The Diverse Activities and Mechanisms of the Acylphloroglucinol Antibiotic Rhodomyrtone: Antibacterial Activity and Beyond
Abstract
:1. Introduction
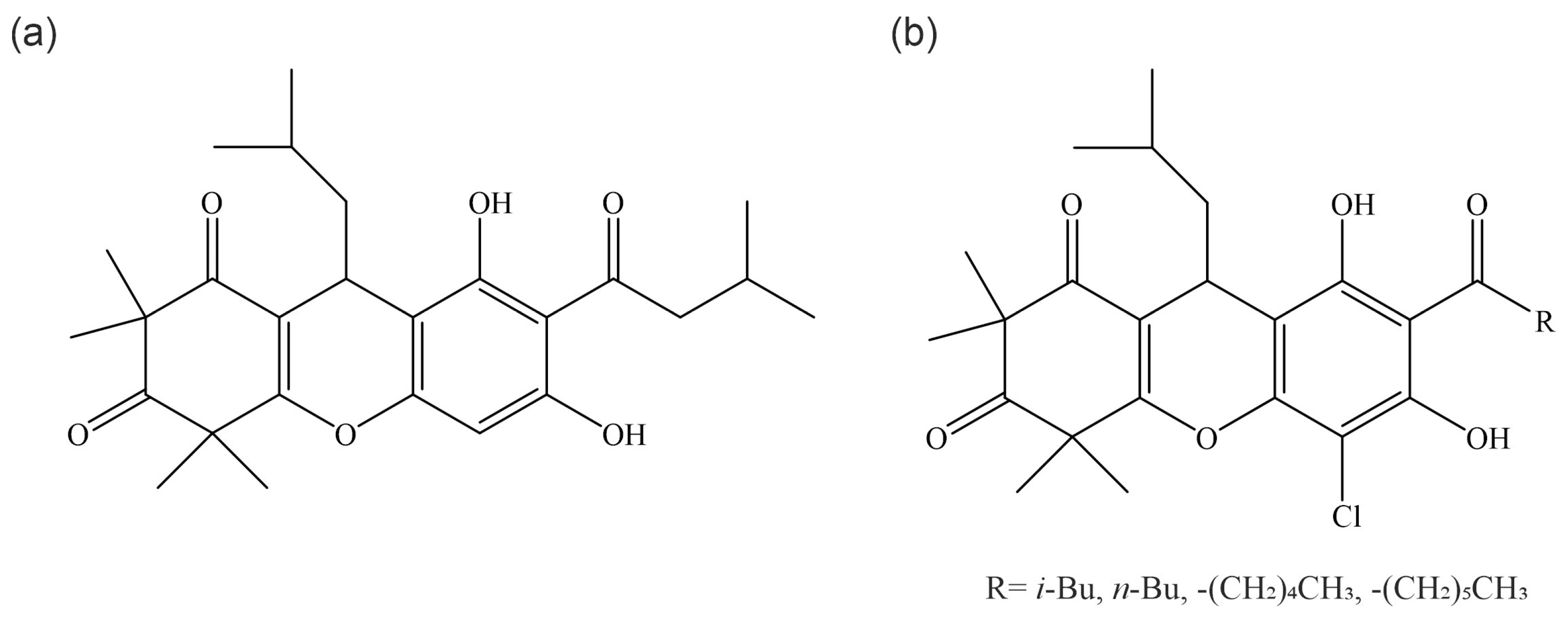
2. Chemical Description of Rhodomyrtone
3. Antimicrobial Activity
| Species | Strain | MIC (µg/mL) | MBC (µg/mL) | References |
|---|---|---|---|---|
| Gram-positive bacteria | ||||
| Staphylococcus aureus | ATCC 29213 | 0.5–2 | 1–16 | [28,29,30,31,32] |
| ATCC 25923 | 0.25–0.78 | 0.39–1 | [18,21,26] | |
| ATCC 6538 | 1.83 | n.d. | [33] | |
| NRIC 1135 | 0.78 | n.d. | [24] | |
| RN4220 (ATCC 35556) | 0.5 | n.d. | [34] | |
| Newman | 0.5 | 0.625 | [35] | |
| COL | 0.5 | 1 | ||
| 75 clinical isolates | 0.25–2 | 0.5–8 | ||
| 110 clinical MRSA isolates | 1 | 4–8 | [36] | |
| ATCC 29740 (bovine MSSA) | 0.5 | 0.5 | [37] | |
| 1158c (bovine MRSA) | 0.5 | 1 | ||
| 4 mastitis isolates | 0.5 | 1 | ||
| NPRC 302 | 1 | 2 | [21] | |
| NPRC 308 | 0.5 | 1 | ||
| NPRC 317 | 0.25 | 1 | ||
| NPRC 322 | 1 | 2 | ||
| NPRC R001 (MRSA) | 0.5–1 | 1–4 | [28,29,38] | |
| EMRSA-16 | 0.5–1 | 0.5–4 | [30,32,39] | |
| EMRSA-15 | 1 | 1 | [30] | |
| EMRSA SOTON9 | 0.5 | 1 | ||
| MRSA BB270 | 0.5 | 0.5 | ||
| MRSA USA300 | 1 | 1 | ||
| VISA Mu3 | 0.5 | 1 | ||
| VISA Mu50 | 0.5 | 0.5 | ||
| 4 MRSA isolates | 0.39–0.78 | 0.39–0.78 | [18] | |
| Staphylococcus epidermidis | ATCC 35984 | 0.25–8 | 2–25 | [18,21,31] |
| NBRC 100911 | 0.78 | n.d. | [24] | |
| NPRC 529 | 0.5 | 1 | [21] | |
| NPRC 537 | 0.5 | 2 | ||
| NPRC 573 | 0.25 | 2 | ||
| NPRC 577 | 0.25 | 1 | ||
| Staphylococcus simulans | 3100-0949 | 0.5 | 1 | [37] |
| Staphylococcus chromogenes | 3140-3115 | 0.25 | 1 | [37] |
| Coagulase-positive staphylococci | BMPOS-31 (mastitis isolate) | 4 | 32 | [31] |
| Coagulase-negative staphylococci | BMNEG-12 (mastitis isolate) | 2 | 16 | [31] |
| Streptococcus pneumoniae | ATCC 700673 | 0.5 | 1 | [40] |
| R6 | 2 | 8 | ||
| TIGR4 | 1 | 2 | ||
| 23 clinical isolates | 0.125–4 | 0.125–8 | ||
| not specified | 0.39 | 1.56 | [18] | |
| Streptococcus pyogenes | 2 clinical isolates | 0.39–0.78 | 1.56 | [18] |
| 47 clinical isolates | 0.39–1.56 | 0.39–1.56 | [13] | |
| Streptococcus mutans | JCM 5175 | 1.56 | n.d. | [24] |
| clinical isolate | 0.39 | n.d. | [26] | |
| not specified | 0.19 | 1.56 | [18] | |
| Streptococcus suis | P1/7 | 0.5 | 1 | [41] |
| Streptococcus gordonii | not specified | 0.19 | 1.56 | [18] |
| Streptococcus salivarius | not specified | 0.39 | 1.56 | [18] |
| Enterococcus faecalis | ATCC 29212 | 2 | 32 | [30] |
| not specified | 1.56 | 12.5 | [18] | |
| Enterococcus spp. | VRE-2 | 2 | >32 | [30] |
| VRE-3 | 1 | 16 | ||
| VRE-4 | 1 | 32 | ||
| VRE-7 | 2 | >32 | ||
| VRE-8 | 2 | >32 | ||
| Propionibacterium acnes | DMST 14916 | 0.25 | 0.25 | [21] |
| NPRC 021 | 0.25 | 0.5 | ||
| NPRC 036 | 0.25 | 0.25 | ||
| NPRC 039 | 0.25 | 0.25 | ||
| 9 clinical isolates | 0.125–0.5 | 0.25–0.5 | [22] | |
| Clostridium difficile | 10 clinical isolates | 0.625–2.5 | 1.25–5 | [42] |
| Bacillus cereus | NBRC 3457 | 0.78 | n.d. | [24] |
| ATCC 11778 | 0.5 | 4 | [43] | |
| 65 food isolates | 0.5 | 2–8 | ||
| not specified | 0.39 | 0.78 | [18] | |
| Bacillus subtilis | 168CA | 0.5 | n.d. | [25,44] |
| JCM 1465 | 0.78 | n.d. | [24] | |
| not specified | 0.39 | 0.39 | [18] | |
| Micrococcus luteus | NBRC 12708 | 0.78 | n.d. | [24] |
| Gram-negative bacteria | ||||
| Escherichia coli | O157 JCM 18426 | >100 | n.d. | [24] |
| Pseudomonas aeruginosa | JCM 5962 | >100 | n.d. | [24] |
| Salmonella typhimurium | NBRC 12529 | >100 | n.d. | [24] |
| Fungi | ||||
| Candida albicans | ATCC 90028 | >100 | n.d. | [26] |
| JCM 2085 | >100 | n.d. | [24] | |
| Saccharomyces cerevisiae | NRIC 1410 | >100 | n.d. | [24] |
4. Antibiofilm Activity
5. Antibacterial Mechanism of Action
5.1. Effects on Cell Division and FtsZ
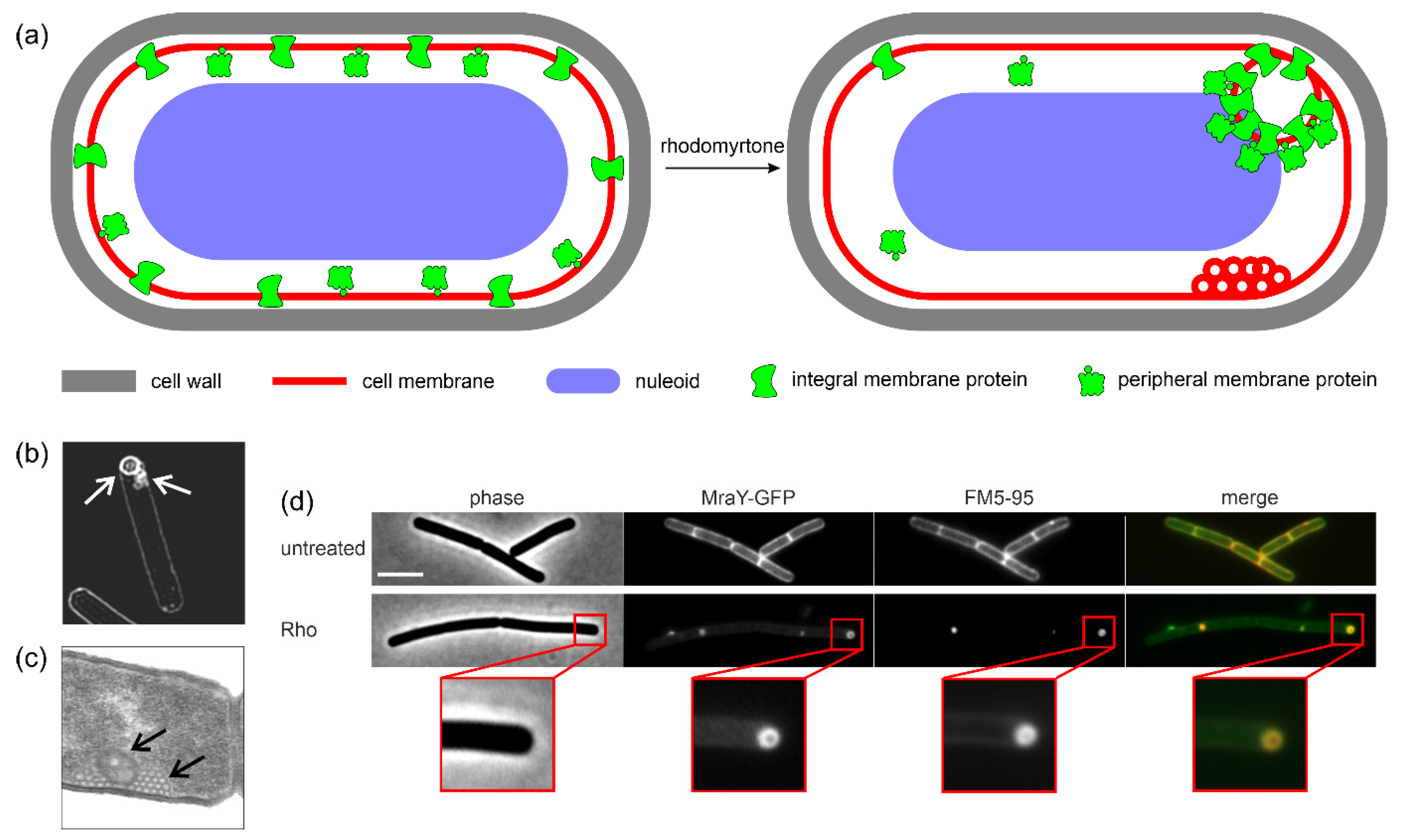
5.2. Interaction with Bacterial Membranes
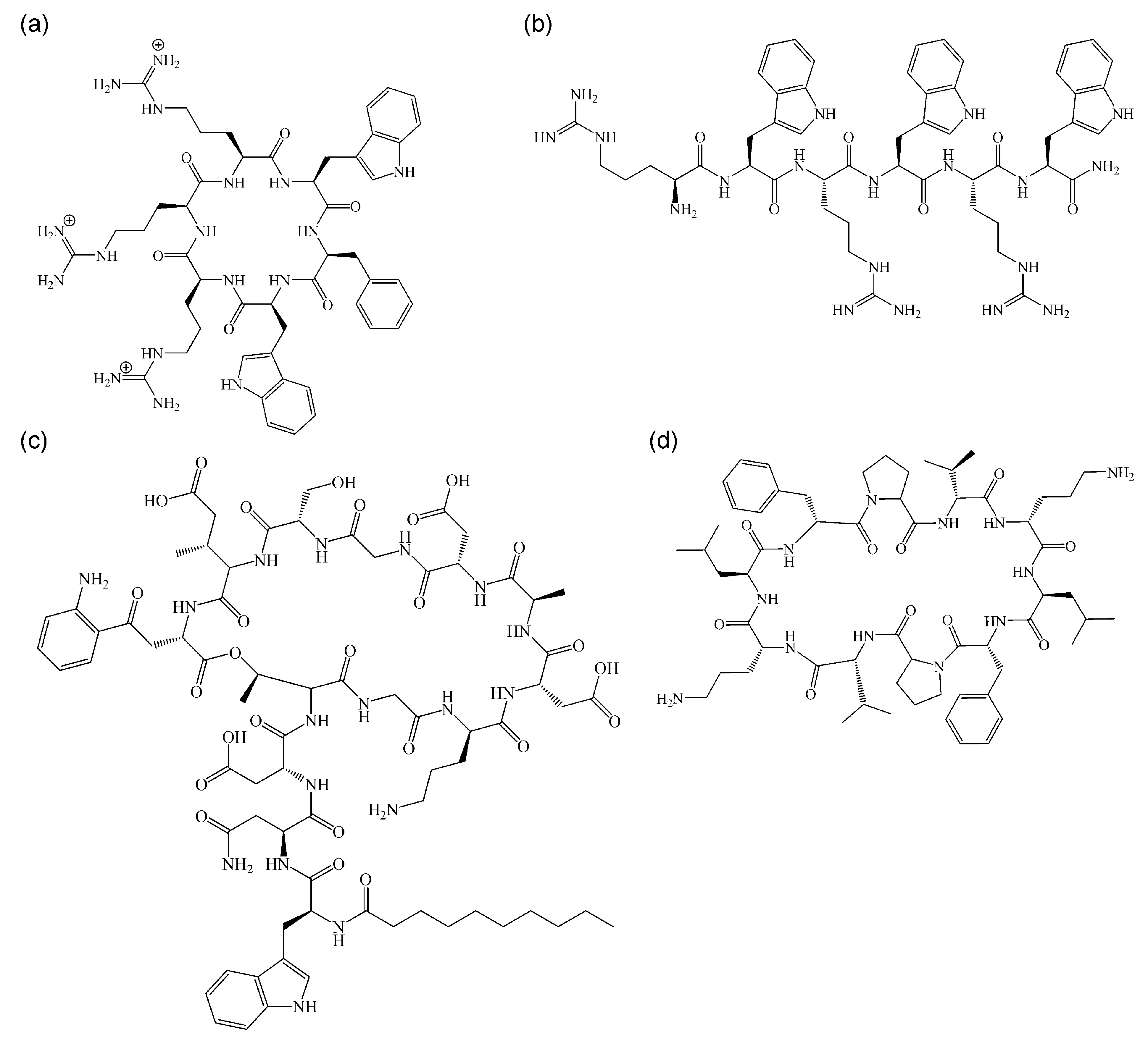
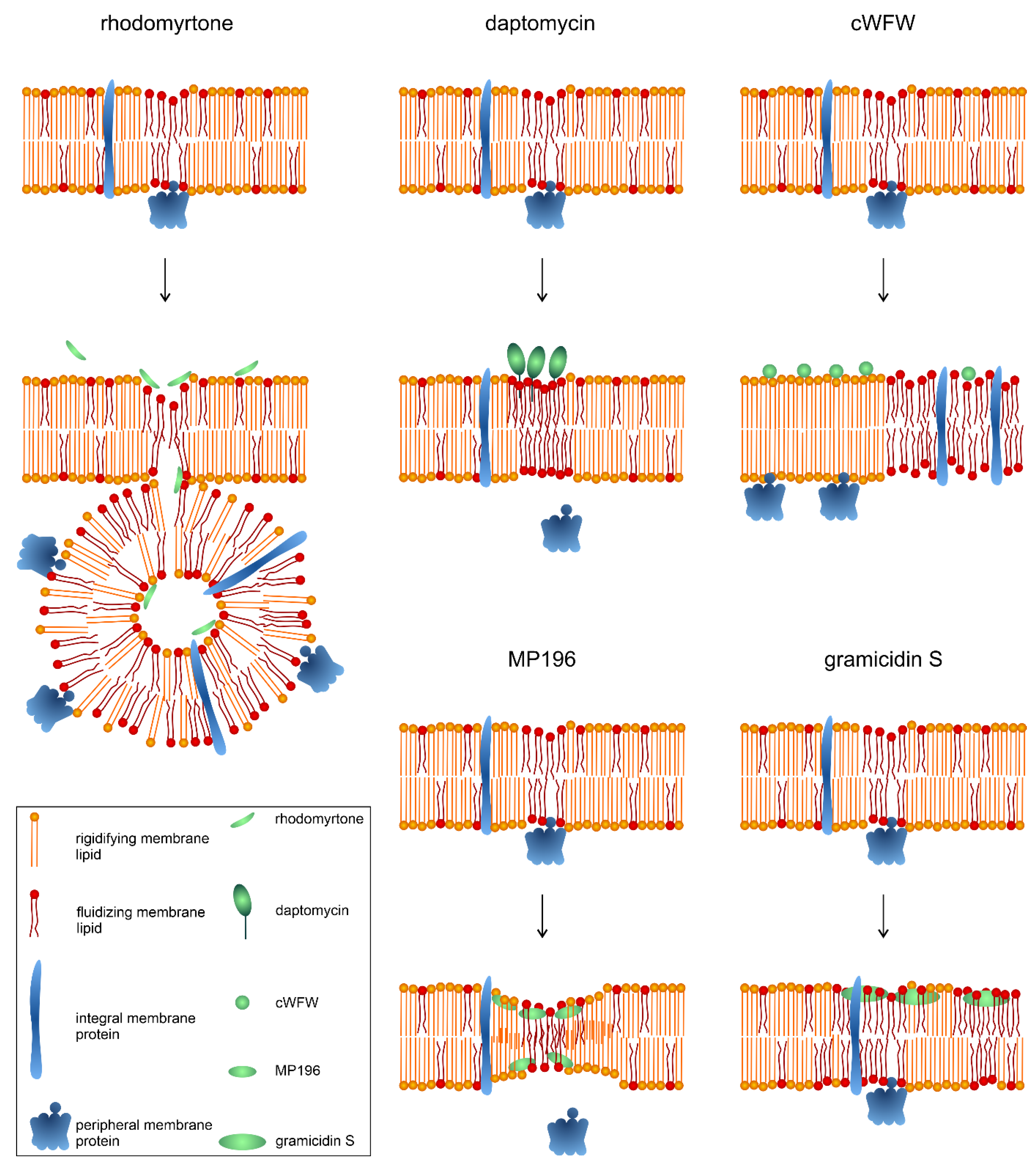
5.3. Comparison with Other Non-Pore-Forming, Membrane-Active Antimicrobials
5.4. Antivirulence Activities
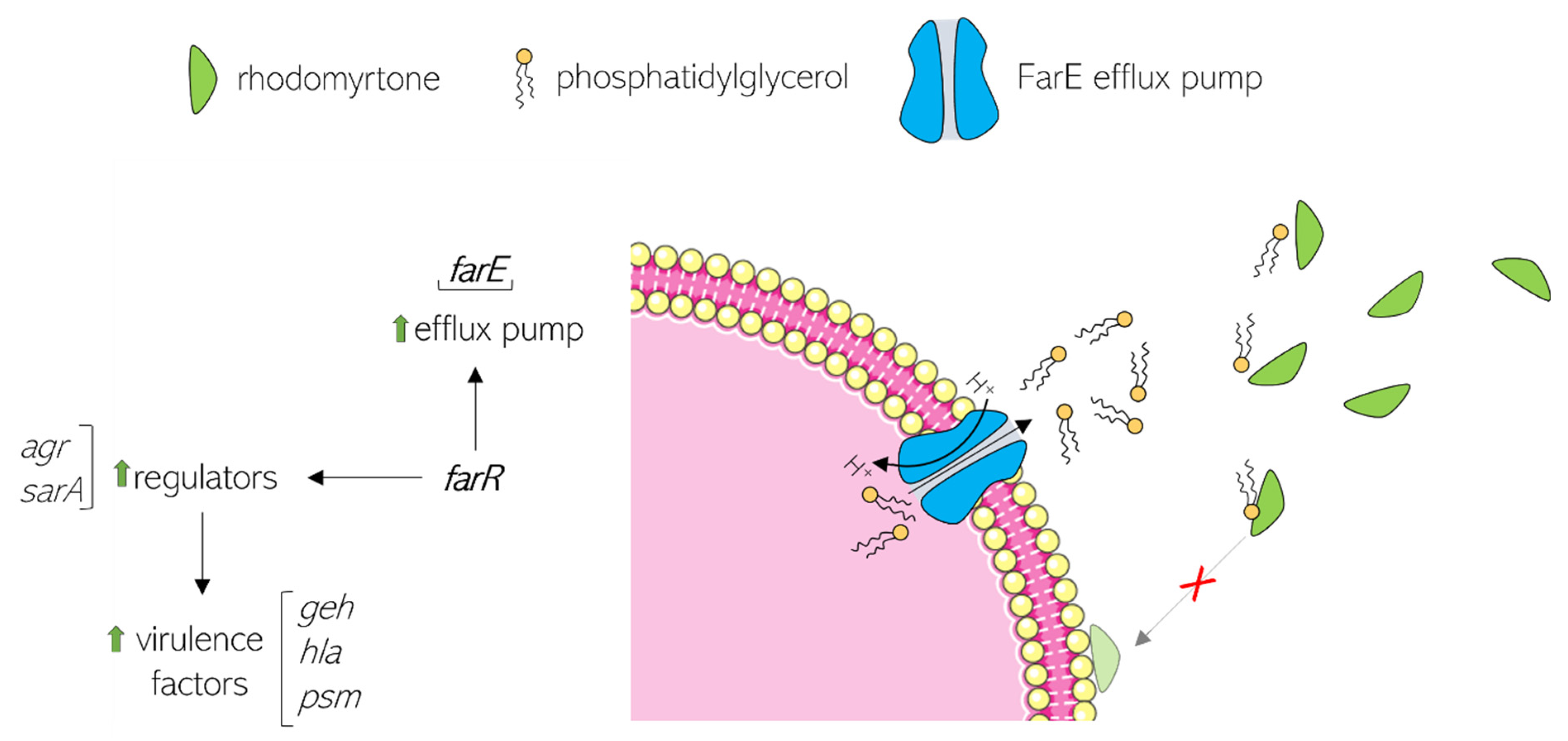
6. Rhodomyrtone Resistance
7. Toxicity
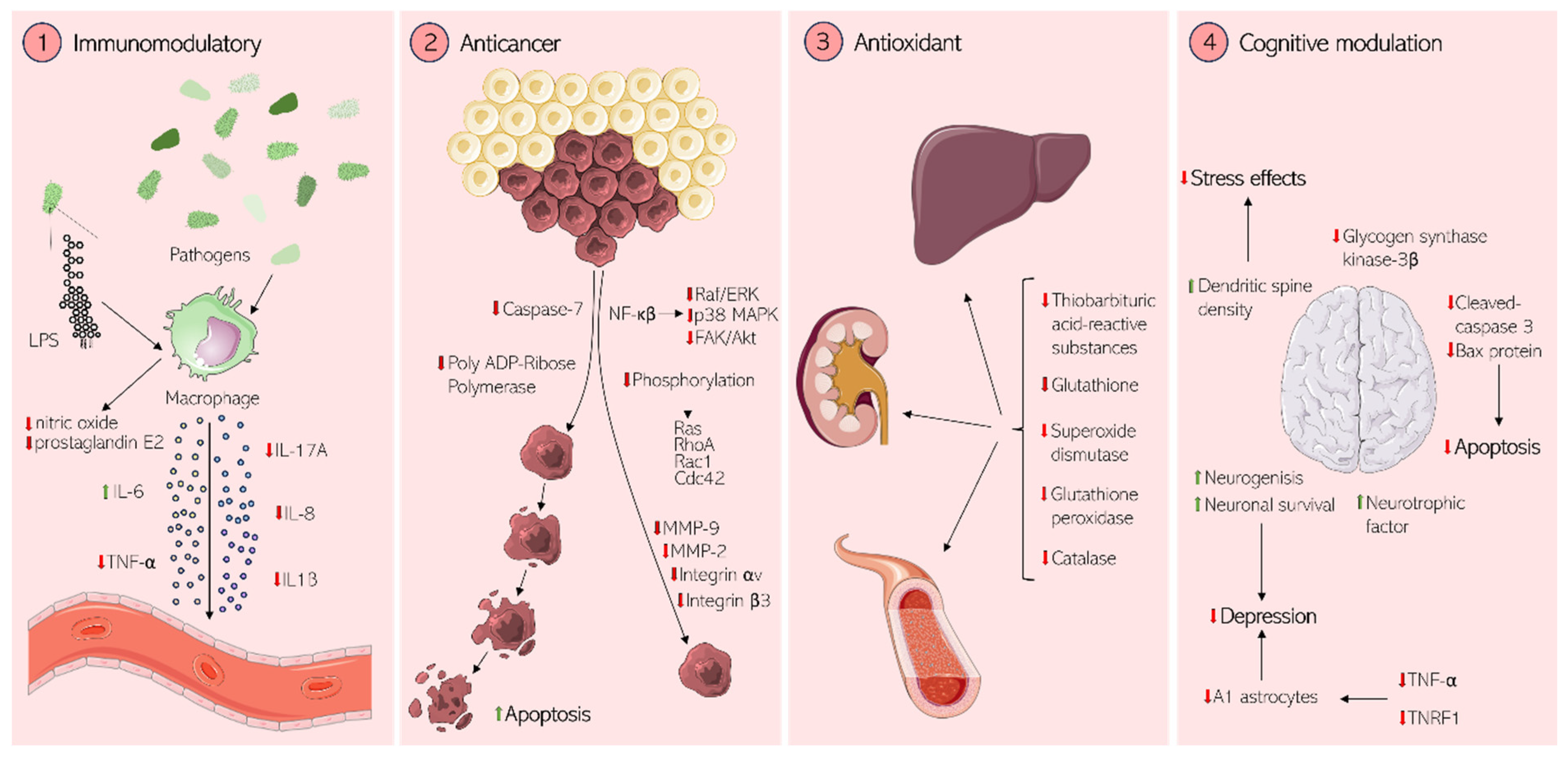
8. Potential Therapeutic Effects in Mammalian Cells
8.1. Immunomodulation
8.2. Anticancer Activity
8.3. Antioxidant Activity
8.4. Cognitive and Neuronal Effects
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Arip, M.; Selvaraja, M.; Rajagopal, M.; Tan, L.F.; Leong, M.Y.; Tan, P.L.; Yap, V.L.; Chinnapan, S.; Tat, N.C.; Abdullah, M.; et al. Review on Plant-Based Management in Combating Antimicrobial Resistance—Mechanistic Perspective. Front. Pharmacol. 2022, 13, 879495. [Google Scholar] [CrossRef]
- World Health Organization. Web Annex A: World Health Organization Model List of Essential Medicines—23rd List, 2023. In The Selection and Use of Essential Medicines 2023: Executive Summary of the Report of the 24th WHO Expert Committee on the Selection and Use of Essential Medic; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1. [Google Scholar]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006024-1. [Google Scholar]
- World Health Organization. Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-004765-5. [Google Scholar]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Ong, H.C.; Nordiana, M. Malay Ethno-Medico Botany in Machang, Kelantan, Malaysia. Fitoterapia 1999, 70, 502–513. [Google Scholar] [CrossRef]
- Lim, T.K. Rhodomyrtus Tomentosa BT—Edible Medicinal and Non Medicinal Plants: Fruits; Springer: Dordrecht, The Netherlands, 2012; Volume 3, pp. 732–737. ISBN 978-94-007-2534-8. [Google Scholar]
- Vo, T.S.; Ngo, D.H. The Health Beneficial Properties of Rhodomyrtus tomentosa as Potential Functional Food. Biomolecules 2019, 9, 76. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, L.; Xie, J.; Feng, Y.; Tian, J.; He, X.; Li, B.; Wang, L.; Wang, X.; Zhang, Y.; et al. Rhodomyrtus tomentosa (Aiton.): A Review of Phytochemistry, Pharmacology and Industrial Applications Research Progress. Food Chem. 2020, 309, 125715. [Google Scholar] [CrossRef]
- Limsuwan, S.; Kayser, O.; Voravuthikunchai, S.P. Antibacterial Activity of Rhodomyrtus tomentosa (Aiton) Hassk. Leaf Extract against Clinical Isolates of Streptococcus pyogenes. J. Evid. Based Complement. Altern. Med. 2012, 2012, 697183. [Google Scholar]
- Harlia Erika Juniar, A.H.A. Cytotoxic and Antioxidant Activity of Karamunting Stem Extract (Rhodomyrtus tomentosa (Aiton) Hassk). J. Equat. Chem. 2017, 6, 37–43. [Google Scholar]
- Noviani, D.; Treasa, A.D.; Tasman Munaf, A.Z.; Winaesih, S.; Nur Hidayati, D.Y.; Nooryanto, M.; Sutrisno, S. In vivo Effect Analysis of Rhodomyrtus tomentosa Leaf Ethanol Extract Against Escherichia coli. Med. Lab. Technol. J. 2023, 7, 83–91. [Google Scholar] [CrossRef]
- Mammino, L. Computational Study of Acylphloroglucinols: An. Investigation with Many. Branches 2019, 91, 597–607. [Google Scholar] [CrossRef]
- Mammino, L. Effects of Complexation with a Metal Ion on the Intramolecular Hydrogen Bonds in Acylphloroglucinols. Theor. Chem. Acc. 2019, 138, 103. [Google Scholar] [CrossRef]
- Limsuwan, S.; Trip, E.N.; Kouwen, T.R.H.M.; Piersma, S.; Hiranrat, A.; Mahabusarakam, W.; Voravuthikunchai, S.P.; van Dijl, J.M.; Kayser, O. Rhodomyrtone: A New Candidate as Natural Antibacterial Drug from Rhodomyrtus tomentosa. Phytomedicine 2009, 16, 645–651. [Google Scholar] [CrossRef]
- Ghosh, C.; Haldar, J. Membrane-Active Small Molecules: Designs Inspired by Antimicrobial Peptides. ChemMedChem 2015, 10, 1606–1624. [Google Scholar] [CrossRef]
- Saising, J.; Voravuthikunchai, S.P. Anti Propionibacterium Acnes Activity of Rhodomyrtone, an Effective Compound from Rhodomyrtus tomentosa (Aiton) Hassk. Leaves. Anaerobe 2012, 18, 400–404. [Google Scholar] [CrossRef]
- Chorachoo, J.; Amnuaikit, T.; Voravuthikunchai, S.P. Liposomal Encapsulated Rhodomyrtone: A Novel Antiacne Drug. Evid. Based. Complement. Altern. Med. 2013, 2013, 157635. [Google Scholar] [CrossRef]
- Wunnoo, S.; Saising, J.; Voravuthikunchai, S.P. Rhodomyrtone Inhibits Lipase Production, Biofilm Formation, and Disorganizes Established Biofilm in Propionibacterium acnes. Anaerobe 2017, 43, 61–68. [Google Scholar] [CrossRef]
- Wunnoo, S.; Bilhman, S.; Amnuaikit, T.; Ontong, J.C.; Singh, S.; Auepemkiate, S.; Voravuthikunchai, S.P. Rhodomyrtone as a New Natural Antibiotic Isolated from Rhodomyrtus tomentosa Leaf Extract: A Clinical Application in the Management of Acne Vulgaris. Antibiotics 2021, 10, 108. [Google Scholar] [CrossRef]
- Kaneshima, T.; Myoda, T.; Toeda, K.; Fujimori, T.; Nishizawa, M. Antimicrobial Constituents of Peel and Seeds of Camu-Camu (Myrciaria dubia). Biosci. Biotechnol. Biochem. 2017, 81, 1461–1465. [Google Scholar] [CrossRef]
- Saeloh, D.; Tipmanee, V.; Jim, K.K.; Dekker, M.P.; Bitter, W.; Voravuthikunchai, S.P.; Wenzel, M.; Hamoen, L.W. The Novel Antibiotic Rhodomyrtone Traps Membrane Proteins in Vesicles with Increased Fluidity. PLoS Pathog. 2018, 14, e1006876. [Google Scholar] [CrossRef]
- Limsuwan, S.; Homlaead, S.; Watcharakul, S.; Chusri, S.; Moosigapong, K.; Saising, J.; Voravuthikunchai, S.P. Inhibition of Microbial Adhesion to Plastic Surface and Human Buccal Epithelial Cells by Rhodomyrtus tomentosa Leaf Extract. Arch. Oral Biol. 2014, 59, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Senadeera, S.P.D.; Duffy, S.; Avery, V.M.; Carroll, A.R. Antiplasmodial β-Triketones from the Flowers of the Australian Tree Angophora woodsiana. Bioorg. Med. Chem. Lett. 2017, 27, 2602–2607. [Google Scholar] [CrossRef] [PubMed]
- Visutthi, M.; Srimanote, P.; Voravuthikunchai, S.P. Responses in the Expression of Extracellular Proteins in Methicillin-Resistant Staphylococcus aureus Treated with Rhodomyrtone. J. Microbiol. 2011, 49, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Sianglum, W.; Srimanote, P.; Wonglumsom, W.; Kittiniyom, K.; Voravuthikunchai, S.P. Proteome Analyses of Cellular Proteins in Methicillin-Resistant Staphylococcus aureus Treated with Rhodomyrtone, a Novel Antibiotic Candidate. PLoS ONE 2011, 6, e16628. [Google Scholar] [CrossRef] [PubMed]
- Leejae, S.; Taylor, P.W.; Voravuthikunchai, S.P. Antibacterial Mechanisms of Rhodomyrtone against Important Hospital-Acquired Antibiotic-Resistant Pathogenic Bacteria. J. Med. Microbiol. 2013, 62, 78–85. [Google Scholar] [CrossRef]
- Mordmuang, A.; Shankar, S.; Chethanond, U.; Voravuthikunchai, S.P. Effects of Rhodomyrtus tomentosa Leaf Extract on Staphylococcal Adhesion and Invasion in Bovine Udder Epidermal Tissue Model. Nutrients 2015, 7, 8503–8517. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Leejae, S.; Voravuthikunchai, S.P. Rhodomyrtone Accumulates in Bacterial Cell Wall and Cell Membrane and Inhibits the Synthesis of Multiple Cellular Macromolecules in Epidemic Methicillin-Resistant Staphylococcus aureus. Antibiotics 2021, 10, 543. [Google Scholar] [CrossRef]
- Liu, H.-X.; Tan, H.-B.; Qiu, S.-X. Antimicrobial Acylphloroglucinols from the Leaves of Rhodomyrtus tomentosa. J. Asian Nat. Prod. Res. 2016, 18, 535–541. [Google Scholar] [CrossRef]
- Saeloh, D.; Tipmanee, V.; Voravuthikunchai, S.P. Rhodomyrtone Target Exploration: Computer Aided Search on Staphylococcus aureus Key Proteins as a Potential Therapeutic Target. Curr. Comput. Aided. Drug Des. 2016, 12, 119–134. [Google Scholar] [CrossRef]
- Mitsuwan, W.; Jiménez-Munguía, I.; Visutthi, M.; Sianglum, W.; Rodríguez-Ortega, M.J.; Voravuthikunchai, S.P. Rhodomyrtone Decreases Staphylococcus aureus SigB Activity during Exponentially Growing Phase and Inhibits Haemolytic Activity within Membrane Vesicles. Microb. Pathog. 2019, 128, 112–118. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Pawlak, J. Rhodomyrtone: A New Anti-Staphylococcus aureus Agent against Resistant Strains. JAC Antimicrob. Resist. 2024, 6, dlae097. [Google Scholar] [CrossRef] [PubMed]
- Mordmuang, A.; Brouillette, E.; Voravuthikunchai, S.P.; Malouin, F. Evaluation of a Rhodomyrtus tomentosa Ethanolic Extract for Its Therapeutic Potential on Staphylococcus aureus Infections Using In Vitro and In Vivo Models of Mastitis. Vet. Res. 2019, 50, 49. [Google Scholar] [CrossRef] [PubMed]
- Sianglum, W.; Saeloh, D.; Tongtawe, P.; Wootipoom, N.; Indrawattana, N.; Voravuthikunchai, S.P. Early Effects of Rhodomyrtone on Membrane Integrity in Methicillin-Resistant Staphylococcus aureus. Microb. Drug Resist. 2018, 24, 882–889. [Google Scholar] [CrossRef]
- Sianglum, W.; Srimanote, P.; Taylor, P.W.; Rosado, H.; Voravuthikunchai, S.P. Transcriptome Analysis of Responses to Rhodomyrtone in Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2012, 7, e45744. [Google Scholar] [CrossRef]
- Mitsuwan, W.; Olaya-Abril, A.; Calderón-Santiago, M.; Jiménez-Munguía, I.; González-Reyes, J.A.; Priego-Capote, F.; Voravuthikunchai, S.P.; Rodríguez-Ortega, M.J. Integrated Proteomic and Metabolomic Analysis Reveals That Rhodomyrtone Reduces the Capsule in Streptococcus pneumoniae. Sci. Rep. 2017, 7, 2715. [Google Scholar] [CrossRef]
- Traithan, A.; Tongtawe, P.; Thanongsaksrikul, J.; Voravuthikunchai, S.; Srimanote, P. Antibacterial Mechanism of Rhodomyrtone Involves the Disruption of Nucleoid Segregation Checkpoint in Streptococcus suis. AMB Express 2020, 10, 110. [Google Scholar] [CrossRef]
- Srisuwan, S.; Mackin, K.E.; Hocking, D.; Lyras, D.; Bennett-Wood, V.; Voravuthikunchai, S.P.; Robins-Browne, R.M. Antibacterial Activity of Rhodomyrtone on Clostridium difficile Vegetative Cells and Spores in Vitro. Int. J. Antimicrob. Agents 2018, 52, 724–729. [Google Scholar] [CrossRef]
- Voravuthikunchai, S.P.; Dolah, S.; Charernjiratrakul, W. Control of Bacillus Cereus in Foods by Rhodomyrtus tomentosa (Ait.) Hassk. Leaf Extract and Its Purified Compound. J. Food Prot. 2010, 73, 1907–1912. [Google Scholar] [CrossRef]
- Saeloh, D.; Wenzel, M.; Rungrotmongkol, T.; Hamoen, L.W.; Tipmanee, V.; Voravuthikunchai, S.P. Effects of Rhodomyrtone on Gram-Positive Bacterial Tubulin Homologue FtsZ. PeerJ 2017, 5, e2962. [Google Scholar] [CrossRef]
- Leejae, S.; Yingyongnarongkul, B.; Suksamrarn, A.; Voravuthikunchai, S.P. Synthesis and Structure–Activity Relationship of Rhodomyrtone Derivatives as Antibacterial Agent. Chin. Chem. Lett. 2012, 23, 1011–1014. [Google Scholar] [CrossRef]
- Morkunas, M.; Dube, L.; Götz, F.; Maier, M.E. Synthesis of the Acylphloroglucinols Rhodomyrtone and Rhodomyrtosone B. Tetrahedron 2013, 69, 8559–8563. [Google Scholar] [CrossRef]
- Morkunas, M.; Maier, M.E. Alternative Routes to the Acylphloroglucinol Rhodomyrtone. Tetrahedron 2015, 71, 9662–9666. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, H.; Huo, L.; Wang, M.; Yang, B.; Zhang, W.; Xu, Z.; Tan, H.; Qiu, S.-X. Structural Optimization and Antibacterial Evaluation of Rhodomyrtosone B Analogues against MRSA Strains. Med. Chem. Commun. 2018, 9, 1698–1707. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Liu, H.-X.; Wang, L.; Xu, Z.-F.; Tan, H.-B.; Qiu, S.-X. Rhodomyrtosone B, a Membrane-Targeting Anti-MRSA Natural Acylgphloroglucinol from Rhodomyrtus tomentosa. J. Ethnopharmacol. 2019, 228, 50–57. [Google Scholar] [CrossRef]
- Wenninger, M.; Maier, M.E.; Viswanathan Ammanath, A.; Huang, L.; Götz, F. Synthesis of Rhodomyrtone Analogs Modified at C7. Eur. J. Org. Chem. 2023, 26, e202300259. [Google Scholar] [CrossRef]
- François, P.; Schrenzel, J.; Götz, F. Biology and Regulation of Staphylococcal Biofilm. Int. J. Mol. Sci. 2023, 24, 5218. [Google Scholar] [CrossRef]
- Saising, J.; Ongsakul, M.; Voravuthikunchai, S.P. Rhodomyrtus tomentosa (Aiton) Hassk. Ethanol Extract and Rhodomyrtone: A Potential Strategy for the Treatment of Biofilm-Forming Staphylococci. J. Med. Microbiol. 2011, 60, 1793–1800. [Google Scholar] [CrossRef]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High Throughput 2018, 7, 24. [Google Scholar] [CrossRef]
- Bach, Q.N.; Hongthong, S.; Quach, L.T.; Pham, L.V.; Pham, T.V.; Kuhakarn, C.; Reutrakul, V.; Nguyen, P.T.M. Antimicrobial Activity of Rhodomyrtone Isolated from Rhodomyrtus tomentosa (Aiton) Hassk. Nat. Prod. Res. 2020, 34, 2518–2523. [Google Scholar] [CrossRef]
- Mitsuwan, W.; Wintachai, P.; Voravuthikunchai, S.P. Rhodomyrtus Tomentosa Leaf Extract and Rhodomyrtone Combat Streptococcus pneumoniae Biofilm and Inhibit Invasiveness to Human Lung Epithelial and Enhance Pneumococcal Phagocytosis by Macrophage. Curr. Microbiol. 2020, 77, 3546–3554. [Google Scholar] [CrossRef]
- Limsuwan, S.; Voravuthikunchai, S.P. Boesenbergia Pandurata (Roxb.) Schltr., Eleutherine americana Merr. and Rhodomyrtus tomentosa (Aiton) Hassk. as Antibiofilm Producing and Antiquorum Sensing in Streptococcus pyogenes. FEMS Immunol. Med. Microbiol. 2008, 53, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Limsuwan, S.; Hesseling-Meinders, A.; Voravuthikunchai, S.P.; van Dijl, J.M.; Kayser, O. Potential Antibiotic and Anti-Infective Effects of Rhodomyrtone from Rhodomyrtus tomentosa (Aiton) Hassk. on Streptococcus pyogenes as Revealed by Proteomics. Phytomedicine 2011, 18, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Saising, J.; Nguyen, M.-T.; Hartner, T.; Ebner, P.; Al Mamun Bhuyan, A.; Berscheid, A.; Muehlenkamp, M.; Schakermann, S.; Kumari, N.; Maier, M.E.; et al. Rhodomyrtone (Rom) Is a Membrane-Active Compound. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Salni, D.; Sargent, M.V.; Skelton, B.W.; Soediro, I.; Sutisna, M.; White, A.H.; Yulinah, E. Rhodomyrtone, an Antibotic from Rhodomyrtus tomentosa. Aust. J. Chem. 2002, 55, 229–232. [Google Scholar] [CrossRef]
- Pilhofer, M.; Ladinsky, M.S.; McDowall, A.W.; Petroni, G.; Jensen, G.J. Microtubules in Bacteria: Ancient Tubulins Build a Five-Protofilament Homolog of the Eukaryotic Cytoskeleton. PLoS Biol. 2011, 9, e1001213. [Google Scholar] [CrossRef]
- de Boer, P.; Crossley, R.; Rothfield, L. The Essential Bacterial Cell-Division Protein FtsZ Is a GTPase. Nature 1992, 359, 254–256. [Google Scholar] [CrossRef]
- Natarajan, K.; Senapati, S. Probing the Conformational Flexibility of Monomeric FtsZ in GTP-Bound, GDP-Bound, and Nucleotide-Free States. Biochemistry 2013, 52, 3543–3551. [Google Scholar] [CrossRef]
- Oliva, M.A.; Trambaiolo, D.; Löwe, J. Structural Insights into the Conformational Variability of FtsZ. J. Mol. Biol. 2007, 373, 1229–1242. [Google Scholar] [CrossRef]
- Bisson-Filho, A.W.; Hsu, Y.-P.; Squyres, G.R.; Kuru, E.; Wu, F.; Jukes, C.; Sun, Y.; Dekker, C.; Holden, S.; VanNieuwenhze, M.S.; et al. Treadmilling by FtsZ Filaments Drives Peptidoglycan Synthesis and Bacterial Cell Division. Science 2017, 355, 739–743. [Google Scholar] [CrossRef]
- Whitley, K.D.; Jukes, C.; Tregidgo, N.; Karinou, E.; Almada, P.; Cesbron, Y.; Henriques, R.; Dekker, C.; Holden, S. FtsZ Treadmilling Is Essential for Z-Ring Condensation and Septal Constriction Initiation in Bacillus subtilis Cell Division. Nat. Commun. 2021, 12, 2448. [Google Scholar] [CrossRef]
- Adams, D.W.; Wu, L.J.; Czaplewski, L.G.; Errington, J. Multiple Effects of Benzamide Antibiotics on FtsZ Function. Mol. Microbiol. 2011, 80, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Bazan, L.; Ruiz-Avila, L.B.; Andreu, D.; Huecas, S.; Andreu, J.M. Cytological Profile of Antibacterial FtsZ Inhibitors and Synthetic Peptide MciZ. Front. Microbiol. 2016, 7, 1558. [Google Scholar] [CrossRef] [PubMed]
- Strahl, H.; Hamoen, L.W. Membrane Potential Is Important for Bacterial Cell Division. Proc. Natl. Acad. Sci. USA 2010, 107, 12281–12286. [Google Scholar] [CrossRef]
- Strahl, H.; Burmann, F.; Hamoen, L.W. The Actin Homologue MreB Organizes the Bacterial Cell Membrane. Nat. Commun. 2014, 5, 3442. [Google Scholar] [CrossRef]
- Gray, D.A.; Wenzel, M. More Than a Pore: A Current Perspective on the In Vivo Mode of Action of the Lipopeptide Antibiotic Daptomycin. Antibiotics 2020, 9, 17. [Google Scholar] [CrossRef]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Krämer, U.; et al. Small Cationic Antimicrobial Peptides Delocalize Peripheral Membrane Proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E1409-18. [Google Scholar] [CrossRef]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.-G.; Schneider, T.; et al. Daptomycin Inhibits Cell Envelope Synthesis by Interfering with Fluid Membrane Microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, e7077–e7086. [Google Scholar] [CrossRef]
- Scheinpflug, K.; Wenzel, M.; Krylova, O.; Bandow, J.E.; Dathe, M.; Strahl, H. Antimicrobial Peptide CWFW Kills by Combining Lipid Phase Separation with Autolysis. Sci. Rep. 2017, 7, 44332. [Google Scholar] [CrossRef]
- Wenzel, M.; Rautenbach, M.; Vosloo, J.A.; Siersma, T.; Aisenbrey, C.H.M.; Zaitseva, E.; Laubscher, W.E.; van Rensburg, W.; Behrends, J.; Bechinger, B.; et al. The Multifaceted Antibacterial Mechanisms of the Pioneering Peptide Antibiotics Tyrocidine and Gramicidin S. mBio 2018, 9, e00802-18. [Google Scholar] [CrossRef]
- Wimley, W.C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef]
- Prenner, E.J.; Lewis, R.N.A.H.; McElhaney, R.N. The Interaction of the Antimicrobial Peptide Gramicidin S with Lipid Bilayer Model and Biological Membranes. Biochim. Biophys. Acta Biomembr. 1999, 1462, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Muraih, J.K.; Tishbi, N.; Herskowitz, J.; Victor, R.L.; Silverman, J.; Uwumarenogie, S.; Taylor, S.D.; Palmer, M.; Mintzer, E. Cardiolipin Prevents Membrane Translocation and Permeabilization by Daptomycin. J. Biol. Chem. 2014, 289, 11584–11591. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M. Membranaktive Antibiotika: Neue Mechanismen Gegen Ein Altbekanntes Ziel. BioSpektrum 2019, 25, 683. [Google Scholar] [CrossRef]
- Grein, F.; Müller, A.; Scherer, K.M.; Liu, X.; Ludwig, K.C.; Klockner, A.; Strach, M.; Sahl, H.-G.; Kubitscheck, U.; Schneider, T. Ca(2+)-Daptomycin Targets Cell Wall Biosynthesis by Forming a Tripartite Complex with Undecaprenyl-Coupled Intermediates and Membrane Lipids. Nat. Commun. 2020, 11, 1455. [Google Scholar] [CrossRef]
- Pogliano, J.; Pogliano, N.; Silverman, J.A. Daptomycin-Mediated Reorganization of Membrane Architecture Causes Mislocalization of Essential Cell Division Proteins. J. Bacteriol. 2012, 194, 4494–4504. [Google Scholar] [CrossRef]
- Berditsch, M.; Lux, H.; Babii, O.; Afonin, S.; Ulrich, A.S. Therapeutic Potential of Gramicidin S in the Treatment of Root Canal Infections. Pharmaceuticals 2016, 9, 56. [Google Scholar] [CrossRef]
- Clauditz, A.; Resch, A.; Wieland, K.-P.; Peschel, A.; Götz, F. Staphyloxanthin Plays a Role in the Fitness of Staphylococcus aureus and Its Ability to Cope with Oxidative Stress. Infect. Immun. 2006, 74, 4950–4953. [Google Scholar] [CrossRef]
- Leejae, S.; Hasap, L.; Voravuthikunchai, S.P. Inhibition of Staphyloxanthin Biosynthesis in Staphylococcus aureus by Rhodomyrtone, a Novel Antibiotic Candidate. J. Med. Microbiol. 2013, 62, 421–428. [Google Scholar] [CrossRef]
- Holland, C.; Mak, T.N.; Zimny-Arndt, U.; Schmid, M.; Meyer, T.F.; Jungblut, P.R.; Brüggemann, H. Proteomic Identification of Secreted Proteins of Propionibacterium acnes. BMC Microbiol. 2010, 10, 230. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Saising, J.; Tribelli, P.M.; Nega, M.; Diene, S.M.; Francois, P.; Schrenzel, J.; Sproer, C.; Bunk, B.; Ebner, P.; et al. Inactivation of FarR Causes High Rhodomyrtone Resistance and Increased Pathogenicity in Staphylococcus aureus. Front. Microbiol. 2019, 10, 1157. [Google Scholar] [CrossRef]
- Alnaseri, H.; Arsic, B.; Schneider, J.E.T.; Kaiser, J.C.; Scinocca, Z.C.; Heinrichs, D.E.; McGavin, M.J. Inducible Expression of a Resistance-Nodulation-Division-Type Efflux Pump in Staphylococcus aureus Provides Resistance to Linoleic and Arachidonic Acids. J. Bacteriol. 2015, 197, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Matsuo, M.; Calderón, C.; Fan, S.-H.; Ammanath, A.V.; Fu, X.; Li, N.; Luqman, A.; Ullrich, M.; Herrmann, F.; et al. Molecular Basis of Rhodomyrtone Resistance in Staphylococcus aureus. MBio 2021, 13, e0383321. [Google Scholar] [CrossRef] [PubMed]
- Pader, V.; Hakim, S.; Painter, K.L.; Wigneshweraraj, S.; Clarke, T.B.; Edwards, A.M. Staphylococcus aureus Inactivates Daptomycin by Releasing Membrane Phospholipids. Nat. Microbiol. 2016, 2, 16194. [Google Scholar] [CrossRef] [PubMed]
- Siriyong, T.; Ontong, J.C.; Leejae, S.; Suwalak, S.; Coote, P.J.; Voravuthikunchai, S.P. In Vivo Safety Assessment of Rhodomyrtone, a Potent Compound, from Rhodomyrtus tomentosa Leaf Extract. Toxicol. Rep. 2020, 7, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Chorachoo, J.; Lambert, S.; Furnholm, T.; Roberts, L.; Reingold, L.; Auepemkiate, S.; Voravuthikunchai, S.P.; Johnston, A. The Small Molecule Rhodomyrtone Suppresses TNF-Alpha and IL-17A-Induced Keratinocyte Inflammatory Responses: A Potential New Therapeutic for Psoriasis. PLoS ONE 2018, 13, e0205340. [Google Scholar] [CrossRef]
- Chai, H.; Liu, B.; Zhan, H.; Li, X.; He, Z.; Ye, J.; Guo, Q.; Chen, J.; Zhang, J.; Li, S. Antidepressant Effects of Rhodomyrtone in Mice with Chronic Unpredictable Mild Stress-Induced Depression. Int. J. Neuropsychopharmacol. 2019, 22, 157–164. [Google Scholar] [CrossRef]
- Suwandecha, T.; Yingyongnarongkul, B.-E.; Towtawin, K.; Voravuthikunchai, S.P.; Sriwiriyajan, S. A Novel Antibiotic, Rhodomyrtone: Pharmacokinetic Studies in a Murine Model and Optimization and Validation of High-Performance Liquid Chromatographic Method for Plasma Analysis. Antibiotics 2024, 13, 156. [Google Scholar] [CrossRef]
- Jeong, D.; Yang, W.S.; Yang, Y.; Nam, G.; Kim, J.H.; Yoon, D.H.; Noh, H.J.; Lee, S.; Kim, T.W.; Sung, G.-H.; et al. In Vitro and In Vivo Anti-Inflammatory Effect of Rhodomyrtus tomentosa Methanol Extract. J. Ethnopharmacol. 2013, 146, 205–213. [Google Scholar] [CrossRef]
- Na-Phatthalung, P.; Teles, M.; Voravuthikunchai, S.P.; Tort, L.; Fierro-Castro, C. Immunomodulatory Effects of Rhodomyrtus tomentosa Leaf Extract and Its Derivative Compound, Rhodomyrtone, on Head Kidney Macrophages of Rainbow Trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2018, 44, 543–555. [Google Scholar] [CrossRef]
- Srisuwan, S.; Tongtawe, P.; Srimanote, P.; Voravuthikunchai, S.P. Rhodomyrtone Modulates Innate Immune Responses of THP-1 Monocytes to Assist in Clearing Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2014, 9, e110321. [Google Scholar] [CrossRef]
- Chorachoo, J.; Saeloh, D.; Srichana, T.; Amnuaikit, T.; Musthafa, K.S.; Sretrirutchai, S.; Voravuthikunchai, S.P. Rhodomyrtone as a Potential Anti-Proliferative and Apoptosis Inducing Agent in HaCaT Keratinocyte Cells. Eur. J. Pharmacol. 2016, 772, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Tayeh, M.; Nilwarangkoon, S.; Tanunyutthawongse, C.; Mahabusarakum, W.; Watanapokasin, R. Apoptosis and Antimigration Induction in Human Skin Cancer Cells by Rhodomyrtone. Exp. Ther. Med. 2018, 15, 5035–5040. [Google Scholar] [CrossRef] [PubMed]
- Tayeh, M.; Nilwarangoon, S.; Mahabusarakum, W.; Watanapokasin, R. Anti-Metastatic Effect of Rhodomyrtone from Rhodomyrtus tomentosa on Human Skin Cancer Cells. Int. J. Oncol. 2017, 50, 1035–1043. [Google Scholar] [CrossRef]
- Tayeh, M.; Watanapokasin, R. Antimetastatic Potential. of Rhodomyrtone on Human Chondrosarcoma SW1353 Cells. Evid. Based Complement. Altern. Med. 2020, 2020, 8180261. [Google Scholar] [CrossRef]
- Lavanya, G.; Voravuthikunchai, S.P.; Towatana, N.H. Acetone Extract from Rhodomyrtus tomentosa: A Potent Natural Antioxidant. Evid. Based Complement. Altern. Med. 2012, 2012, 535479. [Google Scholar] [CrossRef]
- Gao, M.; Song, Y.; Liu, Y.; Miao, Y.; Guo, Y.; Chai, H. TNF-α/TNFR1 Activated Astrocytes Exacerbate Depression-like Behavior in CUMS Mice. Cell Death Discov. 2024, 10, 220. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, R.; Marinho Righetto, G.; Schäfer, A.-B.; Wenzel, M. The Diverse Activities and Mechanisms of the Acylphloroglucinol Antibiotic Rhodomyrtone: Antibacterial Activity and Beyond. Antibiotics 2024, 13, 936. https://doi.org/10.3390/antibiotics13100936
Rani R, Marinho Righetto G, Schäfer A-B, Wenzel M. The Diverse Activities and Mechanisms of the Acylphloroglucinol Antibiotic Rhodomyrtone: Antibacterial Activity and Beyond. Antibiotics. 2024; 13(10):936. https://doi.org/10.3390/antibiotics13100936
Chicago/Turabian StyleRani, Rupa, Gabriela Marinho Righetto, Ann-Britt Schäfer, and Michaela Wenzel. 2024. "The Diverse Activities and Mechanisms of the Acylphloroglucinol Antibiotic Rhodomyrtone: Antibacterial Activity and Beyond" Antibiotics 13, no. 10: 936. https://doi.org/10.3390/antibiotics13100936






