Abstract
Bacteria have evolved and continue to change in response to environmental stressors including antibiotics. Antibiotic resistance and the ability to form biofilms are inextricably linked, requiring the continuous search for alternative compounds to antibiotics that affect biofilm formation. One of the latest drug classes is boron-containing compounds. Over the last several decades, boron has emerged as a prominent element in the field of medicinal chemistry, which has led to an increasing number of boron-containing compounds being considered as potential drugs. The focus of this review is on the developments in boron-containing organic compounds (BOCs) as antimicrobial/anti-biofilm probes and agents.
1. Introduction
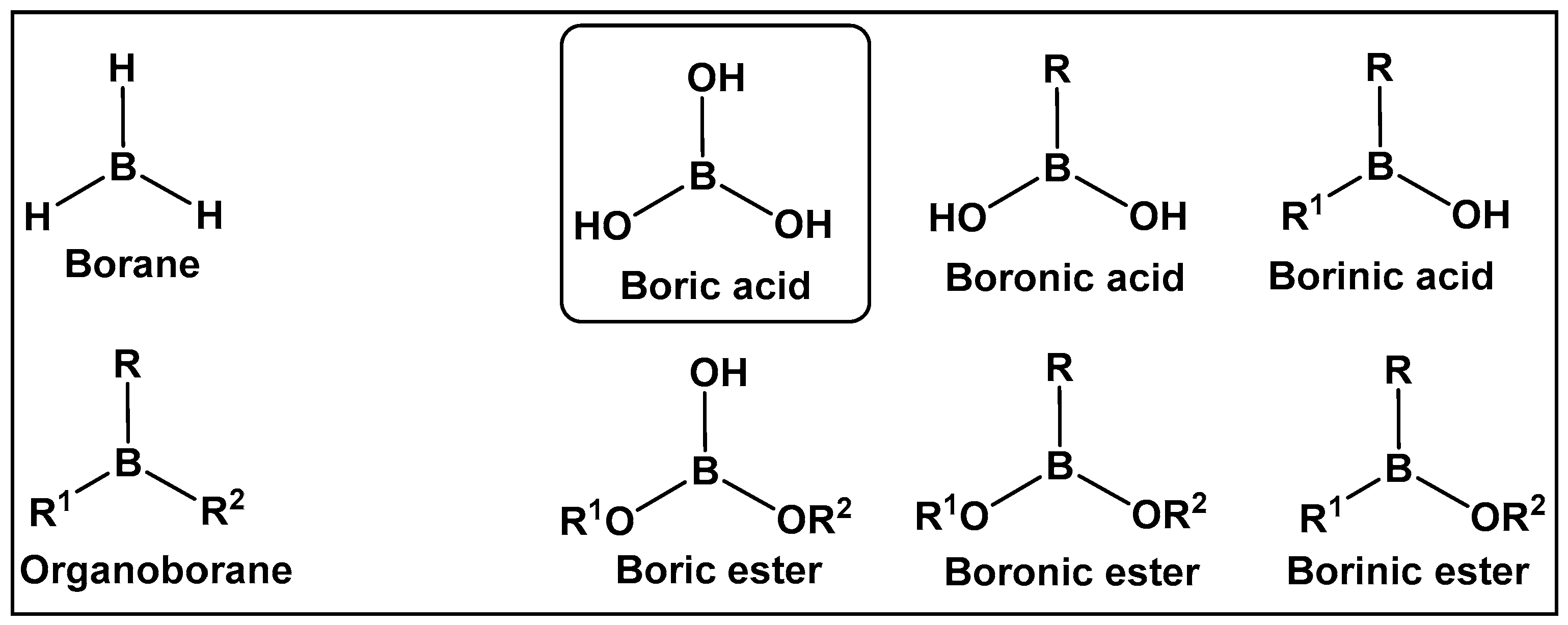
Boron-containing organic compounds. In the periodic table, boron is to the left of carbon, and thus its electronic proximity to carbon has drawn comparisons including the highlighting of their different roles in nature [1]. Both carbon and boron show considerable specialization, as compared to the vast majority of other chemical elements [2]. This functional focus correlates with boron being differentially sequestered across the different organismal domains, due to its ability to dynamically interact with organic molecules. Boron is an exception to the octet rule, in that it is stable with fewer than eight valence electrons. Boric acid (Figure 1 and Figure 2) is the only naturally existing form of boron. It can undergo self-assembly and polymerization to give rise to linear and cyclic hydroxyborons (Figure 2). The only process currently known through which the living organisms prepare boron-containing organic compounds is borylation, that is, the insertion of hydroxyborons [B(OH)n] available in the given organisms’ environment (marine/soil) into predominately small organic molecules [3,4,5,6,7,8,9,10]. Boron is typically incorporated into hydroxy- or amino-rich domains, i.e., those with oxygen/nitrogen Lewis Bases, of a given structure Its presence appears to lead to endogenous functional effects that are unavailable through the known types of “main” bonding in biological systems, based on the organic chemistry of carbon, hydrogen, nitrogen, and oxygen [11].
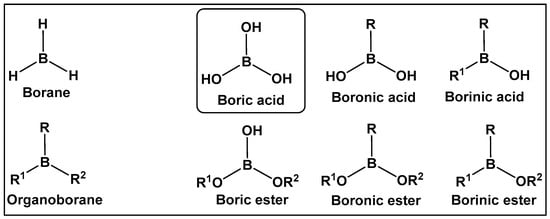
Figure 1.
Introduction to the classes of boron-containing compounds: borane, organoboranes, oxygen-containing boron compounds. To date, fully oxygenated boric acid (BA) is the only naturally occurring form of boron reported.
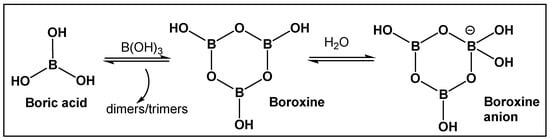
Figure 2.
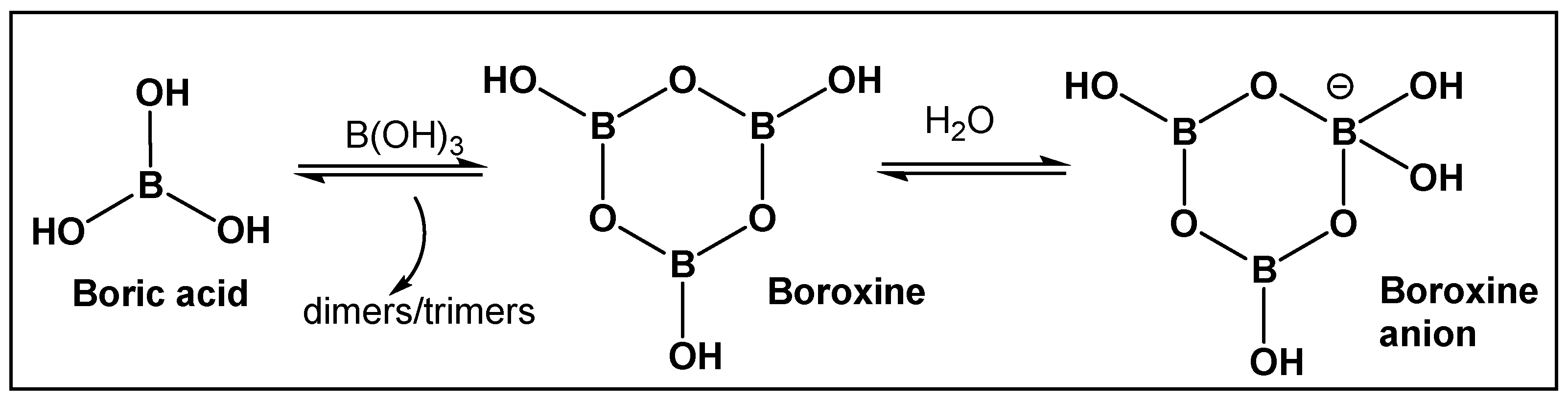
Boric acid and its linear and cyclic forms as a result of self-assembly and polymerization with additional boric acid molecules.
Organisms that incorporate boron into organic compounds via boron–oxygen bonds, are very limited; however, in nature, compounds with carbon–boron bonds (e.g., organoboranes) have not been reported. Until several years ago, the syntheses of organoboranes were the sole prerogative of organic synthetic chemists [12,13,14]. That has changed with the utilization of enzyme-catalyzed borylation leading to the preparation of chiral organoboranes by a genetically encoded platform in Escherichia coli [15]. Since the 1990s, especially following the pioneering work of Suzuki and Miyaura on the utilization of organoboron compounds for C-C bond formation resulting in the production of conjugated systems of alkenes, styrenes, or biaryl compounds, the publications of both synthetic methodology and drug development of boron-containing structures have skyrocketed [16]. The synthetic methodology has paved the way for the use of new boron-containing organic molecules with pharmaceutical applications, e.g., boronic acids and their derivatives. The air-stable organic sp2 boron compounds with a neutral hybridized trivalent boron, such as in boronic acids, have a center with a vacant p orbital. The latter is the reason for the mild Lewis acidic character displayed by boronic acids. They, and other boranol (B-OH)-containing compounds, due to their ability to form tetracoordinate conjugate base (sp3 hybridized) in water, display indirect Brønsted acidity The Lewis acid character and the ability to convert between sp2 and sp3 hybridized boron–hydroxy complexes enable boronic acids/hemiboronic acids, e.g., benzoxaboroles, to participate in a variety of covalent and hydrogen bond interactions with biological membranes/target proteins at physiological conditions thus placing them in the drug class of reversable covalent inhibitors.
There are many excellent in-depth reviews on boron chemistry, and the reader is directed to several excellent research articles and reviews summarizing various aspects of the current state of the synthetic methodology and drug development of boron-containing organic molecules [17,18,19,20,21,22,23,24,25]. The limited number of boron-containing organic molecules found in nature has left chemists with very few natural leads as models for drug development. That differs dramatically from the number of the rest of the known naturally occurring antimicrobials, and perhaps that provided the opportunity for the “de novo” synthesis and use of organoborons as medicinal agents. This review outlines the rationale for boron-containing organic compounds as antimicrobials/anti-biofilm agents. The bibliography contains 216 references from the early examples of organoboron as antimicrobials/anti-biofilm agents (1960s) to the first half of 2024.
A biofilm is a population of microbes that attaches itself to biotic and abiotic surfaces, while producing extracellular polymers to encase itself for protection against environmental assaults. The vast majority of chronic infections and environmental biofouling issues are the result of biofilm formation. Boron-containing small organic molecules are being explored as anti-biofilm compounds, and some have demonstrated promising activity in interfering with the biofilm formation of bacteria and fungi. Historically, they have been developed as inhibitors of the established antimicrobial drug targets, such as β-lactamases. The discussion of the latter is included herein, due to their role as a starting point for their use as anti-biofilm agents. Polymeric structures, utilizing the ability of boron to stabilize crosslinks in these macromolecules, or its binding interactions with different moieties of the polymeric structures of the bacterial surface (e.g., membranes), have been the focus of exploration as anti-biofilm agents, as well and their antimicrobial and antifungal properties are also included herein.
Biofilm. It is estimated that more than 80% of all microbial infections involve biofilm formation. In the last two decades, it has been established that the formation of biofilm is the predominant life-mode of most bacterial species [26]. The studies of the molecular mechanisms underlying biofilm formation and biofilm dispersal have led to the identification of distinct molecules and mechanisms as targets for drugs specifically designed to function as anti-biofilm agents. The molecular target of each anti-biofilm drug class is described below.
The main target for many boron-containing molecules is quorum sensing (QS), a regulatory process that has a role in biofilm formation and is an overall regulator of virulence factor expression in pathogenic bacteria [27]. The development of specific anti-biofilm drugs is a strategy that is expected to alleviate the current antibiotic crisis due to the development of drug resistance. It should be noted that antimicrobial resistance (AMR) alone will add an expected annual cost of $100 trillion in the USA alone by 2050 [28]. Potential anti-biofilm drugs could be classified as next-generation antimicrobials (NGA) since the target, i.e., biofilm formation, is different from the existing targets of the currently used clinically relevant antibiotics. Ideally, NGAs should also possess anti-virulence properties at concentrations that do not impact bacterial viability, thereby minimizing the selective pressure they apply and the probability of developing bacterial resistance towards them [29].
2. Utilizing the Boric Acids’ Scaffold by Following Nature’s Pragmatism
2.1. Naturally Occurring Bacterial Boron–Oxygen Bond-Containing Compounds (Boric Acids and Derivatives)
2.1.1. The Unique Antimicrobials Containing Boron
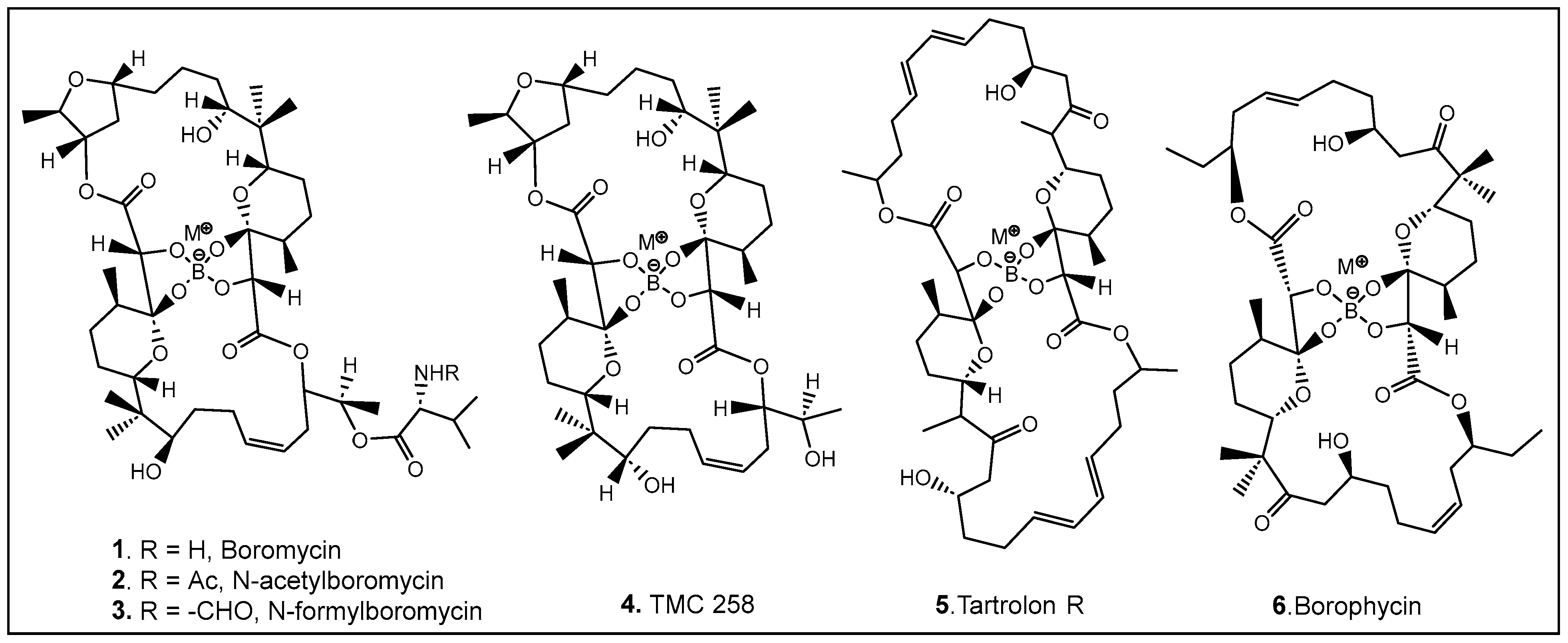
The most common naturally occurring boron-containing molecules are the complexes of boric acid with the diol moiety of biomolecules. About 6 decades after the discovery of the importance of boron as microelement for higher plants [30,31,32,33,34,35,36,37,38], the first organic compound containing boron was reported. Boromycin 1 (Figure 3) was isolated from the Streptomyces species [3,5,39,40,41]. A structurally similar compound named tartrolon 5 (Figure 3) [42,43] was subsequently isolated from Sorangium cellulosum, a myxobacterium [8] and from the symbiotic cellulose-degrading bacteria in shipworm gills [5]. Derivatives on the amino group of boromycin N-acetyl- (2, Figure 3) and N-formyl- (3, Figure 3) boromycin have also been isolated from the boromycin-producing Streptomyces antibioticus [4], as well as another boromycin derivative—desvalinoboromycin or TMC 25B 4 (Figure 3) from soil Streptomyces spp. [44]. Boromycin, together with several other polyketide antibiotics (Figure 3), belongs to the family of the boron-containing antibiotics produced by Gram-negative bacteria, which were initially found to be effective against Gram-positive bacteria [3,4,5,41]. Several other activities of boromycin and its derivatives were later determined, i.e., antiviral, specifically anti-HIV [44,45], coccidiostatic [46], anti-toxoplasmitic/anti-cryptosporidic [41,47], and anti-mycobacterial [48]. Boromycin, a K+ ionophore [48], has also been found to have effect on Ca2+ homeostasis [49]. In boromycin and structurally related antibiotics, boron plays a structural role, inducing the folding of the polyols into compact structures [17]. The potent cytotoxin, borophycin, was isolated from the marine cyanobacteria Nostoc spongiaeforme var. tenue [50] and Nostoc linckia [51] and hasbeen investigated for its anticancer activity [52]. The structural elucidation, biosynthesis, synthesis, and biological activities of boron-containing antibiotics have been extensively reviewed [9,10,53,54].
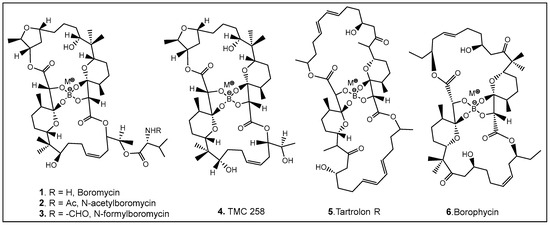
Figure 3.
Representative examples of currently known boron-containing antibiotics forming a Böeseken complex (a complex formed between alcohols/α-hydroxy acids and boron) [51,53].
2.1.2. Autoinducer-2 (AI-2) Quorum Sensing (QS) Inducer: A Borate Diester
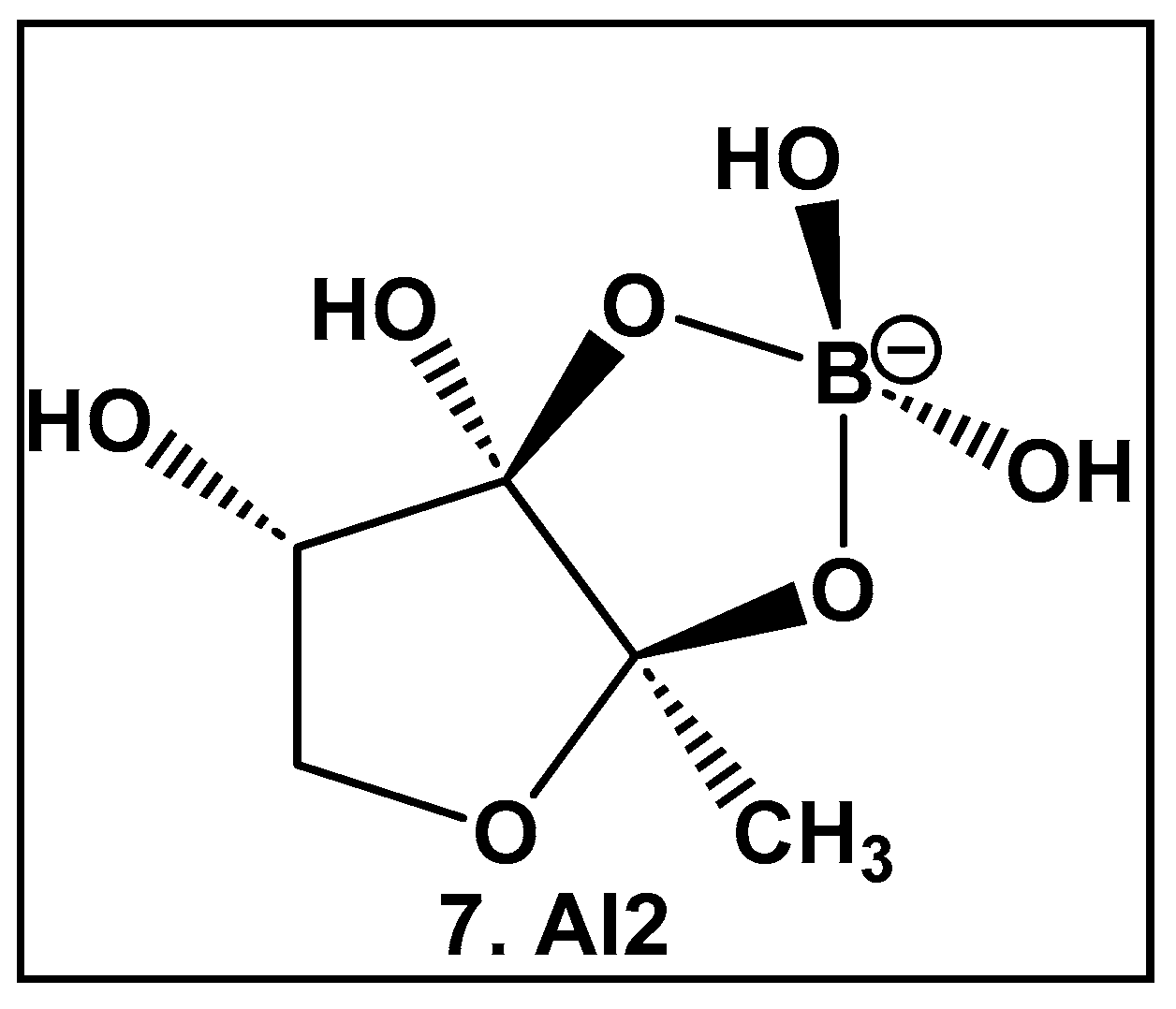
AI-2 furanosyl borate diester complex 7 (Figure 4), which highly resembles the core of the larger boron-containing antibiotics (Figure 3), was isolated from the bioluminescent marine bacterium Vibrio harveyii as one of two autoinducers that regulate light production in this bacterium in response to cell density (quorum molecule) [55,56]. Bacteria communicate using extracellular (hormone-like) signal molecules termed as autoinducers in a process called quorum sensing, which is typically dependent on the cell population size. It has been found that both Gram-negative and Gram-positive bacteria have the necessary enzymatic machinery to produce AI-2 [57]. Thus, the boron-containing AI-2 has been suggested to be the “universal” bacterial QS signal in interbacterial communication [55,56,57,58,59,60,61,62]. That realization has led to the design and synthesis of AI-2 analogs [60,61], as well as various potential AI-2 inhibitors [63,64,65,66,67,68,69,70,71,72]. To date, there are no FDA-approved drugs as AI-2 autoinducer inhibitors, most likely due to the complexity of QS signaling and promiscuity of the receptors which are involved in the synergistic agonism of AI-2 and analogs [62].
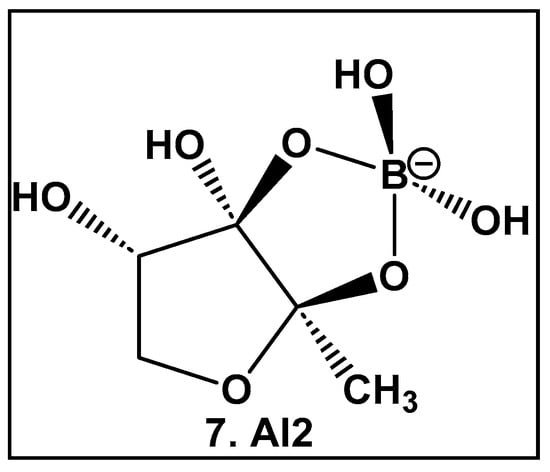
Figure 4.
Structure of AI-2, the universal QS autoinducer: a boron-diester.
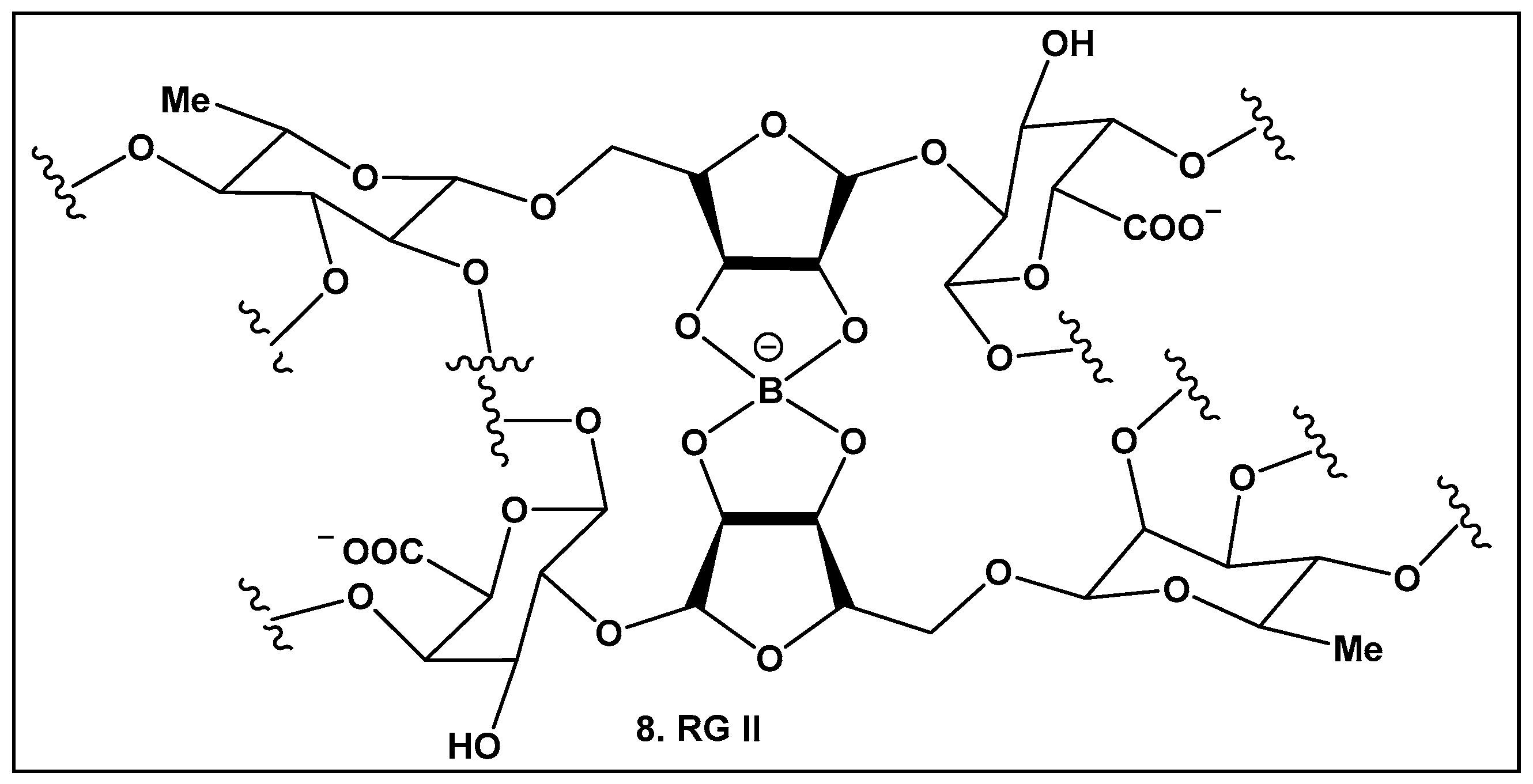
2.1.3. Rhamnogalacturonan II (RG-II)—a Boron–Sugar–Alcohol Complex
Boron carbohydrate complexes have been isolated from bacteria, seaweed, lichens, and fungi [9,10], with boron alcohol complexes from higher plants discovered close to half a century ago [73]. Their representative, Rhamnogalacturan II (RG-II) (8, Figure 5), is one of the most complex carbohydrates ever found in nature [73]. RG-II is found in the primary cell walls of all vascular plants [74,75,76,77,78,79]. The borate crosslinking in this carbohydrate is required for the normal plant development and growth, and its production requires an array of different proteins [74,75,76,77,78,79,80,81]. Mutations leading to alterations in the RG-II structure also affect crosslinking and could lead to severe impaired growth or even to plant death [82]. Beyond plants, RG-II has been recently identified as a Toll-like receptor 4 agonist that inhibits tumor growth by activating dendritic cell-mediated CD8+ T cells [83] in mammals.
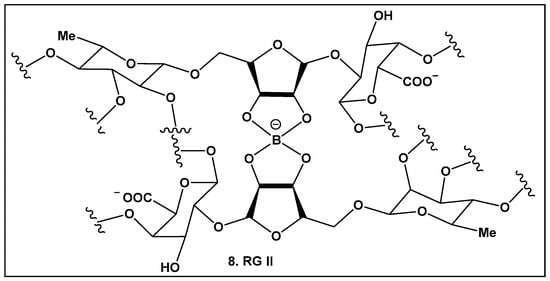
Figure 5.
Boron-containing compounds in higher plants: Partial structure of Rhamnogalacturonan II (RG-II) with focus on boron–oxygen complex at the center of the RG-II molecule.
Regardless of the type of organisms in which they are present, natural boron-containing compounds are those with boron–oxygen bonds. The rapid improvement in many spectroscopy techniques, such as nuclear magnetic resonance (NMR) and, more specifically, Boron11 NMR, has allowed for the detection of a higher percentage of naturally occurring boron-containing compounds than expected [84]. It will be interesting to see whether the yet to-be-discovered boron-containing compounds will have more variety in boron-bonding, or whether they will confirm nature’s pragmatism in binding.
3. Compounds Prepared by Organic Syntheses: Not Yet Found in Nature
3.1. “Nonclassical” Anti-Bacterial Chemotypes: Synthetic Boron—Carbon Bond-Containing Compounds
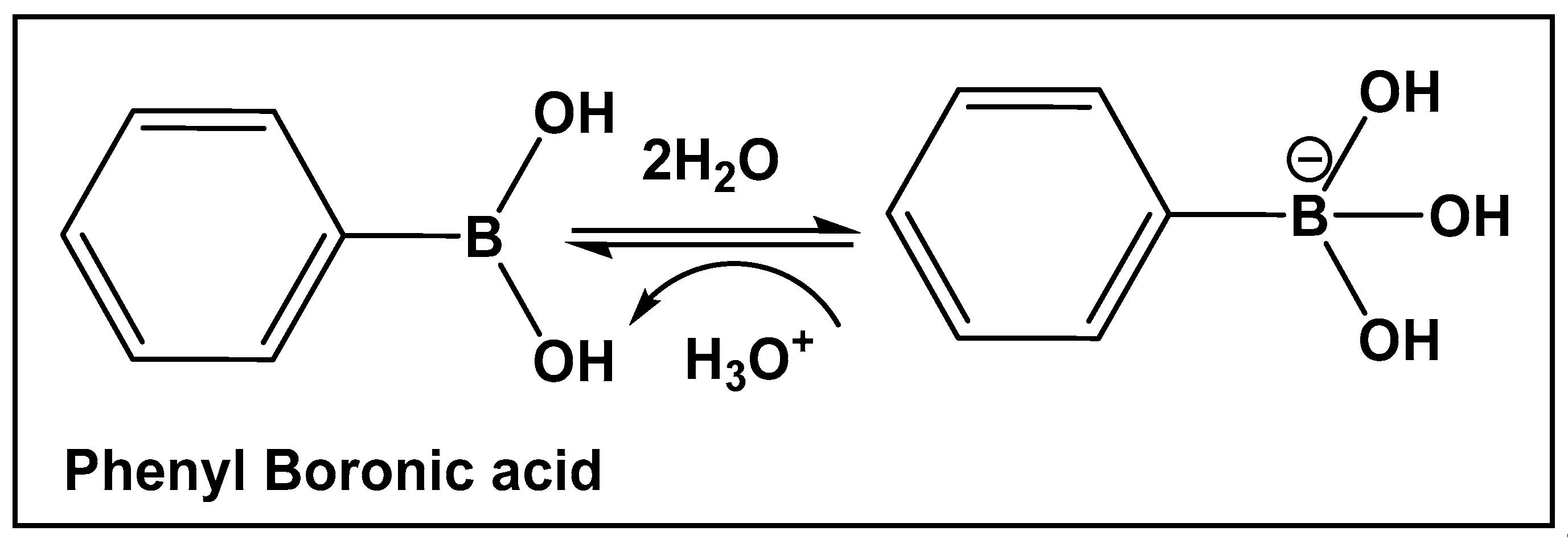
Boron-carbon bond-containing compounds’ reversible interconversion from sp2 to sp3 in the presence of nucleophiles is shown in Figure 6.
Figure 6.
Illustrative example of the interconversion of the hybridization of boronic acids in the presence of water.
3.1.1. Boronic Acids and Their Cyclic Hemiesters: β-Lactamase Inhibitors
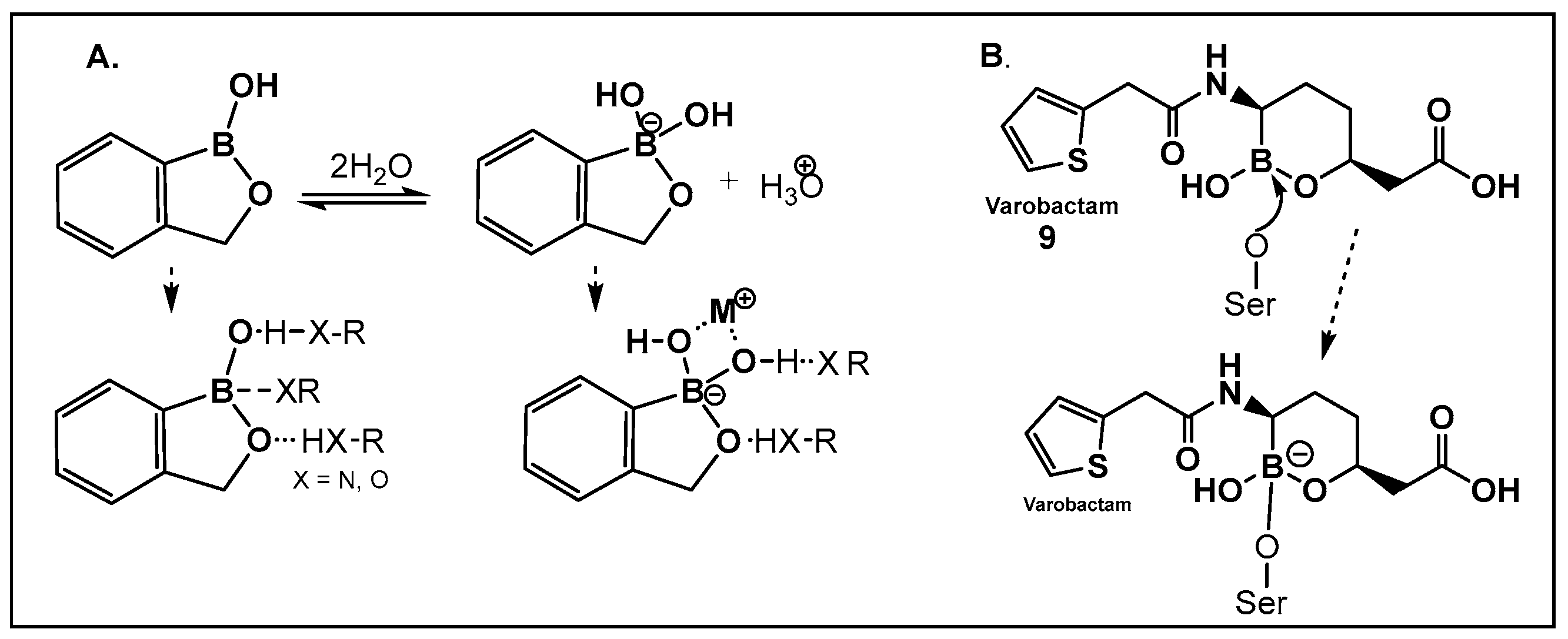
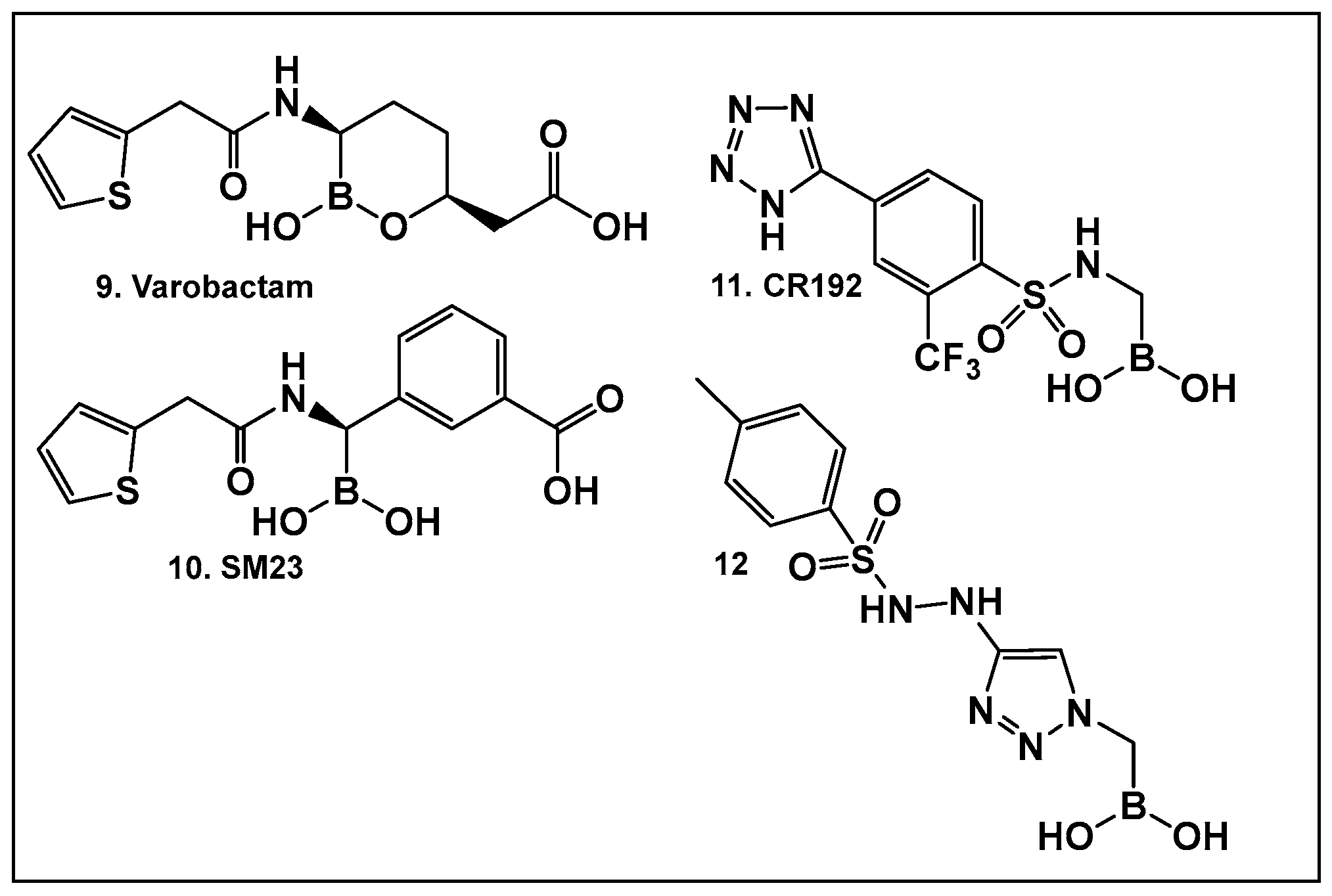
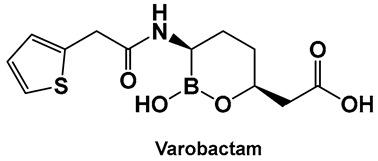
Vaborbactam (9, Figure 7 and Figure 8) is a boron-containing compound approved by the FDA (2017) as a β-lactamase inhibitor [85]. It is considered the most potent inhibitor of its class, with activity against class A serine carbapenemases. Specifically, vaborbactam’s inhibitory function is restricted to Klebsiella pneumoniae carbapenemase (KPC), while exhibiting no activity against mammalian serine proteases. The potential of boron-containing compounds and specifically that of boronic acids (BAs) as antimicrobials was demonstrated in the 1970s, with the use of the boronic acid (BA) as a reversible inhibitor of the serine β-lactamase from Bacillus cereus [86]. The hydrolysis of β-lactams by the β-lactamases involves the well-documented formation of a tetrahedral intermediate. Boronic acids are often described as “transition state analogs” (BATSIs), since they have a Lewis Acid (LA) property, i.e., they can act as electrophiles, a quality that permits the formation of tetrahedral adducts with alcohols/(serine) enzymes by interconversion between their sp2 and sp3 forms (Figure 7) [85,86,87,88,89,90,91,92,93]. Securing target selectivity, i.e., maximizing the selectivity for a given enzyme, e.g., β-lactamases, leading to diminishing/evading the off-target effects, is of great importance for the success of a given structure as a drug candidate.
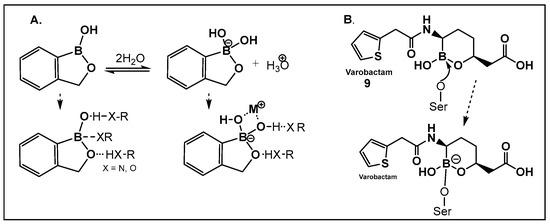
Figure 7.
(A). Equilibrium dissociation and biologically relevant interactions of benzoxaborole in water; (B). Molecular mode of inhibition of vaborbactam, 9, a hemiboronic acid drug by the active site serine.
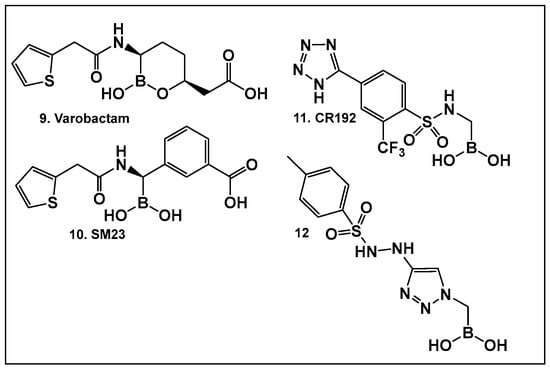
Figure 8.
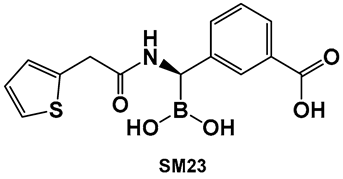
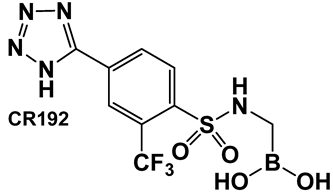
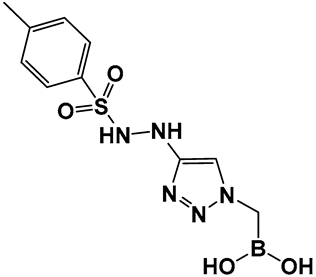
Examples of boronic acids and their hemiesters targeting β-lactamses: Boronic acids 10–12 and the cyclic hemiester of boronic acid, and FDA-approved Vaborbactam 9 as β-lactamase inhibitors.
The utilization of α-aminoalkyl boronic acids, classified amongst the α-amino acid analogs, led to the synthesis of peptidyl derivatives of the boronic acid as selective β-lactamase inhibitors. These included SM23, 10 (Figure 8) which has activity against Acinetobacter baumannii cephalosporinase (ADC-7, Ki 21 nM) [94,95,96], as well as class C β-lactamase (AmpC, Ki 1nM) [88,89,90]. SM23 10 exhibits an improved inhibition (>2-fold) as compared to the analogous glycylboronic acids [90], including RPX7009, (Vaborbactam) 9, (Figure 8). Preparation of a cyclic boronic acid derivative RPX7009, (Vaborbactam) 9, (Figure 8) led to high potency and selectivity for the β-lactamases vs. the human proteases, which have an affinity to acyclic substrates/molecules [85]. Vaborbactam has been approved by the FDA for use in combination with meropenem to selectively target pathogens producing serine carbapenemases [93,94]. This narrow FDA usage approval is despite its inhibitory activity against many other types of serine β-lactamases [94], with it demonstrating particularly potent activity against class A enzymes, Klebsiella pneumoniae carbapenemase (KPC), extended spectrum β-lactamases (ESBLs), Cefotaximase-Munich (CTX-M), sufhydryl reagent variable (SHV), and class C cephamycin β-lactamase (CMY) enzymes [91,92,93,94,95,96]. In addition, it has better activity as compared to the β-lactamase inhibitors clavulanic acid and tazobactam against the class A carbapenemase KPC-2 (Ki 0.069 mM), as well as the class C enzymes P99 and CMY-2 (Ki 0.053; Ki 0.03 mM, respectively) [85]. Both SM3, 10, and RPX7009 (Vaborbactam), 9 (Figure 8), activities are based on an amide scaffold, which gives them the ability to mirror the β-lactams’ amide chain, a structural feature that makes them highly selective and potent as β-lactamase inhibitors.
The replacement of the amide moiety with sulfonamide as in CR192, 11 (Figure 8), [95] was intended to make the structure a better fit for the β-lactamase active site. Amide replacement with its non-classical bioisostere—1,2,3-triazole as in 12 (Figure 8)—was explored to prepare both lactamase inhibitors and molecular probes [96]. Both compounds in CR192, 11 (Figure 8, Ki 0.45 nM) [95] and 12 (Figure 8, Ki 90 nM for ADC-7) [96], demonstrated β-lactamase activity better than Vaborbactam (Ki 0.72 mM) against that enzyme.
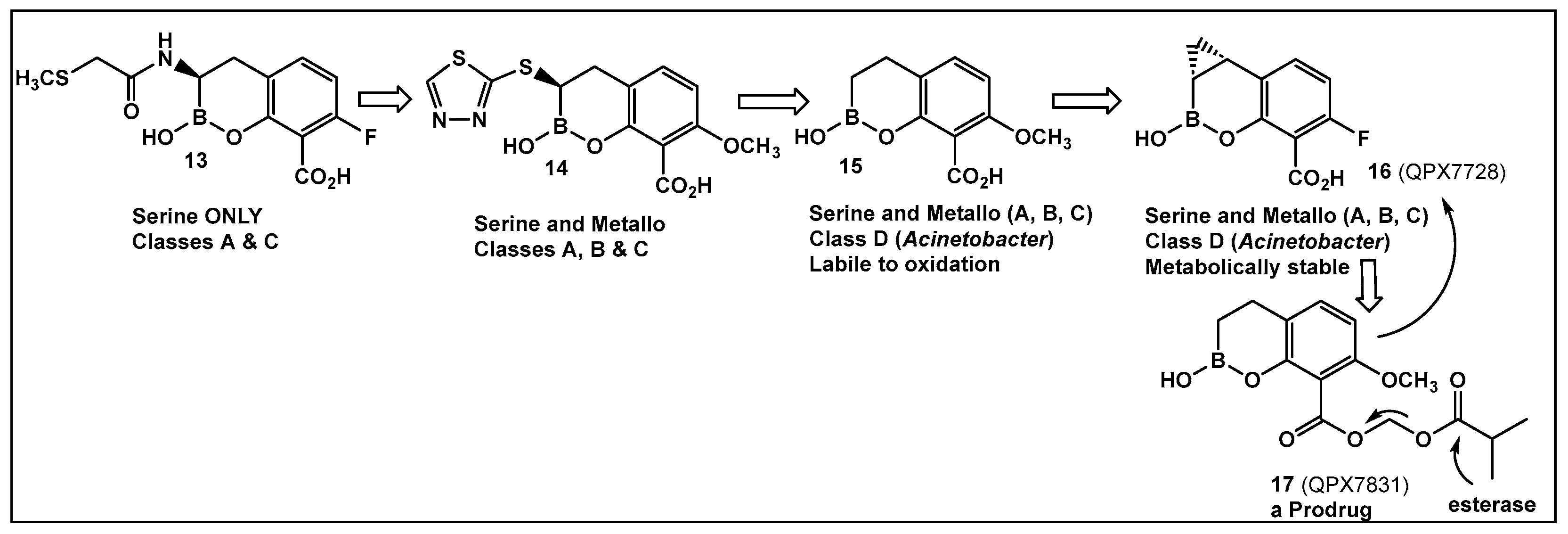
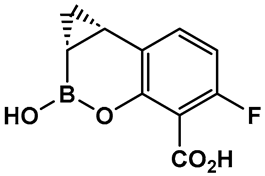
Further efforts toward both broadening the spectrum and improving the metabolic stability of vaborbactam have led to the synthesis of QPX7728 (16, Figure 9). The latter has a broad spectrum of inhibition, including class B and class D enzymes, and is considerably resistant to porin modifications and efflux [97]. The class B (metallo) enzyme NDM-1 of Enterobacteriaceae and the class D (OXA) enzymes of Acinetobacter baumannii continue to be refractory to inhibition, despite the success of vaborbactam as a broad-spectrum β-lactamase inhibitor. Therefore, the discovery of cyclic boronic acid QPX7728 (16, Figure 9), an ultra-broad-spectrum inhibitor of serine and metallo-β-lactamases, is a very important advancement in the development of β-lactamase inhibitors [97]. Compound QPX7728 (16, Figure 9), appears to be a promising agent for use in combination with a β-lactam antibiotics for the treatment of a wide range of multidrug-resistant Gram-negative bacteria [98]. The clinical development of QPX7728 (16, Figure 9) is still in progress (Phase III Clinical Trials) [99].
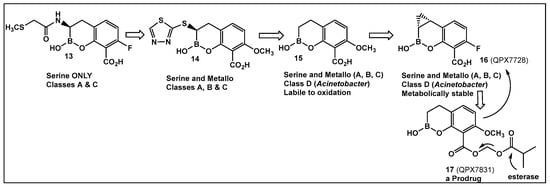
Figure 9.
Evolution of the hemiboronic acids toward broadening their spectrum as β-lactamase inhibitors (serine- and metallo-β-lactamases) and improving their metabolic stability [97,98,99,100].
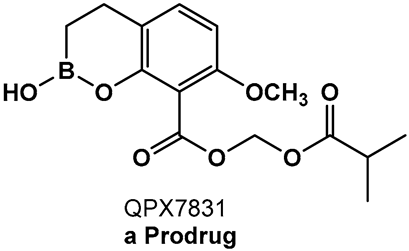
Addressing the need for effective oral therapies to treat Gram-negative bacterial infections, efforts were directed toward identifying an oral prodrug of the β-lactamase inhibitor clinical candidate QPX7728 (16, Figure 9). From the 17 prodrugs synthesized, compound QPX7831 (17, Figure 9) emerged with optimal properties across all key attributes—rates of cleavage to the active form in vitro, pharmacokinetics across species, and crystallinity [100].
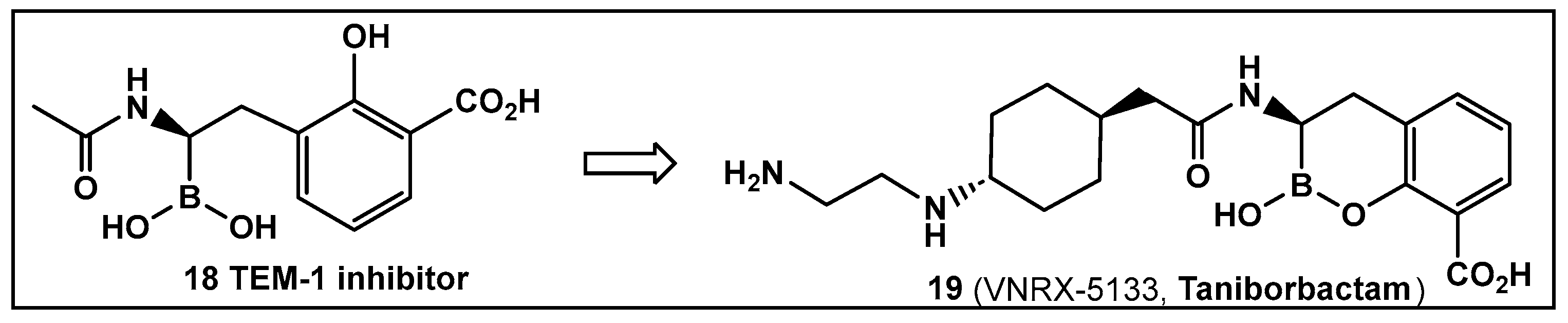
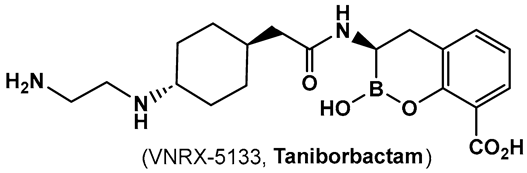
Another direction in the evolution of the hemiboronic acids as broad-spectrum inhibitors led to the synthesis of Taniborbactam (VNRX-5133, 19, Figure 10). Its design strategy is based on the amide 18 (Figure 10), which is a TEM-1 β-lactamase inhibitor [101]. The lead optimization of the narrow spectrum amide 18 (Figure 10), led to preparation of compounds with a cyclic boronate scaffold. The initial library of hemiboronic acids were weakly active, with narrower spectrum than the desired inhibitors of β-lactamase enzymes [102]. Based on the observed type of binding of this initial generation of hemiboronic acids within the β-lactamase active site, a hydrophobic group was added to enhance the van der Waals interactions between the compounds and the hydrophobic residues with the β-lactamases. This change led to VNRX-5133 (19, Figure 10, Taniborbactam) [102]. Taniborbactam is selective for bacterial enzymes and is nontoxic to mammalian cells, making it the first pan-spectrum b-lactamase inhibitor to enter clinical development.
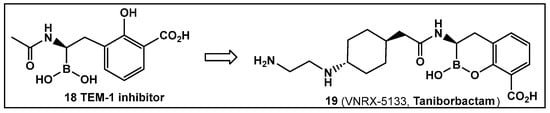
Figure 10.
Hemiboronic acid 19 VNRX-5133, Taniborbactam), a pan-β-lactamase inhibitor developed based on the binding of the boronic acid 18 as TEM-1 inhibitor [101,102].
Its N-(2-aminoethyl)- cyclohexylamine side chain proved to be critical for broad-spectrum β-lactamase inhibition and enhanced Gram-negative outer membrane permeability and periplasmic accumulation. Structural studies revealed that the pan-β lactamase inhibition VNRX-5133 (19, Figure 10) mimics the tetrahedral intermediates of both the serine-based and zinc based enzymatic hydrolysis processes [102].
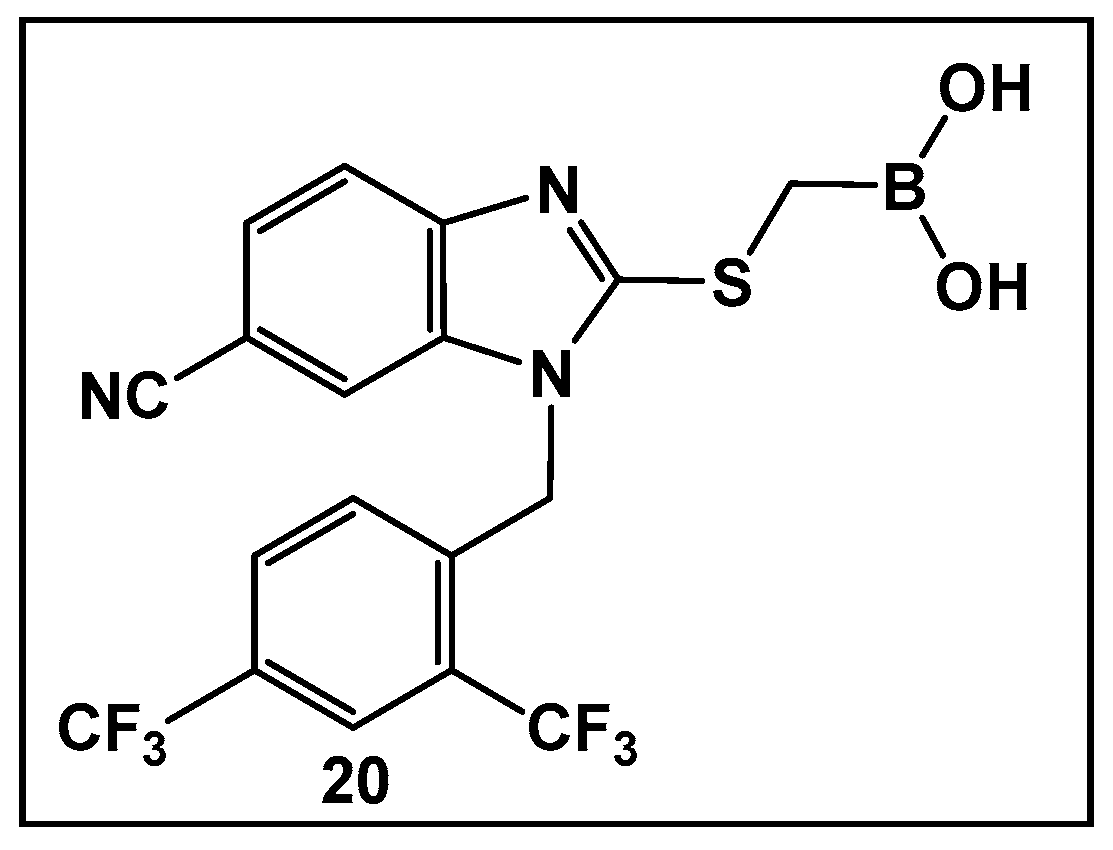
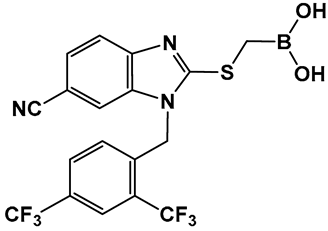
The development of boronic acids as inhibitors of other microbial defense systems against the β-lactam antibiotics, such as the sensor domain of BlaR in methicillin-resistant S. aureus (MRSA), has been recently reported 20 (Figure 11) [103]. Sensor domains of BlaR and/or MecR detect the presence of β-lactam antibiotics, and the information is transmitted to the cytoplasm, leading to reactivation of the antibiotic-resistance genes. Boronic acid 20 (Figure 10) is based on a benzimidazole-containing hit out of 11 million compounds screened in silico. The crystal structure confirms the engagement of 20 (Figure 10) with the serine residue of BlaR, inhibiting the BlaR induction 99% and suppressing bacterial growth by 24%.
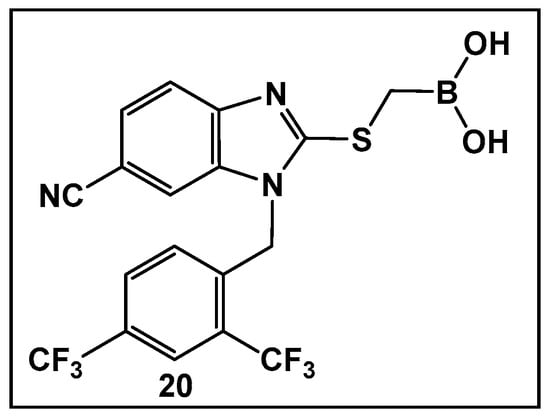
Figure 11.
Examples of boronic acids targeting microbial defense systems: boronic acid 20 as an inhibitor of the sensor domain of BlaR in MRSA [103].
3.1.2. Cyclic Hemiesters of Boronic Acids (Oxaboroles) as Antifungals
As the syntheses of numerous boronic acids and their esters have progressed, the versatility of hemiboronic acids as a pharmacophore has been unveiled. The FDA approval of an antifungal agent of this class validates the importance for the diversification of chemotypes in search of new alternative drug targets to aid in alleviating the current state of drug resistance [104,105,106].
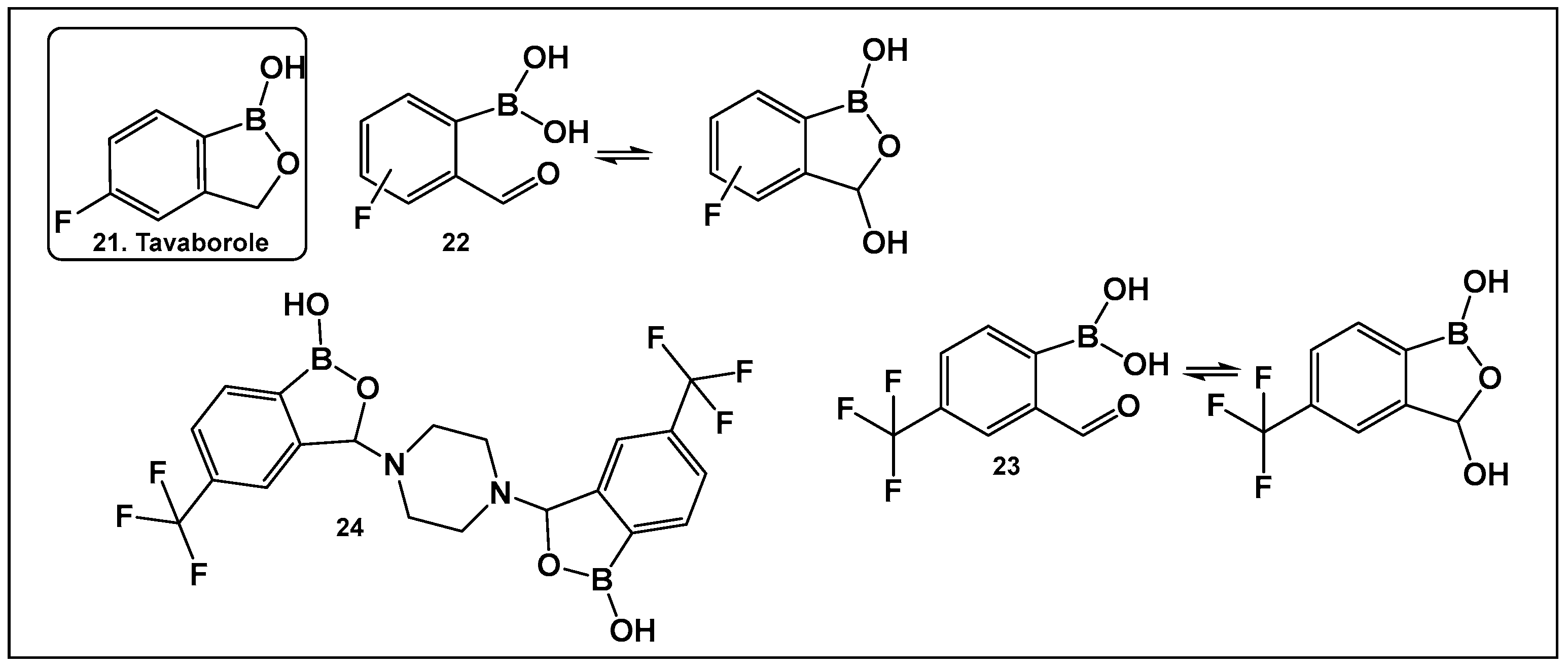
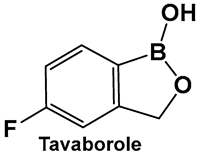
Tavaborole 21 (Figure 12) exerts antifungal activity by inhibiting the fungal aminoacyl-tRNA synthetase. Tavaborole binds to the diol, or terminal adenosine of the enzyme, leading to effective inhibition of fungal protein synthesis [107]. This drug (AN2690, Kerydin) was approved by the FDA in 2014 for the treatment of onychomycosis, a fungal infection of the nail [108]. The FDA approval of Vaborbactam, 9, (Figure 7 and Figure 8), another cyclic hemiester of boronic acid, took place in 2017.
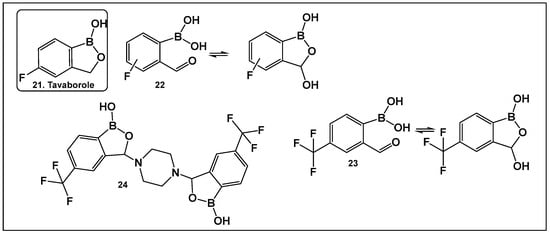
Figure 12.
Structures of oxaboroles: FDA-approved antifungal agent Tavaborole, 21; fluorinated derivatives of 2-formylphenyl boronic acids, 23, 24, designed as antibacterials and antifungals.
Other scaffolds, such as 2-formylphenylboronic acids 22 (Figure 12), have been evaluated for activity against the fungi Aspergillus, Fusarium, Penicillium and Candida. The ortho position of the formyl group is absolutely necessary for the antifungal activity of these compounds, most likely due to the presence of their tautomer (the cyclic 3-hydroxybenzooxaborole) in solution [109]. The para-fluoro analog of 22 (Figure 12), similarly to tavaborole, demonstrated the best activity against the majority of the fungi tested [109].
The effect of the replacement of fluorine with trifluoromethyl group as a substituent of the 2-formylphenylboronic 23 (Figure 12) has also been explored [110]. Both benzoxaborole and bis(benzoxaborole) derivatives of 2-formyl-4-(trifluoromethyl)phenylboronic acid have been evaluated for activity as antifungals and antimicrobials. The most potent antifungal compound is piperazine bis(benzoxaborole) 24 (Figure 12), which demonstrated MIC values of 7.8 μg/mL against Candida albicans and 3.9 μg/mL against Aspergillus niger [110]. Docking studies performed with the members in this compound library showed that binding LeuRS of C. albicans was analogous to that of tavaborole, 24, (Figure 12). In addition, 23 and 24 (Figure 12) have antimicrobial activity against Bacillus cereus with MIC values of 7.8 μg/mL, for both compounds [110].
3.1.3. AI-2 Bioisosteres as Biofilm Inhibitors: Phenyl Boronic Acids
Although originally designed as a β-lactamase inhibitor, SM23, 10 (Figure 8), has also demonstrated the inhibition of Pseudomonas aeruginosa, a Gram-negative microbe that is part of the ESKAPE group of pathogens [111], with biofilm production in the 0.390–25 μM range [111]. SM23, 10 (Figure 8), drastically hinders the release of the QS factors vital to P. aeruginosa, such as pyocyanin and elastase, and substantially downregulates the QS autoinducers. The authors report that the boronic acid derivative SM23 mimics the pathogen-produced autoinducers, downregulating both lasI and lasR gene transcripts and thus affecting the QS system, together with biofilm formation and virulence expression [111].
SM23, 10 (Figure 8), decreases the levels of biofilm formation (at 0.780–6.250 μM, about 50% of biomass reduction is observed) and that of pyoverdine production by P. aeruginosa, as assessed by a new in vitro model closely mimicking clinical settings (endotracheal tube contamination) [111]. It is interesting to note that even though the planktonic cells of P. aeruginosa strongly associate with SM23, 10, (Figure 8), they are not affected by the compound, which might be a sign of optimism that this compound could be resistant to the known drug resistance mechanisms, in addition to its dual action as a β-lactamase and biofilm inhibitor [111].
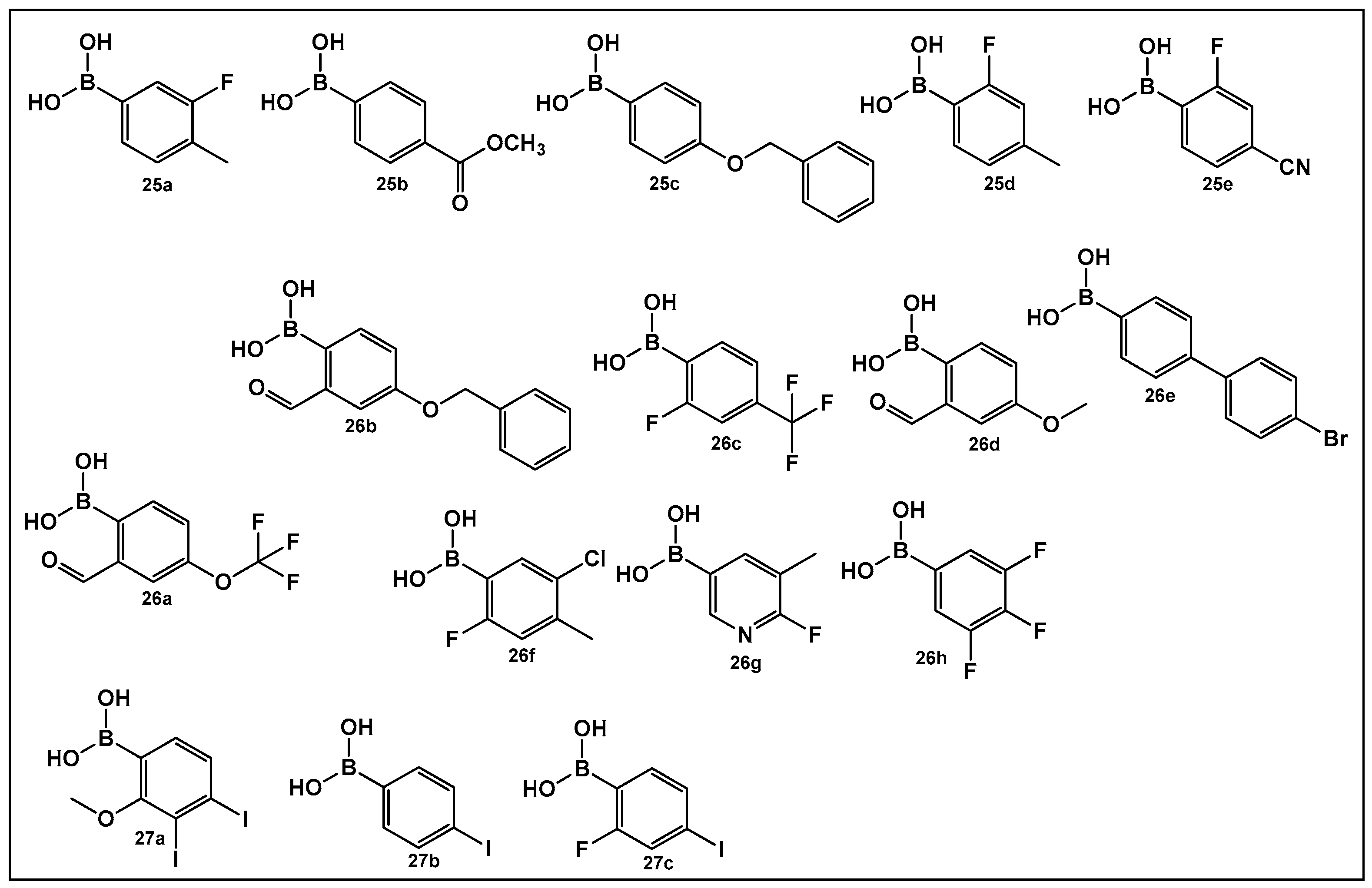
The activity of phenyl boronic acids, many containing mono- and poly- fluorinated or trifluoromethylated phenyl rings, belonging to different boronic acid libraries (the 25, 26 and 27 series, Figure 13), as inhibitors of biofilm formation in Vibrio harveyi were reported a decade earlier than the SM23, 10 (Figure 8) [112,113]. The IC50 values of these inhibitors (representative examples 25–27 shown in Figure 13) in whole bacterial cells are in the low to submicromolar range. Five boronic acids of the 25 series (25a–25e, Figure 13) showed significant inhibitory activities, with IC50 in the single digit micromolar range as follows: 25a, (IC50: 9 ± 5 μM); 25b, (IC50: 5 ± 2 μM); 25c, (IC50: 4 ± 1 μM); 25d, (IC50: 4 ± 1 μM), and 25e, (IC50: 6 ± 4 μM) [112]. Similarly, 11 compounds of the 26 series, representatives of which shown in Figure 13, (26a–26h) demonstrated activities at or below the single-digit range as follows: 26a, (IC50: 5 ± 2 μM); 26b, (IC50: 0.7 ± 0.1 μM); 26c, (IC50: 2 ± 0.3 μM); 26d, (IC50: 6 ± 2 μM); 26e, (IC50: 3 ± 1 μM); and 26f, (IC50: 6 ± 2 μM), and they also appear to not have significant inhibition of bacterial growth. At the concentrations that showed significant QS inhibition, no general cytotoxicity was observed. The rest of the compounds such as boronic acids 26g and 26h (Figure 13) showed IC50 between 11 and 100 μM, and several boronic acids compounds having IC50 above 100 μM [113].
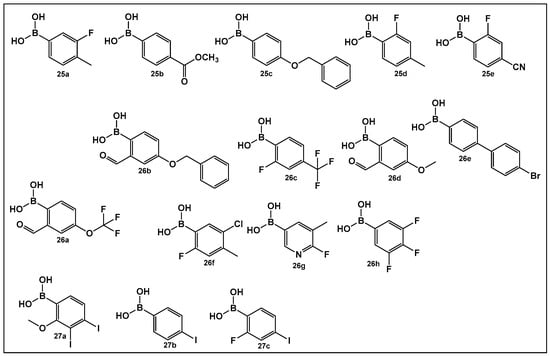
Figure 13.
Phenyl boronic acids as QS modulators.
A recent study has expanded the exploration of phenyl-containing halogenated acids, including three boronic acids 27a–27c (Figure 13), as biofilm inhibitors of two Vibrio species, V. harveyi and V. parahaemolyticus, by introducing iodine into the phenyl ring [114]. The phenyl boronic acids 27b and 27c (Figure 13) demonstrated both antibacterial activity against Vibrio planktonic cells at the 100 μg/mL concentration and prevented biofilm formation in a dose-dependent manner. These two compounds prevent biofilm on the surfaces of both squid and shrimp models as well [114].
3.1.4. AI-2 Bioisosteres as Efflux Pump Inhibitors/QS Inhibitors: Pyridine Boronic Acids
Overexpression of efflux pumps (EP) in bacteria historically has been associated with resistance to fluoroquinolone antibiotics. The established role of the EPs is the removal of substances toxic to the bacterium, such as antibiotics, metabolites, and QS compounds. In the last decade or so, a connection between the secretion of QS compounds and the EPs has been noted, based on the observation that some EPs’ inhibitors sensitize bacteria to antibiotics and can prevent biofilm formation [115,116,117,118,119,120,121,122].
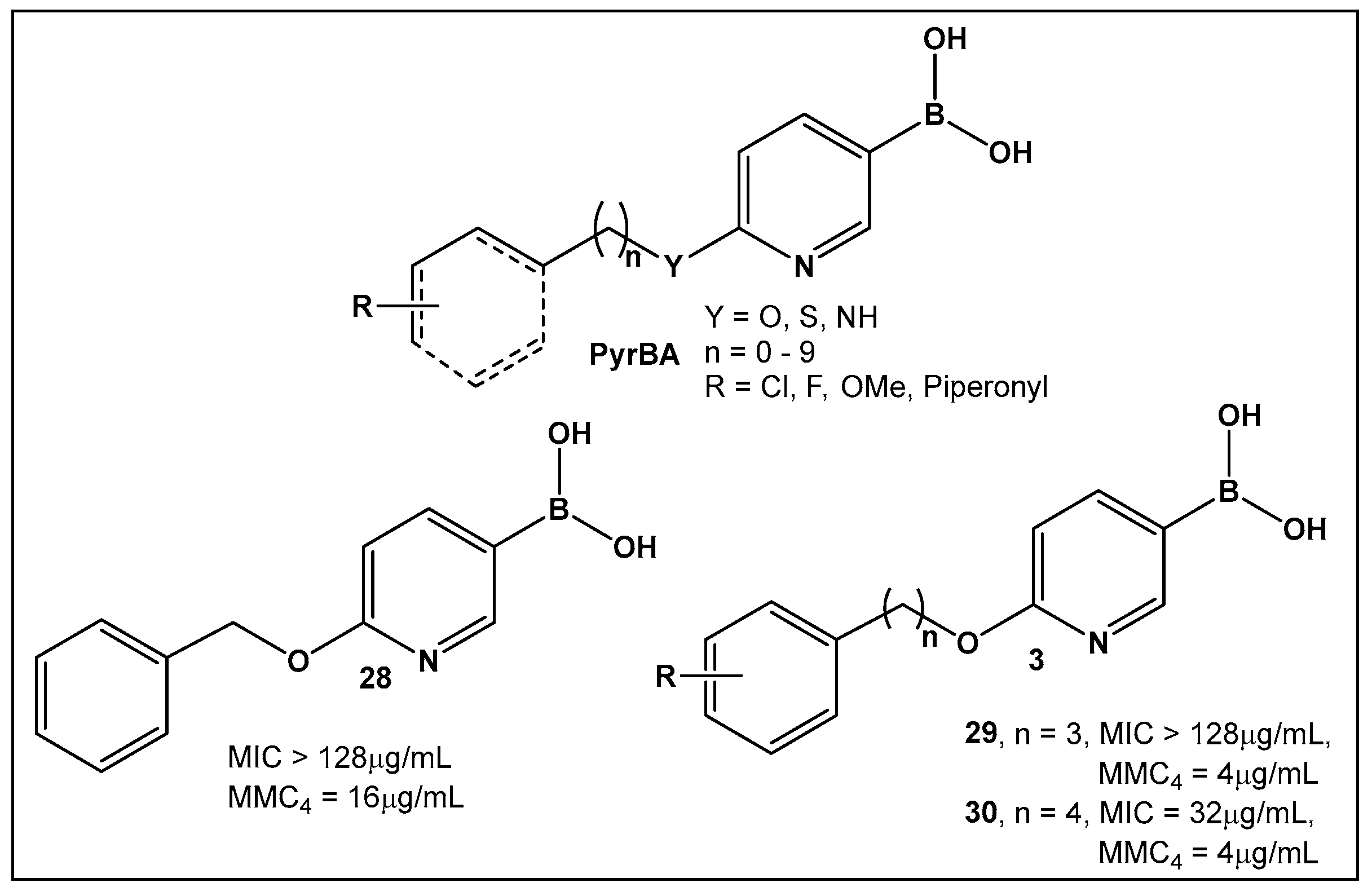
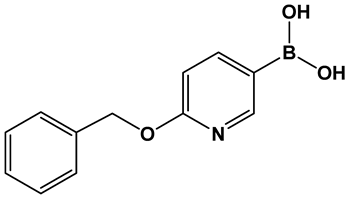
Potential inhibitors of the NorA efflux pump of Staphylococcus aureus were screened using a library of 150 heterocyclic boronic acids. Those with a pyridine-3-boronic acid scaffold (PyrBA, Figure 14) were selected for further development [123,124]. The most promising compound from the initial screen shown to potentiate ciprofloxacin activity in a NorA-overexpressing SA-1199B strain (4-fold increase) was 6-benzyloxypyridine-3-boronic acid 28 (Figure 14). Further structural modification of 28 (Figure 14) led to the second-generation derivatives of pyridine-3-boronic acids, from which compounds 29 and 30 (Figure 14) showed a further 4-fold increase in activity as compared to 28 (Figure 14). These two compounds potentiate the activity of ciprofloxacin and norfloxacin, while exhibiting no significant intrinsic antibacterial activity. Importantly, they do not inhibit the mammalian P-gp efflux pump.
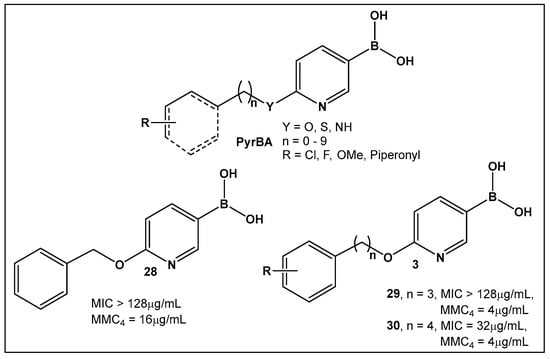
Figure 14.
3-Pyridine boronic acid-based inhibitors of NorA efflux pump of S. aureus, 29 and 30, demonstrate the best inhibitory activities and potentiate ciprofloxacin against resistant S. aureus by a 4-fold increase at MMC4 of 4 μg/mL.
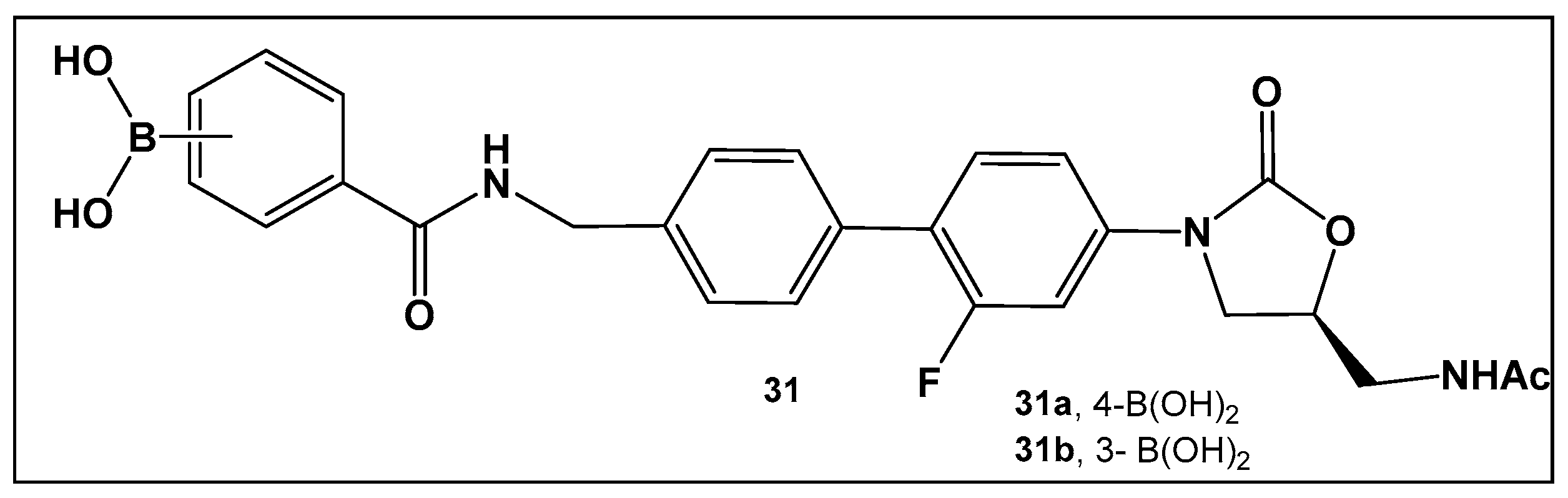
The external incorporation of phenyl boronic acid as a Lewis acid unit in the structure of oxazolidinone, 31 (Figure 15) has led to derivatives based on the N-aryl-oxazolidinones Linezolid and Radezolid with better activity than that of the two antimicrobials [125], with Linezolid considered as the drug of last resort against Gram-positive bacteria-induced infections [126,127,128,129]. Compounds of this type, i.e., 31 (Figure 15), have the unique binding mode of oxazolidinones structural type to the A-site pocket of the 50S subunit of bacterial ribosomes, peptidyl transferase center (PTC), negatively affecting bacterial protein synthesis and growth [130]. It is interesting to note that the Linezolid derivative, compound 31a (Figure 15), demonstrated levels of antimicrobial activity that were eight-fold to thirty-two-fold higher than Linezolid against a panel of Gram-positive strains, and a near to one hundred-fold activity against the Gram-negative E. coli JW5503, a mutant strain with a defective efflux capability (MIC of 0.78 μM for 31a vs. >50 μM for LZD), thus underscoring the potential of efflux pump incapacitation in antimicrobial/anti-biofilm drug development [125].
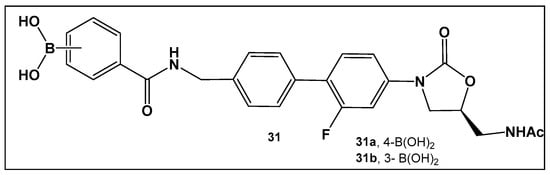
Figure 15.
Boronic acid derivative of N-aryl-oxazolidinones as inhibitor in Gram-positive bacteria.
3.1.5. AI-2 Bioisosteres as QS Modulators/Biofilm Modulators: Oxazaborolidines—Representatives of Boron–Nitrogen Bond-Containing Compounds
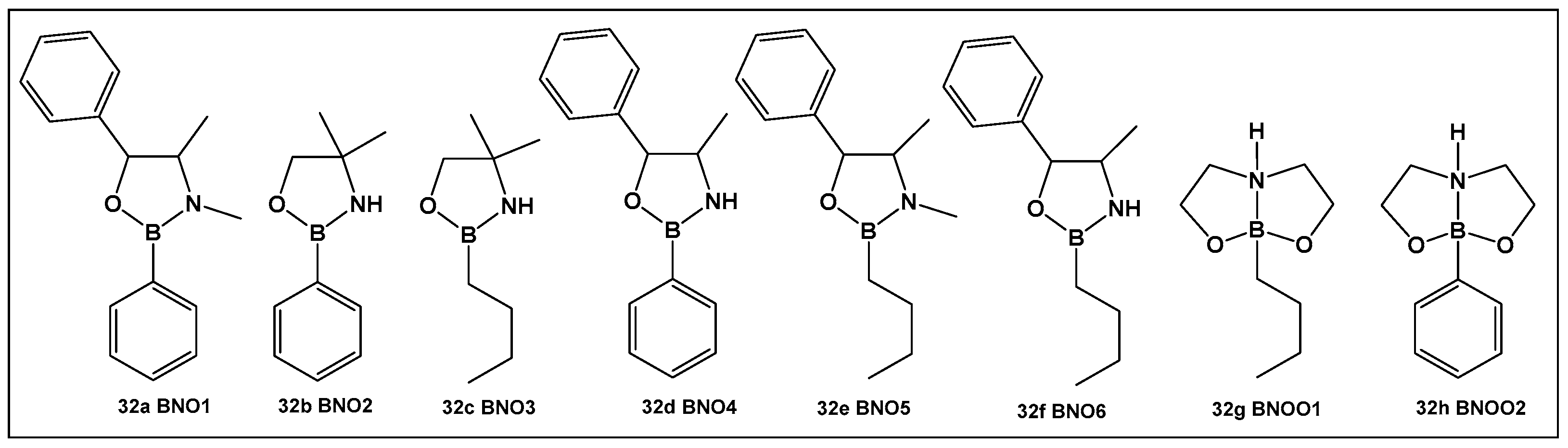
The first oxazaborolidines were introduced as catalysts for enantioselective synthesis since they can form chiral cations derived by various activation procedures [131,132,133,134]. These compounds can be readily obtained by the reaction of boronic acid with amino alcohols [131,132,133,134]. Several representative oxazaborolidines have been prepared and their antibacterial activity against Streptococcus mutans evaluated [135,136,137]. Since S. mutans plays a key role in dental caries and biofilm formation, identifying effective treatments against oral biofilm formation would be impactful for most of the human population.
From the compounds tested (32a–32h, Figure 16), the most active compound against Streptococcus mutans is 32e, MIC 0.53 μM. It has alkyl groups on the nitrogen and the boron, a methyl on the nitrogen and an n-butyl on the boron, which sets it apart from the rest of the compounds. Compounds that have one of these groups but lack the others (32a, 32c and 32f, Figure 16) have lower activity (compound 32d having the lowest MIC of 1.33 μM). In comparison with boronic acid at its maximal solubility in water (10 mM), these oxazaborolidines demonstrated antibacterial activity against S. mutans at much lower concentrations. When the compounds (32a–32h, Figure 16) were tested for their anti-biofilm activity, the boron–butyl moiety proved to be of great importance for the anti-adhesion effect for all the derivatives that possess it (32c, 32e–32g, Figure 16), with diminished effect upon the incorporation of the boron atom in a fused heterocyclic ring (32g, Figure 16) [9,10,136].
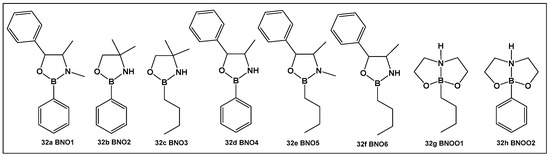
Figure 16.
Oxazaborolidines as AI-2 bioisosteres.
Oxazaborolidines 32a–32e (Figure 16), based on their structural resemblance to AI-2 QS autoinducer (Figure 4), have also been evaluated as QS modulators [138] in V. harveyi. Compounds 32a and 32e (Figure 16) strongly induced the bioluminescence in the V. harveyi mutant (BB170), which was lacking autoinducer 1 (AI-1), in a dose-dependent manner (0–600 μM). The latter, together with AI-2 and V. cholerae autoinducer 1 (CAI-1), mediates the bioluminescence of V. harveyi. The same two oxazaborolidines 32a and 32e (Figure 16) had no effect on V. harveyi’s (BB886) bioluminescence while lacking AI-2 [138]. Additional experiments using spent medium containing AI-2 or adding a synthetic precursor of AI-2 (termed DPD) in the presence of the compounds tested on a V. harveyi (MM77) mutant, which does not produce either AI-1 or AI-2, demonstrated that AI-2 is essential for the effect of 32a and 32e (Figure 16) on V. harveyi’s induction of bioluminescence. Thus, 32a and 32e (Figure 16) appear to be co-agonists of AI-2, since they enhance signal transduction only in the presence of AI-2 (or pre-AI-2) and only through the AI-2 cascade. That makes these two compounds the first oxazaborolidines to influence AI-2 activity [9,10,138].
3.1.6. Aromatic Boron-Containing Heterocycles: Hemiboronic Naphthoids (i.e., Benzodiazaborines)
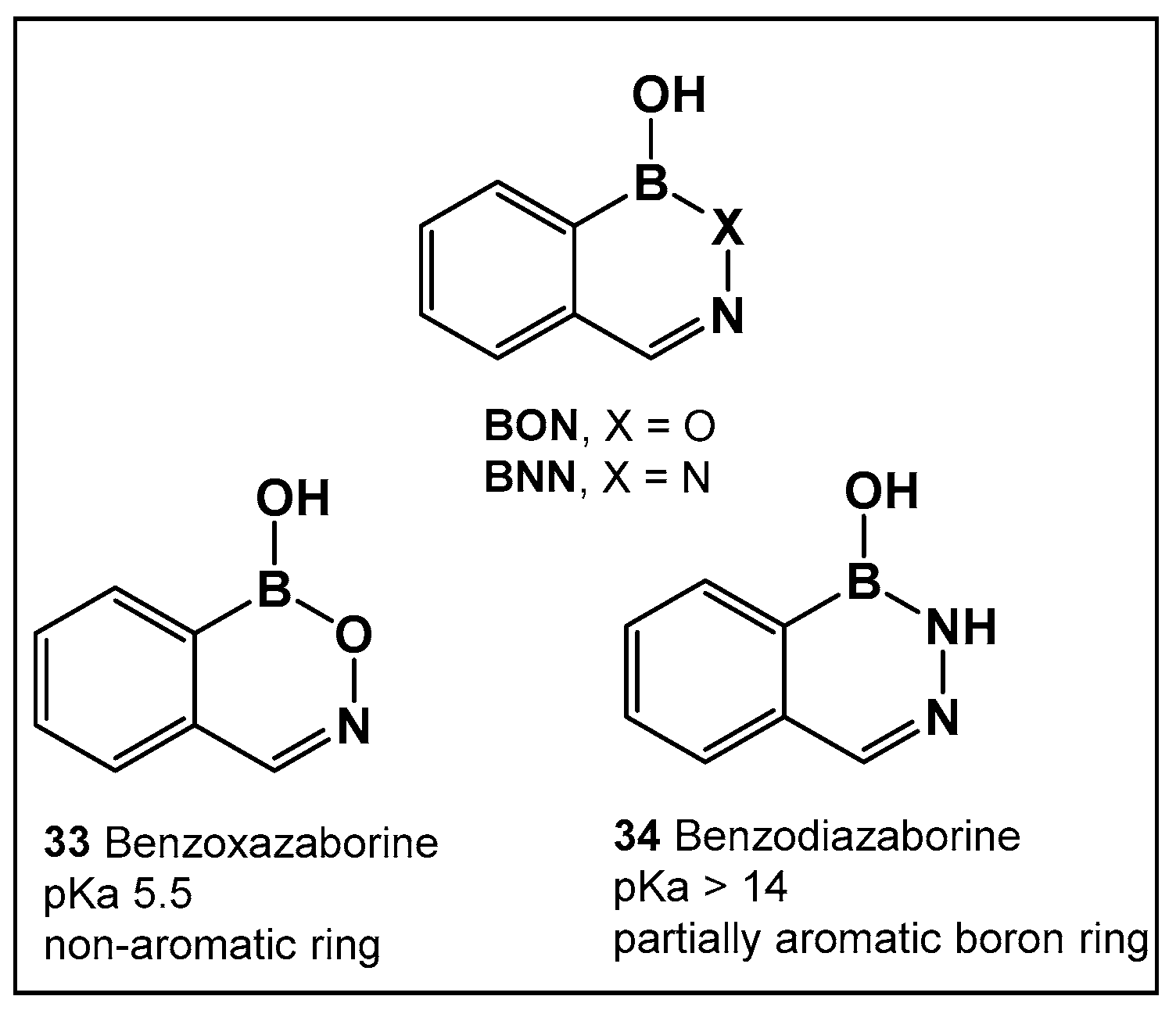
Boron compounds with a Boron–Oxygen–Nitrogen complex (B–O–N motif/BON heterocycles, Figure 17) are much less explored, even though their first representatives were prepared in the early 1960s. In recent years, there has been a growing interest in BON heterocycles as new chemotypes for drug design. The exocyclic B–O–N motif, which is readily formed under mild conditions, is unexpectedly hydrolytic and thermally stable [24,139,140].
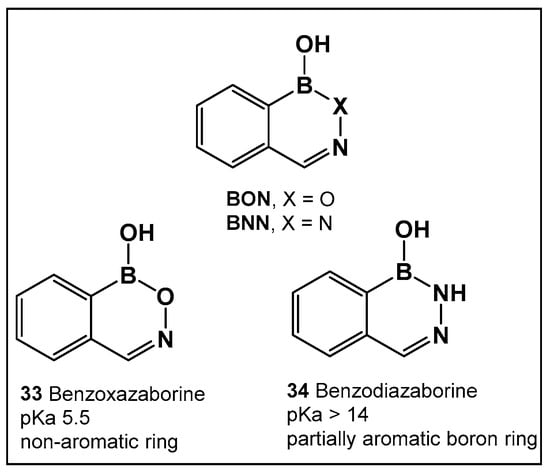
Figure 17.
Structures of benzoxazaborines (BONs) and benzodiazaborines (BNNs) and their different chemical characteristics.
Further exploration of the electronic proximity of boron to carbon resulted in the synthesis of a new chemical scaffold BNN (Figure 17) and the determination of its physicochemical properties [24,139,140]. While benzoxazaborines, BONs (Figure 17), with a boron-containing six-membered ring lack an appreciable aromatic character, they are chemically related to benzoxaboroles due to their moderate acidity (pKa 5.5 for 1a vs. 7.4 for benzoxaborole) [24]. Replacement of the oxygen with a nitrogen atom, however, leads to BNN (Figure 17), the aza-analogs, i.e., benzodiazaborines, which display partially aromatic characteristics, and a much higher pKa. Thus, benzodiazaborines are considered a naphthoid isostere [24,140]. Both compound classes (benzoxazaborines and benzodiazaborines) are soluble and stable in aqueous solutions, a physicochemical property that aids the biological screens [24,140]. Their differences, however, allow for these two compound classes (BON and BNN, Figure 17) to serve as functional mimics of two different “established” chemotypes, namely benzoxaboroles and aromatic scaffolds, e.g., hydroxyquinolines, naphthols, and phthalazinones, respectively.
The 1960s marked the beginning of the exploration into benzodiazaborines as a chemotype in medicinal chemistry [141]. The antimicrobial activity of 1,2-dihydro-1 -hydroxy- 2-(organosulfonyl)benzo-, furo-, and -thieno[d][l,2,3]diazaborines have been established a decade later [142,143]. Synthesis of differently substituted benzo- and heterocyclo-substituted diazaborines and their evaluation as antimicrobials continues to draw interest [144,145,146,147].
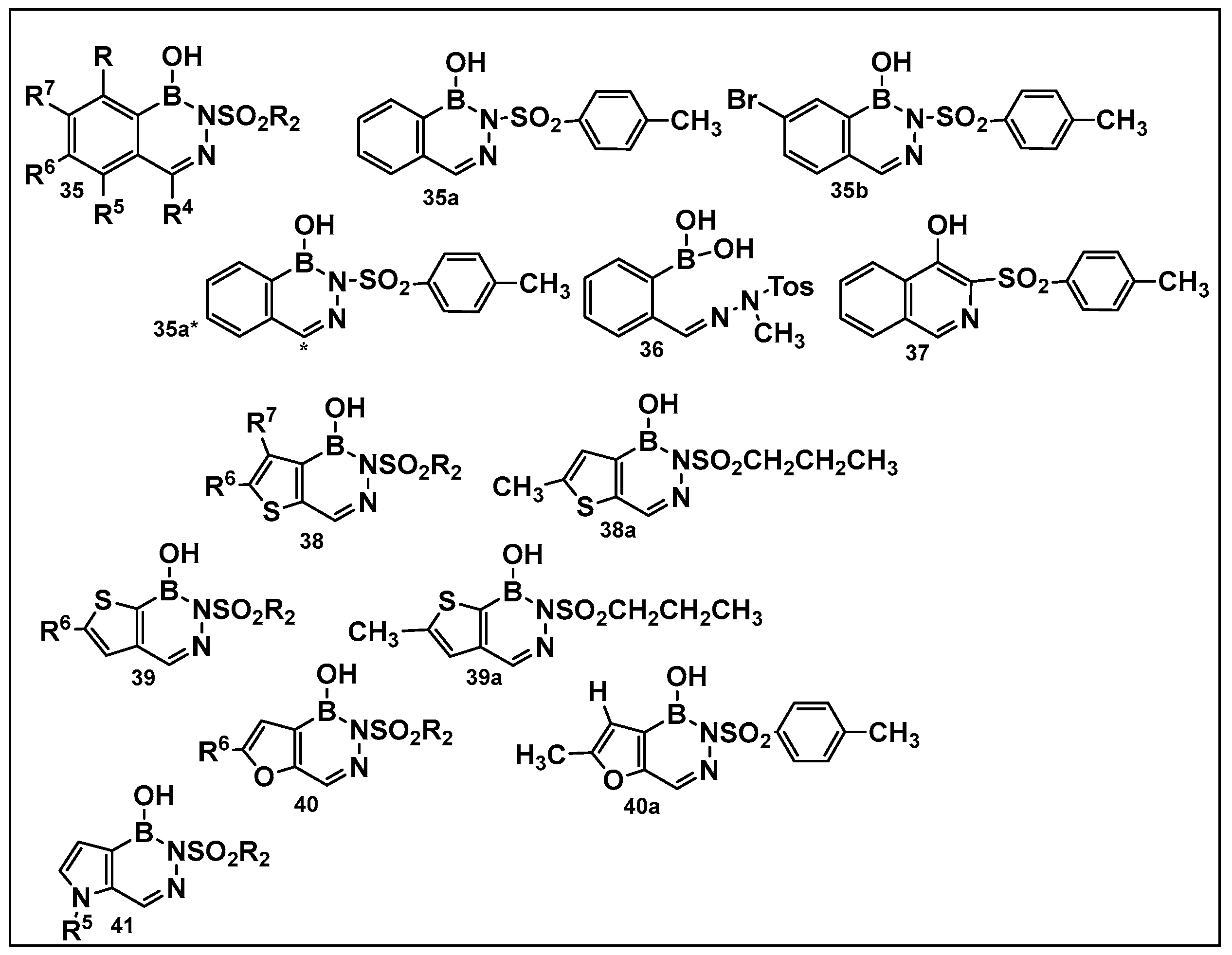
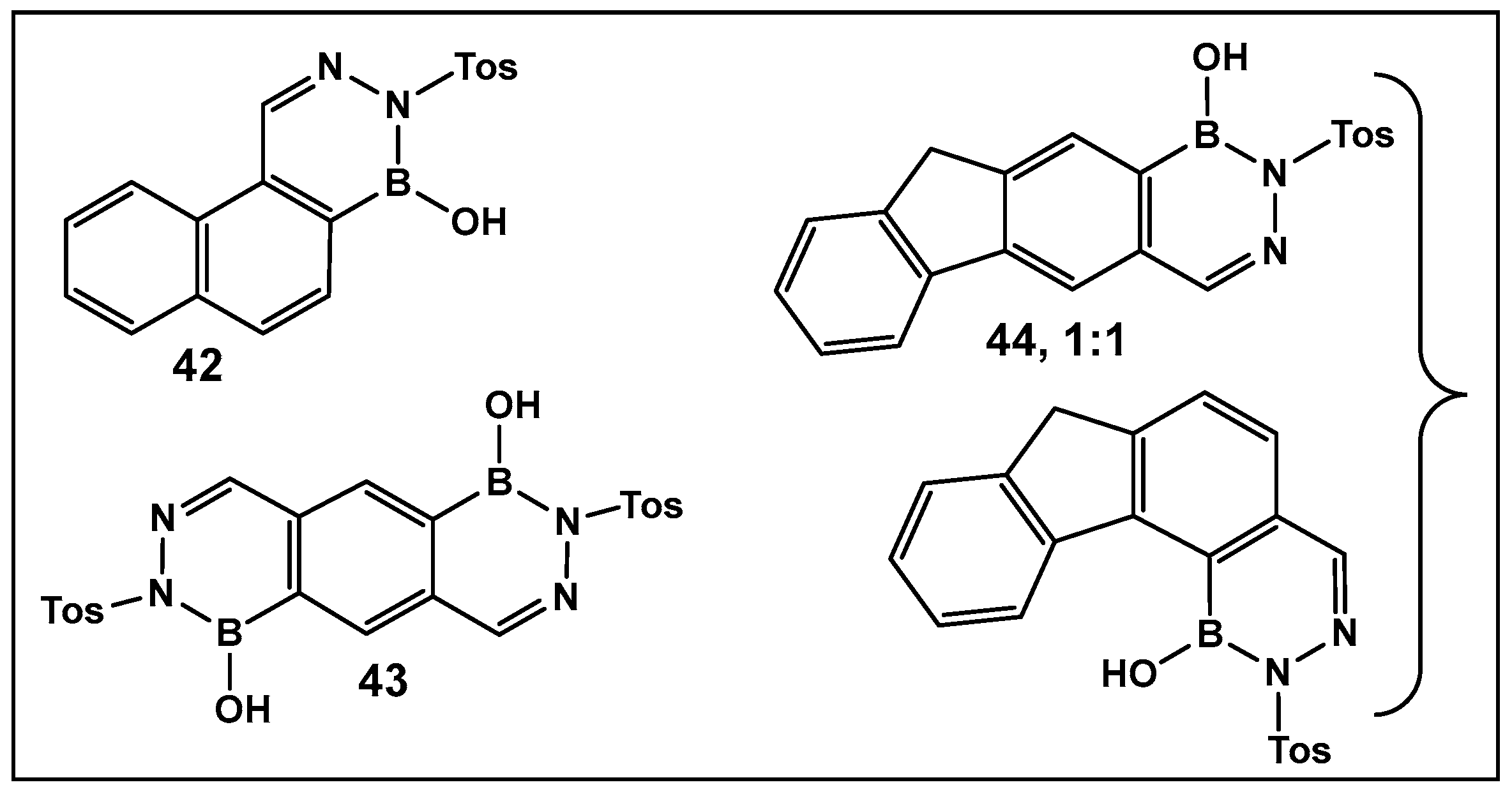
Diazaborines continue to be explored as antimicrobial agents due to their exclusive activity against Gram-negative bacteria, which are notoriously harder to treat. The diazaborine “specialization” is attributed to their well-documented mechanism of action—the inhibition of lipopolysaccharide biosynthesis in Gram-negative bacteria [141]. The SAR of derivatives of the aryl-substituted diazaborines (Figure 18) have been determined. Based on its characteristic, compound 35a (Figure 18) has been chosen for further examination [144,145]. To address the question of whether the bicyclic diazaborines are the active antimicrobial species, the ring-opened analog 36 (Figure 18) and the isoquinoline 37 (Figure 18) were prepared as stable analogs of 35a (Figure 15). Neither of the two derivatives (36 and 37, Figure 18) demonstrated antimicrobial activity similar to 35a (Figure 18). Furthermore, neither the changes in the size or position of the aryl moiety nor the addition of a second diazaborine (compounds 42–44, Figure 19) demonstrated the antimicrobial activity of their mono-aryl counterparts [144].
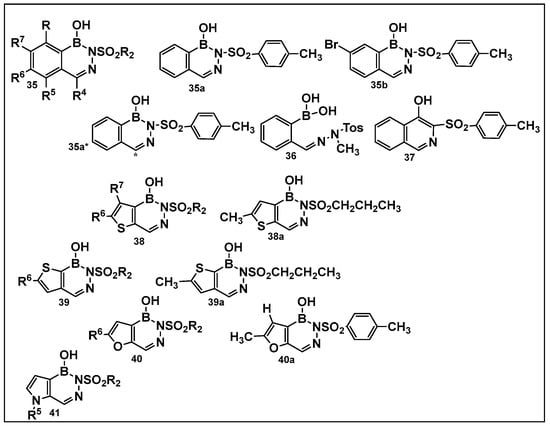
Figure 18.
Differently substituted aromatic ring-containing diazaborines: 35-sulfonylbenzodiazaborines,with 35a and 35b as the most active compound of this series against Proteus (12.5 μg/mL), Klebsiella (3.12 μg/mL)and Salmonella ( 6.25 μg/mL), Neisseria gonorrhoeae (2–8 μg/mL) and, to a lesser extent, against Escherichia coli (25 μg/mL and Enterobacter (>50 μg/mL) [144]; 38, thieno[2,3-d]diazaborines are slightly more active than 39, thieno[3, 2-d]-diazaborines in general; the 2-alkyl-sulfonyl derivatives of both 38 and 39 have good activities in vitro and in vivo; compound 38a was chosen for further evaluation. Proteus (0.78 μg/mL), Klebsiella (0.39 μg/mL), and Salmonella (0.78 μg/mL), N. gonorrhoeae (1 μg/mL), E. coli (1.56 μg/mL), and Enterobacter (3.12 μg/mL); 40, the furodiazaborines series follow a similar SAR as the 40 series; the methyl-substituted 40a demonstrates good activity: Proteus (3.12 μg/mL), Klebsiella (1.56 μg/mL), and Salmonella (3.12 μg/mL), N. gonorrhoeae (1–8 μg/mL), E. coli (12.5 μg/mL), and Enterobacter (25 μg/mL); the pyrrolodiazaborines 41 are inactive [144]. * means radioactive-labeled compound.
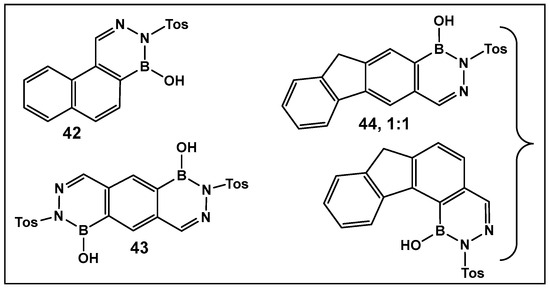
Figure 19.
Oligocyclic diazaborine derivatives (42–44) demonstrate very low antimicrobial activity in vitro [144].
The radiolabeled analog of 35a, compound 35a* (Figure 18), and compound 38a have been further examined to determine their molecular mechanism for inhibition of E. coli protein EnvM [145]. Protein EnvM inhibits the enoyl-ACP and enoyl-CoA reductase activity of EnvM by binding to the protein in the presence of NAD+ or NADH. Based on this data, it was concluded that EnvM is the NADH-dependent enoyl-ACP reductase (EC 1.3.1.9) of E. coli, and proposed to rename the corresponding gene fabI [145]. It was also determined that the target and mechanism by which the diazaborines, using 35a (Figure 18), inhibit the ribosome biogenesis by blocking in eukaryotic cells the large subunit formation [146].
The 2-Acylated benzodiazaborines as well as the heterocyclo-diazaborines (Figure 20) were also prepared and their antimicrobial activity evaluated [147]. From the prepared library of compounds, several demonstrated activity against E. coli. Two others were active against Mycobacterium smegmatis (45b and 47, Figure 20). The finding that these two compounds have isoniazid covalently embedded in their structures suggests that they might be acting as isoniazid prodrugs for M. smegmatis [147].
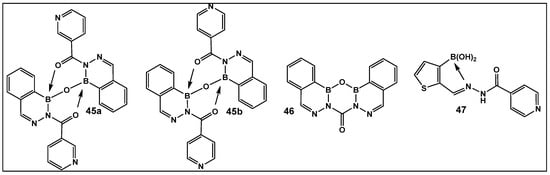
Figure 20.
2-Acylated 2,3,1-benzodiazaborines and 2-acyl-2,3,1-diazaborine heterocycles with hydration/dehydration abilities. Compounds 45b and 47 demonstrated the best antimicrobial activity against Mycobacterium smegmatis and Escherichia coli, with MIC values of 2–32 (μg/mL) [147].
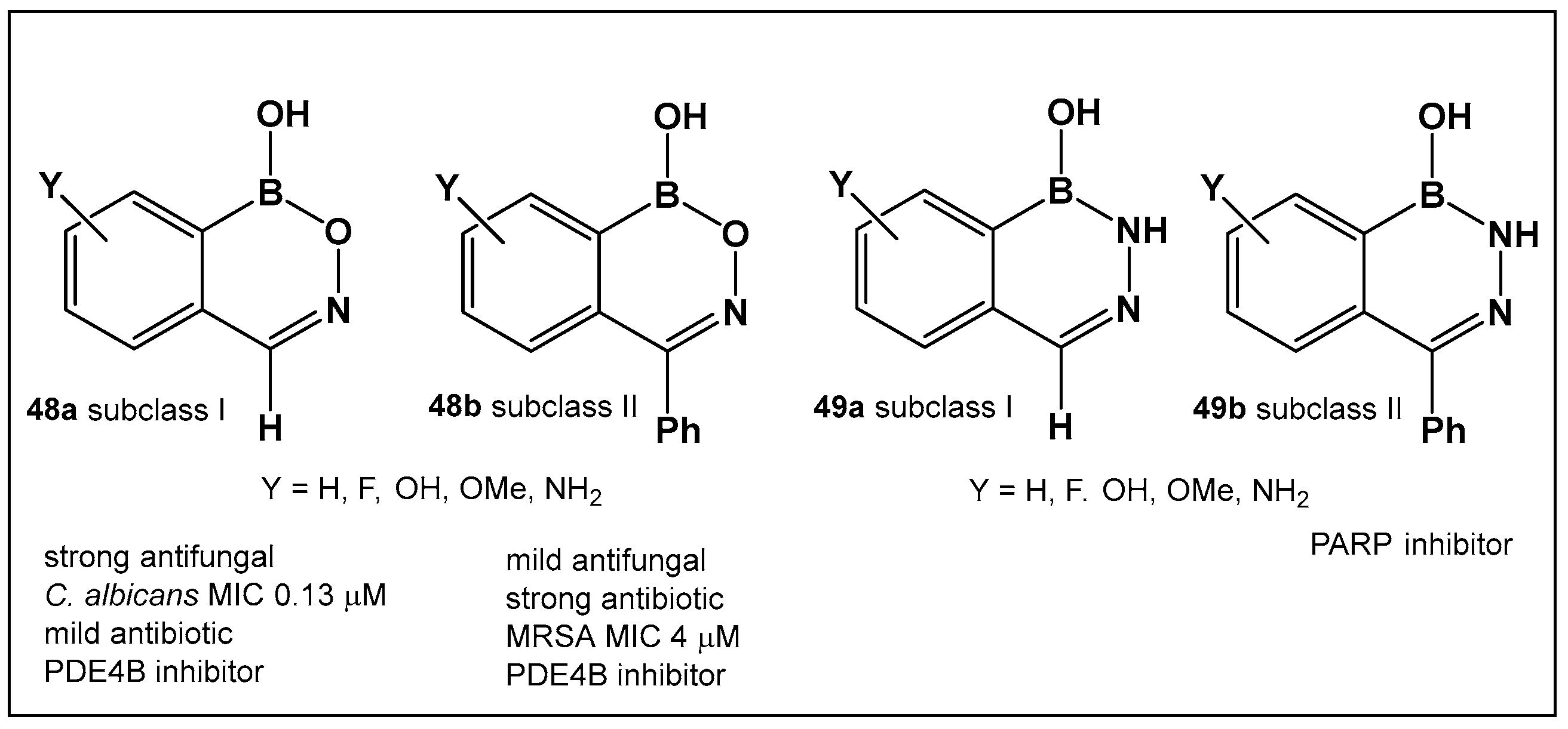
The evaluation of the biological activity of benzodiazaborines (BNN, Figure 17, Figure 18, Figure 19 and Figure 20), in addition to the antimicrobial described above, has been summarized in several reports since the 1980s as inhibitors of lipopolysaccharide biosynthesis and human neutrophil elastase, as well as estrogen mimics [148,149,150]; however, that of the benzoxazaborines has remained unexplored until very recently [24,140]. A library composed of about 70 derivatives of differently substituted derivatives from both chemotypes have been characterized and screened for biological activity [106]. Differently substituted benzoxazaborines 48a and 48b (Figure 21) demonstrated antimicrobial activity against MRSA (MIC 4 μM) and a mild activity against C. albicans when the substituent on the boron-containing ring is phenyl (48b, Figure 21), [24]. The activity “switches” to a strong antifungal activity (C. albicans, MIC 0.13 μM) and mild antibiotic against MRSA (48a, Figure 21) in the absence of the phenyl substituent. Both subclasses (benzoxazaborines 48a and 48b Figure 21) are also phosphodiesterase (PDE4B) inhibitors [24]. While their benzodiazaborine counter analogs 49a and 49b (Figure 21) have no antimicrobial activity, they do possess anticancer activity (poly ADP ribose polymerase (PARP) inhibitors) [24].
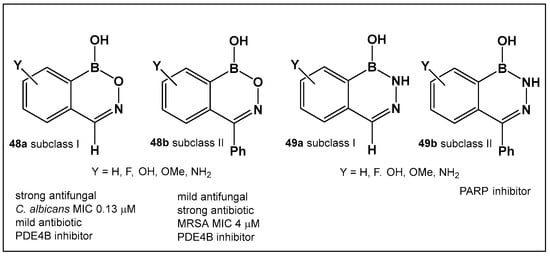
Figure 21.
Structures of benzoxazaborines 48 (BONs) and benzodiazaborines 49 (BNNs) and their different bioactivities [24,140].
3.1.7. Dynamic Covalent Chemistry: Polymeric Structures, Hydrogels, and Photosensitizers Based on Boronic Acids
Exploration of the dynamic interconversion between boronic acids and their esters through diols (1,2- and 1,3- diols forming 5- and 6-membered esters, respectively) has been translated into the design of responsive biocompatible materials [151,152,153,154,155,156,157].
Boronic Acid-Containing Hydrogels—Schiff Bases Stabilized by Boronic Acids
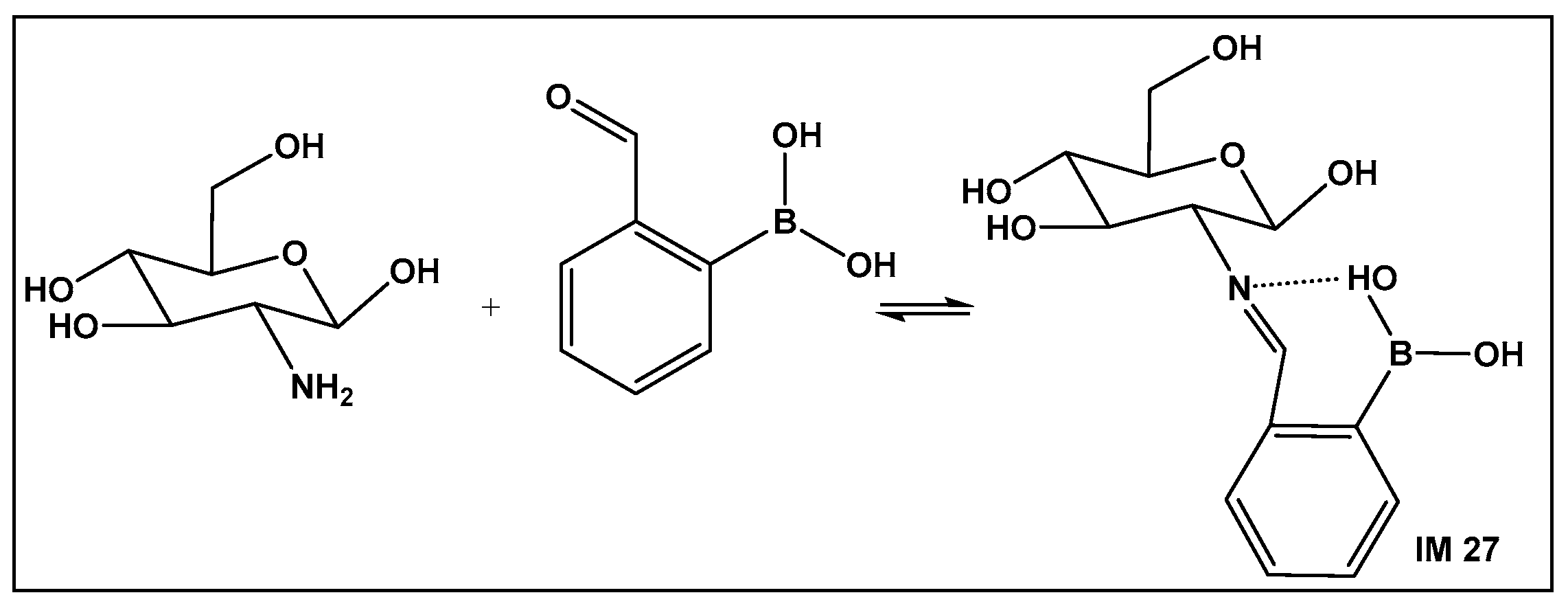
Recent approaches for wound healing include the design of moldable, self-healing hydrogels. The latter are three-dimensional polymeric networks, considered highly biocompatible due to their high degree of hydration [158,159,160,161,162,163,164,165,166,167,168]. One of the most common drawbacks of the traditional wound dressing, as well as the traditional hydrogel dressing, is that they are made of relatively rigid materials that keep them somewhat embedded in the wound throughout the healing process. Removal of these dressings can trigger further pain, as well as tissue damage hence the need for self-removable/degradable materials. One of the natural polymers, chitosan, due to its physicochemical and therapeutic properties, is one of the recent examples of hydrogels that utilize the reversibility of the boronic esters under physiological conditions. In addition, chitosan has intrinsic antimicrobial and fungicidal activity [163]. Crosslinking of the natural polymer to prepare a hydrogel can be accomplished via chemical or physical means. The former gives greater stability of the crosslinked structure. It involves imine formation from the primary amino groups of the chitosan with dialdehydes (e.g., glutaraldehyde). The imine bond is easily hydrolysable, which makes the hydrogels responsive to changes in pH and biodegradable. The toxicity of dialdehydes, however, restricts their use for biomedical applications. Replacement of the dialdehydes with 2-formyl boronic acids has led to new chitosan–boron-containing crosslinks, where the boronic acid helps stabilize the imine (IM 27; Figure 22) formed from the aldehyde moiety of the boronic acid and the amine moiety of the chitosan, i.e., imino–boronate formation (Figure 22). The imino–boronate formation has been also employed for construction of hydrogels as drug delivery systems [164], and it is an established strategy in preparation of bioconjugates and kinase inhibitors [165,166]. The chitosan crosslinked via imine, stabilized by the boronic acid (Figure 22) hydrogel, demonstrated fungicidal activity at a low concentration of 0.142% of 2-formyl boronic acid in hydrogel against C. albicans and C. glabrata both in their planktonic state. In addition, the inhibition of the metabolic activity of the corresponding Candida biofilms by more than 99.5% occurred, which makes these hydrogels promising candidates for the treatment of vulvovaginitis infections [162].
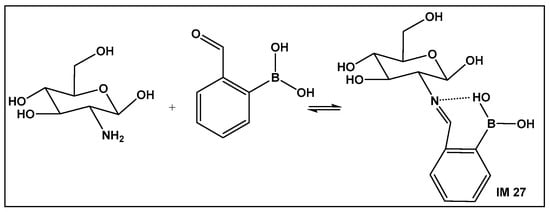
Figure 22.
Imine–boronic acid crosslink of chitosan: a fragment depicting the amino sugar unit of chitosan reacting with 2-formyl-boronic acid to form an imine IM 27.
Mesoporous chitosan nanofibers loaded with norfloxacin and coated with the phenylboronic acid used to aid in burn wound healing have also been reported [167].
Boronic Acids-Containing Nanoparticles—Exploration of Boron–Alcohol–Sugar Complexes
Bacteria can overexpress polysaccharides on their cell surface, therefore boronic acids are used to target and selectively exert an antimicrobial effect [169]. Materials capable of binding to the main components of the biofilm matrix, such as polysaccharides, can disrupt its architecture. After dispersion of the biofilm, bacterial infection can be treated by “conventional means”, e.g., antibiotics. Boronic acids’ incorporation/coating of different types of molecules from peptides to nanoparticles, designed as drug carriers, is predominately used as the affinity ligand for binding to different classes of microorganisms [170,171,172]. A library of dual-targeting micelles loaded with nitric oxide (NO) and modified with phenylboronic acid and quaternary ammonium salts against cyclodextrins has been prepared and evaluated for their antimicrobial activity against E. coli [171]. The idea behind the combination of the aforementioned functionalities is that the boronic acid group has the ability to interact strongly and specifically with the surface of E. coli, while the quaternary amine salt can interact electrostatically with bacteria, due to the bacteria’s negatively charged cell membrane. These two functionalities were hypothesized to be able to alter the molecular structure of the cell membrane as well as increase its permeability, leading to cell lysis [171]. The results showed enhanced binding and killing efficiency of this type of antimicrobial agents against E. coli infection [171].
Similarly, the improved binding to both the Gram-negative and Gram-positive bacteria provided by boronic acid has been utilized in the preparation of chloramphenicol-imprinted polymer particles [172]. The latter has a high antibiotic loading and a slow release of the antibiotic payload. An additional feature of such boronic acid-coated polymers to their enhanced antibacterial efficiency is their potential to act as scavengers for removal of the unused antibiotic from the environment [172].
Boronic Acids-Containing Photosensitizers—Exploration of Boron–Alcohol–Sugar Complexes
Exploration of the application of photodynamic therapy (PDT, where photosensitizing molecules, PS, are activated by light irradiation) has led to several strategies to obtain photo-activated microbiocidal compounds. These toxic species include reactive oxygen species (ROS), an area that has been vastly explored [173,174,175,176,177], as well as antimicrobial peptides (AMP), produced in the plant and animal kingdoms as part of their defensive strategies against pathogenic bacteria [178,179]. These approaches are relatively safe and effective and appear to not easily induce bacterial resistance, while exhibiting success in combating multidrug-resistant (MDR) bacteria [180,181,182]. However, even very effective PSs require high PS concentrations and light doses to treat biofilms [183].
Each of the approaches has drawbacks, which include the following: the light-generated ROS as a highly reactive species can damage mammalian cell components [184], and their bactericidal activity is limited by short diffusion distance of the ROS [185,186,187]; using predominately hydrophobic photosensitizers covalently bound to the AMP could have a negative effect on the AMP’s targeting efficiency [188,189]. To improve the PS’s bacteria-binding selectivity, the introduction of binding ligands with bacterial binding affinity [190,191,192,193,194] is often used to enhance the affinity of the material interfaces toward bacteria. Incorporation of boronic acids in PS, such as the use of AGA405, a silicon (IV) phthalocyanine (SiPc), has also been achieved [195]. The boronic acid–PS complex was designed to target both planktonic and biofilm-producing Gram-negative bacteria. Testing of these new PS has been performed on E. coli [195]. Photoactivation of 10 mm AGA405 has demonstrated significant changes in the bacterial cell membrane. Detection of the bacterial biofilm, as well as non-aggregated single bacterial cells at that concentration, has clearly demonstrated the superiority of AGA405 as compared with its counterpart, phthalocyanine, which is lacking the boronic acid substituents (Pc1). Another study using a Zn-based boronic acid-functionalized phthalocyanine, PcN4-BA, has been reported [196]. This PS demonstrated highly efficient ROS generation, leading to excellent photodynamic antimicrobial activity against the antibiotic-resistant bacterial strains [196]. The dependence of the uptake of PS and the photo-bactericidal activity in planktonic cultures and biofilms from bacterial glycans suggest an important role of the nature of polysaccharides in antimicrobial photodynamic therapy (aPDT) [195]. Still, the very elaborate organic synthesis of the aforementioned PS, covalently bound to the molecules for use as chemical weapons against bacteria, is a limiting factor for their application. While noncovalently bound biological binding agents secure more dynamic/unstable binding exist, they also suffer from a high cost. Hybrid organic–inorganic materials, termed metal−organic frameworks (MOFs), are promising photosensitizing materials [197].
Recently, a combination of boronic acid as the ligand and photosensitized porphyrin Cu(II) with one single Zr-MOF was prepared in order to enhance the MOF’s antibacterial capability by improving its binding to the saccharides on the multidrug-resistant bacterial surfaces by the boronic acids [198]. This MOF demonstrated good antibacterial activity against planktonic cells of S. aureus, including MRSA and E. coli [198]. The authors also predicted that a combination of MOF and a hydrogel as a wound dressing should have an improved controlled released and thus enhance the safety of MOF use [198].
Boron Clusters—Exploration of Boron-Hydrophobic Interactions Biological Membranes
Boron is less electronegative than hydrogen or carbon, therefore B–H bonds in boron cluster compounds exhibit very little polarization. Consequently, borohydrides form dihydrogen bonds [199,200], which contrasts with C–H, N–H, O–H, or S–H bonds, where the degree of bond polarization follows the electronegativity (and size) of the atom bonded to the hydrogen. As a result, the surface of the electroneutral boron clusters, and their derivatives, are hydrophobic [201,202]. Therefore, their interaction with components of biological systems is via lipid membranes and proteins [203].
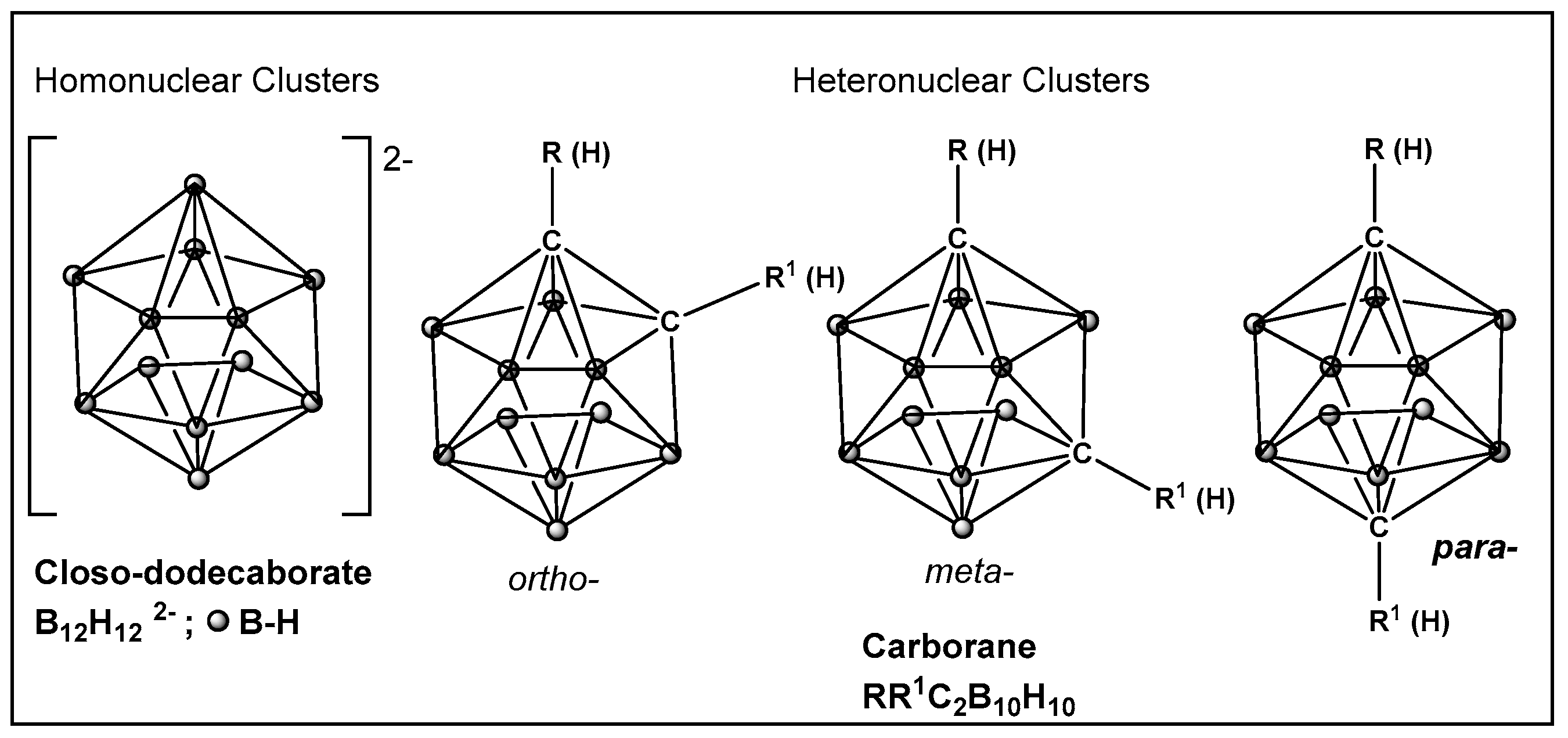
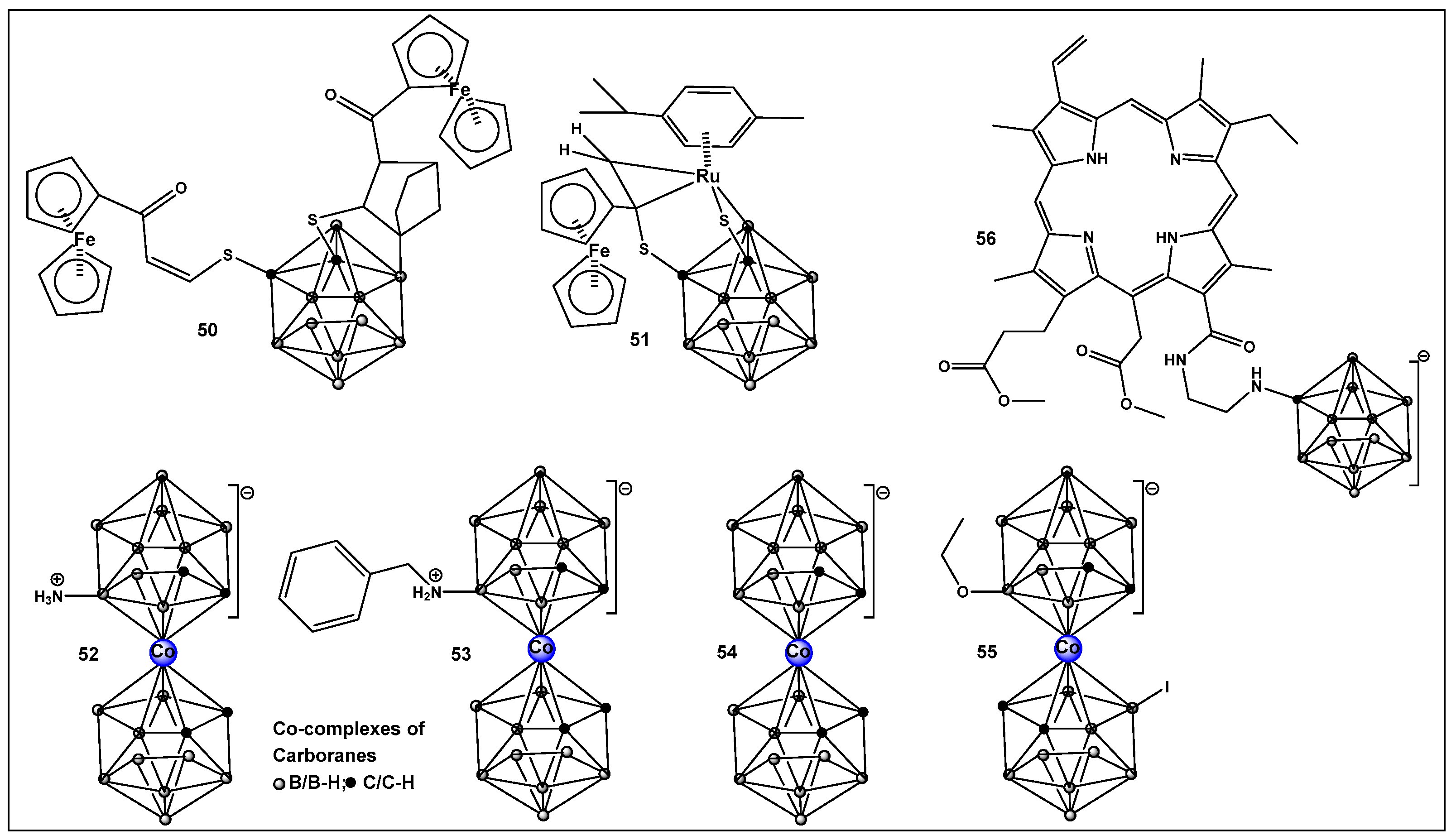
Boron clusters are another example of compounds obtained synthetically, where their ability to form bonds between their own atoms (self-catenation), similarly to carbon, has resulted in a large family of boron hydrides and heteroatom derivatives of hydrides (Figure 23). The structure of boron cluster compounds (borohydride clusters, carboranes, and metallacarboranes) have a polyhedral cage-like structure, which sets them apart from typical organic compounds’ structures that consist of chains and rings. The boron structures termed icosahedral boron cluster compounds are the most stable amongst the variety of differently shaped boron clusters. The three-dimensional aromaticity of the icosahedral boron clusters (similarly to the two-dimensional, planar aromaticity of the carbon-based aromatic rings) is responsible for their stability. The stability of the icosahedral boron clusters (i.e., 50–56, Figure 24), in addition to their good bioavailability, makes them the most commonly used structures in drug design/development [203,204,205,206,207,208,209,210]. The boron clusters exploration as standalone structures or for use in conjunction with known classes of antimicrobials, e.g., β-lactams, has been on the rise since the turn of the century, and several compounds have already been identified to have antimicrobial activity against both Gram-positive and Gram-negative organisms [203]. One example is Fc2SBCp1, 50 (Figure 24), an o-carborane derivative which demonstrated activity against two multidrug-resistant (MDR) clinical isolates of S. aureus and P. aeruginosa, (with MIC and minimal bactericidal concentration (MBC) values of 36 and 72 μg/mL, respectively) [208]. Bacterial cell wall damage by Fc2SBCp1, 50 (Figure 24), resulting in cellular content leakage was observed for both bacterial species. In addition, Fc2SBCp1, 50, (Figure 24) inhibited the biofilm formation by S. aureus and P. aeruginosa at a concentration of 0.25 × MIC (9 μg/mL), and 0.5 × MIC (18 μg/mL) of Fc2SBCp1, respectively [211].
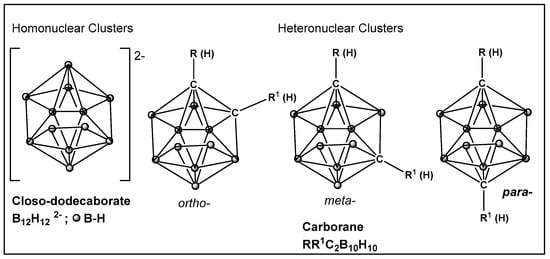
Figure 23.
Boronic clusters—closo-dodecanoboarate and carborane.
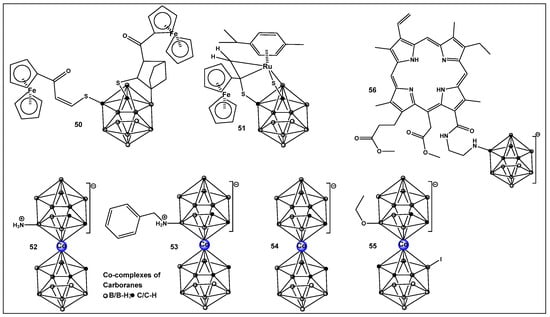
Figure 24.
Structures of boron clusters with antimicrobial/anti-biofilm activity.
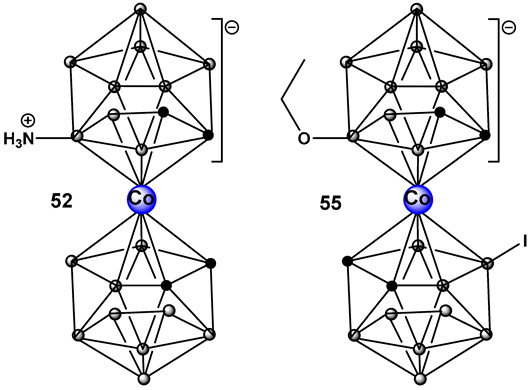
Another ferrocene-substituted carborane, the ruthenium(II)–arene complex FcRuSB, 51 (Figure 24), evaluated by the same research group, also significantly inhibited S. aureus and P. aeruginosa biofilm formation at a concentration of 8 μg/mL [212]. Biofilm inhibition, in turn, restored the sensitivity of the two pathogens to common antibiotics, including cefuroxime sodium, penicillin G, norfloxacin, streptomycin, and roxithromycin. The mechanism of biofilm inhibition by compound 51 (Figure 24) FcRuSB was through the inhibition of extracellular matrix proteins (EMP) expression, leading to the reduced adhesion of bacteria cells. Cobalt containing boron clusters, compounds 52–55, (Figure 24) were also prepared and evaluated for their anti-biofilm activity.
Compound 52, NH3–[COSAN] (Figure 24) demonstrated anti-biofilm activity against Gram-positive Staphylococcus epidermidis and S. aureus and fungi (Trichosporon cutaneum). Its benzyl derivative, PhNH2-[COSAN], 53 (Figure 24) anti-biofilm activity is highly selective against T. cutaneum (80% inhibition of biofilm at 1 mg/mL [213]. Compound Na[COSAN] (54, Figure 24) demonstrated better activity than erythromycin in the inhibition of the biofilm formation of the Gram-positive S. epidermidis, with the best activity of 50% biofilm inhibition at a concentration of 1 mg/mL [213]. At 10 mg/mL, compound 54 (Figure 24) also has anti-biofilm formation activity against Gram-negative E. coli and the fungi Candida parapsilosis and, to a lesser extent, T. cutaneum (50 μg/mL) but has no effect on biofilm formation by P. aeruginosa [213].
The alkoxy derivative, 55, (Figure 24) demonstrated the inhibition of MRSA biofilm formation by more than 80%, at a concentration of 0.25 × MIC (2 mg/mL), and by more than 97% at a concentration of 0.5 × MIC (4 mg/mL) thus placing compound 55 (Figure 18) among the compounds with excellent anti-biofilm activity [214].
Carboranes, have also been used in building the structure of carboranyl-chlorin e6, 56 (Figure 24), a potent photosensitizer for use in PDT [215], (chlorin e6 is an FDA-approved second-generation photosensitizer used in anticancer therapy). Compound 56 complex demonstrates considerably better photodynamic inactivation-induced bacterial cell death, as compared to the parent compound chlorin e6 against Gram-positive bacteria Bacillus subtilis, S. aureus, and Mycobacterium sp. However, neither chlorin e6 nor its boron derivative 56, (Figure 24) have activity against the Gram-negative E. coli when illuminated at 4 J/cm−2. Upon increasing the illumination to 20 J/cm−2, only compound 56 (Figure 24) demonstrated antibacterial activity against E. coli, as well as against B. subtilis. Interestingly compound 56 (Figure 24) possesses higher antibacterial activity than chlorin e6 when the samples are not illuminated (i.e., dark toxicity) [215].
4. Summary/Outlook/Future Direction
Over the past two decades, advances in drug discovery of boron compounds have led to the development of the successful benzoxaborole drug class, the FDA-approved serine β-lactamase inhibitor (vaborbactam), and the antifungal (tavaborole). Several compounds, such as the antitrypanosomal acoziborole, antituberculosis GSK3036665, anti-MAC lung disease (Mycobacterium avium complex lung disease) epetraborole and the polymerase inhibitor GSK 2878175, are currently undergoing clinical trials. Examples of boron-containing antimicrobials are summarized in Table 1.

Table 1.
Examples of the exploration and evolution of organoborons as antimicrobials/anti-biofilm agents from 1980s to 2024.
The FDA approval of boron-containing drugs represent a milestone that shows the opportunities presented by incorporation of an element that provides air-stable organic compounds with a mild Lewis acid character. The ability of benzoxaboroles, under physiological conditions, to form a variety of covalent interactions and hydrogen bonds with biological targets, similar to the boron–oxygen complexes exploited by nature, the ability to inhibit biofilm formation, and their general safety (low toxicity profile) is the basis for their success as drugs. Additionally, we expect to see an increase in research that explores the use of other boron-containing scaffolds such as the ones discussed herein. They are broadening the chemotypes of the reversible covalent binders and appear to have the ability to strike a balance between potency and selectivity in different biological systems. Covalent (irreversible) modulators of therapeutic targets have been an integral part of antimicrobial drug discovery since the discovery of Penicillin. Reversible covalent compounds provide minimization of the off-target effects. Boron-containing electrophiles, as reversible covalent modulators, represent a direction for a more tailored reactivity, which is especially important for drug development of antimicrobials. It is imperative to use drugs to which drug resistance is less likely to develop, such as those that interfere with biofilm formation.
Author Contributions
Conceptualization, writing—original draft preparation, M.I.K.; writing—review and editing, B.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank the American University and the Midwestern University Offices of Research and Sponsored Programs and Midwestern University College of Graduate Studies for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jemmis, E.D.; Jayasree, E.G. Analogies between boron and carbon. Acc. Chem. Res. 2003, 36, 816–824. [Google Scholar] [CrossRef]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Hütter, R.; Keller-Schierlein, W.; Knüsel, F.; Prelog, V.; Rodgers, G.C.; Suter, P.; Vogel, G.; Voser, W.; Zähner, H. The metabolic products of microorganisms. Boromycin. Helv. Chim. Acta 1967, 50, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Chen, T.S.; Chang, C.J.; Fenselau, C.; Floss, H.G. Isolation of two minor components of the boromycin fermentation: N-acetylboromycin and N-formylboromycin. J. Antibiot. 1985, 38, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Irschik, H.; Schummer, D.; Gerth, K.; Höfle, G.; Reichenbach, H. The tartrolons, new boron-containing antibiotics from a myxobacterium Sorangium cellulosum. J. Antibiot. 1995, 48, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Wolkenstein, K.; Sun, H.; Falk, H.; Griesinger, C. Structure and absolute configuration of Jurassic polyketide-derived spiroborate pigments obtained from microgram quantities. J. Am. Chem. Soc. 2015, 137, 13460–13463. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Elshahawi, S.I.; Trindade-Silva, A.E.; Hanora, A.; Han, A.W.; Flores, M.S.; Vizzoni, V.; Schrago, C.G.; Soares, C.A.; Concepcion, G.P.; Distel, D.L.; et al. Boronated tartrolon antibiotic produced by symbiotic cellulose-degrading bacteria in shipworm gills. Proc. Natl Acad. Sci. USA 2013, 110, E295–E304. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Al Quntar, A.A.; Srebnik, M. Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chem. Rev. 2011, 111, 209–237. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A. Naturally Occurring Boron Containing Compounds and Their Biological Activities. J. Nat. Prod. Resour. 2017, 3, 147–154. [Google Scholar]
- Mollner, T.A.; Isenegger, P.G.; Josephson, B.; Buchanan, C.; Lercher, L.; Oehlrich, D.; Hansen, D.F.; Mohammed, S.; Baldwin, A.J.; Gouverneur, V.; et al. Post-translational insertion of boron in proteins to probe and modulate function. Nat. Chem. Biol. 2021, 17, 1245–1261. [Google Scholar] [CrossRef]
- Cheng, Q.-Q.; Zhu, S.-F.; Zhang, Y.-Z.; Xie, X.-L.; Zhou, Q.-L. Copper-catalyzed B–H bond insertion reaction: A highly efficient and enantioselective C–B bond-forming reaction with amine–borane and phosphine–borane adducts. J. Am. Chem. Soc. 2013, 135, 14094–14097. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Qi, W.-Y.; Xu, B.; Xu, M.-H. Rhodium(i)-catalyzed asymmetric carbene insertion into B–H bonds: Highly enantioselective access to functionalized organoboranes. J. Am. Chem. Soc. 2015, 137, 5268–5271. [Google Scholar] [CrossRef] [PubMed]
- Hyde, S.; Veliks, J.; Liégault, B.; Grassi, D.; Taillefer, M.; Gouverneur, V. Copper-catalyzed insertion into heteroatom–hydrogen bonds with trifluorodiazoalkanes. Angew. Chem. Int. Ed. 2016, 55, 3785–3789. [Google Scholar] [CrossRef]
- Kan, S.B.J.; Huang, X.; Gumulya, Y.; Chen, K.; Arnold, F.H. Genetically programmed chiral organoborane synthesis. Nature 2017, 552, 132–136. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Silva, M.P.; Saraiva, L.; Pinto, M.; Sousa, M.E. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules 2020, 25, 4323. [Google Scholar] [CrossRef]
- Molloy, J.J.; Schäfer, M.; Wienhold, M.; Morack, T.; Daniliuc, C.G.; Gilmour, R. Boron-enabled geometric isomerization of alkenes via selective energy-transfer catalysis. Science 2020, 369, 302–306. [Google Scholar] [CrossRef]
- Plescia, J.; Moitessier, N. Design and discovery of boronic acid drugs. Eur. J. Med. Chem. 2020, 195, 112270. [Google Scholar] [CrossRef]
- Volochnyuk, D.M.; Gorlova, A.O.; Grygorenko, O.O. Saturated Boronic Acids, Boronates, and Trifluoroborates: An Update on Their Synthetic and Medicinal Chemistry. Chem. Eur. J. 2021, 27, 15277–15326. [Google Scholar] [CrossRef]
- Messner, K.; Vuong, B.; Tranmer, G.K. The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry. Pharmaceuticals 2022, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Shareef, M.A.; Das, S.; Nandwana, N.K.; Das, Y.; Saito, M.; Weiss, L.M. Boron-Containing heterocycles as promising pharmacological agents. Bioorg. Med. Chem. 2022, 63, 116748. [Google Scholar] [CrossRef] [PubMed]
- Grams, R.J.; Santos, W.L.; Scorei, I.R.; Abad-García, A.; Rosenblum, C.A.; Bita, A.; Cerecetto, H.; Viñas, C.; Soriano-Ursúa, M.A. The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology. Chem. Rev. 2024, 124, 2441–2511. [Google Scholar] [CrossRef]
- Kazmi, M.Z.H.; Schneider, O.M.; Hall, D.G. Expanding the Role of Boron in New Drug Chemotypes: Properties, Chemistry, Pharmaceutical Potential of Hemiboronic Naphthoids. J. Med. Chem. 2023, 66, 13768–13787. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wu, J.; Song, L.; Zhang, M.; Hipolito, C.J.; Wu, C.; Wang, S.; Zhang, Y.; Yin, Y. Merging the Versatile Functionalities of Boronic Acid with Peptides. Int. J. Mol. Sci. 2021, 22, 12958. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Juhas, M.; Eberl, L.; Tümmler, B. Quorum sensing: The power of cooperation in the world of Pseudomonas. Environ. Microbiol. 2005, 7, 459–471. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Gadar, K.; McCarthy, R.R. Using next generation antimicrobials to target the mechanisms of infection. NPJ Antimicrob. Resist. 2023, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, H. Presence and utility of boron in plants. Ann. Inst. Pasteur 1910, 24, 321–329. [Google Scholar]
- Warington, K. The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot. 1923, 37, 629–672. [Google Scholar] [CrossRef]
- Street, R.A.; Kulkarni, M.G.; Stirk, W.A.; Southway, C.; Van Staden, J. Variation in heavy metals and microelements in South African medicinal plants obtained from street markets. Food Addit. Contam. Part A 2008, 25, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Peng, J. Interaction between Boron and Other Elements in Plants. Genes 2023, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.A.; Abreu, I.; Bell, R.W.; Bienert, M.D.; Brown, P.H.; Dell, B.; Fujiwara, T.; Goldbach, H.E.; Lehto, T.; Mock, H.-P.; et al. Boron: An essential element for vascular plants. New Phytol. 2020, 226, 1232–1237. [Google Scholar] [CrossRef]
- Maldonado, P.; Aquea, F.; Reyes-Díaz, M.; Cárcamo-Fincheira, P.; Soto-Cerda, B.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Role of boron and its interaction with other elements in plants. Front. Plant Sci. 2024, 15, 1332459. [Google Scholar]
- Bolanos, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef]
- Läuchli, A. Functions of Boron in Higher Plants: Recent Advances and Open Questions. Plant Biol. 2002, 4, 190–192. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Rangavajla, N.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; Ciocîlteu, M.V.; Dincă, L.; Neamţu, J.; Bunaciu, A.; et al. Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound. Inorganics 2023, 11, 112. [Google Scholar] [CrossRef]
- Davies, D.H.; Norris, G.L.F. Growth Promotion Means for Ruminant Animals. European Patent EP 2893 19790711, 11 July 1979. [Google Scholar]
- Dunitz, J.D.; Hawley, D.M.; Miklos, D.; White, D.N.J.; Berlin, Y.; Morusic, R.; Prelog, V. Structure of boromycin. Helv. Chim. Acta 1971, 54, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Pache, W.; Zahner, H. Metabolic products of microorganisms: 77. Studies on the mechanism of action of boromycin. Arch. Mikrobiol. 1969, 67, 156–165. [Google Scholar] [CrossRef]
- De Carvalho, L.P.; Groeger-Otero, S.; Kreidenweiss, A.; Kremsner, P.G.; Mordmüller, B.; Held, J. Boromycin has Rapid-Onset Antibiotic Activity against Asexual and Sexual Blood Stages of Plasmodium falciparum. Front. Cell. Infect. Microbiol. 2022, 11, 802294. [Google Scholar] [CrossRef] [PubMed]
- Schummer, D.; Schomburg, D.; Irschik, H.; Reichenbach, H.; Hoefle, G. Antibiotics from gliding bacteria. Part LXXV. Absolute configuration and biosynthesis of tartrolon B, a boron containing macrodiolide from Sorangium cellulosum. Liebigs Ann. 1996, 1996, 965–969. [Google Scholar] [CrossRef]
- Kohno, J.; Kawahata, T.; Otake, T.; Morimoto, M.; Mori, H.; Ueba, N.; Nishio, M.; Kinumaki, A.; Komatsubara, S.; Kawashima, K. Boromycin, an anti-HIV antibiotic. Biosci. Biotechnol. Biochem. 1996, 60, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.S.; Chang, C.-J.; Floss, H.G. Biosynthesis of Boromycin. J. Org. Chem. 1981, 46, 2661–2665. [Google Scholar] [CrossRef]
- Miller, M.B.; Burg, R.W. Boromycin as a Coccidiostat. U.S. Patent 3864479, 4 February 1975. [Google Scholar]
- Abenoja, J.; Cotto-Rosario, A.; O’Connor, R. Boromycin Has Potent Anti-Toxoplasma and Anti-Cryptosporidium Activity. Antimicrob. Agents Chemother. 2021, 65, e01278-20. [Google Scholar] [CrossRef] [PubMed]
- Moreira, W.; Aziz, D.B.; Dick, T. Boromycin kills mycobacterial persisters without detectable resistance. Front. Microbiol. 2016, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, B.; Kaiserova, K.; Simkovic, M.; Orlicky, J.; Knezl, V.; Varecka, L. The effect of boromycin on the. Ca2+ homeostasis. Mol. Cell. Biochem. 2002, 231, 15–22. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S. Tenuecyclamides A–D, cyclic hexapeptides from the cyanobacterium Nostoc spongiaeforme var. tenue. J. Nat. Prod. 1998, 61, 1248–1251. [Google Scholar] [CrossRef]
- Hemscheidt, T.; Puglisi, M.P.; Larsen, L.K.; Patterson, G.M.L.; Moore, R.E.; Rios, J.L.; Clardy, J. Structure and biosynthesis of borophycin, a new boeseken complex of boric acid from a marine strain of the blue-green alga Nostoc linckia. J. Org. Chem. 1995, 59, 3467–3471. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef] [PubMed]
- Böeseken, J. The use of Boric Acid for the Determination of the Configuration of Carbohydrates. J. Adv. Carbohydr. Chem. 1949, 4, 189–210. [Google Scholar]
- Dembitsky, V.M.; Smoum, R.; Al-Quntar, A.A.; Abu Ali, H.; Pergament, I.; Srebnik, M. Natural occurrence of boron-containing compounds in plants, algae and microorganisms. Plant Sci. 2002, 163, 931–942. [Google Scholar] [CrossRef]
- Bassler, B.L.; Wright, M.; Showalker, R.E.; Silverman, M.R. Intercellular signalling in Vibrio harveyi: Sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 1993, 3, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Wright, M.; Silverman, M.R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: Sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 1994, 13, 273–286. [Google Scholar] [CrossRef]
- Surette, M.G.; Miller, M.B.; Bassler, B.L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: A new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 1999, 96, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Daniel, R.; Wagner-Döbler, I.; Zeng, A.P. Is autoinducer-2 a universal signal for interspecies communication: A comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol. Biol. 2004, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Konaklieva, M.I.; Plotkin, B.J. Chemical communication—Do we have a quorum? Mini Rev. Med. Chem. 2006, 6, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, J.T.; Welch, M.; Spring, D.R. Learning the language of bacteria. ACS Chem. Biol. 2007, 2, 715–717. [Google Scholar] [CrossRef]
- Miller, S.T.; Xavier, K.B.; Campagna, S.R.; Taga, M.E.; Semmelhack, M.F.; Bassler, B.L.; Hughson, F.M. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol. Cell 2004, 15, 677–687. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Liu, X.; Wang, Z.; Jiang, M.; Wang, R.; Xie, L.; Liu, Q.; Xie, X.; Shang, D.; et al. Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat. Commun. 2020, 11, 5371. [Google Scholar] [CrossRef]
- Lowery, C.A.; Park, J.; Kaufmann, G.F.; Janda, K.D. An unexpected switch in the modulation of AI-2-based quorum sensing discovered through synthetic 4,5-dihydroxy-2,3-pentanedione analogues. J. Am. Chem. Soc. 2008, 130, 9200–9201. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.V.; Kis, P.; Xavier, K.B.; Ventura, M.R. Synthesis and Potential of Autoinducer-2 and Analogs to Manipulate Inter-Species Quorum Sensing. Isr. J. Chem. 2023, 63, e202200091. [Google Scholar] [CrossRef]
- Smith, J.A.I.; Wang, J.; Nguen-Mau, S.-M.; Lee, V.; Herman, O.S. Biological screening of a diverse set of AI-2 analogues in Vibrio harveyi suggests that receptors which are involved in synergistic agonism of AI-2 and analogues are promiscuous. Chem. Commun. 2009, 45, 7033–7035. [Google Scholar] [CrossRef]
- Guo, M.; Gamby, S.; Zheng, Y.; Sintim, H.O. Small molecule inhibitors of AI-2 signaling in bacteria: State-of-the-art and future perspectives for anti-quorum sensing agents. Int. J. Mol. Sci. 2013, 14, 7694–7728. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, Y.; Yuan, B.; Yue, Y.; Zhao, M.; Luo, R.; Wu, H.; Wang, L.; Zhang, Y.; Xiao, J.; et al. Effect of Autoinducer-2 Quorum Sensing Inhibitor on Interspecies Quorum Sensing. Front. Microbiol. 2022, 13, 791802. [Google Scholar] [CrossRef]
- Escobar-Muciño, E.; Arenas-Hernández, M.M.P.; Luna-Guevara, M.L. Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella. Microorganisms 2022, 10, 884. [Google Scholar] [CrossRef]
- Meijler, M.M.; Hom, L.G.; Kaufmann, G.F.; McKenzie, K.M.; Sun, C.; Moss, J.A.; Matsushita, M.; Janda, K.D. Synthesis and biological validation of a ubiquitous quorum-sensing molecule. Angew. Chem. Int. Ed. 2004, 43, 2106–2108. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Kergaravat, B.; Jacquet, P.; Billot, R.; Grizard, D.; Chabrière, É.; Plener, L.; Daudé, D. Disrupting quorum sensing as a strategy to inhibit bacterial virulence in human, animal, and plant pathogens. Pathog. Dis. 2024, 82, ftae009. [Google Scholar] [CrossRef]
- Tsuchikama, K.; Zhu, J.; Lowery, C.A.; Kaufmann, G.F.; Janda, K.D. C4-alkoxy-HPD: A potent class of synthetic modulators surpassing nature in AI-2 quorum sensing. J. Am. Chem. Soc. 2012, 134, 13562–13564. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; Lowery, C.A.; Janda, K.D. Probing autoinducer-2 based quorum sensing: The biological consequences of molecules unable to traverse equilibrium states. J. Org. Chem. 2011, 76, 6981–6989. [Google Scholar] [CrossRef]
- Darvill, A.G.; McNeil, M.; Albersheim, P. Structure of Plant Cell Walls: VIII. A New Pectic Polysaccharide. Plant Physiol. 1978, 62, 418–422. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.; Darvill, A.G.; Albersheim, P. Structure of plant cell walls: X. Rhamnogalacturonan I, a structurally complex pectic polysaccharide in the walls of suspension-cultured sycamore cells. Plant Physiol. 1980, 66, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Franz, G. Structure-Activity Relation of Polysaccharides with Antitumor activity. Farm. Tijdschr. Belg. 1987, 64, 301. [Google Scholar]
- Kameda, T.; Ishii, T.; Matsunaga, T.; Ashida, J. 11B Solid-state NMR Investigation of the Rhamnogalacturonan II-borate Complex in Plant Cell Walls. Anal. Sci. 2006, 22, 321–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bewley, J.D. Handbook of Plant Science; John Wiley & Sons Ltd: Chichester, UK, 2007; Volume 2, p. 892. [Google Scholar]
- Feldheim, W. Oligofructose and inulin—Dietary fibre and probiotic. Ernaehrung 2000, 24, 162–164. [Google Scholar]
- Dexter, S.T. Growth, Organic nitrogen reactions, and buffer capacity in relation to hardiness of Plants. Plant Physiol. 1935, 10, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, P.; Carlier, M.; Mollet, J.-C.; Lehner, A. The cell wall pectic rhamnogalacturonan II, an enigma in plant glycobiology. In Carbohydrate Chemistry; The Royal Society of Chemistry: London, UK, 2021; pp. 553–571. [Google Scholar]
- Begum, R.A.; Fry, S.C. Boron bridging of rhamnogalacturonan-II in Rosa and arabidopsis cell cultures occurs mainly in the endo-membrane system and continues at a reduced rate after secretion. Ann. Bot. 2022, 130, 703–715. [Google Scholar]
- Zhang, Y.; Deepak Sharma, D.; Liang, Y.; Downs, N.; Dolman, F.; Thorne, K.; Black, I.M.; Pereira, J.H.; Adams, P.; Scheller, H.V.; et al. A putative rhamnogalacturonan-II CMP-β-Kdo transferase identified using CRISPR/Cas9 gene edited callus to circumvent embryo lethality. bioRxiv 2023. [Google Scholar] [CrossRef]
- Park, S.N.; Tae Noh, K.; Jeong, Y.I.; Duk Jung, I.; Kyu Kang, H.; Sun Cha, G.; Lee, S.J.; Seo, J.K.; Kang, D.H.; Hwang, T.-H.; et al. Rhamnogalacturonan II is a Toll-like receptor 4 agonist that inhibits tumor growth by activating dendritic cell-mediated CD8+ T cells. Exp. Mol. Med. 2013, 45, e8. [Google Scholar] [CrossRef]
- Macho, J.M.; Blue, R.M.; Lee, H.W.; MacMillan, J.B. Boron NMR as a Method to Screen Natural Product Libraries for B-Containing Compounds. Org. Lett. 2022, 24, 3161–3166. [Google Scholar] [CrossRef]
- Hecker, S.J.; Reddy, K.R.; Totrov, M.; Hirst, G.C.; Lomovskaya, O.; Griffith, D.C.; King, P.; Tsivkovski, R.; Sun, D.; Sabet, M.; et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs. class A serine carbapenemases. J. Med. Chem. 2015, 58, 3682–3692. [Google Scholar] [CrossRef] [PubMed]
- Kiener, P.A.; Waley, S.G. Reversible inhibitors of penicillinases. Biochem. J. 1978, 169, 197–204. [Google Scholar] [CrossRef]
- Beesley, T.; Gascoyne, N.; Knott-Hunziker, V.; Petursson, S.; Waley, S.G.; Jaurin, B.; Grundström, T. The inhibition of class C β-lactamases by boronic acids. Biochem. J. 1983, 209, 229–233. [Google Scholar] [CrossRef]
- Morandi, S.; Morandi, F.; Caselli, E.; Shoichet, B.K.; Prati, F. Structure-based optimization of cephalothin-analogue boronic acids as β-lactamase inhibitors. Bioorg. Med. Chem. 2008, 16, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Weston, G.S.; Blázquez, J.; Baquero, F.; Shoichet, B.K. Structure-based enhancement of boronic acid-based inhibitors of AmpC β-lactamase. J. Med. Chem. 1998, 41, 4577–4586. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Caselli, E.; Morandi, S.; Focia, P.J.; Blázquez, J.; Shoichet, B.K.; Prati, F. Nanomolar inhibitors of AmpC β-lactamase. J. Am. Chem. Soc. 2003, 125, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.L.; Rodkey, E.A.; Taracila, M.A.; Drawz, S.M.; Bethel, C.R.; Papp-Wallace, K.M.; Smith, K.M.; Xu, Y.; Dwulit-Smith, J.R.; Romagnoli, C.; et al. Design and exploration of novel boronic acid inhibitors reveals important interactions with a clavulanic acid-resistant sulfhydryl-variable (SHV) β-lactamase. J. Med. Chem. 2013, 56, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Sampson, J.M.; Ori, C.; Prati, F.; Drawz, S.M.; Bethel, C.R.; Bonomo, R.A.; van den Akker, F. Novel insights into the mode of inhibition of class A SHV-1 β-lactamases revealed by boronic acid transition state inhibitors. Antimicrob. Agents Chemother. 2011, 55, 174–183. [Google Scholar] [CrossRef][Green Version]
- Alsenani, T.A.; Rodríguez, M.M.; Ghiglione, B.; Taracila, M.A.; Mojica, M.F.; Rojas, L.J.; Hujer, A.M.; Gutkind, G.; Bethel, C.R.; Rather, P.N.; et al. Boronic Acid Transition State Inhibitors as Potent Inactivators of KPC and CTX-M β-Lactamases: Biochemical and Structural Analyses. Antimicrob. Agents Chemother. 2023, 67, e00930-22. [Google Scholar] [CrossRef] [PubMed]
- Lapuebla, A.; Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Quale, J.; Landman, D. Activity of meropenem combined with RPX7009, a novel β-lactamase inhibitor, against Gram-negative clinical isolates in New York City. Antimicrob. Agents Chemother. 2015, 59, 4856–4860. [Google Scholar] [CrossRef]
- Bouza, A.A.; Swanson, H.C.; Smolen, K.A.; VanDine, A.L.; Taracila, M.A.; Romagnoli, C.; Caselli, E.; Prati, F.; Bonomo, R.A.; Powers, R.A.; et al. Structure-Based Analysis of Boronic Acids as Inhibitors of Acinetobacter-Derived Cephalosporinase-7, a Unique Class C β-Lactamase. ACS Infect. Dis. 2018, 4, 325–336. [Google Scholar] [CrossRef]
- Caselli, E.; Fini, F.; Introvigne, M.L.; Stucchi, M.; Taracila, M.A.; Fish, E.R.; Smolen, K.A.; Rather, P.N.; Powers, R.A.; Wallar, B.J.; et al. 1,2,3-Triazolylmethaneboronate: A Structure Activity Relationship Study of a Class of β-Lactamase Inhibitors against Acinetobacter baumannii Cephalosporinase. ACS Infect. Dis. 2020, 6, 1965–1975. [Google Scholar] [CrossRef]
- Hecker, S.J.; Reddy, K.R.; Lomovskaya, O.; Griffith, D.C.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Sun, D.; Sabet, M.; Tarazi, Z.; et al. Discovery of Cyclic Boronic Acid QPX7728, an Ultrabroad-Spectrum Inhibitor of Serine and Metallo-β-lactamases. J. Med. Chem. 2020, 63, 7491–7507. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Tsivkovski, R.; Sun, D.; Reddy, R.; Totrov, M.; Hecker, S.; Griffith, D.; Loutit, J.; Dudley, M. QPX7728, An Ultra-Broad-Spectrum B-Lactamase Inhibitor for Intravenous and Oral Therapy: Overview of Biochemical and Microbiological Characteristics. Front. Microbiol. 2021, 12, 697180. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Rubio-Aparicio, D.; Tsivkovski, R.; Loutit, J.; Dudley, M. The Ultrabroad-Spectrum Beta-Lactamase Inhibitor QPX7728 Restores the Potency of Multiple Oral Beta-Lactam Antibiotics against Beta-Lactamase-Producing Strains of Resistant Enterobacterales. Antimicrob. Agents Chemother. 2022, 66, e0216821. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Parkinson, J.; Sabet, M.; Tarazi, Z.; Boyer, S.H.; Lomovskaya, O.; Griffith, D.C.; Hecker, S.J.; Dudley, M.N. Orally Bioavailable Prodrug of Boronic b-Lactamase Acid Inhibitor QPX7728. J. Med. Chem. 2021, 64, 17523–17529. [Google Scholar] [CrossRef] [PubMed]
- Ness, S.; Martin, R.; Kindler, A.M.; Paetzel, M.; Gold, M.; Jensen, S.E.; Jones, J.B.; Strynadka, N.C. Structure-based design guides the improved efficacy of deacylation transition state analogueinhibitors of TEM-1 beta-lactamase. Biochemistry 2000, 39, 5312–5321. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Trout, R.E.L.; Chu, G.-H.; McGarry, D.; Jackson, R.W.; Hamrick, J.C.; Daigle, D.M.; Cusick, S.M.; Pozzi, C.; De Luca, F.; et al. Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-b-lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. J. Med. Chem. 2020, 63, 2789–2801. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Birhanu, B.T.; Miguel-Ruano, V.; Kim, C.; Batuecas, M.; Yang, J.; El-Araby, A.M.; Jiménez-Faraco, E.; Schroeder, V.A.; Alba, A.; et al. Restoring susceptibility to β-lactam antibiotics in methicillin-resistant Staphylococcus aureus. Nat. Chem. Biol. 2024. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Shearer, J.; Castro, J.L.; Lawson, A.D.G.; MacCoss, M.; Taylor, R.D. Rings in Clinical Trials and Drugs: Present and Future. J. Med. Chem. 2022, 65, 8699–8712. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.M.; Adamczyk-Woźniak, A.; Madura, I.D. Effect of substituents in novel bioactive tavaborole derivatives on the intermolecular interaction hierarchy. CrystEngComm 2022, 24, 3586–3596. [Google Scholar] [CrossRef]
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crepin, T.; Zhou, H.; Zhang, Y.-K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An Antifungal Agent Inhibits an Aminoacyl-tRNA Synthetase by Trapping tRNA in the Editing Site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef]
- Baker, S.J.; Zhang, Y.-K.; Akama, T.; Lau, A.; Zhou, H.; Hernandez, V.; Mao, W.; Alley, M.R.K.; Sanders, V.; Plattner, J.J. Discovery of a New Boron-Containing Antifungal Agent, 5-Fluoro- 1,3-Dihydro-1-Hydroxy-2,1- Benzoxaborole (AN2690), for the Potential Treatment of Onychomycosis. J. Med. Chem. 2006, 49, 4447–4450. [Google Scholar] [CrossRef] [PubMed]
- Borys, K.M.; Wieczorek, D.; Pecura, K.; Lipok, J.; Adamczyk-Woźniak, A. Antifungal activity and tautomeric cyclization equilibria of formylphenylboronic acids. Bioorg. Chem. 2019, 91, 103081. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Tarkowska, M.; Lazar, Z.; Kaczorowska, E.; Madura, I.D.; Dąbrowska, M.A.; Lipok, J.; Wieczorek, D. Synthesis, structure, properties and antimicrobial activity of para trifluoromethyl phenylboronic derivatives. Bioorg. Chem. 2022, 119, 105560. [Google Scholar] [CrossRef]
- Peppoloni, S.; Pericolini, E.; Colombari, B.; Pinetti, D.; Cermelli, C.; Fini, F.; Prati, F.; Caselli, E.; Blasi, E. The β-Lactamase Inhibitor Boronic Acid Derivative SM23 as a New Anti-Pseudomonas aeruginosa Biofilm. Front. Microbiol. 2020, 11, 35. [Google Scholar] [CrossRef]
- Ni, N.; Chou, H.T.; Wang, J.; Li, M.; Lu, C.D.; Tai, P.C.; Wang, B. Identification of boronic acids as antagonists of bacterial quorum sensing in Vibrio harveyi. Biochem. Biophys. Res. Commun. 2008, 369, 590–594. [Google Scholar] [CrossRef]
- Ni, N.; Choudhary, G.; Peng, H.; Li, M.; Chou, H.-T.; Lu, C.-D.; Gilbert, E.S.; Wang, B. Inhibition of Quorum Sensing in Vibrio harveyi by Boronic Acids. Chem. Biol. Drug Des. 2009, 74, 51–56. [Google Scholar] [CrossRef]
- Sathiyamoorthi, E.; Lee, J.-H.; Lee, J. Antibacterial and antibiofilm activity of halogenated phenylboronic acids against Vibrio parahaemolyticus and Vibrio harveyi. Front. Cell. Infect. Microbiol. 2024, 14, 1340910. [Google Scholar] [CrossRef]
- Rezaie, P.; Pourhajibagher, M.; Chiniforush, N.; Hosseini, N.; Bahador, A. The Effect of Quorum-Sensing and Efflux Pumps Interactions in Pseudomonas aeruginosa against Photooxidative Stress. J. Lasers Med. Sci. 2018, 9, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Rico, M.; Olivares-Pacheco, J.; Alvarez-Ortega, C.; Cámara, M.; Martínez, J.L. Role of the multidrug resistance efflux pump MexCD-OprJ in the Pseudomonas aeruginosa quorum sensing response. Front. Microbiol. 2018, 9, 2752. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Gupta, J.; Sharma, S.; Sharma, D.; Sharma, S. Focused review on dual inhibition of quorum sensing and efflux pumps: A potential way to combat multi drug resistant Staphylococcus aureus infections. Int. J. Biol. Macromol. 2021, 190, 33–43. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Moore-Machacek, A.; Gloe, A.; O’Leary, N.; Reen, F.J. Efflux, Signaling and Warfare in a Polymicrobial World. Antibiotics 2023, 12, 731. [Google Scholar] [CrossRef]
- Hajiagha, M.N.; Kafil, H.S. Efflux pumps and microbial biofilm formation. Infect. Genet. Evol. 2023, 112, 105459. [Google Scholar] [CrossRef]
- Ren, J.; Wang, M.; Zhou, W.; Liu, Z. Efflux pumps as potential targets for biofilm inhibition. Front. Microbiol. 2024, 15, 1315238. [Google Scholar] [CrossRef]
- Laborda, P.; Lolle, S.; Hernando-Amado, S.; Alcalde-Rico, M.; Aanæs, K.; Martínez, J.L.; Molin, S.; Johansen, H.K. Mutations in the efflux pump regulator MexZ shift tissue colonization by Pseudomonas aeruginosa to a state of antibiotic tolerance. Nat. Commun. 2024, 15, 2584. [Google Scholar] [CrossRef]
- Fontaine, F.; Hequet, A.; Voisin-Chiret, A.S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. First identification of boronic species as novel potential inhibitors of the Staphylococcus aureus NorA efflux pump. J. Med. Chem. 2014, 57, 2536–2548. [Google Scholar] [CrossRef]
- Fontaine, F.; Héquet, A.; Voisin-Chiret, A.-S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. Boronic species as promising inhibitors of the Staphylococcus aureus NorA efflux pump: Study of 6-substituted pyridine-3-boronic acid derivatives. Eur. J. Med. Chem. 2015, 95, 185–198. [Google Scholar] [CrossRef]
- Cruz, C.D.; Wrigstedt, P.; Moslova, K.; Iashin, V.; Mäkkylä, H.; Ghemtio, L.; Heikkinen, S.; Tammela, P.; Perea-Buceta, J.E. Installation of an aryl boronic acid function into the external section of N-aryl-oxazolidinones: Synthesis and antimicrobial evaluation. Eur. J. Med. Chem. 2021, 211, 113002. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.; Zurenko, G.; Barbachyn, M. The discovery of linezolid, the first oxazolidinone antibacterial agent. Curr. Drug Targets-Infect. Disord. 2001, 1, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Jones, R.N. Oxazolidinone antibiotics. Lancet 2001, 358, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Barbachyn, M.R. The oxazolidinones: Past, present, and future: The oxazolidinones: Past, present, and future. Ann. N. Y. Acad. Sci. 2011, 1241, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Kanyo, Z.F.; Wang, D.; Franceschi, F.J.; Moore, P.B.; Steitz, T.A.; Duffy, E.M. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 2008, 51, 3353–3356. [Google Scholar] [CrossRef]
- Corey, E.J.; Helal, C. Reduction of Carbonyl Compounds with Chiral Oxazaborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method. Angew. Chem. Int. Ed. 1988, 37, 1986–2012. [Google Scholar] [CrossRef]
- Srebnik, M.; Deloux, L. Asymmetric boron-catalyzed reactions. Chem. Rev. 1993, 93, 763–784. [Google Scholar]
- Harada, T.; Kusukawa, T. Development of Highly Enantioselective Oxazaborolidinone Catalysts for the Reactions of Acyclic α,β-Unsaturated Ketones. Synlett 2007, 12, 1823–1835. [Google Scholar] [CrossRef]
- Butenschön, H. Oxazaborolidines as Catalysts for Enantioselective Cycloadditions: Now [2+2]! Angew. Chem. Int. Ed. 2008, 47, 3492–3495. [Google Scholar] [CrossRef]
- Corey, E.J. Enantioselective Catalysis Based on Cationic Oxazaborolidines. Angew. Chem. Int. Ed. 2009, 48, 2100–2117. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, A.; Steinberg, D.; Dembitsky, V.; Moussaieff, A.; Zaks, B.; Srebnik, M. Synthesis and Evaluation of Oxazaborolidines for Antibacterial Activity against Streptococcus mutans. J. Med. Chem. 2004, 47, 2409–2410. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, A.; Srebnik, M.; Zaks, B.; Dembitsky, V.; Steinberg, D. Evaluation of oxazaborolidine activity on Streptococcus mutans biofilm formation. Int. J. Antimicrob. Agents 2005, 26, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, A.; Smoum, R.; Takrouri, K.; Shalom, E.; Zaks, B.; Steinberg, D.; Rubinstein, A.; Goldberg, I.; Katzhendler, J.; Srebnik, M. Pharmacologically active boranes. Pure Appl. Chem. 2006, 78, 1425–1453. [Google Scholar] [CrossRef]
- Aharoni, R.; Bronstheyn, M.; Jabbour, A.; Zaks, B.; Srebnik, M.; Steinberg, D. Oxazaborolidine derivatives inducing autoinducer-2 signal transduction in Vibrio harveyi. Bioorg. Med. Chem. 2008, 16, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Golovanov, I.S.; Sukhorukov, A.Y. Merging Boron with Nitrogen-Oxygen Bonds: A Review on BON Heterocycles. Top. Curr. Chem. 2021, 379, 8. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, M.Z.H.; Rygus, J.P.G.; Ang, H.T.; Paladino, M.; Johnson, M.A.; Ferguson, M.J.; Hall, D.G. Lewis or Bronsted? A Rectification of the Acidic and Aromatic Nature of Boranol-Containing Naphthoid Heterocycles. J. Am. Chem. Soc. 2021, 143, 10143–10156. [Google Scholar] [CrossRef]
- Mueckter, H.; Huemer, H.; Sous, H.; Poszich, H. 6th International Congress of Chemotherapy; June 1967 Proceedings 1/2, 805; International Society for Chemotherapy: Vienna, Austria, 1967. [Google Scholar]
- Gronowitz, S.; Dhlgren, T.; Namtvedt, J.; Roos, C.; Sjöberg, B.; Forsgren, U. Antibacterial borazaro derivatives. I. 5-Arylsulphonyl-4-hydroxy-4,5-borazarothieno(2,3-c)pyridines and 6-arylsulphonyl-7-hydroxy-7,6-borazaro-thieno(3,2-c)pyridines. Acta Pharm. Suec. 1971, 8, 377–390. [Google Scholar]
- Gronowitz, S.; Dhlgren, T.; Namtvedt, J.; Roos, C.; Sjöberg, B.; Forsgren, U. Antibacterial borazaro derivatives. II. Effect of substituents on the antibacterial activity of 5-arylsulfonyl-4hydroxy-4,5-borazarothieno(2,3-c)pyridines and 6-arylsulfonyl-7-hydroxy-7,6-borazarothieno(3,2-c)pyridines. Acta Pharm. Suec. 1971, 8, 623–638. [Google Scholar]
- Grassberger, M.A.; Turnowsky, F.; Hildebrandt, J. Preparation and antibacterial activities of new 1,2,3-diazaborine derivatives and analogs. J. Med. Chem. 1984, 8, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Berglert, H.; Wallnert, P.; Ebelingt, A.; Leitingert, B.; Fuchsbichler, S.; Aschaued, H.; Kollenzll, G.; Hogenauert, G.; Turnowsky, F. Protein EnvM Is the NADH-dependent Enoyl-ACP Reductase (FabI) of Escherichia coli. J. Biol. Chem. 1994, 269, 5493–5496. [Google Scholar] [CrossRef]
- Loibl, M.; Klein, I.; Prattes, M.; Schmidt, C.; Kappel, L.; Zisser, G.; Gungl, A.; Krieger, E.; Pertschy, B.; Bergler, H. The Drug Diazaborine Blocks Ribosome Biogenesis by Inhibiting the AAA-ATPase Drg1. J. Biol. Chem. 2014, 289, 3913–3922. [Google Scholar] [CrossRef]
- Kanichar, D.; Roppiyakuda, L.; Ewa Kosmowska, E.; Faust, M.A.; Kim, P.; Tran, K.P.; Chowa, F.; Buglo, E.; Groziak, M.P.; Sarina, E.A.; et al. Synthesis, Characterization, and Antibacterial Activity of Structurally Complex 2-Acylated 2,3,1-Benzodiazaborines and Related Compounds. Chem. Bidivers. 2014, 11, 1381–1397. [Google Scholar] [CrossRef]
- Hogenauer, G.; Woisetschlager, M. A Diazaborine Derivative Inhibits Lipopolysaccharide Biosynthesis. Nature 1981, 293, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.P.M.; Goncalves, L.M.; Guedes, R.C.; Moreira, R.; Gois, M.P. Diazaborines as New Inhibitors of Human Neutrophil Elastase. ACS Omega 2018, 3, 7418–7423. [Google Scholar] [CrossRef] [PubMed]
- Dukes, A.; Carroll, X.B.; Groziak, M.P. Design, development, synthesis, and crystal structure of the prototype of a new class of deep blue-fluorescing boron heterocycle estrogen mimics. Bioorg. Med. Chem. Lett. 2022, 72, 128864. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.Q.; Guo, B.; Ma, Y.; Cui, W.; He, F.; Li, B.; Yang, H.; Shea, K.J. Dynamic introduction of cell adhesive factor via reversible multicovalent phenylboronic acid/cis-diol polymeric complexes. J. Am. Chem. Soc. 2014, 136, 6203–6206. [Google Scholar] [CrossRef]
- Polsky, R.; Harper, J.C.; Wheeler, D.R.; Arango, D.C.; Brozik, S.M. Electrically addressable cell immobilization using phenylboronic acid diazonium salts. Angew. Chem. Int. Ed. 2008, 120, 2671–2674. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Ayoob, A.M.; Appel, E.A.; Borenstein, J.T.; Langer, R.; Anderson, D.G. Mixed reversible covalent crosslink kinetics enable precise, hierarchical mechanical tuning of hydrogel networks. Adv. Mater. 2017, 29, 1605947. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, A.; Schiller, R.; Dobrindt, U. Boronic acid-functionalized photosensitizers: A straightforward strategy to target the sweet site of bacteria and implement active agents in polymer coating. Angew. Chem. Int. Ed. 2017, 56, 10362–10366. [Google Scholar] [CrossRef]
- McGann, C.L.; Streifel, B.C.; Lundin, J.G.; Wynne, J.H. Multifunctional polyHIPE wound dressings for the treatment of severe limb trauma. Polymer 2017, 126, 408–418. [Google Scholar] [CrossRef]
- Wu, R.R.; Ma, Y.; Pan, J.M.; Lee, S.H.; Liu, J.X.; Zhu, H.J.; Gu, R.X.; Shea, K.J.; Pan, G.Q. Efficient capture, rapid killing and ultrasensitive detection of bacteria by a nanodecorated multi-functional electrode sensor. Biosens. Bioelectron. 2018, 101, 52–59. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Z.; Luo, J.; Wang, P.; Lu, G.; Pan, J. Engineered reversible adhesive biofoams for accelerated dermal wound healing: Intriguing multi-covalent phenylboronic acid/cis-diol interaction. Colloids Surf. B Biointerfaces 2023, 221, 112987. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Chawla, P.; Ranjan Srivastava, A.; Pandey, P.; Chawla, V. Hydrogels: A journey from diapers to gene delivery. Mini-Rev. Med. Chem. 2014, 14, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Bisyrul, M.; Othman, H.; Javed, F.; Ahmad, Z.; Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.S.; Fiorica, C.; Di Stefano, M.; Pitarresi, G.; Gulino, A.; Agnello, S.; Giammona, G. In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration. Carbohydr. Polym. 2015, 122, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual crosslinked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Ma, R.; Xu, Y.; Zhang, Y.; Li, B.; An, Y.; Shi, L. A G-Quadruplex Hydrogel via Multicomponent Self-Assembly: Formation and Zero-Order Controlled Release. ACS Appl. Mater. Interfaces 2017, 9, 13056–13067. [Google Scholar] [CrossRef]
- António, J.P.M.; Russo, R.; Carvalho, C.P.; Cal, P.M.S.D.; Gois, P.M.P. Boronic acids as building blocks for the construction of therapeutically useful bioconjugates. Chem. Soc. Rev. 2019, 48, 3513–3536. [Google Scholar] [CrossRef]
- Ailincai, D.; Cibotaru, S.; Anisiei, A.; Coman, C.G.; Pasca, A.S.; Rosca, I.; Sandu, A.-I.; Mititelu-Tartau, L.; Marin, L. Mesoporous chitosan nanofibers loaded with norfloxacin and coated with phenylboronic acid perform as bioabsorbable active dressings to accelerate the healing of burn wounds. Carbohydr. Polym. 2023, 318, 121135. [Google Scholar] [CrossRef]
- Mi, F.L.; Kuan, C.Y.; Shyu, S.S.; Lee, S.T.; Chang, S.F. Study of gelation kinetics and chain-relaxation properties of glutaraldehyde-cross-linked chitosan gel and their effects on microspheres preparation and drug release. Carbohydr. Polym. 2000, 41, 389–396. [Google Scholar] [CrossRef]
- Mikhailov, S.N.; Zakharova, A.N.; Drenichev, M.S.; Ershov, A.V.; Kasatkina, M.A.; Vladimirov, L.V.; Novikov, V.V.; Kildeeva, N.R. Crosslinking of chitosan with dialdehyde derivatives of nucleosides and nucleotides. Mechanism and comparison with glutaraldehyde. Nucleosides. Nucleotides Nucleic Acids 2016, 35, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.E.; Zhang, W.; Falkinham, J.O., 3rd; Santos, W.L. Branched Peptides: Acridine and Boronic Acid Derivatives as Antimicrobial Agents. ACS Med. Chem. Lett. 2017, 8, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Cui, H.; Liang, X.; Yu, J.; Wang, J.; Zhao, G. Quaternary Ammonium-Tethered Phenylboronic Acids Appended Supramolecular Nanomicelles as a Promising Bacteria Targeting Carrier for Nitric Oxide Delivery. Polymers 2022, 14, 4451. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Liu, W.; Carlquist, M.; Ye, L. Boronic Acid Modified Polymer Nanoparticles for Enhanced Bacterial Deactivation. Chembiochem 2019, 20, 2991–2995. [Google Scholar] [CrossRef] [PubMed]
- Noimark, S.; Dunnill, C.W.; Parkin, I.P. The role of surfaces in catheter-associated infections. Adv. Drug Deliv. Rev. 2013, 65, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Wang, B.; Wang, M.; Mikhailovsky, A.; Wang, S.; Bazan, G.C. A Membrane-Intercalating Conjugated Oligoelectrolyte with High-Efficiency Photodynamic Antimicrobial Activity. Angew. Chem. Int. Ed. 2017, 56, 5031–5034. [Google Scholar] [CrossRef]
- Park, J.H.; Ahn, M.Y.; Kim, Y.C.; Kim, S.A.; Moon, Y.H.; Ahn, S.G.; Yoon, J.H. In vitro and in vivo antimicrobial effect of photodynamic therapy using a highly pure chlorin e6 against Staphylococcus aureus Xen29. Biol. Pharm. Bull. 2012, 35, 509–514. [Google Scholar] [CrossRef]
- Han, H.H.; Jin, Q.; Wang, H.B.; Teng, W.Z.; Wu, J.N.; Tong, H.X.; Chen, T.T.; Ji, J. Intracellular Dual Fluorescent Lightup Bioprobes for Image-Guided Photodynamic Cancer Therapy. Small 2016, 12, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Muthukrishnan, N.; Pellois, J.P. Photoinactivation of Gram Positive and Gram Negative Bacteria with the Antimicrobial Peptide (KLAKLAK)2 Conjugated to the Hydrophilic Photosensitizer Eosin Y. Bioconjug. Chem. 2013, 24, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Dosselli, R.; Tampieri, C.; Ruiz-González, R.; De Munari, S.; Ragàs, X.; Sánchez-García, D.; Agut, M.; Nonell, S.; Reddi, E.; Gobbo, M. Synthesis, characterization, and photoinduced antibacterial activity of porphyrin-type photosensitizers conjugated to the antimicrobial peptide apidaecin 1b. J. Med. Chem. 2013, 56, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Z.; Zhang, J.; Kwok, R.T.K.; Xie, S.; Tang, R.; Jia, Y.; Yang, J.; Wang, L.; Lam, J.W.Y.; et al. A Bifunctional Aggregation-Induced Emission Luminogen for Monitoring and Killing of Multidrug-Resistant Bacteria. Adv. Funct. Mater. 2018, 28, 1804632. [Google Scholar] [CrossRef]
- Courtney, C.M.; Goodman, S.M.; Nagy, T.A.; Levy, M.; Bhusal, P.; Madinger, N.E.; Detweiler, C.S.; Nagpal, P.; Chatterjee, A. Potentiating Antibiotics in Drug-Resistant Clinical Isolates via Stimuli-Activated Superoxide Generation. Sci. Adv. 2017, 3, e1701776. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gopal, A.; Karaosmanoglu, S.; Lin, J.; Munshi, T.; Zhang, W.; Chen, X.; Yan, L. Photosensitizer Doped Zeolitic Imidazolate Framework-8 Nanocomposites for Combined Antibacterial Therapy to Overcome Methicillin-Resistant Staphylococcus aureus (MRSA). Colloids Surf. B Biointerfaces 2020, 190, 110900. [Google Scholar] [CrossRef] [PubMed]
- De Melo, W.C.; Avci, P.; de Oliveria, M.N.; Gupta, A.; Vecchio, D.; Sadasivam, M.; Chandran, R.; Huan, Y.-Y.; Yin, R.; Perussi, L.R.; et al. Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection. Expert Rev. Anti-Infect. Ther. 2013, 11, 669–693. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, Z.H.; Zheng, Q.Q.; Zhou, X.Y.; Zhang, Y.; Shi, Y.F.; Lin, J.T.; Xue, W. Star-shaped polymer consisting of a porphyrin core and poly (L-lysine) dendron arms: Synthesis, drug delivery, and in vitro chemo/photodynamic therapy. Macromol. Rapid Commun. 2013, 34, 548–552. [Google Scholar] [CrossRef]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.P. Tailoring photosensitive ROS for advanced photodynamic therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.C.; Lin, J.H.; Zhang, S.; Lou, C.W.; Li, T.-T. Synergistic antibacterial strategy based on photodynamic therapy: Progress and perspectives. Chem. Eng. J. 2022, 450, 138129. [Google Scholar] [CrossRef]
- Wu, B.; Hu, K.F. Targeted antibacterial photodynamic therapy with aggregation-induced emission photosensitizers. Interdiscip. Med. 2024, 2, e20230038. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Wu, J.; Xie, S.; Zhou, Z.; Liu, L.; Huang, M.; Jiang, L.; Xu, P.; Li, J. Exploring the mechanism of photosensitizer conjugation on membrane perturbation of antimicrobial peptide: A multiscale molecular simulation study. Int. J. Biol. Macromol. 2023, 247, 125698. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Z.Y.; Liu, Y.; Yang, J.C.; Liu, Y.; Hu, F.P.; Zhu, K.; Jiang, X.Y. Gold Nanoclusters for Targeting Methicillin-Resistant Staphylococcus aureus in vivo. Angew. Chem. Int. Ed. 2018, 57, 3958–3962. [Google Scholar] [CrossRef]
- Hu, X.-L.; Chu, L.; Dong, X.; Chen, C.-R.; Tang, T.; Chen, D.; He, X.-P.; Tian, H. Multivalent Glycosheets for Double Light Driven Therapy of Multidrug-Resistant Bacteria on Wounds. Adv. Funct. Mater. 2019, 29, 1806986. [Google Scholar] [CrossRef]
- Pieters, R.J. Maximising Multivalency Effects in Protein- Carbohydrate Interactions. Org. Biomol. Chem. 2009, 7, 2013–2025. [Google Scholar] [CrossRef]
- Gast, D.; Koller, F.; Krafczyk, R.; Bauer, L.; Wunder, S.; Lassak, J.; Hoffmann-Roeder, A. A Set of Rhamnosylation-Specific Antibodies Enables Detection of Novel Protein Glycosylations in Bacteria. Org. Biomol. Chem. 2020, 18, 6823–6828. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Zhang, L.; Miron, R.J.; Liang, J.; Shi, M.; Mo, W.; Zheng, S.; Zhao, Y.; Zhang, Y. Pretreated Macrophage- Membrane-Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Adv. Mater. 2018, 30, 1804023. [Google Scholar] [CrossRef]
- Van der Vlies, A.J.; Hasegawa, U. Functionalization of Framboidal Phenylboronic Acid-Containing Nanoparticles via Aqueous Suzuki–Miyaura Coupling Reactions. Molecules 2023, 28, 3602. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Li, X.; Oh, J.; Kwon, N.; Kim, G.; Kim, D.; Yoon, J. A Boronic Acid-Functionalized Phthalocyanine with an Aggregation- Enhanced Photodynamic Effect for Combating Antibiotic-Resistant Bacteria. Chem. Sci. 2020, 11, 5735–5739. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations. Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Qi, J.; Dong, R.; Liu, H.; Wu, D.; Shao, H.; Jiang, X. Boronic Acid-Decorated Multivariate Photosensitive Metal-Organic Frameworks for Combating Multi-Drug-Resistant Bacteria. ACS Nano 2022, 16, 7732–7744. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Lepsík, M.; Horinek, D.; Havlas, Z.; Hobza, P. Interaction of Carboranes with Biomolecules: Formation of Dihydrogen Bonds. ChemPhysChem 2006, 7, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Farras, P.; Juarez-Perez, E.J.; Lepsík, M.; Luque, R.; Nunez, R.; Teixidor, F. Metallacarboranes and their interactions: Theoretical insights and their applicability. Chem. Soc. Rev. 2012, 41, 3445–3463. [Google Scholar] [CrossRef] [PubMed]
- Plesek, J. Potential Applications of the Boron Cluster Compounds. Chem. Rev. 1992, 92, 269–278. [Google Scholar] [CrossRef]
- FeFernandez-Alvarez, R.; Hlavatovičová, E.; Rodzeń, K.; Strachota, A.; Kereïche, S.; Matějíček, P.; Cabrera-González, J.; Núñez, R.; Uchman, M. Synthesis and self-assembly of a carborane-containing ABC triblock terpolymer: Morphology control on a dual-stimuli responsive system. Polym. Chem. 2019, 10, 2774–2780. [Google Scholar] [CrossRef]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684. [Google Scholar] [CrossRef]
- Eberhardt, W.H.; Crawford, B., Jr.; Lipscomb, W.N. The Valence Structure of the Boron Hydrides. J. Chem. Phys. 1954, 22, 989–1001. [Google Scholar] [CrossRef]
- Axtell, J.C.; Saleh, L.M.A.; Qian, E.A.; Wixtrom, A.I.; Spokoyny, A.M. Synthesis and Applications of Perfunctionalized Boron Clusters. Inorg. Chem. 2018, 57, 2333–2350. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Polyhedral Boranes for Medical Applications: Current Status and Perspectives. Eur. J. Inorg. Chem. 2009, 2009, 1433–1450. [Google Scholar] [CrossRef]
- Kaim, W.; Hosmane, N.S.; Záliš, S.; Maguire, J.A.; Lipscomb, W.N. Boron Atoms as Spin Carriers in Two- and Three-Dimensional Systems. Angew. Chem. Int. Ed. 2009, 48, 5082–5091. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; de Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
- Li, S.H.; Wang, Z.J.; Wei, Y.F.; Wu, C.Y.; Gao, S.P.; Jiang, H.; Zhao, X.Q.; Yan, H.; Wang, X.M. Antimicrobial activity of a ferrocene-substituted carborane derivative targeting multidrug-resistant infection. Biomaterials 2013, 34, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, C.; Tang, X.; Gao, S.; Zhao, X.; Yan, H.; Wang, X. New strategy for reversing biofilm-associated antibiotic resistance through ferrocene-substituted carborane ruthenium(II)-arene complex. Sci. China Chem. 2013, 56, 595–603. [Google Scholar] [CrossRef]
- Vanková, E.; Lokocová, K.; Matátková, O.; Krízová, I.; Masák, J.; Grüner, B.; Kaule, P.; Cermák, J.; Šícha, V. Cobalt bis-dicarbollide and its ammonium derivatives are effective antimicrobial and antibiofilm agents. J. Organomet. Chem. 2019, 899, 120891–120898. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Liu, W.W.; Chen, Y.; Jiang, H.; Yan, H.; Kosenko, I.; Chekulaeva, L.; Sivaev, I.; Bregadze, V.; Wang, X.M. A highly potent antibacterial agent targeting methicillin-resistant Staphylococcus aureus based on cobalt bis (1,2-dicarbollide) alkoxy derivative. Organometallics 2017, 36, 3484–3490. [Google Scholar] [CrossRef]
- Omarova, E.O.; Nazarov, P.A.; Firsov, A.M.; Strakhovskaya, M.G.; Arkhipova, A.Y.; Moisenovich, M.M.; Agapov, I.I.; Ol’shevskaya, V.A.; Zaitsev, A.V.; Kalinin, V.N.; et al. Carboranyl-Chlorin e6 as a Potent Antimicrobial Photosensitizer. PLoS ONE 2015, 10, e0141990. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).