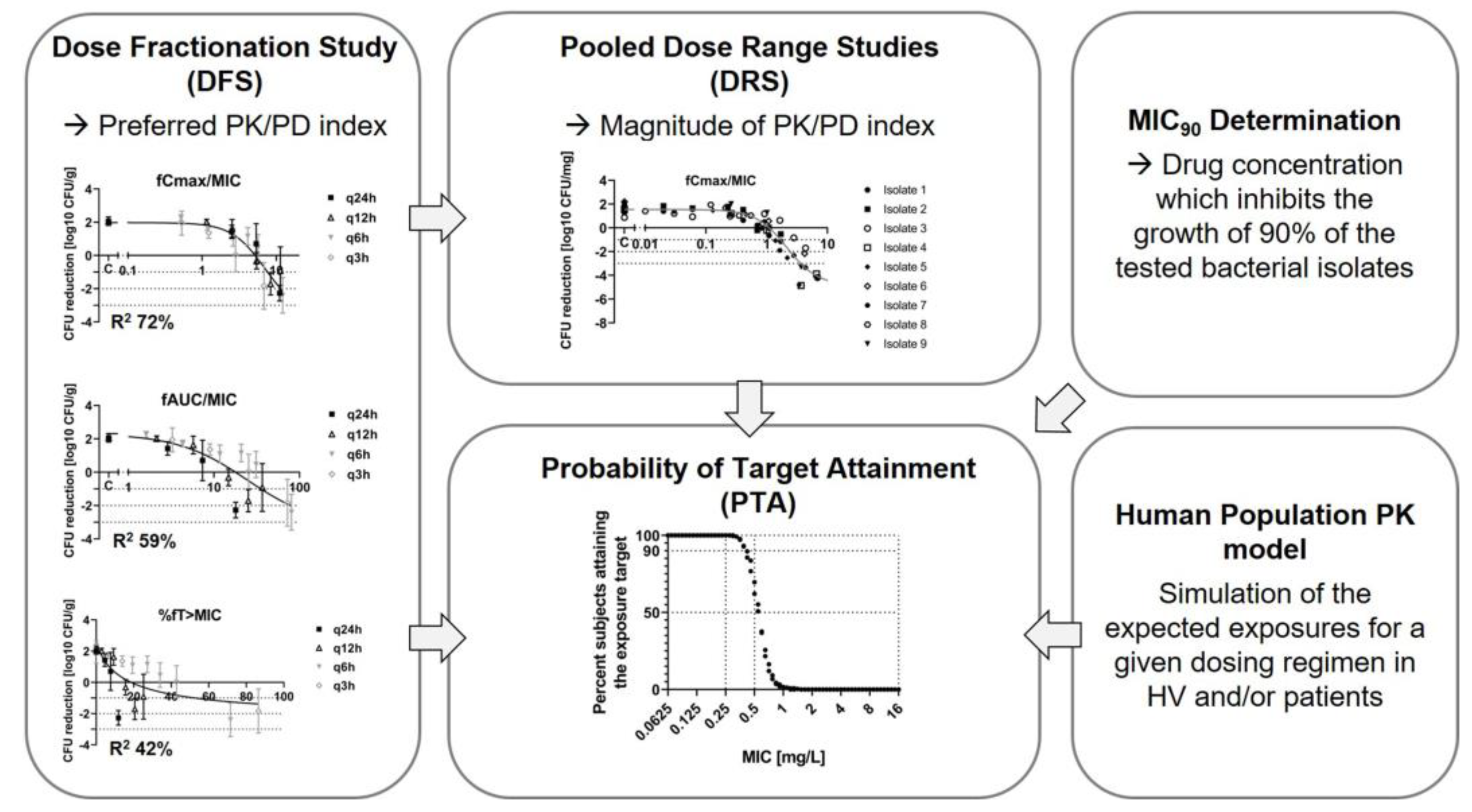


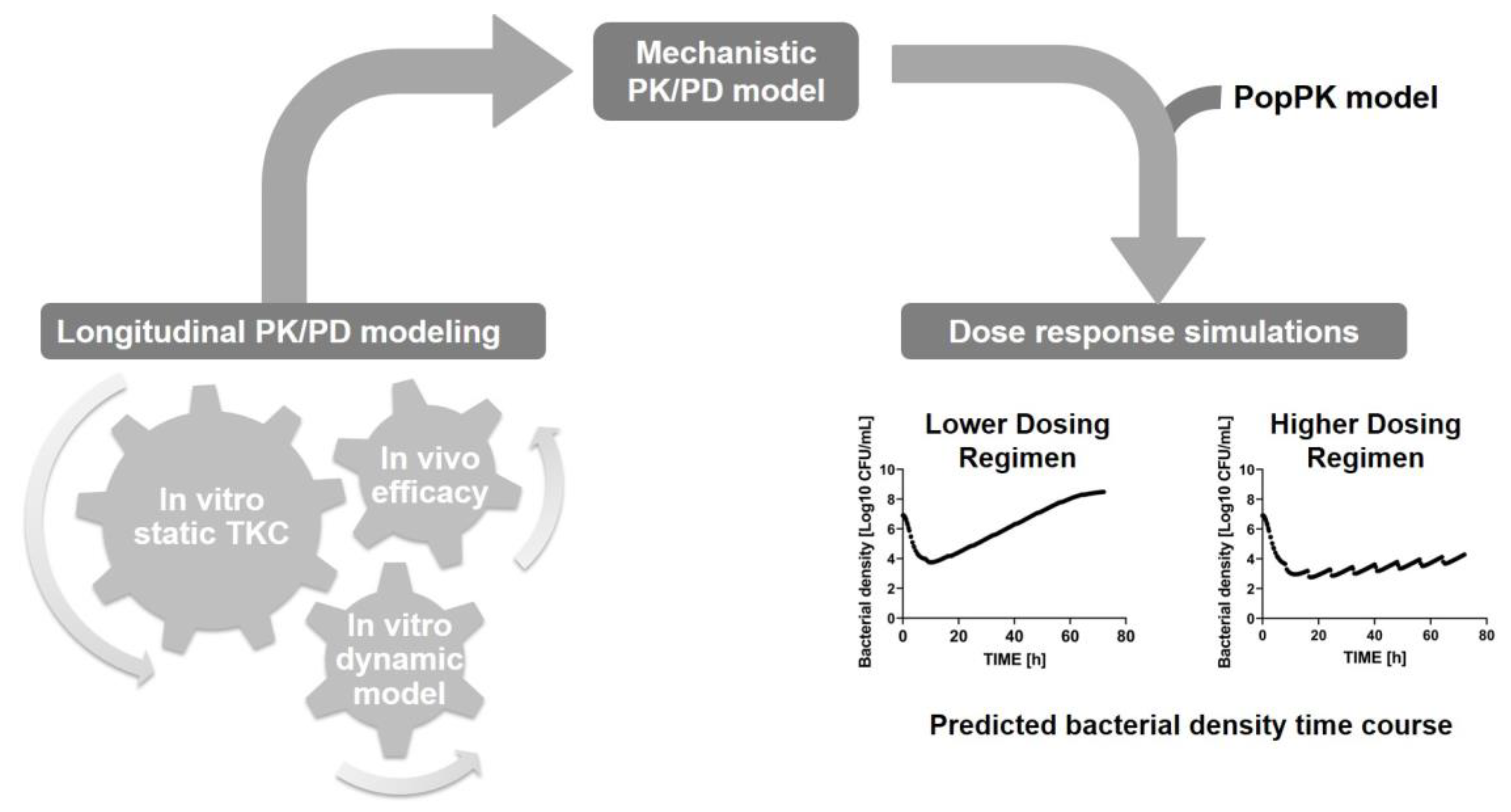
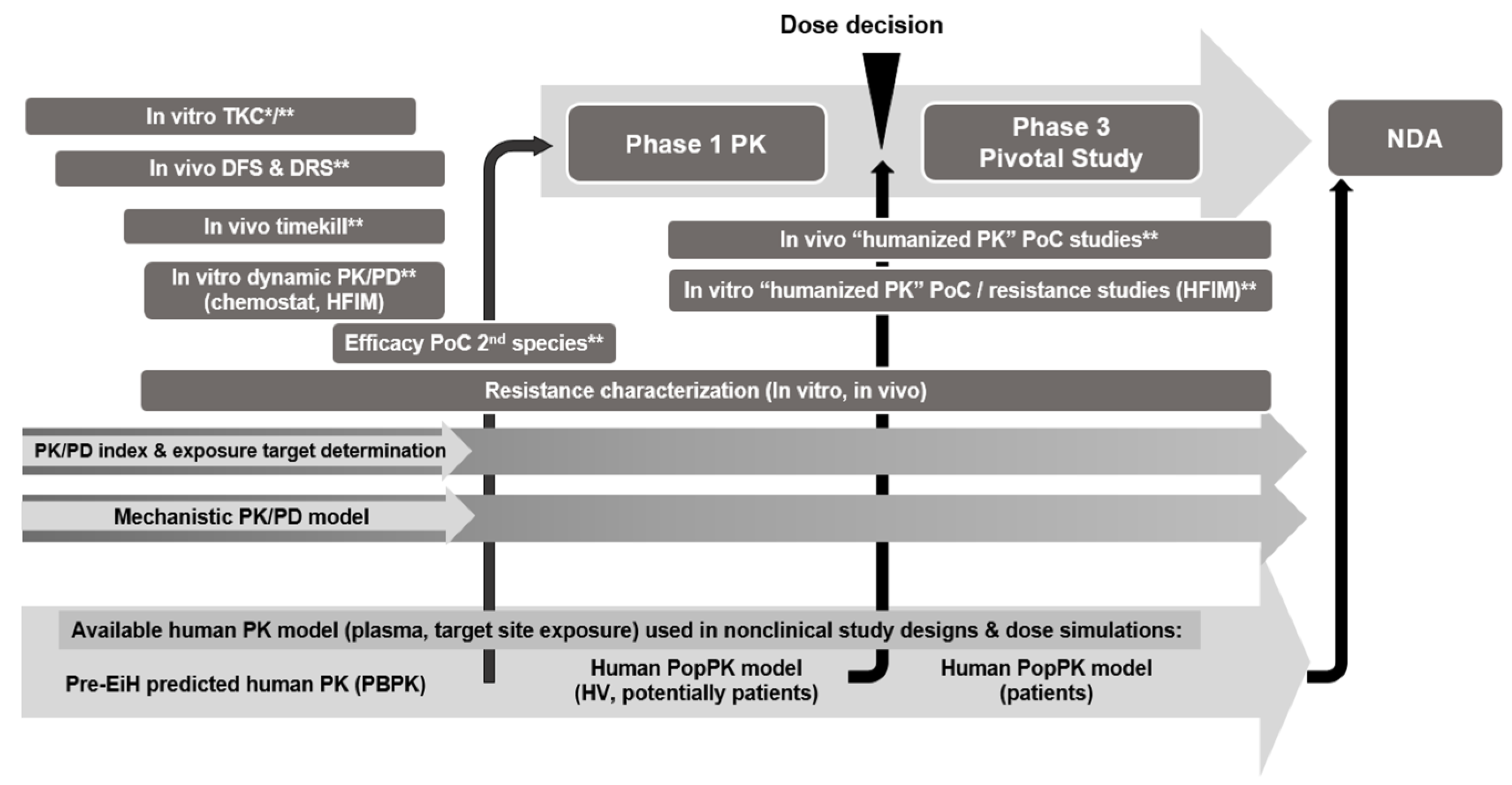

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bissantz, C.; Zampaloni, C.; David-Pierson, P.; Dieppois, G.; Guenther, A.; Trauner, A.; Winther, L.; Stubbings, W. Translational PK/PD for the Development of Novel Antibiotics—A Drug Developer’s Perspective. Antibiotics 2024, 13, 72. https://doi.org/10.3390/antibiotics13010072
Bissantz C, Zampaloni C, David-Pierson P, Dieppois G, Guenther A, Trauner A, Winther L, Stubbings W. Translational PK/PD for the Development of Novel Antibiotics—A Drug Developer’s Perspective. Antibiotics. 2024; 13(1):72. https://doi.org/10.3390/antibiotics13010072
Chicago/Turabian StyleBissantz, Caterina, Claudia Zampaloni, Pascale David-Pierson, Guennaelle Dieppois, Andreas Guenther, Andrej Trauner, Lotte Winther, and William Stubbings. 2024. "Translational PK/PD for the Development of Novel Antibiotics—A Drug Developer’s Perspective" Antibiotics 13, no. 1: 72. https://doi.org/10.3390/antibiotics13010072
APA StyleBissantz, C., Zampaloni, C., David-Pierson, P., Dieppois, G., Guenther, A., Trauner, A., Winther, L., & Stubbings, W. (2024). Translational PK/PD for the Development of Novel Antibiotics—A Drug Developer’s Perspective. Antibiotics, 13(1), 72. https://doi.org/10.3390/antibiotics13010072





