Activity of Organoboron Compounds against Biofilm-Forming Pathogens
Abstract
1. Introduction
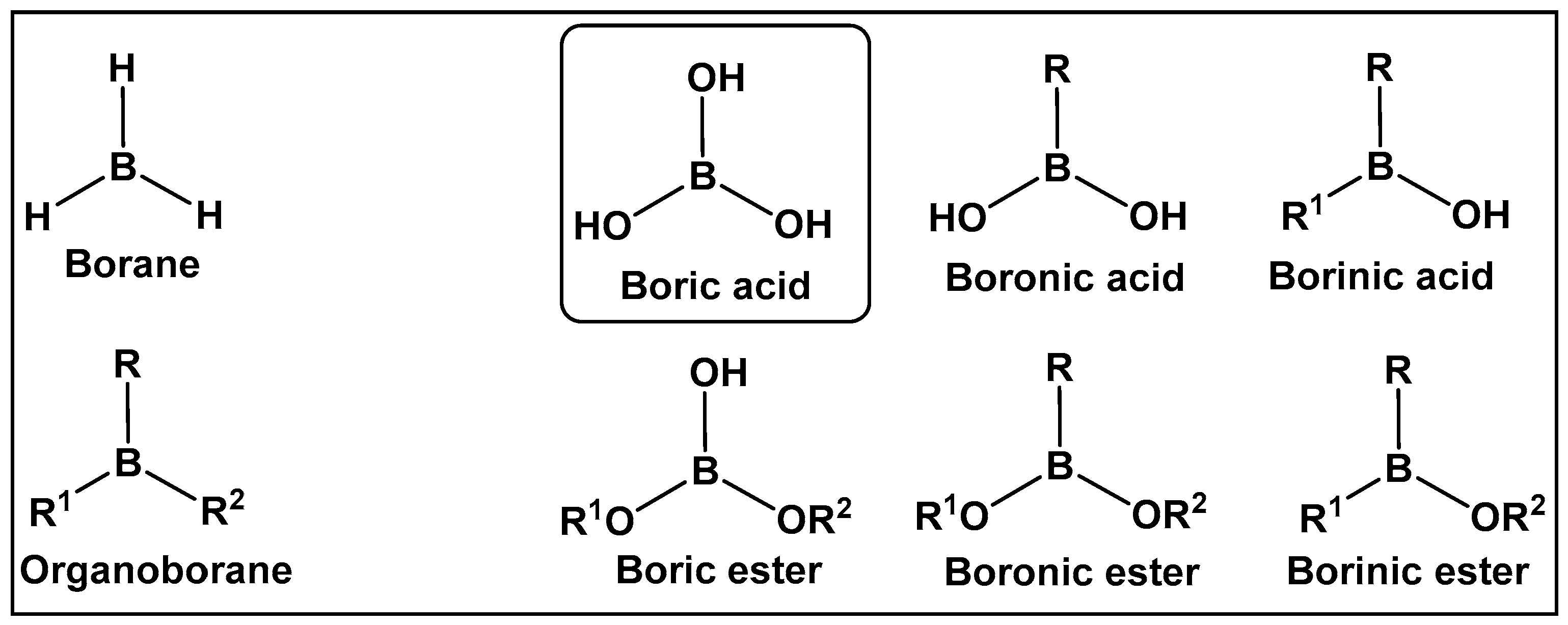
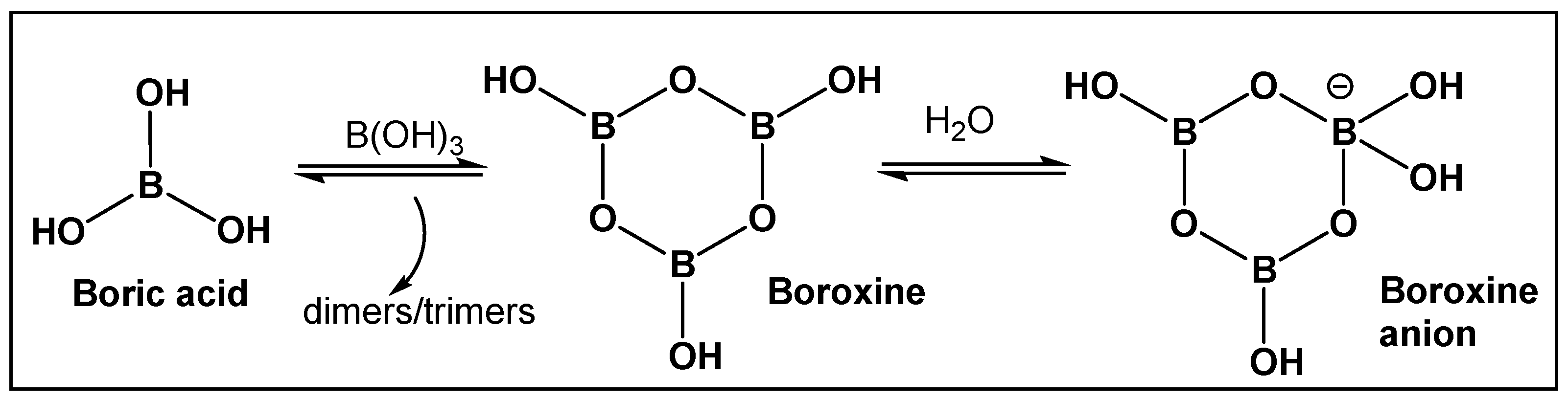
2. Utilizing the Boric Acids’ Scaffold by Following Nature’s Pragmatism
2.1. Naturally Occurring Bacterial Boron–Oxygen Bond-Containing Compounds (Boric Acids and Derivatives)
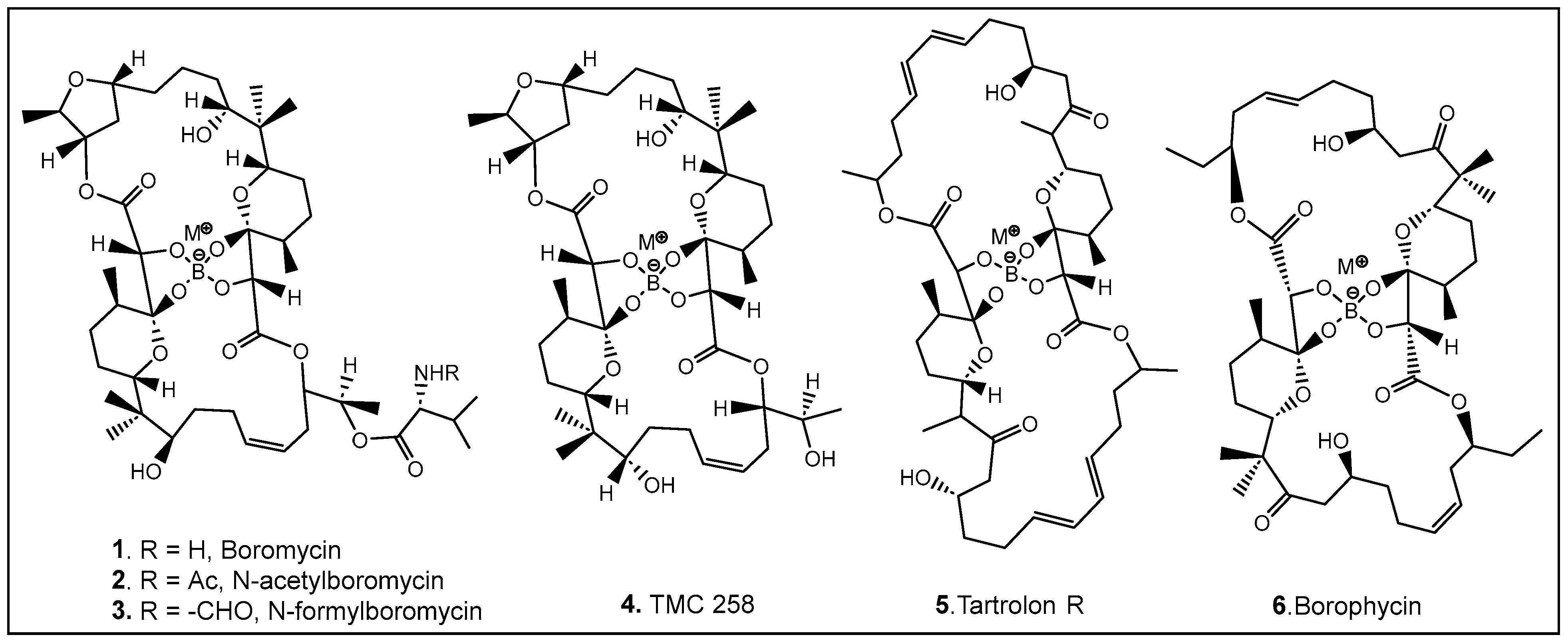
2.1.1. The Unique Antimicrobials Containing Boron
2.1.2. Autoinducer-2 (AI-2) Quorum Sensing (QS) Inducer: A Borate Diester
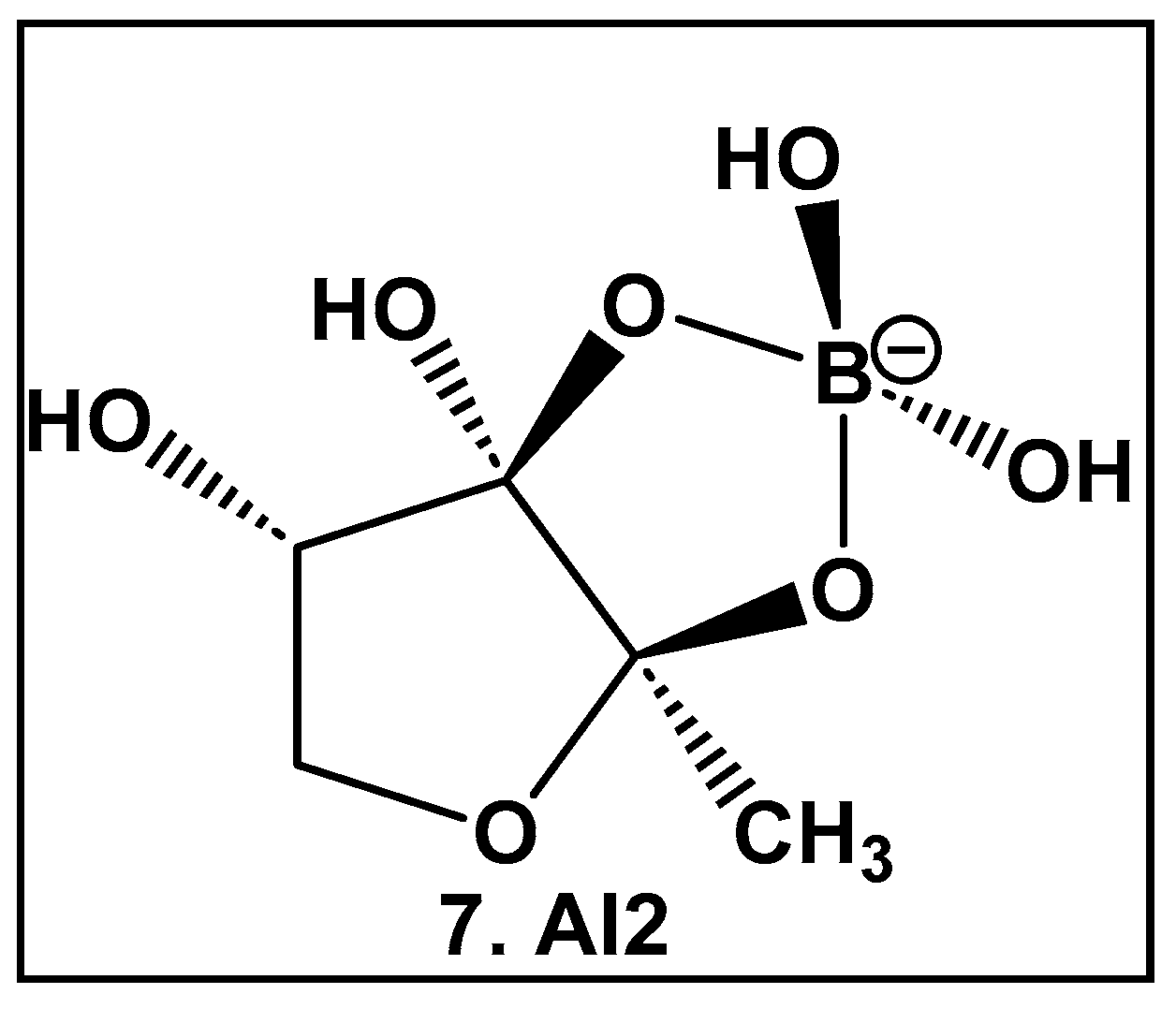
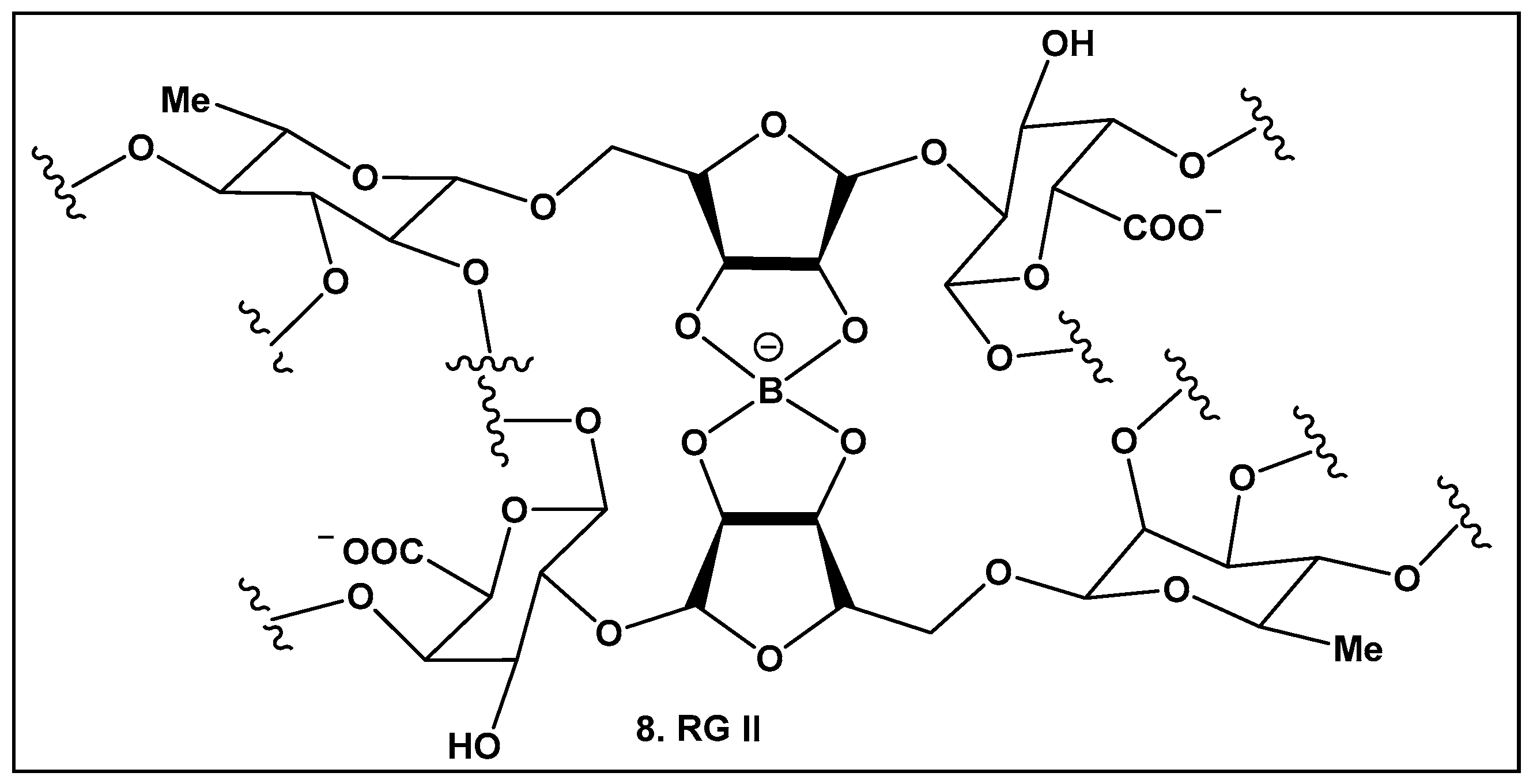
2.1.3. Rhamnogalacturonan II (RG-II)—a Boron–Sugar–Alcohol Complex
3. Compounds Prepared by Organic Syntheses: Not Yet Found in Nature
3.1. “Nonclassical” Anti-Bacterial Chemotypes: Synthetic Boron—Carbon Bond-Containing Compounds
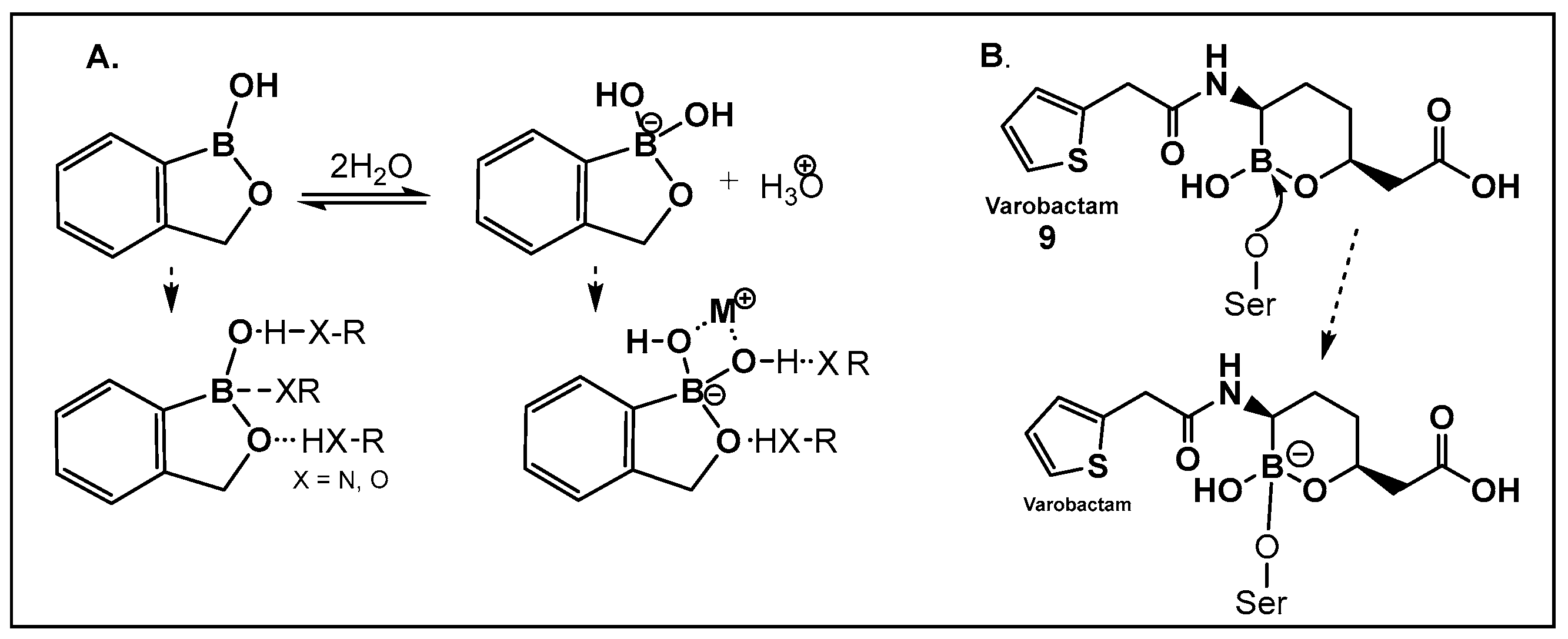
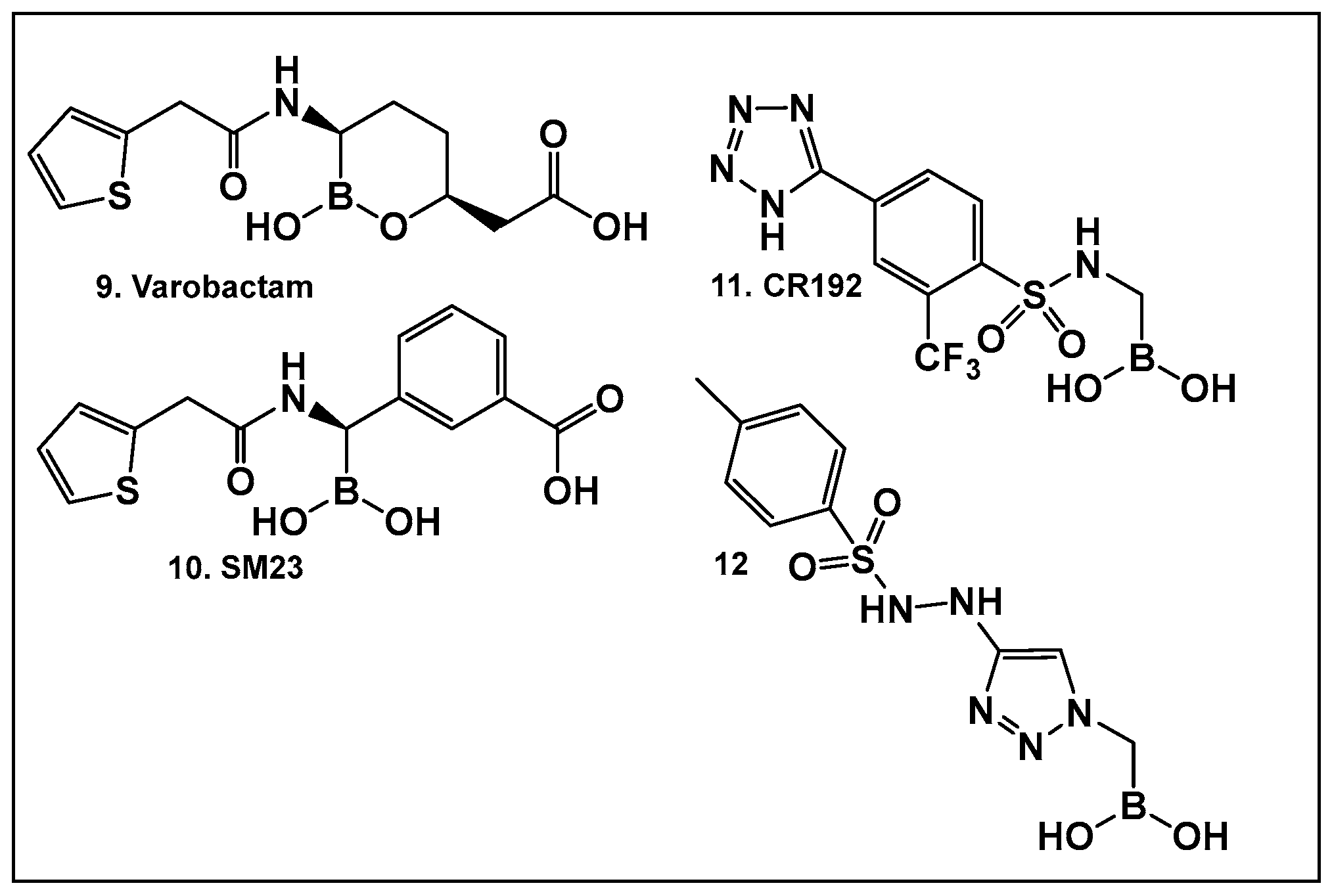
3.1.1. Boronic Acids and Their Cyclic Hemiesters: β-Lactamase Inhibitors
3.1.2. Cyclic Hemiesters of Boronic Acids (Oxaboroles) as Antifungals
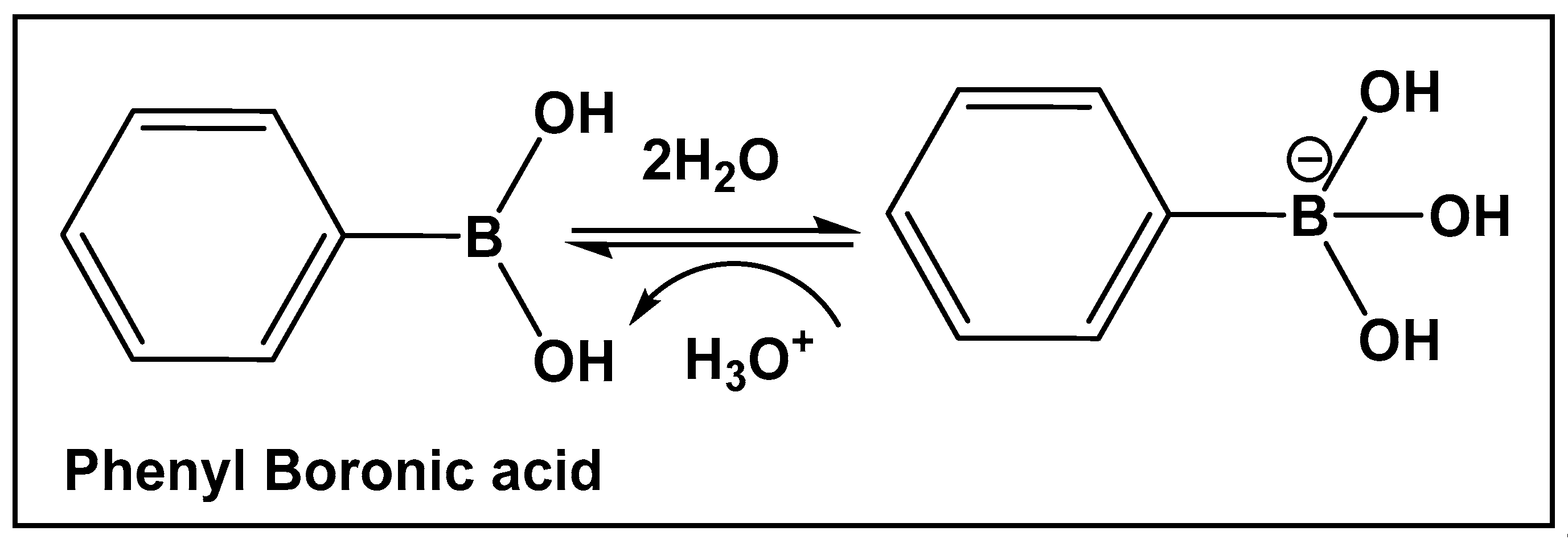
3.1.3. AI-2 Bioisosteres as Biofilm Inhibitors: Phenyl Boronic Acids
3.1.4. AI-2 Bioisosteres as Efflux Pump Inhibitors/QS Inhibitors: Pyridine Boronic Acids
3.1.5. AI-2 Bioisosteres as QS Modulators/Biofilm Modulators: Oxazaborolidines—Representatives of Boron–Nitrogen Bond-Containing Compounds
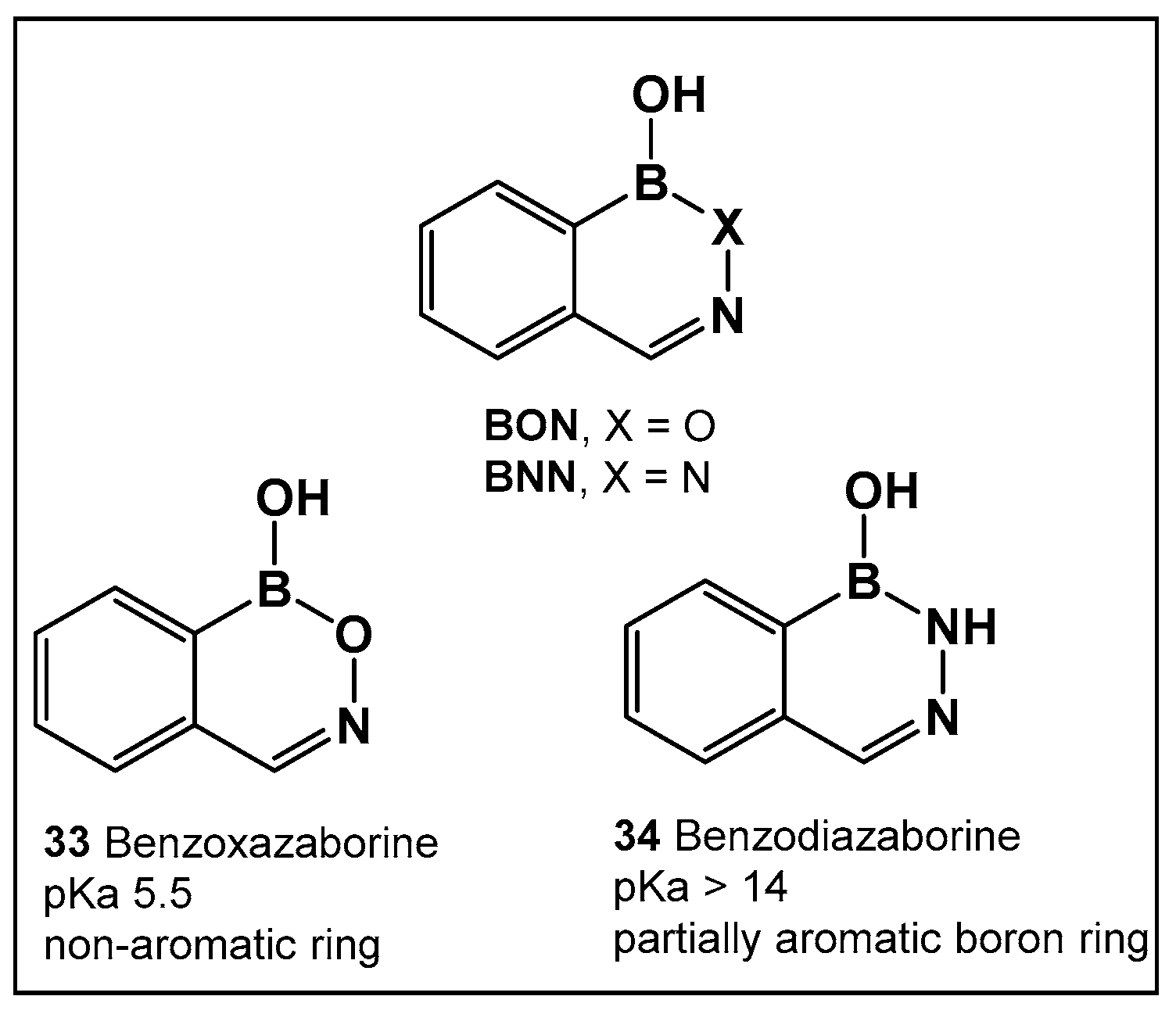
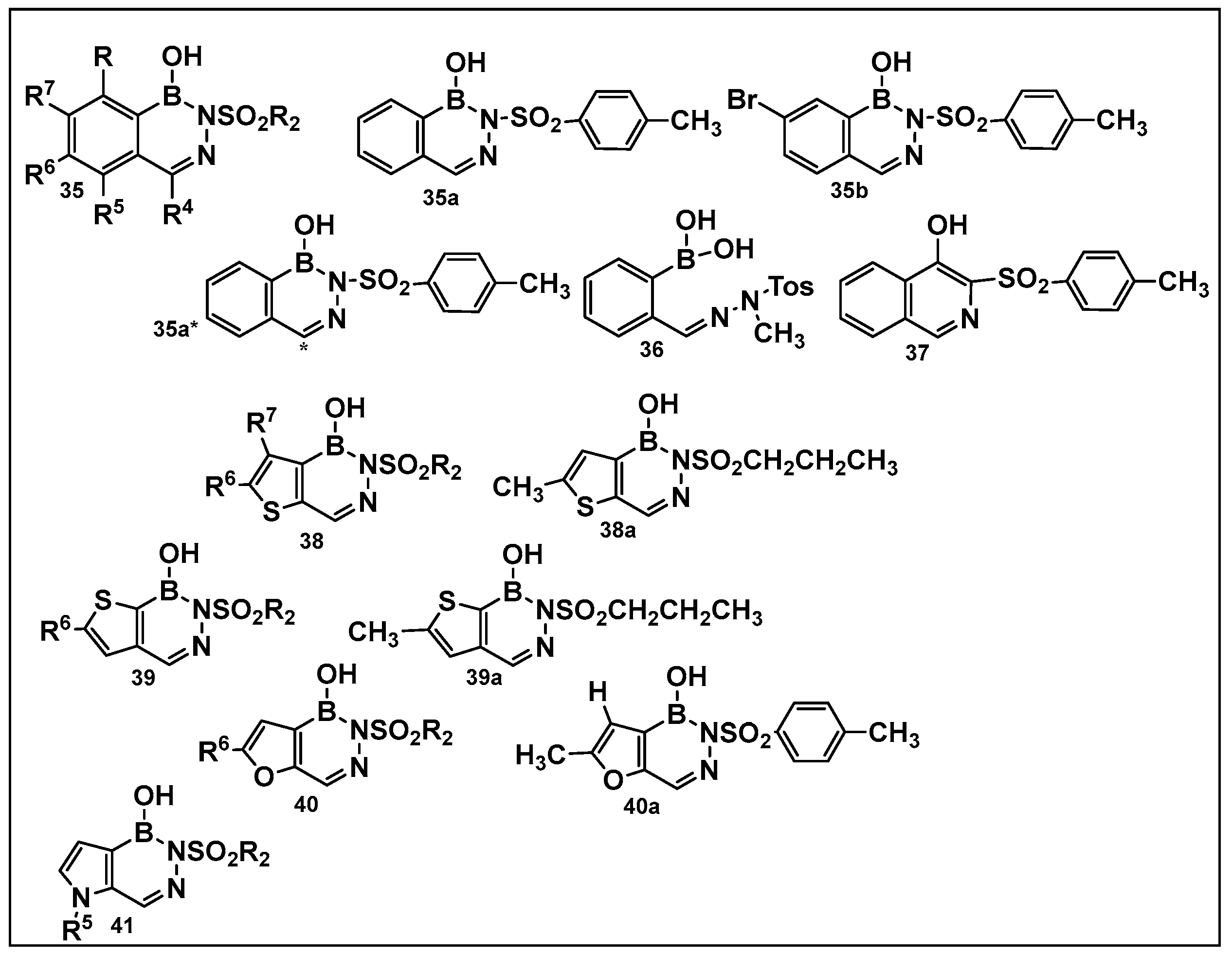
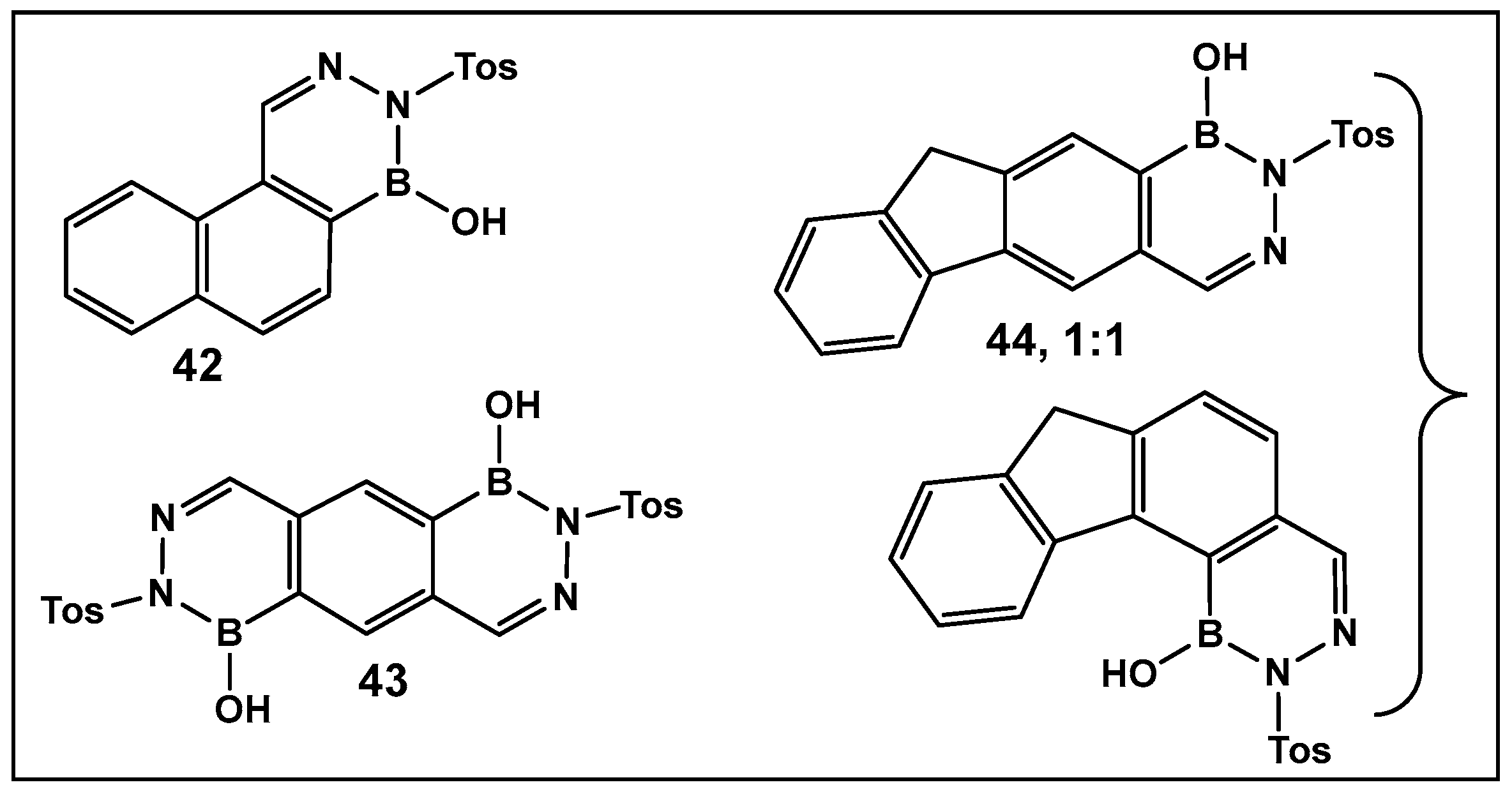
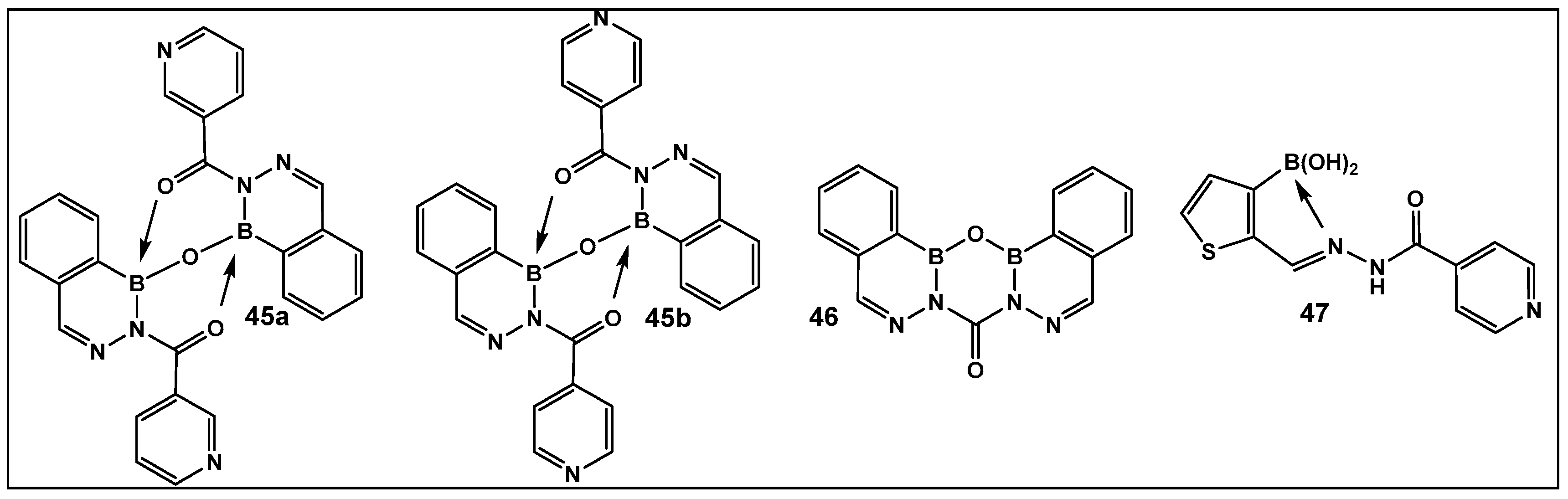
3.1.6. Aromatic Boron-Containing Heterocycles: Hemiboronic Naphthoids (i.e., Benzodiazaborines)
3.1.7. Dynamic Covalent Chemistry: Polymeric Structures, Hydrogels, and Photosensitizers Based on Boronic Acids
Boronic Acid-Containing Hydrogels—Schiff Bases Stabilized by Boronic Acids
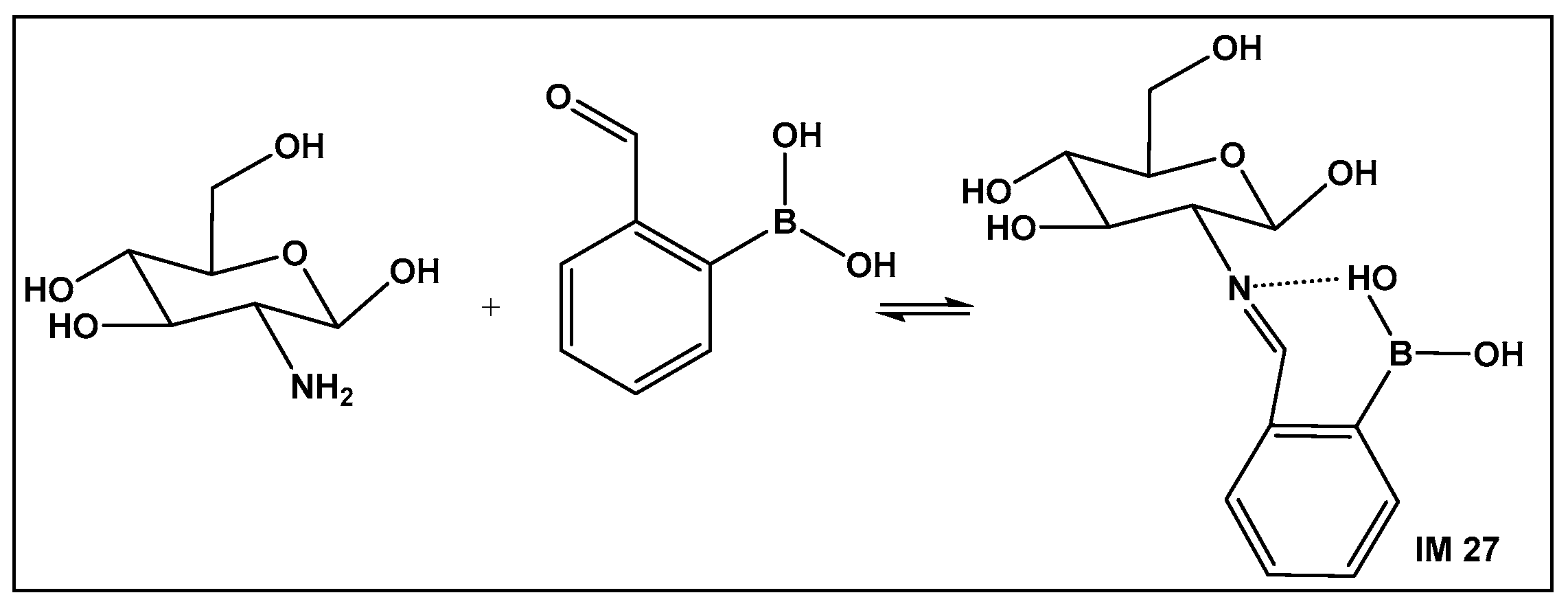
Boronic Acids-Containing Nanoparticles—Exploration of Boron–Alcohol–Sugar Complexes
Boronic Acids-Containing Photosensitizers—Exploration of Boron–Alcohol–Sugar Complexes
Boron Clusters—Exploration of Boron-Hydrophobic Interactions Biological Membranes
4. Summary/Outlook/Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemmis, E.D.; Jayasree, E.G. Analogies between boron and carbon. Acc. Chem. Res. 2003, 36, 816–824. [Google Scholar] [CrossRef]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Hütter, R.; Keller-Schierlein, W.; Knüsel, F.; Prelog, V.; Rodgers, G.C.; Suter, P.; Vogel, G.; Voser, W.; Zähner, H. The metabolic products of microorganisms. Boromycin. Helv. Chim. Acta 1967, 50, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Chen, T.S.; Chang, C.J.; Fenselau, C.; Floss, H.G. Isolation of two minor components of the boromycin fermentation: N-acetylboromycin and N-formylboromycin. J. Antibiot. 1985, 38, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Irschik, H.; Schummer, D.; Gerth, K.; Höfle, G.; Reichenbach, H. The tartrolons, new boron-containing antibiotics from a myxobacterium Sorangium cellulosum. J. Antibiot. 1995, 48, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Wolkenstein, K.; Sun, H.; Falk, H.; Griesinger, C. Structure and absolute configuration of Jurassic polyketide-derived spiroborate pigments obtained from microgram quantities. J. Am. Chem. Soc. 2015, 137, 13460–13463. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Elshahawi, S.I.; Trindade-Silva, A.E.; Hanora, A.; Han, A.W.; Flores, M.S.; Vizzoni, V.; Schrago, C.G.; Soares, C.A.; Concepcion, G.P.; Distel, D.L.; et al. Boronated tartrolon antibiotic produced by symbiotic cellulose-degrading bacteria in shipworm gills. Proc. Natl Acad. Sci. USA 2013, 110, E295–E304. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Al Quntar, A.A.; Srebnik, M. Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chem. Rev. 2011, 111, 209–237. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A. Naturally Occurring Boron Containing Compounds and Their Biological Activities. J. Nat. Prod. Resour. 2017, 3, 147–154. [Google Scholar]
- Mollner, T.A.; Isenegger, P.G.; Josephson, B.; Buchanan, C.; Lercher, L.; Oehlrich, D.; Hansen, D.F.; Mohammed, S.; Baldwin, A.J.; Gouverneur, V.; et al. Post-translational insertion of boron in proteins to probe and modulate function. Nat. Chem. Biol. 2021, 17, 1245–1261. [Google Scholar] [CrossRef]
- Cheng, Q.-Q.; Zhu, S.-F.; Zhang, Y.-Z.; Xie, X.-L.; Zhou, Q.-L. Copper-catalyzed B–H bond insertion reaction: A highly efficient and enantioselective C–B bond-forming reaction with amine–borane and phosphine–borane adducts. J. Am. Chem. Soc. 2013, 135, 14094–14097. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Qi, W.-Y.; Xu, B.; Xu, M.-H. Rhodium(i)-catalyzed asymmetric carbene insertion into B–H bonds: Highly enantioselective access to functionalized organoboranes. J. Am. Chem. Soc. 2015, 137, 5268–5271. [Google Scholar] [CrossRef] [PubMed]
- Hyde, S.; Veliks, J.; Liégault, B.; Grassi, D.; Taillefer, M.; Gouverneur, V. Copper-catalyzed insertion into heteroatom–hydrogen bonds with trifluorodiazoalkanes. Angew. Chem. Int. Ed. 2016, 55, 3785–3789. [Google Scholar] [CrossRef]
- Kan, S.B.J.; Huang, X.; Gumulya, Y.; Chen, K.; Arnold, F.H. Genetically programmed chiral organoborane synthesis. Nature 2017, 552, 132–136. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Silva, M.P.; Saraiva, L.; Pinto, M.; Sousa, M.E. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules 2020, 25, 4323. [Google Scholar] [CrossRef]
- Molloy, J.J.; Schäfer, M.; Wienhold, M.; Morack, T.; Daniliuc, C.G.; Gilmour, R. Boron-enabled geometric isomerization of alkenes via selective energy-transfer catalysis. Science 2020, 369, 302–306. [Google Scholar] [CrossRef]
- Plescia, J.; Moitessier, N. Design and discovery of boronic acid drugs. Eur. J. Med. Chem. 2020, 195, 112270. [Google Scholar] [CrossRef]
- Volochnyuk, D.M.; Gorlova, A.O.; Grygorenko, O.O. Saturated Boronic Acids, Boronates, and Trifluoroborates: An Update on Their Synthetic and Medicinal Chemistry. Chem. Eur. J. 2021, 27, 15277–15326. [Google Scholar] [CrossRef]
- Messner, K.; Vuong, B.; Tranmer, G.K. The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry. Pharmaceuticals 2022, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Shareef, M.A.; Das, S.; Nandwana, N.K.; Das, Y.; Saito, M.; Weiss, L.M. Boron-Containing heterocycles as promising pharmacological agents. Bioorg. Med. Chem. 2022, 63, 116748. [Google Scholar] [CrossRef] [PubMed]
- Grams, R.J.; Santos, W.L.; Scorei, I.R.; Abad-García, A.; Rosenblum, C.A.; Bita, A.; Cerecetto, H.; Viñas, C.; Soriano-Ursúa, M.A. The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology. Chem. Rev. 2024, 124, 2441–2511. [Google Scholar] [CrossRef]
- Kazmi, M.Z.H.; Schneider, O.M.; Hall, D.G. Expanding the Role of Boron in New Drug Chemotypes: Properties, Chemistry, Pharmaceutical Potential of Hemiboronic Naphthoids. J. Med. Chem. 2023, 66, 13768–13787. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wu, J.; Song, L.; Zhang, M.; Hipolito, C.J.; Wu, C.; Wang, S.; Zhang, Y.; Yin, Y. Merging the Versatile Functionalities of Boronic Acid with Peptides. Int. J. Mol. Sci. 2021, 22, 12958. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Juhas, M.; Eberl, L.; Tümmler, B. Quorum sensing: The power of cooperation in the world of Pseudomonas. Environ. Microbiol. 2005, 7, 459–471. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Gadar, K.; McCarthy, R.R. Using next generation antimicrobials to target the mechanisms of infection. NPJ Antimicrob. Resist. 2023, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, H. Presence and utility of boron in plants. Ann. Inst. Pasteur 1910, 24, 321–329. [Google Scholar]
- Warington, K. The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot. 1923, 37, 629–672. [Google Scholar] [CrossRef]
- Street, R.A.; Kulkarni, M.G.; Stirk, W.A.; Southway, C.; Van Staden, J. Variation in heavy metals and microelements in South African medicinal plants obtained from street markets. Food Addit. Contam. Part A 2008, 25, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Peng, J. Interaction between Boron and Other Elements in Plants. Genes 2023, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.A.; Abreu, I.; Bell, R.W.; Bienert, M.D.; Brown, P.H.; Dell, B.; Fujiwara, T.; Goldbach, H.E.; Lehto, T.; Mock, H.-P.; et al. Boron: An essential element for vascular plants. New Phytol. 2020, 226, 1232–1237. [Google Scholar] [CrossRef]
- Maldonado, P.; Aquea, F.; Reyes-Díaz, M.; Cárcamo-Fincheira, P.; Soto-Cerda, B.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Role of boron and its interaction with other elements in plants. Front. Plant Sci. 2024, 15, 1332459. [Google Scholar]
- Bolanos, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef]
- Läuchli, A. Functions of Boron in Higher Plants: Recent Advances and Open Questions. Plant Biol. 2002, 4, 190–192. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Rangavajla, N.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; Ciocîlteu, M.V.; Dincă, L.; Neamţu, J.; Bunaciu, A.; et al. Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound. Inorganics 2023, 11, 112. [Google Scholar] [CrossRef]
- Davies, D.H.; Norris, G.L.F. Growth Promotion Means for Ruminant Animals. European Patent EP 2893 19790711, 11 July 1979. [Google Scholar]
- Dunitz, J.D.; Hawley, D.M.; Miklos, D.; White, D.N.J.; Berlin, Y.; Morusic, R.; Prelog, V. Structure of boromycin. Helv. Chim. Acta 1971, 54, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Pache, W.; Zahner, H. Metabolic products of microorganisms: 77. Studies on the mechanism of action of boromycin. Arch. Mikrobiol. 1969, 67, 156–165. [Google Scholar] [CrossRef]
- De Carvalho, L.P.; Groeger-Otero, S.; Kreidenweiss, A.; Kremsner, P.G.; Mordmüller, B.; Held, J. Boromycin has Rapid-Onset Antibiotic Activity against Asexual and Sexual Blood Stages of Plasmodium falciparum. Front. Cell. Infect. Microbiol. 2022, 11, 802294. [Google Scholar] [CrossRef] [PubMed]
- Schummer, D.; Schomburg, D.; Irschik, H.; Reichenbach, H.; Hoefle, G. Antibiotics from gliding bacteria. Part LXXV. Absolute configuration and biosynthesis of tartrolon B, a boron containing macrodiolide from Sorangium cellulosum. Liebigs Ann. 1996, 1996, 965–969. [Google Scholar] [CrossRef]
- Kohno, J.; Kawahata, T.; Otake, T.; Morimoto, M.; Mori, H.; Ueba, N.; Nishio, M.; Kinumaki, A.; Komatsubara, S.; Kawashima, K. Boromycin, an anti-HIV antibiotic. Biosci. Biotechnol. Biochem. 1996, 60, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.S.; Chang, C.-J.; Floss, H.G. Biosynthesis of Boromycin. J. Org. Chem. 1981, 46, 2661–2665. [Google Scholar] [CrossRef]
- Miller, M.B.; Burg, R.W. Boromycin as a Coccidiostat. U.S. Patent 3864479, 4 February 1975. [Google Scholar]
- Abenoja, J.; Cotto-Rosario, A.; O’Connor, R. Boromycin Has Potent Anti-Toxoplasma and Anti-Cryptosporidium Activity. Antimicrob. Agents Chemother. 2021, 65, e01278-20. [Google Scholar] [CrossRef] [PubMed]
- Moreira, W.; Aziz, D.B.; Dick, T. Boromycin kills mycobacterial persisters without detectable resistance. Front. Microbiol. 2016, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, B.; Kaiserova, K.; Simkovic, M.; Orlicky, J.; Knezl, V.; Varecka, L. The effect of boromycin on the. Ca2+ homeostasis. Mol. Cell. Biochem. 2002, 231, 15–22. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S. Tenuecyclamides A–D, cyclic hexapeptides from the cyanobacterium Nostoc spongiaeforme var. tenue. J. Nat. Prod. 1998, 61, 1248–1251. [Google Scholar] [CrossRef]
- Hemscheidt, T.; Puglisi, M.P.; Larsen, L.K.; Patterson, G.M.L.; Moore, R.E.; Rios, J.L.; Clardy, J. Structure and biosynthesis of borophycin, a new boeseken complex of boric acid from a marine strain of the blue-green alga Nostoc linckia. J. Org. Chem. 1995, 59, 3467–3471. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef] [PubMed]
- Böeseken, J. The use of Boric Acid for the Determination of the Configuration of Carbohydrates. J. Adv. Carbohydr. Chem. 1949, 4, 189–210. [Google Scholar]
- Dembitsky, V.M.; Smoum, R.; Al-Quntar, A.A.; Abu Ali, H.; Pergament, I.; Srebnik, M. Natural occurrence of boron-containing compounds in plants, algae and microorganisms. Plant Sci. 2002, 163, 931–942. [Google Scholar] [CrossRef]
- Bassler, B.L.; Wright, M.; Showalker, R.E.; Silverman, M.R. Intercellular signalling in Vibrio harveyi: Sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 1993, 3, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Wright, M.; Silverman, M.R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: Sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 1994, 13, 273–286. [Google Scholar] [CrossRef]
- Surette, M.G.; Miller, M.B.; Bassler, B.L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: A new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 1999, 96, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Daniel, R.; Wagner-Döbler, I.; Zeng, A.P. Is autoinducer-2 a universal signal for interspecies communication: A comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol. Biol. 2004, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Konaklieva, M.I.; Plotkin, B.J. Chemical communication—Do we have a quorum? Mini Rev. Med. Chem. 2006, 6, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, J.T.; Welch, M.; Spring, D.R. Learning the language of bacteria. ACS Chem. Biol. 2007, 2, 715–717. [Google Scholar] [CrossRef]
- Miller, S.T.; Xavier, K.B.; Campagna, S.R.; Taga, M.E.; Semmelhack, M.F.; Bassler, B.L.; Hughson, F.M. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol. Cell 2004, 15, 677–687. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Liu, X.; Wang, Z.; Jiang, M.; Wang, R.; Xie, L.; Liu, Q.; Xie, X.; Shang, D.; et al. Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat. Commun. 2020, 11, 5371. [Google Scholar] [CrossRef]
- Lowery, C.A.; Park, J.; Kaufmann, G.F.; Janda, K.D. An unexpected switch in the modulation of AI-2-based quorum sensing discovered through synthetic 4,5-dihydroxy-2,3-pentanedione analogues. J. Am. Chem. Soc. 2008, 130, 9200–9201. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.V.; Kis, P.; Xavier, K.B.; Ventura, M.R. Synthesis and Potential of Autoinducer-2 and Analogs to Manipulate Inter-Species Quorum Sensing. Isr. J. Chem. 2023, 63, e202200091. [Google Scholar] [CrossRef]
- Smith, J.A.I.; Wang, J.; Nguen-Mau, S.-M.; Lee, V.; Herman, O.S. Biological screening of a diverse set of AI-2 analogues in Vibrio harveyi suggests that receptors which are involved in synergistic agonism of AI-2 and analogues are promiscuous. Chem. Commun. 2009, 45, 7033–7035. [Google Scholar] [CrossRef]
- Guo, M.; Gamby, S.; Zheng, Y.; Sintim, H.O. Small molecule inhibitors of AI-2 signaling in bacteria: State-of-the-art and future perspectives for anti-quorum sensing agents. Int. J. Mol. Sci. 2013, 14, 7694–7728. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, Y.; Yuan, B.; Yue, Y.; Zhao, M.; Luo, R.; Wu, H.; Wang, L.; Zhang, Y.; Xiao, J.; et al. Effect of Autoinducer-2 Quorum Sensing Inhibitor on Interspecies Quorum Sensing. Front. Microbiol. 2022, 13, 791802. [Google Scholar] [CrossRef]
- Escobar-Muciño, E.; Arenas-Hernández, M.M.P.; Luna-Guevara, M.L. Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella. Microorganisms 2022, 10, 884. [Google Scholar] [CrossRef]
- Meijler, M.M.; Hom, L.G.; Kaufmann, G.F.; McKenzie, K.M.; Sun, C.; Moss, J.A.; Matsushita, M.; Janda, K.D. Synthesis and biological validation of a ubiquitous quorum-sensing molecule. Angew. Chem. Int. Ed. 2004, 43, 2106–2108. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Kergaravat, B.; Jacquet, P.; Billot, R.; Grizard, D.; Chabrière, É.; Plener, L.; Daudé, D. Disrupting quorum sensing as a strategy to inhibit bacterial virulence in human, animal, and plant pathogens. Pathog. Dis. 2024, 82, ftae009. [Google Scholar] [CrossRef]
- Tsuchikama, K.; Zhu, J.; Lowery, C.A.; Kaufmann, G.F.; Janda, K.D. C4-alkoxy-HPD: A potent class of synthetic modulators surpassing nature in AI-2 quorum sensing. J. Am. Chem. Soc. 2012, 134, 13562–13564. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; Lowery, C.A.; Janda, K.D. Probing autoinducer-2 based quorum sensing: The biological consequences of molecules unable to traverse equilibrium states. J. Org. Chem. 2011, 76, 6981–6989. [Google Scholar] [CrossRef]
- Darvill, A.G.; McNeil, M.; Albersheim, P. Structure of Plant Cell Walls: VIII. A New Pectic Polysaccharide. Plant Physiol. 1978, 62, 418–422. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.; Darvill, A.G.; Albersheim, P. Structure of plant cell walls: X. Rhamnogalacturonan I, a structurally complex pectic polysaccharide in the walls of suspension-cultured sycamore cells. Plant Physiol. 1980, 66, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Franz, G. Structure-Activity Relation of Polysaccharides with Antitumor activity. Farm. Tijdschr. Belg. 1987, 64, 301. [Google Scholar]
- Kameda, T.; Ishii, T.; Matsunaga, T.; Ashida, J. 11B Solid-state NMR Investigation of the Rhamnogalacturonan II-borate Complex in Plant Cell Walls. Anal. Sci. 2006, 22, 321–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bewley, J.D. Handbook of Plant Science; John Wiley & Sons Ltd: Chichester, UK, 2007; Volume 2, p. 892. [Google Scholar]
- Feldheim, W. Oligofructose and inulin—Dietary fibre and probiotic. Ernaehrung 2000, 24, 162–164. [Google Scholar]
- Dexter, S.T. Growth, Organic nitrogen reactions, and buffer capacity in relation to hardiness of Plants. Plant Physiol. 1935, 10, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, P.; Carlier, M.; Mollet, J.-C.; Lehner, A. The cell wall pectic rhamnogalacturonan II, an enigma in plant glycobiology. In Carbohydrate Chemistry; The Royal Society of Chemistry: London, UK, 2021; pp. 553–571. [Google Scholar]
- Begum, R.A.; Fry, S.C. Boron bridging of rhamnogalacturonan-II in Rosa and arabidopsis cell cultures occurs mainly in the endo-membrane system and continues at a reduced rate after secretion. Ann. Bot. 2022, 130, 703–715. [Google Scholar]
- Zhang, Y.; Deepak Sharma, D.; Liang, Y.; Downs, N.; Dolman, F.; Thorne, K.; Black, I.M.; Pereira, J.H.; Adams, P.; Scheller, H.V.; et al. A putative rhamnogalacturonan-II CMP-β-Kdo transferase identified using CRISPR/Cas9 gene edited callus to circumvent embryo lethality. bioRxiv 2023. [Google Scholar] [CrossRef]
- Park, S.N.; Tae Noh, K.; Jeong, Y.I.; Duk Jung, I.; Kyu Kang, H.; Sun Cha, G.; Lee, S.J.; Seo, J.K.; Kang, D.H.; Hwang, T.-H.; et al. Rhamnogalacturonan II is a Toll-like receptor 4 agonist that inhibits tumor growth by activating dendritic cell-mediated CD8+ T cells. Exp. Mol. Med. 2013, 45, e8. [Google Scholar] [CrossRef]
- Macho, J.M.; Blue, R.M.; Lee, H.W.; MacMillan, J.B. Boron NMR as a Method to Screen Natural Product Libraries for B-Containing Compounds. Org. Lett. 2022, 24, 3161–3166. [Google Scholar] [CrossRef]
- Hecker, S.J.; Reddy, K.R.; Totrov, M.; Hirst, G.C.; Lomovskaya, O.; Griffith, D.C.; King, P.; Tsivkovski, R.; Sun, D.; Sabet, M.; et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs. class A serine carbapenemases. J. Med. Chem. 2015, 58, 3682–3692. [Google Scholar] [CrossRef] [PubMed]
- Kiener, P.A.; Waley, S.G. Reversible inhibitors of penicillinases. Biochem. J. 1978, 169, 197–204. [Google Scholar] [CrossRef]
- Beesley, T.; Gascoyne, N.; Knott-Hunziker, V.; Petursson, S.; Waley, S.G.; Jaurin, B.; Grundström, T. The inhibition of class C β-lactamases by boronic acids. Biochem. J. 1983, 209, 229–233. [Google Scholar] [CrossRef]
- Morandi, S.; Morandi, F.; Caselli, E.; Shoichet, B.K.; Prati, F. Structure-based optimization of cephalothin-analogue boronic acids as β-lactamase inhibitors. Bioorg. Med. Chem. 2008, 16, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Weston, G.S.; Blázquez, J.; Baquero, F.; Shoichet, B.K. Structure-based enhancement of boronic acid-based inhibitors of AmpC β-lactamase. J. Med. Chem. 1998, 41, 4577–4586. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Caselli, E.; Morandi, S.; Focia, P.J.; Blázquez, J.; Shoichet, B.K.; Prati, F. Nanomolar inhibitors of AmpC β-lactamase. J. Am. Chem. Soc. 2003, 125, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.L.; Rodkey, E.A.; Taracila, M.A.; Drawz, S.M.; Bethel, C.R.; Papp-Wallace, K.M.; Smith, K.M.; Xu, Y.; Dwulit-Smith, J.R.; Romagnoli, C.; et al. Design and exploration of novel boronic acid inhibitors reveals important interactions with a clavulanic acid-resistant sulfhydryl-variable (SHV) β-lactamase. J. Med. Chem. 2013, 56, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Sampson, J.M.; Ori, C.; Prati, F.; Drawz, S.M.; Bethel, C.R.; Bonomo, R.A.; van den Akker, F. Novel insights into the mode of inhibition of class A SHV-1 β-lactamases revealed by boronic acid transition state inhibitors. Antimicrob. Agents Chemother. 2011, 55, 174–183. [Google Scholar] [CrossRef][Green Version]
- Alsenani, T.A.; Rodríguez, M.M.; Ghiglione, B.; Taracila, M.A.; Mojica, M.F.; Rojas, L.J.; Hujer, A.M.; Gutkind, G.; Bethel, C.R.; Rather, P.N.; et al. Boronic Acid Transition State Inhibitors as Potent Inactivators of KPC and CTX-M β-Lactamases: Biochemical and Structural Analyses. Antimicrob. Agents Chemother. 2023, 67, e00930-22. [Google Scholar] [CrossRef] [PubMed]
- Lapuebla, A.; Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Quale, J.; Landman, D. Activity of meropenem combined with RPX7009, a novel β-lactamase inhibitor, against Gram-negative clinical isolates in New York City. Antimicrob. Agents Chemother. 2015, 59, 4856–4860. [Google Scholar] [CrossRef]
- Bouza, A.A.; Swanson, H.C.; Smolen, K.A.; VanDine, A.L.; Taracila, M.A.; Romagnoli, C.; Caselli, E.; Prati, F.; Bonomo, R.A.; Powers, R.A.; et al. Structure-Based Analysis of Boronic Acids as Inhibitors of Acinetobacter-Derived Cephalosporinase-7, a Unique Class C β-Lactamase. ACS Infect. Dis. 2018, 4, 325–336. [Google Scholar] [CrossRef]
- Caselli, E.; Fini, F.; Introvigne, M.L.; Stucchi, M.; Taracila, M.A.; Fish, E.R.; Smolen, K.A.; Rather, P.N.; Powers, R.A.; Wallar, B.J.; et al. 1,2,3-Triazolylmethaneboronate: A Structure Activity Relationship Study of a Class of β-Lactamase Inhibitors against Acinetobacter baumannii Cephalosporinase. ACS Infect. Dis. 2020, 6, 1965–1975. [Google Scholar] [CrossRef]
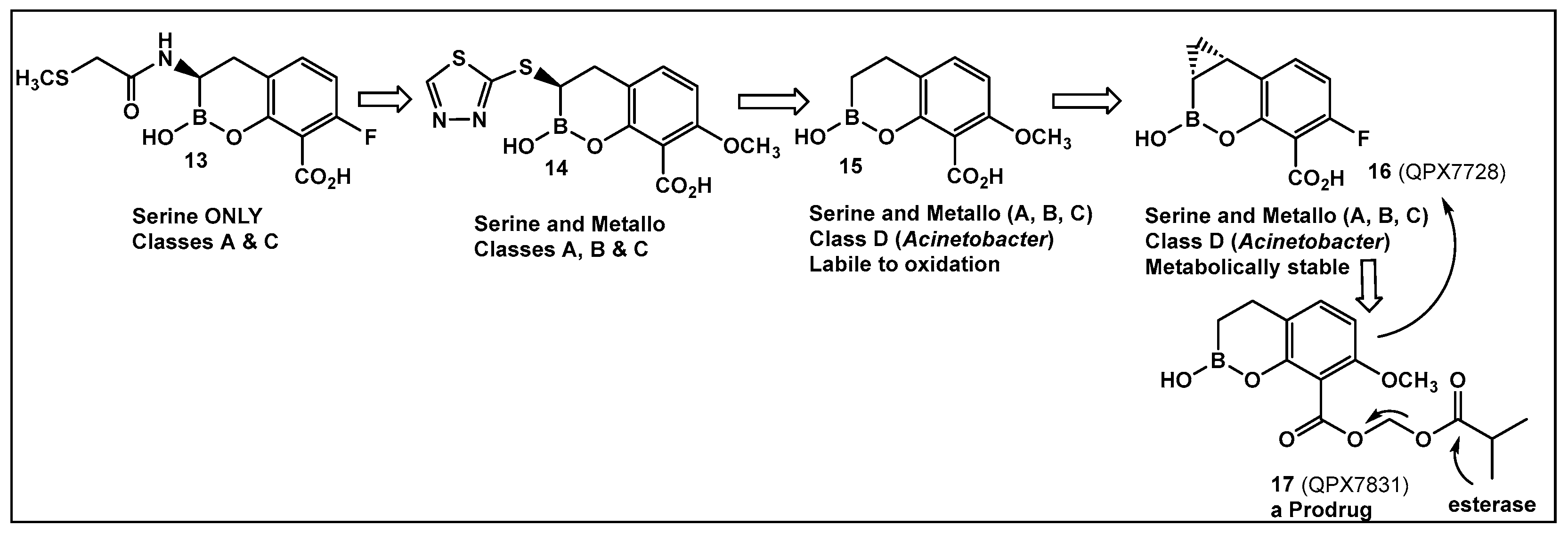
- Hecker, S.J.; Reddy, K.R.; Lomovskaya, O.; Griffith, D.C.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Sun, D.; Sabet, M.; Tarazi, Z.; et al. Discovery of Cyclic Boronic Acid QPX7728, an Ultrabroad-Spectrum Inhibitor of Serine and Metallo-β-lactamases. J. Med. Chem. 2020, 63, 7491–7507. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Tsivkovski, R.; Sun, D.; Reddy, R.; Totrov, M.; Hecker, S.; Griffith, D.; Loutit, J.; Dudley, M. QPX7728, An Ultra-Broad-Spectrum B-Lactamase Inhibitor for Intravenous and Oral Therapy: Overview of Biochemical and Microbiological Characteristics. Front. Microbiol. 2021, 12, 697180. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Rubio-Aparicio, D.; Tsivkovski, R.; Loutit, J.; Dudley, M. The Ultrabroad-Spectrum Beta-Lactamase Inhibitor QPX7728 Restores the Potency of Multiple Oral Beta-Lactam Antibiotics against Beta-Lactamase-Producing Strains of Resistant Enterobacterales. Antimicrob. Agents Chemother. 2022, 66, e0216821. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Parkinson, J.; Sabet, M.; Tarazi, Z.; Boyer, S.H.; Lomovskaya, O.; Griffith, D.C.; Hecker, S.J.; Dudley, M.N. Orally Bioavailable Prodrug of Boronic b-Lactamase Acid Inhibitor QPX7728. J. Med. Chem. 2021, 64, 17523–17529. [Google Scholar] [CrossRef] [PubMed]
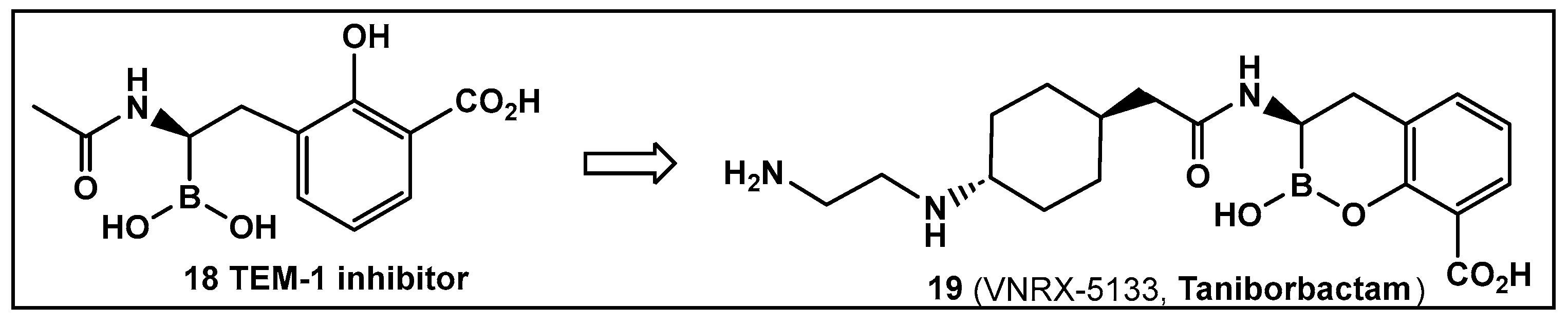
- Ness, S.; Martin, R.; Kindler, A.M.; Paetzel, M.; Gold, M.; Jensen, S.E.; Jones, J.B.; Strynadka, N.C. Structure-based design guides the improved efficacy of deacylation transition state analogueinhibitors of TEM-1 beta-lactamase. Biochemistry 2000, 39, 5312–5321. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Trout, R.E.L.; Chu, G.-H.; McGarry, D.; Jackson, R.W.; Hamrick, J.C.; Daigle, D.M.; Cusick, S.M.; Pozzi, C.; De Luca, F.; et al. Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-b-lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. J. Med. Chem. 2020, 63, 2789–2801. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Birhanu, B.T.; Miguel-Ruano, V.; Kim, C.; Batuecas, M.; Yang, J.; El-Araby, A.M.; Jiménez-Faraco, E.; Schroeder, V.A.; Alba, A.; et al. Restoring susceptibility to β-lactam antibiotics in methicillin-resistant Staphylococcus aureus. Nat. Chem. Biol. 2024. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Shearer, J.; Castro, J.L.; Lawson, A.D.G.; MacCoss, M.; Taylor, R.D. Rings in Clinical Trials and Drugs: Present and Future. J. Med. Chem. 2022, 65, 8699–8712. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.M.; Adamczyk-Woźniak, A.; Madura, I.D. Effect of substituents in novel bioactive tavaborole derivatives on the intermolecular interaction hierarchy. CrystEngComm 2022, 24, 3586–3596. [Google Scholar] [CrossRef]
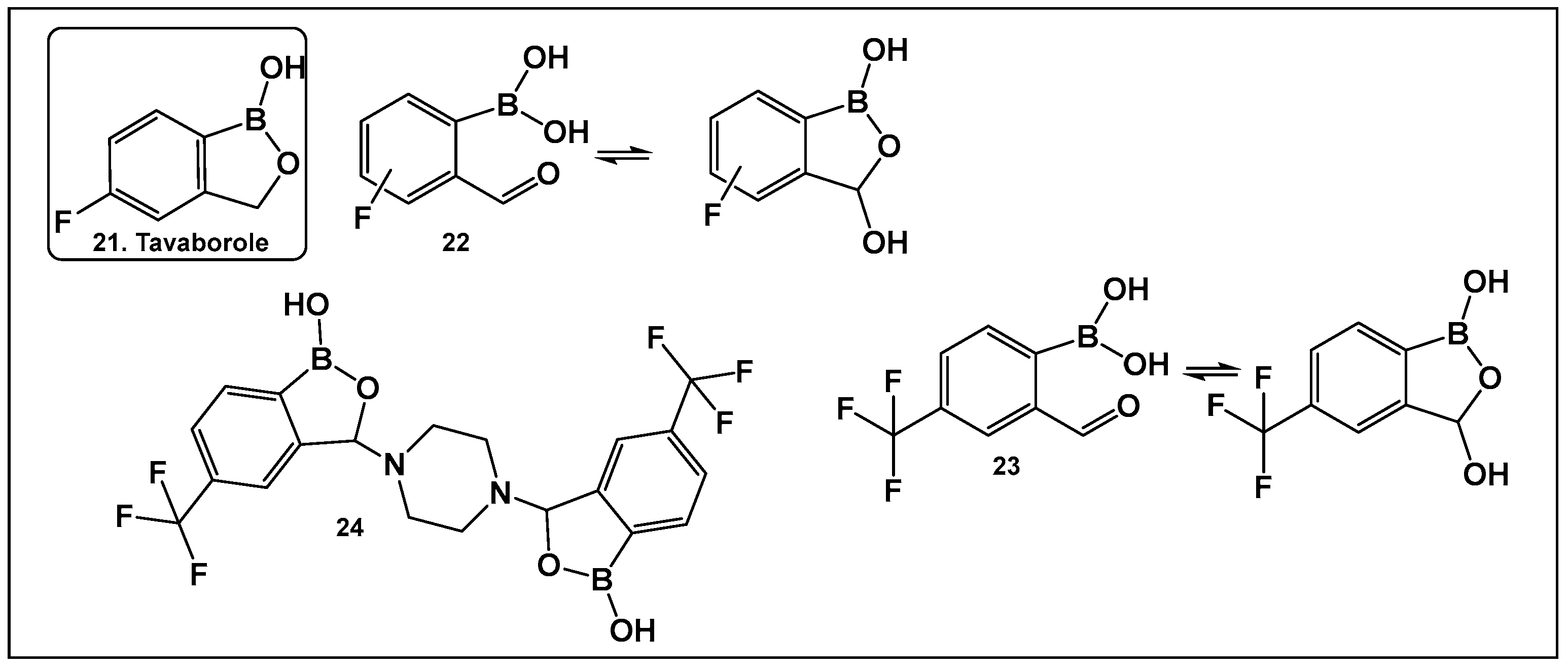
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crepin, T.; Zhou, H.; Zhang, Y.-K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An Antifungal Agent Inhibits an Aminoacyl-tRNA Synthetase by Trapping tRNA in the Editing Site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef]
- Baker, S.J.; Zhang, Y.-K.; Akama, T.; Lau, A.; Zhou, H.; Hernandez, V.; Mao, W.; Alley, M.R.K.; Sanders, V.; Plattner, J.J. Discovery of a New Boron-Containing Antifungal Agent, 5-Fluoro- 1,3-Dihydro-1-Hydroxy-2,1- Benzoxaborole (AN2690), for the Potential Treatment of Onychomycosis. J. Med. Chem. 2006, 49, 4447–4450. [Google Scholar] [CrossRef] [PubMed]
- Borys, K.M.; Wieczorek, D.; Pecura, K.; Lipok, J.; Adamczyk-Woźniak, A. Antifungal activity and tautomeric cyclization equilibria of formylphenylboronic acids. Bioorg. Chem. 2019, 91, 103081. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Tarkowska, M.; Lazar, Z.; Kaczorowska, E.; Madura, I.D.; Dąbrowska, M.A.; Lipok, J.; Wieczorek, D. Synthesis, structure, properties and antimicrobial activity of para trifluoromethyl phenylboronic derivatives. Bioorg. Chem. 2022, 119, 105560. [Google Scholar] [CrossRef]
- Peppoloni, S.; Pericolini, E.; Colombari, B.; Pinetti, D.; Cermelli, C.; Fini, F.; Prati, F.; Caselli, E.; Blasi, E. The β-Lactamase Inhibitor Boronic Acid Derivative SM23 as a New Anti-Pseudomonas aeruginosa Biofilm. Front. Microbiol. 2020, 11, 35. [Google Scholar] [CrossRef]
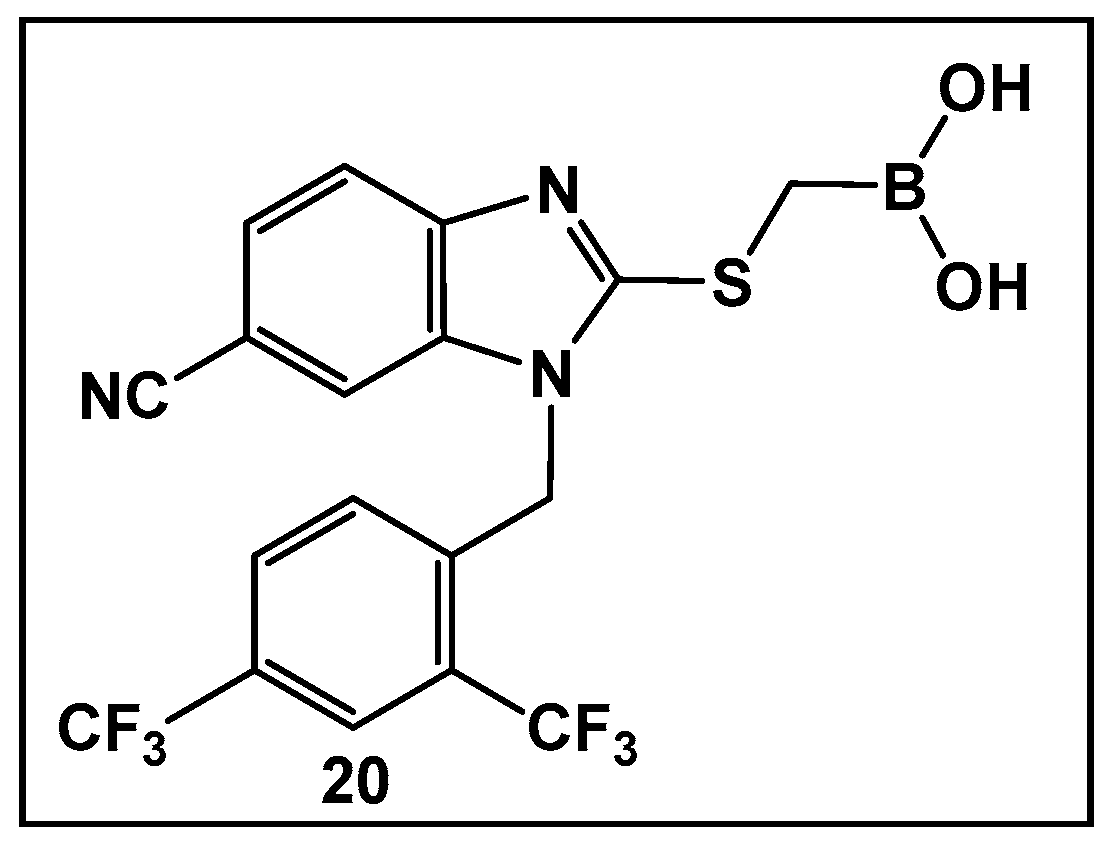
- Ni, N.; Chou, H.T.; Wang, J.; Li, M.; Lu, C.D.; Tai, P.C.; Wang, B. Identification of boronic acids as antagonists of bacterial quorum sensing in Vibrio harveyi. Biochem. Biophys. Res. Commun. 2008, 369, 590–594. [Google Scholar] [CrossRef]
- Ni, N.; Choudhary, G.; Peng, H.; Li, M.; Chou, H.-T.; Lu, C.-D.; Gilbert, E.S.; Wang, B. Inhibition of Quorum Sensing in Vibrio harveyi by Boronic Acids. Chem. Biol. Drug Des. 2009, 74, 51–56. [Google Scholar] [CrossRef]
- Sathiyamoorthi, E.; Lee, J.-H.; Lee, J. Antibacterial and antibiofilm activity of halogenated phenylboronic acids against Vibrio parahaemolyticus and Vibrio harveyi. Front. Cell. Infect. Microbiol. 2024, 14, 1340910. [Google Scholar] [CrossRef]
- Rezaie, P.; Pourhajibagher, M.; Chiniforush, N.; Hosseini, N.; Bahador, A. The Effect of Quorum-Sensing and Efflux Pumps Interactions in Pseudomonas aeruginosa against Photooxidative Stress. J. Lasers Med. Sci. 2018, 9, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Rico, M.; Olivares-Pacheco, J.; Alvarez-Ortega, C.; Cámara, M.; Martínez, J.L. Role of the multidrug resistance efflux pump MexCD-OprJ in the Pseudomonas aeruginosa quorum sensing response. Front. Microbiol. 2018, 9, 2752. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Gupta, J.; Sharma, S.; Sharma, D.; Sharma, S. Focused review on dual inhibition of quorum sensing and efflux pumps: A potential way to combat multi drug resistant Staphylococcus aureus infections. Int. J. Biol. Macromol. 2021, 190, 33–43. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Moore-Machacek, A.; Gloe, A.; O’Leary, N.; Reen, F.J. Efflux, Signaling and Warfare in a Polymicrobial World. Antibiotics 2023, 12, 731. [Google Scholar] [CrossRef]
- Hajiagha, M.N.; Kafil, H.S. Efflux pumps and microbial biofilm formation. Infect. Genet. Evol. 2023, 112, 105459. [Google Scholar] [CrossRef]
- Ren, J.; Wang, M.; Zhou, W.; Liu, Z. Efflux pumps as potential targets for biofilm inhibition. Front. Microbiol. 2024, 15, 1315238. [Google Scholar] [CrossRef]
- Laborda, P.; Lolle, S.; Hernando-Amado, S.; Alcalde-Rico, M.; Aanæs, K.; Martínez, J.L.; Molin, S.; Johansen, H.K. Mutations in the efflux pump regulator MexZ shift tissue colonization by Pseudomonas aeruginosa to a state of antibiotic tolerance. Nat. Commun. 2024, 15, 2584. [Google Scholar] [CrossRef]
- Fontaine, F.; Hequet, A.; Voisin-Chiret, A.S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. First identification of boronic species as novel potential inhibitors of the Staphylococcus aureus NorA efflux pump. J. Med. Chem. 2014, 57, 2536–2548. [Google Scholar] [CrossRef]
- Fontaine, F.; Héquet, A.; Voisin-Chiret, A.-S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. Boronic species as promising inhibitors of the Staphylococcus aureus NorA efflux pump: Study of 6-substituted pyridine-3-boronic acid derivatives. Eur. J. Med. Chem. 2015, 95, 185–198. [Google Scholar] [CrossRef]
- Cruz, C.D.; Wrigstedt, P.; Moslova, K.; Iashin, V.; Mäkkylä, H.; Ghemtio, L.; Heikkinen, S.; Tammela, P.; Perea-Buceta, J.E. Installation of an aryl boronic acid function into the external section of N-aryl-oxazolidinones: Synthesis and antimicrobial evaluation. Eur. J. Med. Chem. 2021, 211, 113002. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.; Zurenko, G.; Barbachyn, M. The discovery of linezolid, the first oxazolidinone antibacterial agent. Curr. Drug Targets-Infect. Disord. 2001, 1, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Jones, R.N. Oxazolidinone antibiotics. Lancet 2001, 358, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Barbachyn, M.R. The oxazolidinones: Past, present, and future: The oxazolidinones: Past, present, and future. Ann. N. Y. Acad. Sci. 2011, 1241, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Kanyo, Z.F.; Wang, D.; Franceschi, F.J.; Moore, P.B.; Steitz, T.A.; Duffy, E.M. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 2008, 51, 3353–3356. [Google Scholar] [CrossRef]
- Corey, E.J.; Helal, C. Reduction of Carbonyl Compounds with Chiral Oxazaborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method. Angew. Chem. Int. Ed. 1988, 37, 1986–2012. [Google Scholar] [CrossRef]
- Srebnik, M.; Deloux, L. Asymmetric boron-catalyzed reactions. Chem. Rev. 1993, 93, 763–784. [Google Scholar]
- Harada, T.; Kusukawa, T. Development of Highly Enantioselective Oxazaborolidinone Catalysts for the Reactions of Acyclic α,β-Unsaturated Ketones. Synlett 2007, 12, 1823–1835. [Google Scholar] [CrossRef]
- Butenschön, H. Oxazaborolidines as Catalysts for Enantioselective Cycloadditions: Now [2+2]! Angew. Chem. Int. Ed. 2008, 47, 3492–3495. [Google Scholar] [CrossRef]
- Corey, E.J. Enantioselective Catalysis Based on Cationic Oxazaborolidines. Angew. Chem. Int. Ed. 2009, 48, 2100–2117. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, A.; Steinberg, D.; Dembitsky, V.; Moussaieff, A.; Zaks, B.; Srebnik, M. Synthesis and Evaluation of Oxazaborolidines for Antibacterial Activity against Streptococcus mutans. J. Med. Chem. 2004, 47, 2409–2410. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, A.; Srebnik, M.; Zaks, B.; Dembitsky, V.; Steinberg, D. Evaluation of oxazaborolidine activity on Streptococcus mutans biofilm formation. Int. J. Antimicrob. Agents 2005, 26, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, A.; Smoum, R.; Takrouri, K.; Shalom, E.; Zaks, B.; Steinberg, D.; Rubinstein, A.; Goldberg, I.; Katzhendler, J.; Srebnik, M. Pharmacologically active boranes. Pure Appl. Chem. 2006, 78, 1425–1453. [Google Scholar] [CrossRef]
- Aharoni, R.; Bronstheyn, M.; Jabbour, A.; Zaks, B.; Srebnik, M.; Steinberg, D. Oxazaborolidine derivatives inducing autoinducer-2 signal transduction in Vibrio harveyi. Bioorg. Med. Chem. 2008, 16, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Golovanov, I.S.; Sukhorukov, A.Y. Merging Boron with Nitrogen-Oxygen Bonds: A Review on BON Heterocycles. Top. Curr. Chem. 2021, 379, 8. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, M.Z.H.; Rygus, J.P.G.; Ang, H.T.; Paladino, M.; Johnson, M.A.; Ferguson, M.J.; Hall, D.G. Lewis or Bronsted? A Rectification of the Acidic and Aromatic Nature of Boranol-Containing Naphthoid Heterocycles. J. Am. Chem. Soc. 2021, 143, 10143–10156. [Google Scholar] [CrossRef]
- Mueckter, H.; Huemer, H.; Sous, H.; Poszich, H. 6th International Congress of Chemotherapy; June 1967 Proceedings 1/2, 805; International Society for Chemotherapy: Vienna, Austria, 1967. [Google Scholar]
- Gronowitz, S.; Dhlgren, T.; Namtvedt, J.; Roos, C.; Sjöberg, B.; Forsgren, U. Antibacterial borazaro derivatives. I. 5-Arylsulphonyl-4-hydroxy-4,5-borazarothieno(2,3-c)pyridines and 6-arylsulphonyl-7-hydroxy-7,6-borazaro-thieno(3,2-c)pyridines. Acta Pharm. Suec. 1971, 8, 377–390. [Google Scholar]
- Gronowitz, S.; Dhlgren, T.; Namtvedt, J.; Roos, C.; Sjöberg, B.; Forsgren, U. Antibacterial borazaro derivatives. II. Effect of substituents on the antibacterial activity of 5-arylsulfonyl-4hydroxy-4,5-borazarothieno(2,3-c)pyridines and 6-arylsulfonyl-7-hydroxy-7,6-borazarothieno(3,2-c)pyridines. Acta Pharm. Suec. 1971, 8, 623–638. [Google Scholar]
- Grassberger, M.A.; Turnowsky, F.; Hildebrandt, J. Preparation and antibacterial activities of new 1,2,3-diazaborine derivatives and analogs. J. Med. Chem. 1984, 8, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Berglert, H.; Wallnert, P.; Ebelingt, A.; Leitingert, B.; Fuchsbichler, S.; Aschaued, H.; Kollenzll, G.; Hogenauert, G.; Turnowsky, F. Protein EnvM Is the NADH-dependent Enoyl-ACP Reductase (FabI) of Escherichia coli. J. Biol. Chem. 1994, 269, 5493–5496. [Google Scholar] [CrossRef]
- Loibl, M.; Klein, I.; Prattes, M.; Schmidt, C.; Kappel, L.; Zisser, G.; Gungl, A.; Krieger, E.; Pertschy, B.; Bergler, H. The Drug Diazaborine Blocks Ribosome Biogenesis by Inhibiting the AAA-ATPase Drg1. J. Biol. Chem. 2014, 289, 3913–3922. [Google Scholar] [CrossRef]
- Kanichar, D.; Roppiyakuda, L.; Ewa Kosmowska, E.; Faust, M.A.; Kim, P.; Tran, K.P.; Chowa, F.; Buglo, E.; Groziak, M.P.; Sarina, E.A.; et al. Synthesis, Characterization, and Antibacterial Activity of Structurally Complex 2-Acylated 2,3,1-Benzodiazaborines and Related Compounds. Chem. Bidivers. 2014, 11, 1381–1397. [Google Scholar] [CrossRef]
- Hogenauer, G.; Woisetschlager, M. A Diazaborine Derivative Inhibits Lipopolysaccharide Biosynthesis. Nature 1981, 293, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.P.M.; Goncalves, L.M.; Guedes, R.C.; Moreira, R.; Gois, M.P. Diazaborines as New Inhibitors of Human Neutrophil Elastase. ACS Omega 2018, 3, 7418–7423. [Google Scholar] [CrossRef] [PubMed]
- Dukes, A.; Carroll, X.B.; Groziak, M.P. Design, development, synthesis, and crystal structure of the prototype of a new class of deep blue-fluorescing boron heterocycle estrogen mimics. Bioorg. Med. Chem. Lett. 2022, 72, 128864. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.Q.; Guo, B.; Ma, Y.; Cui, W.; He, F.; Li, B.; Yang, H.; Shea, K.J. Dynamic introduction of cell adhesive factor via reversible multicovalent phenylboronic acid/cis-diol polymeric complexes. J. Am. Chem. Soc. 2014, 136, 6203–6206. [Google Scholar] [CrossRef]
- Polsky, R.; Harper, J.C.; Wheeler, D.R.; Arango, D.C.; Brozik, S.M. Electrically addressable cell immobilization using phenylboronic acid diazonium salts. Angew. Chem. Int. Ed. 2008, 120, 2671–2674. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Ayoob, A.M.; Appel, E.A.; Borenstein, J.T.; Langer, R.; Anderson, D.G. Mixed reversible covalent crosslink kinetics enable precise, hierarchical mechanical tuning of hydrogel networks. Adv. Mater. 2017, 29, 1605947. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, A.; Schiller, R.; Dobrindt, U. Boronic acid-functionalized photosensitizers: A straightforward strategy to target the sweet site of bacteria and implement active agents in polymer coating. Angew. Chem. Int. Ed. 2017, 56, 10362–10366. [Google Scholar] [CrossRef]
- McGann, C.L.; Streifel, B.C.; Lundin, J.G.; Wynne, J.H. Multifunctional polyHIPE wound dressings for the treatment of severe limb trauma. Polymer 2017, 126, 408–418. [Google Scholar] [CrossRef]
- Wu, R.R.; Ma, Y.; Pan, J.M.; Lee, S.H.; Liu, J.X.; Zhu, H.J.; Gu, R.X.; Shea, K.J.; Pan, G.Q. Efficient capture, rapid killing and ultrasensitive detection of bacteria by a nanodecorated multi-functional electrode sensor. Biosens. Bioelectron. 2018, 101, 52–59. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Z.; Luo, J.; Wang, P.; Lu, G.; Pan, J. Engineered reversible adhesive biofoams for accelerated dermal wound healing: Intriguing multi-covalent phenylboronic acid/cis-diol interaction. Colloids Surf. B Biointerfaces 2023, 221, 112987. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Chawla, P.; Ranjan Srivastava, A.; Pandey, P.; Chawla, V. Hydrogels: A journey from diapers to gene delivery. Mini-Rev. Med. Chem. 2014, 14, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Bisyrul, M.; Othman, H.; Javed, F.; Ahmad, Z.; Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.S.; Fiorica, C.; Di Stefano, M.; Pitarresi, G.; Gulino, A.; Agnello, S.; Giammona, G. In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration. Carbohydr. Polym. 2015, 122, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual crosslinked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Ma, R.; Xu, Y.; Zhang, Y.; Li, B.; An, Y.; Shi, L. A G-Quadruplex Hydrogel via Multicomponent Self-Assembly: Formation and Zero-Order Controlled Release. ACS Appl. Mater. Interfaces 2017, 9, 13056–13067. [Google Scholar] [CrossRef]
- António, J.P.M.; Russo, R.; Carvalho, C.P.; Cal, P.M.S.D.; Gois, P.M.P. Boronic acids as building blocks for the construction of therapeutically useful bioconjugates. Chem. Soc. Rev. 2019, 48, 3513–3536. [Google Scholar] [CrossRef]
- Ailincai, D.; Cibotaru, S.; Anisiei, A.; Coman, C.G.; Pasca, A.S.; Rosca, I.; Sandu, A.-I.; Mititelu-Tartau, L.; Marin, L. Mesoporous chitosan nanofibers loaded with norfloxacin and coated with phenylboronic acid perform as bioabsorbable active dressings to accelerate the healing of burn wounds. Carbohydr. Polym. 2023, 318, 121135. [Google Scholar] [CrossRef]
- Mi, F.L.; Kuan, C.Y.; Shyu, S.S.; Lee, S.T.; Chang, S.F. Study of gelation kinetics and chain-relaxation properties of glutaraldehyde-cross-linked chitosan gel and their effects on microspheres preparation and drug release. Carbohydr. Polym. 2000, 41, 389–396. [Google Scholar] [CrossRef]
- Mikhailov, S.N.; Zakharova, A.N.; Drenichev, M.S.; Ershov, A.V.; Kasatkina, M.A.; Vladimirov, L.V.; Novikov, V.V.; Kildeeva, N.R. Crosslinking of chitosan with dialdehyde derivatives of nucleosides and nucleotides. Mechanism and comparison with glutaraldehyde. Nucleosides. Nucleotides Nucleic Acids 2016, 35, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.E.; Zhang, W.; Falkinham, J.O., 3rd; Santos, W.L. Branched Peptides: Acridine and Boronic Acid Derivatives as Antimicrobial Agents. ACS Med. Chem. Lett. 2017, 8, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Cui, H.; Liang, X.; Yu, J.; Wang, J.; Zhao, G. Quaternary Ammonium-Tethered Phenylboronic Acids Appended Supramolecular Nanomicelles as a Promising Bacteria Targeting Carrier for Nitric Oxide Delivery. Polymers 2022, 14, 4451. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Liu, W.; Carlquist, M.; Ye, L. Boronic Acid Modified Polymer Nanoparticles for Enhanced Bacterial Deactivation. Chembiochem 2019, 20, 2991–2995. [Google Scholar] [CrossRef] [PubMed]
- Noimark, S.; Dunnill, C.W.; Parkin, I.P. The role of surfaces in catheter-associated infections. Adv. Drug Deliv. Rev. 2013, 65, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Wang, B.; Wang, M.; Mikhailovsky, A.; Wang, S.; Bazan, G.C. A Membrane-Intercalating Conjugated Oligoelectrolyte with High-Efficiency Photodynamic Antimicrobial Activity. Angew. Chem. Int. Ed. 2017, 56, 5031–5034. [Google Scholar] [CrossRef]
- Park, J.H.; Ahn, M.Y.; Kim, Y.C.; Kim, S.A.; Moon, Y.H.; Ahn, S.G.; Yoon, J.H. In vitro and in vivo antimicrobial effect of photodynamic therapy using a highly pure chlorin e6 against Staphylococcus aureus Xen29. Biol. Pharm. Bull. 2012, 35, 509–514. [Google Scholar] [CrossRef]
- Han, H.H.; Jin, Q.; Wang, H.B.; Teng, W.Z.; Wu, J.N.; Tong, H.X.; Chen, T.T.; Ji, J. Intracellular Dual Fluorescent Lightup Bioprobes for Image-Guided Photodynamic Cancer Therapy. Small 2016, 12, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Muthukrishnan, N.; Pellois, J.P. Photoinactivation of Gram Positive and Gram Negative Bacteria with the Antimicrobial Peptide (KLAKLAK)2 Conjugated to the Hydrophilic Photosensitizer Eosin Y. Bioconjug. Chem. 2013, 24, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Dosselli, R.; Tampieri, C.; Ruiz-González, R.; De Munari, S.; Ragàs, X.; Sánchez-García, D.; Agut, M.; Nonell, S.; Reddi, E.; Gobbo, M. Synthesis, characterization, and photoinduced antibacterial activity of porphyrin-type photosensitizers conjugated to the antimicrobial peptide apidaecin 1b. J. Med. Chem. 2013, 56, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Z.; Zhang, J.; Kwok, R.T.K.; Xie, S.; Tang, R.; Jia, Y.; Yang, J.; Wang, L.; Lam, J.W.Y.; et al. A Bifunctional Aggregation-Induced Emission Luminogen for Monitoring and Killing of Multidrug-Resistant Bacteria. Adv. Funct. Mater. 2018, 28, 1804632. [Google Scholar] [CrossRef]
- Courtney, C.M.; Goodman, S.M.; Nagy, T.A.; Levy, M.; Bhusal, P.; Madinger, N.E.; Detweiler, C.S.; Nagpal, P.; Chatterjee, A. Potentiating Antibiotics in Drug-Resistant Clinical Isolates via Stimuli-Activated Superoxide Generation. Sci. Adv. 2017, 3, e1701776. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gopal, A.; Karaosmanoglu, S.; Lin, J.; Munshi, T.; Zhang, W.; Chen, X.; Yan, L. Photosensitizer Doped Zeolitic Imidazolate Framework-8 Nanocomposites for Combined Antibacterial Therapy to Overcome Methicillin-Resistant Staphylococcus aureus (MRSA). Colloids Surf. B Biointerfaces 2020, 190, 110900. [Google Scholar] [CrossRef] [PubMed]
- De Melo, W.C.; Avci, P.; de Oliveria, M.N.; Gupta, A.; Vecchio, D.; Sadasivam, M.; Chandran, R.; Huan, Y.-Y.; Yin, R.; Perussi, L.R.; et al. Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection. Expert Rev. Anti-Infect. Ther. 2013, 11, 669–693. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, Z.H.; Zheng, Q.Q.; Zhou, X.Y.; Zhang, Y.; Shi, Y.F.; Lin, J.T.; Xue, W. Star-shaped polymer consisting of a porphyrin core and poly (L-lysine) dendron arms: Synthesis, drug delivery, and in vitro chemo/photodynamic therapy. Macromol. Rapid Commun. 2013, 34, 548–552. [Google Scholar] [CrossRef]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.P. Tailoring photosensitive ROS for advanced photodynamic therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.C.; Lin, J.H.; Zhang, S.; Lou, C.W.; Li, T.-T. Synergistic antibacterial strategy based on photodynamic therapy: Progress and perspectives. Chem. Eng. J. 2022, 450, 138129. [Google Scholar] [CrossRef]
- Wu, B.; Hu, K.F. Targeted antibacterial photodynamic therapy with aggregation-induced emission photosensitizers. Interdiscip. Med. 2024, 2, e20230038. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Wu, J.; Xie, S.; Zhou, Z.; Liu, L.; Huang, M.; Jiang, L.; Xu, P.; Li, J. Exploring the mechanism of photosensitizer conjugation on membrane perturbation of antimicrobial peptide: A multiscale molecular simulation study. Int. J. Biol. Macromol. 2023, 247, 125698. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Z.Y.; Liu, Y.; Yang, J.C.; Liu, Y.; Hu, F.P.; Zhu, K.; Jiang, X.Y. Gold Nanoclusters for Targeting Methicillin-Resistant Staphylococcus aureus in vivo. Angew. Chem. Int. Ed. 2018, 57, 3958–3962. [Google Scholar] [CrossRef]
- Hu, X.-L.; Chu, L.; Dong, X.; Chen, C.-R.; Tang, T.; Chen, D.; He, X.-P.; Tian, H. Multivalent Glycosheets for Double Light Driven Therapy of Multidrug-Resistant Bacteria on Wounds. Adv. Funct. Mater. 2019, 29, 1806986. [Google Scholar] [CrossRef]
- Pieters, R.J. Maximising Multivalency Effects in Protein- Carbohydrate Interactions. Org. Biomol. Chem. 2009, 7, 2013–2025. [Google Scholar] [CrossRef]
- Gast, D.; Koller, F.; Krafczyk, R.; Bauer, L.; Wunder, S.; Lassak, J.; Hoffmann-Roeder, A. A Set of Rhamnosylation-Specific Antibodies Enables Detection of Novel Protein Glycosylations in Bacteria. Org. Biomol. Chem. 2020, 18, 6823–6828. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Zhang, L.; Miron, R.J.; Liang, J.; Shi, M.; Mo, W.; Zheng, S.; Zhao, Y.; Zhang, Y. Pretreated Macrophage- Membrane-Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Adv. Mater. 2018, 30, 1804023. [Google Scholar] [CrossRef]
- Van der Vlies, A.J.; Hasegawa, U. Functionalization of Framboidal Phenylboronic Acid-Containing Nanoparticles via Aqueous Suzuki–Miyaura Coupling Reactions. Molecules 2023, 28, 3602. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Li, X.; Oh, J.; Kwon, N.; Kim, G.; Kim, D.; Yoon, J. A Boronic Acid-Functionalized Phthalocyanine with an Aggregation- Enhanced Photodynamic Effect for Combating Antibiotic-Resistant Bacteria. Chem. Sci. 2020, 11, 5735–5739. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations. Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Qi, J.; Dong, R.; Liu, H.; Wu, D.; Shao, H.; Jiang, X. Boronic Acid-Decorated Multivariate Photosensitive Metal-Organic Frameworks for Combating Multi-Drug-Resistant Bacteria. ACS Nano 2022, 16, 7732–7744. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Lepsík, M.; Horinek, D.; Havlas, Z.; Hobza, P. Interaction of Carboranes with Biomolecules: Formation of Dihydrogen Bonds. ChemPhysChem 2006, 7, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Farras, P.; Juarez-Perez, E.J.; Lepsík, M.; Luque, R.; Nunez, R.; Teixidor, F. Metallacarboranes and their interactions: Theoretical insights and their applicability. Chem. Soc. Rev. 2012, 41, 3445–3463. [Google Scholar] [CrossRef] [PubMed]
- Plesek, J. Potential Applications of the Boron Cluster Compounds. Chem. Rev. 1992, 92, 269–278. [Google Scholar] [CrossRef]
- FeFernandez-Alvarez, R.; Hlavatovičová, E.; Rodzeń, K.; Strachota, A.; Kereïche, S.; Matějíček, P.; Cabrera-González, J.; Núñez, R.; Uchman, M. Synthesis and self-assembly of a carborane-containing ABC triblock terpolymer: Morphology control on a dual-stimuli responsive system. Polym. Chem. 2019, 10, 2774–2780. [Google Scholar] [CrossRef]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684. [Google Scholar] [CrossRef]
- Eberhardt, W.H.; Crawford, B., Jr.; Lipscomb, W.N. The Valence Structure of the Boron Hydrides. J. Chem. Phys. 1954, 22, 989–1001. [Google Scholar] [CrossRef]
- Axtell, J.C.; Saleh, L.M.A.; Qian, E.A.; Wixtrom, A.I.; Spokoyny, A.M. Synthesis and Applications of Perfunctionalized Boron Clusters. Inorg. Chem. 2018, 57, 2333–2350. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Polyhedral Boranes for Medical Applications: Current Status and Perspectives. Eur. J. Inorg. Chem. 2009, 2009, 1433–1450. [Google Scholar] [CrossRef]
- Kaim, W.; Hosmane, N.S.; Záliš, S.; Maguire, J.A.; Lipscomb, W.N. Boron Atoms as Spin Carriers in Two- and Three-Dimensional Systems. Angew. Chem. Int. Ed. 2009, 48, 5082–5091. [Google Scholar] [CrossRef] [PubMed]
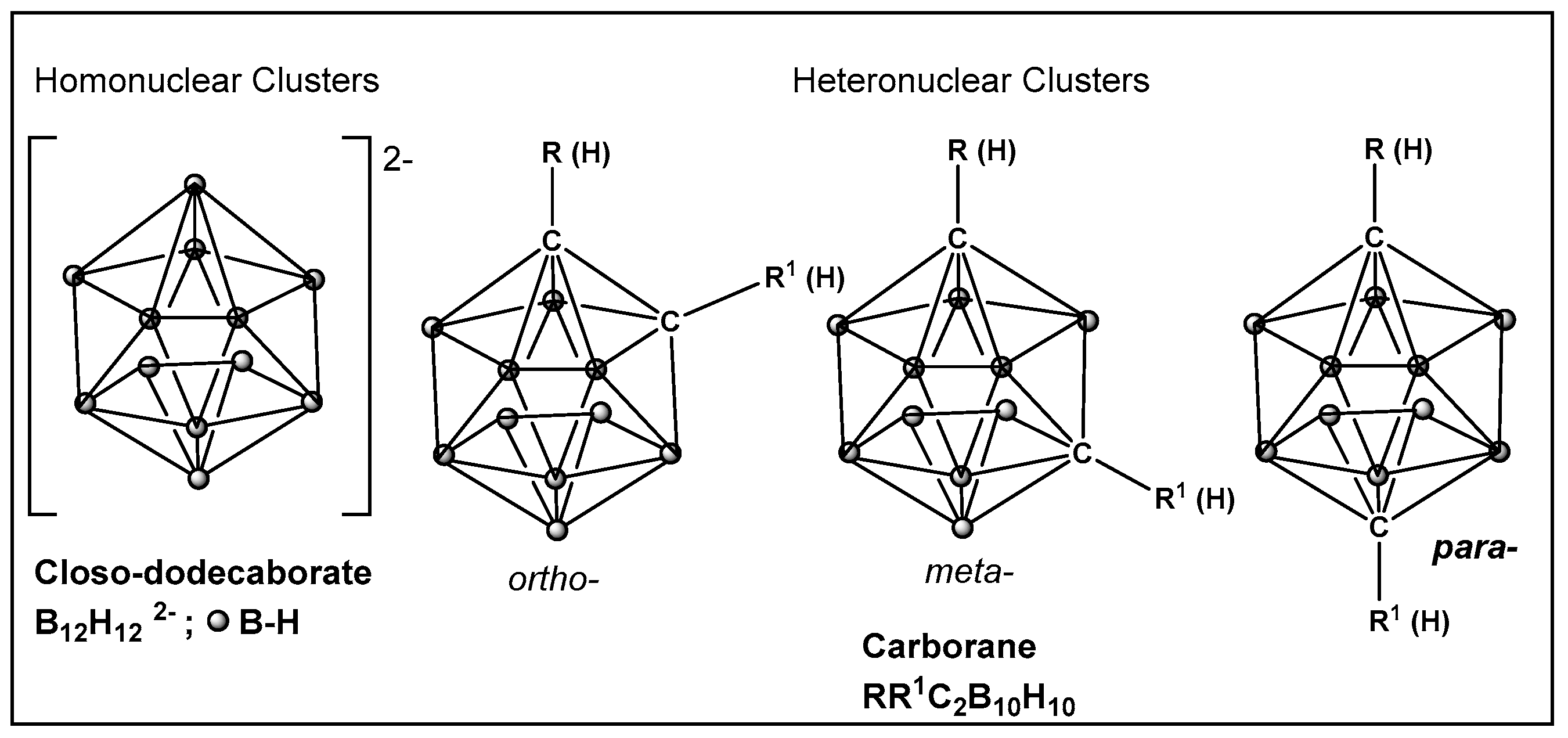
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; de Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
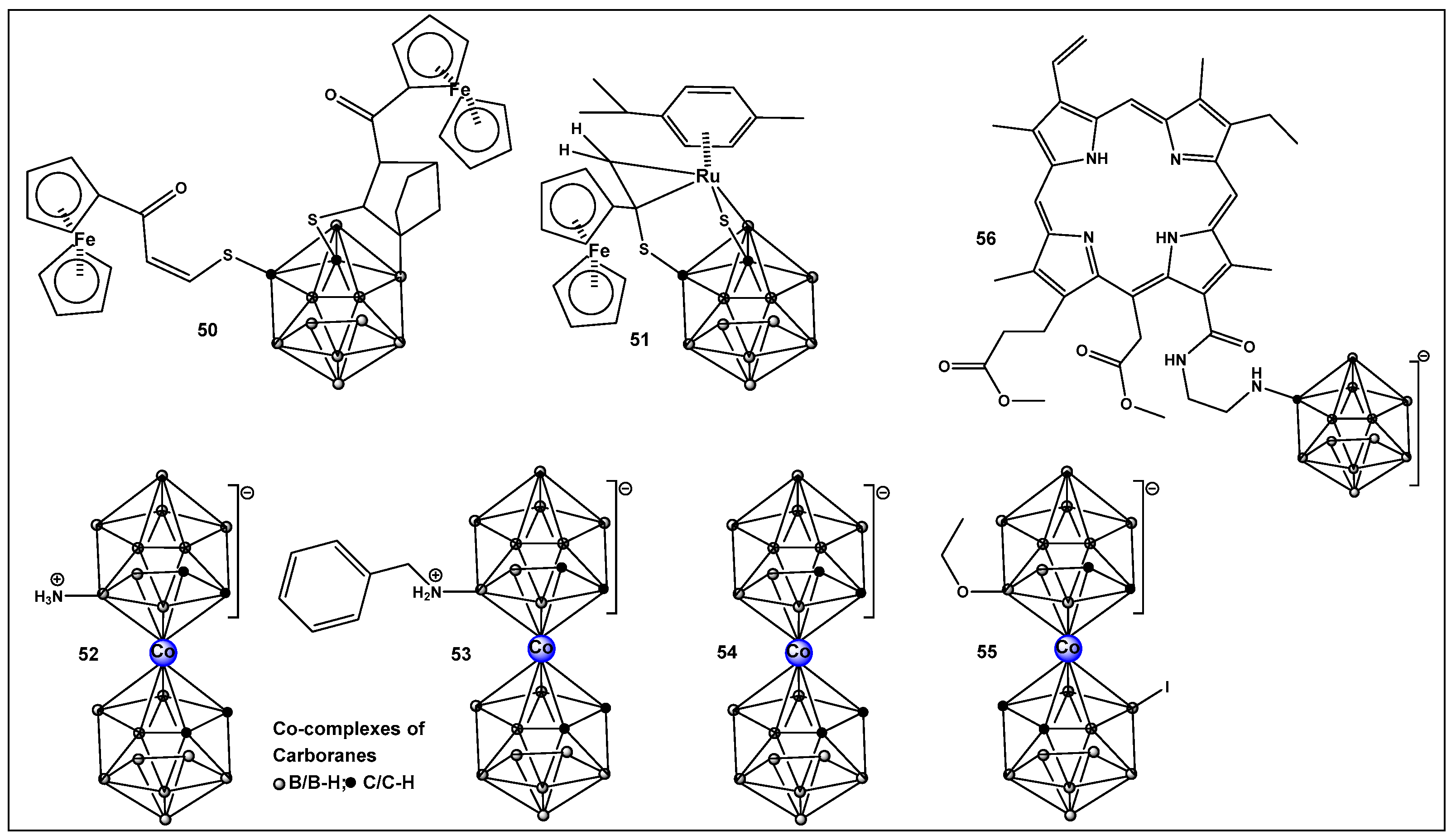
- Li, S.H.; Wang, Z.J.; Wei, Y.F.; Wu, C.Y.; Gao, S.P.; Jiang, H.; Zhao, X.Q.; Yan, H.; Wang, X.M. Antimicrobial activity of a ferrocene-substituted carborane derivative targeting multidrug-resistant infection. Biomaterials 2013, 34, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, C.; Tang, X.; Gao, S.; Zhao, X.; Yan, H.; Wang, X. New strategy for reversing biofilm-associated antibiotic resistance through ferrocene-substituted carborane ruthenium(II)-arene complex. Sci. China Chem. 2013, 56, 595–603. [Google Scholar] [CrossRef]
- Vanková, E.; Lokocová, K.; Matátková, O.; Krízová, I.; Masák, J.; Grüner, B.; Kaule, P.; Cermák, J.; Šícha, V. Cobalt bis-dicarbollide and its ammonium derivatives are effective antimicrobial and antibiofilm agents. J. Organomet. Chem. 2019, 899, 120891–120898. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Liu, W.W.; Chen, Y.; Jiang, H.; Yan, H.; Kosenko, I.; Chekulaeva, L.; Sivaev, I.; Bregadze, V.; Wang, X.M. A highly potent antibacterial agent targeting methicillin-resistant Staphylococcus aureus based on cobalt bis (1,2-dicarbollide) alkoxy derivative. Organometallics 2017, 36, 3484–3490. [Google Scholar] [CrossRef]
- Omarova, E.O.; Nazarov, P.A.; Firsov, A.M.; Strakhovskaya, M.G.; Arkhipova, A.Y.; Moisenovich, M.M.; Agapov, I.I.; Ol’shevskaya, V.A.; Zaitsev, A.V.; Kalinin, V.N.; et al. Carboranyl-Chlorin e6 as a Potent Antimicrobial Photosensitizer. PLoS ONE 2015, 10, e0141990. [Google Scholar] [CrossRef]
| Entry &Ref. | Antimicrobial Activity | Chemotype/Structure | Under Clinical Development/FDA Approval |
|---|---|---|---|
| 9. [93,94] | β-lactamase inhibitor—serine enzymes | 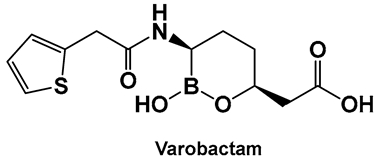 Hemiboronic acid | FDA approved (carbapenemases only, in combination with meropenem) 2017 |
| 10. [88,89,90,111] | β-lactamase inhibitor (incl. Acinetobacter baumannii cephalosporinase); anti-biofilm | 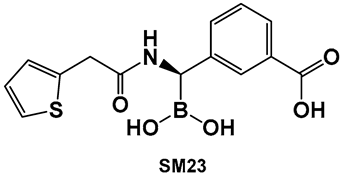 Boronic acid | |
| 11. [95,96] | β-lactamase inhibitor—serine enzymes | 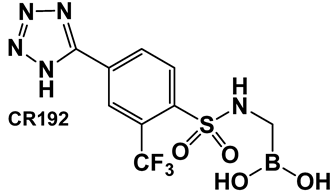 Boronic acid | |
| 12. [95,96] | β-lactamase inhibitor—serine enzymes | 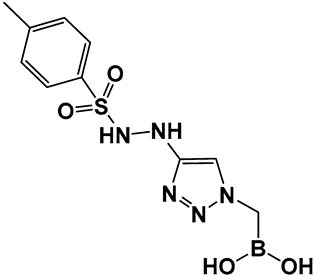 Boronic acid | |
| 16. [97,98,99] | β-lactamase inhibitor—ultra-broad-spectrum serine and metallo-enzymes | 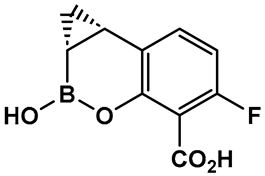 Hemiboronic acid | Under Clinical development (Phase I Clinical Trials) |
| 17. [100] | β-lactamase inhibitor—ultra-broad-spectrum serine and metallo-enzymes | 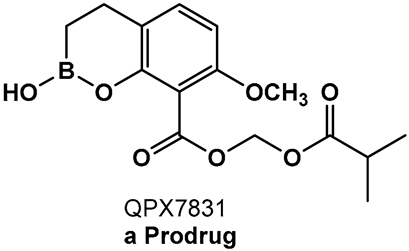 Hemiboronic acid | |
| 19. [102] | β-lactamase inhibitor—pan spectrum | 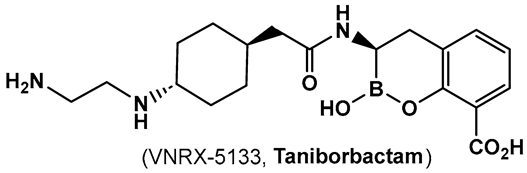 Hemiboronic acid | Under Clinical development (Phase III Clinical Trials) |
| 20. [103] | Inhibitor of the sensor domain of BlaR in MRSA | 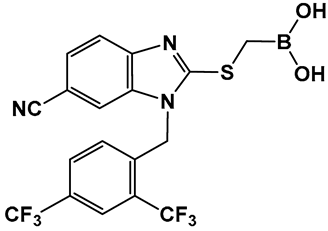 Boronic acid | |
| 21. [107,108] | Antifungal activity by inhibiting the fungal aminoacyl-tRNA synthetase | 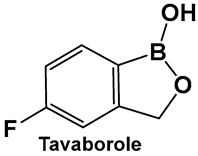 Hemiboronic acid | FDA approved for treatment of onychomycosis 2014 |
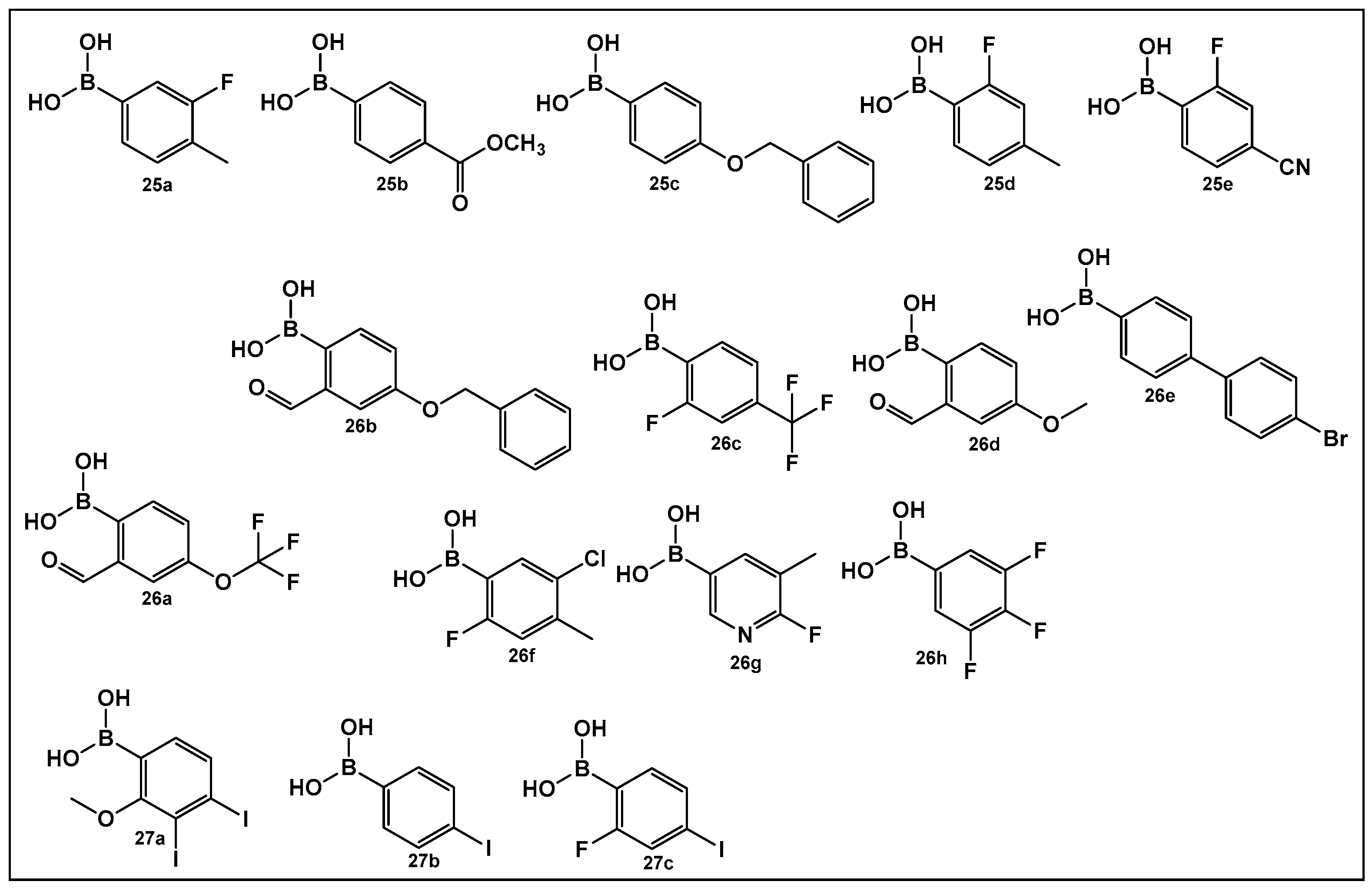
| 25–27. [112,113,114] | Inhibitors of biofilm formation in Vibrio harveyi (nonhalogenated/halogenated) | 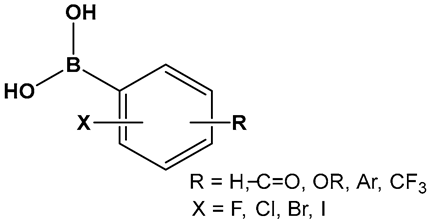 Boronic acid | |
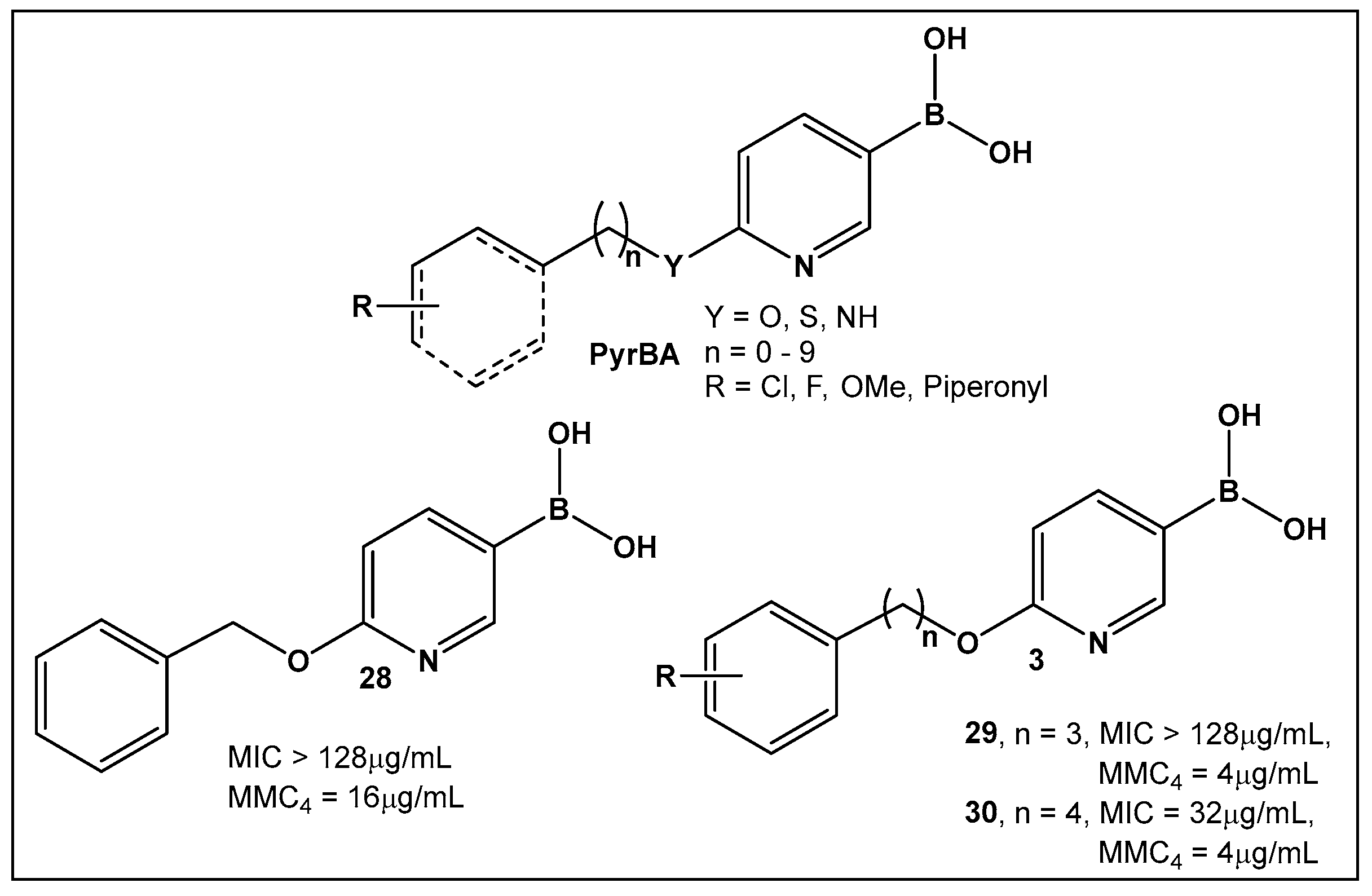
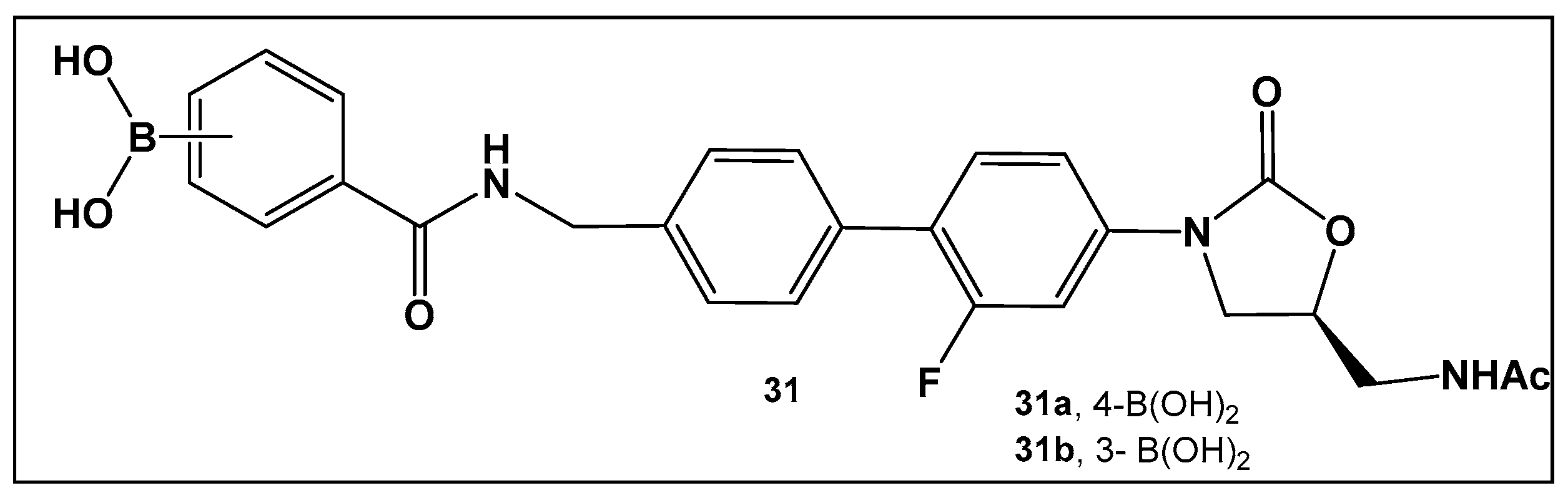
| 28. [123,124] | Bacterial efflux pump inhibitor | 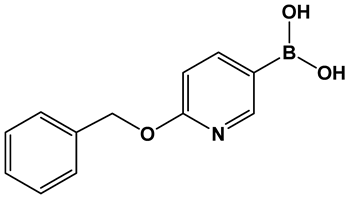 Boronic acid | |
| 31. [135,136,137] | Bacterial protein synthesis inhibitor A-site pocket of the 50S subunit of bacterial ribosomes, peptidyl transferase center (PTC) | 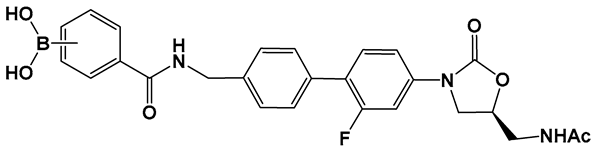 Boronic acid | |
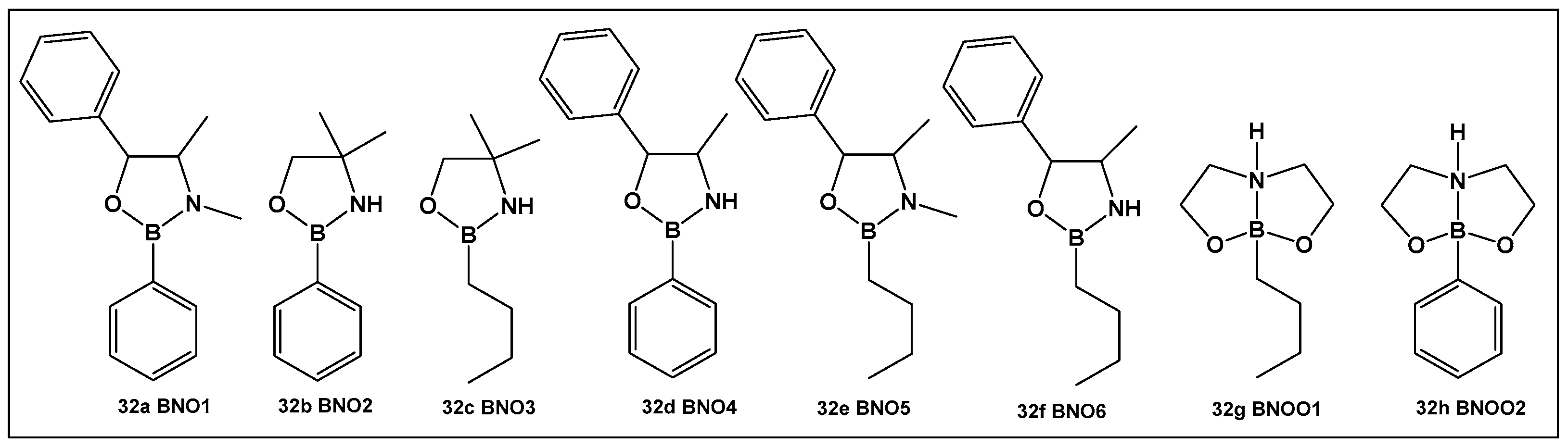
| 32e. [135,136,137] | Antimicrobial, against Streptococcus, anti-biofilm agent | 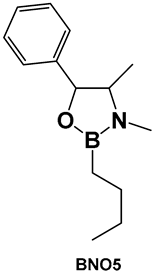 Oxazaborolidines | |
| 35–40. [144,145,146] | Antimicrobial against E. coli, Enterobacter, Proteus, Neisseria, Klebsiella, Salmonella | 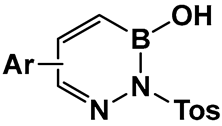 Benzodiazaborines | |
| 45a, 47. [147] | Antimicrobial activity against E. coli and Mycobacterium smegmatis |  2-Acylated benzodiazaborines | |
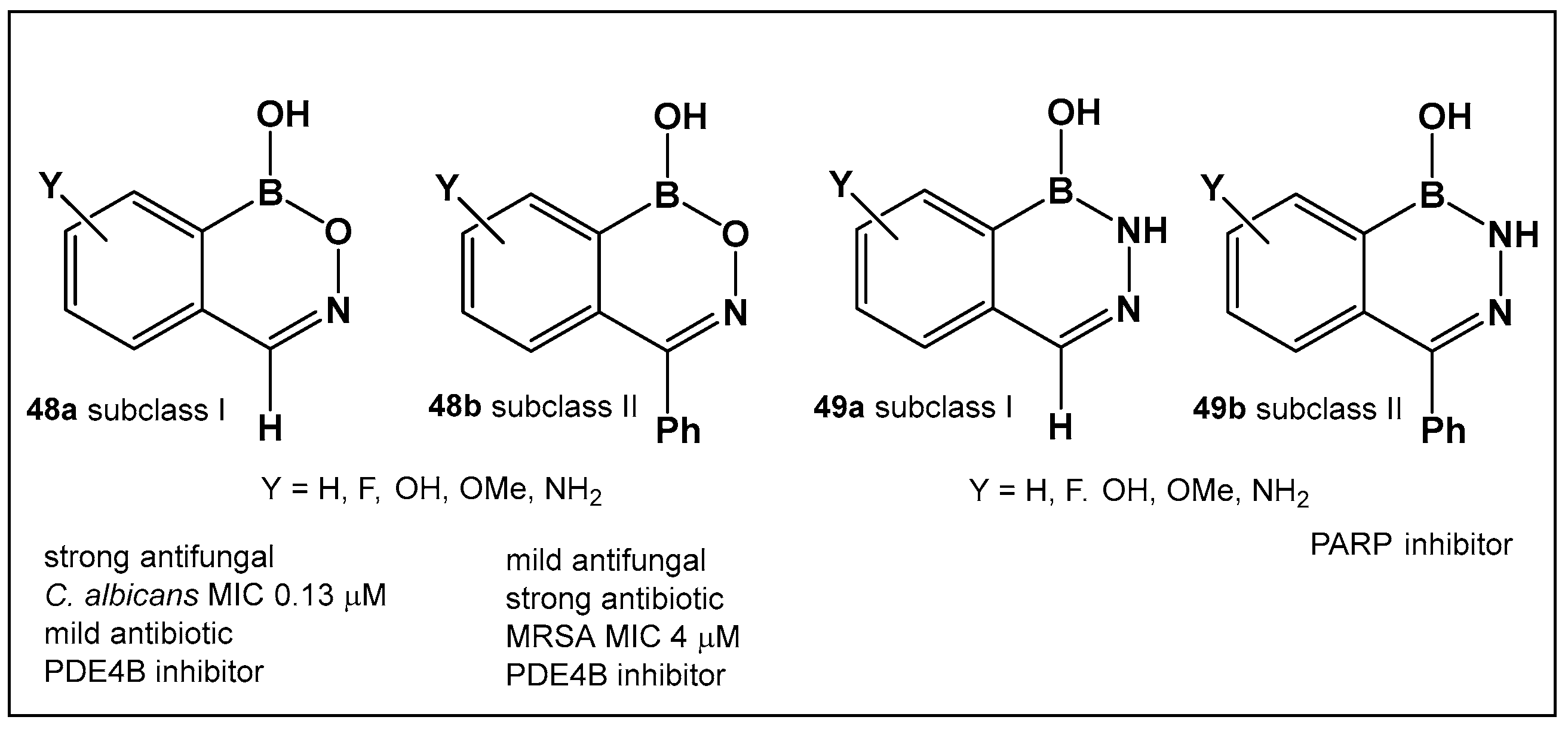
| 48a, 48b. [24,140] | 48a—Antifungal—Candida 48b—Antimicrobial—methicillin-resistant Staphylococcus aureus (MRSA) | 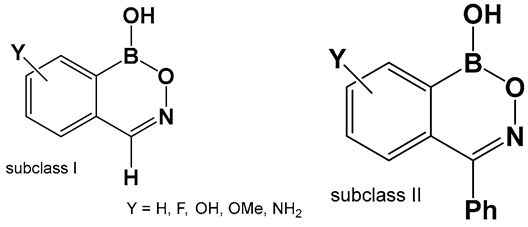 Benzoxazaborines | |
| 52–55. [213,214] | 52. Antimicrobial/antifungal Gram-positive Staphylococcus epidermidis and S. aureus and fungus Trichosporon cutaneum) 55. Anti-biofilm MRSA | 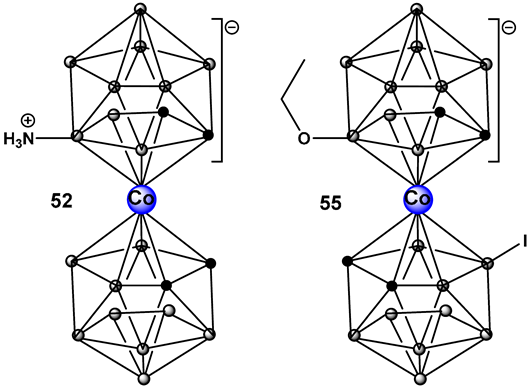 Boron clusters |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konaklieva, M.I.; Plotkin, B.J. Activity of Organoboron Compounds against Biofilm-Forming Pathogens. Antibiotics 2024, 13, 929. https://doi.org/10.3390/antibiotics13100929
Konaklieva MI, Plotkin BJ. Activity of Organoboron Compounds against Biofilm-Forming Pathogens. Antibiotics. 2024; 13(10):929. https://doi.org/10.3390/antibiotics13100929
Chicago/Turabian StyleKonaklieva, Monika I., and Balbina J. Plotkin. 2024. "Activity of Organoboron Compounds against Biofilm-Forming Pathogens" Antibiotics 13, no. 10: 929. https://doi.org/10.3390/antibiotics13100929
APA StyleKonaklieva, M. I., & Plotkin, B. J. (2024). Activity of Organoboron Compounds against Biofilm-Forming Pathogens. Antibiotics, 13(10), 929. https://doi.org/10.3390/antibiotics13100929





