Antimicrobial and Cytotoxic Potential of Eucalyptus Essential Oil-Based Nanoemulsions for Mouthwashes Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Eucalyptus Essential Oil
2.2. Physicochemical Characterization of Nanoemulsions
2.2.1. Particle Size and Polydispersity Index
2.2.2. Zeta Potential and pH
2.2.3. Transmission Electron Microscopy
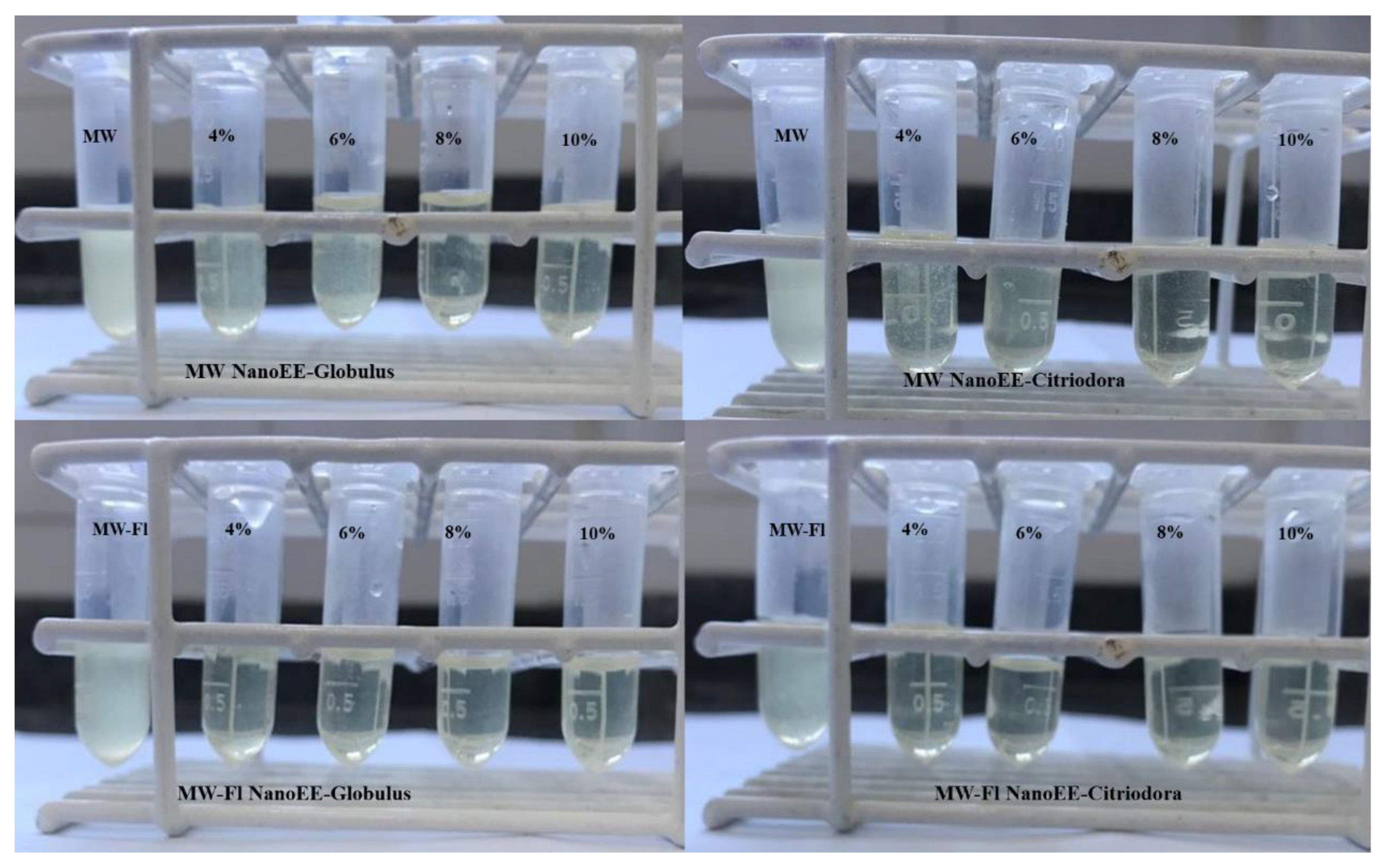
2.2.4. Antimicrobial Activity Tests: Inhibitory Concentration of Nanoemulsions
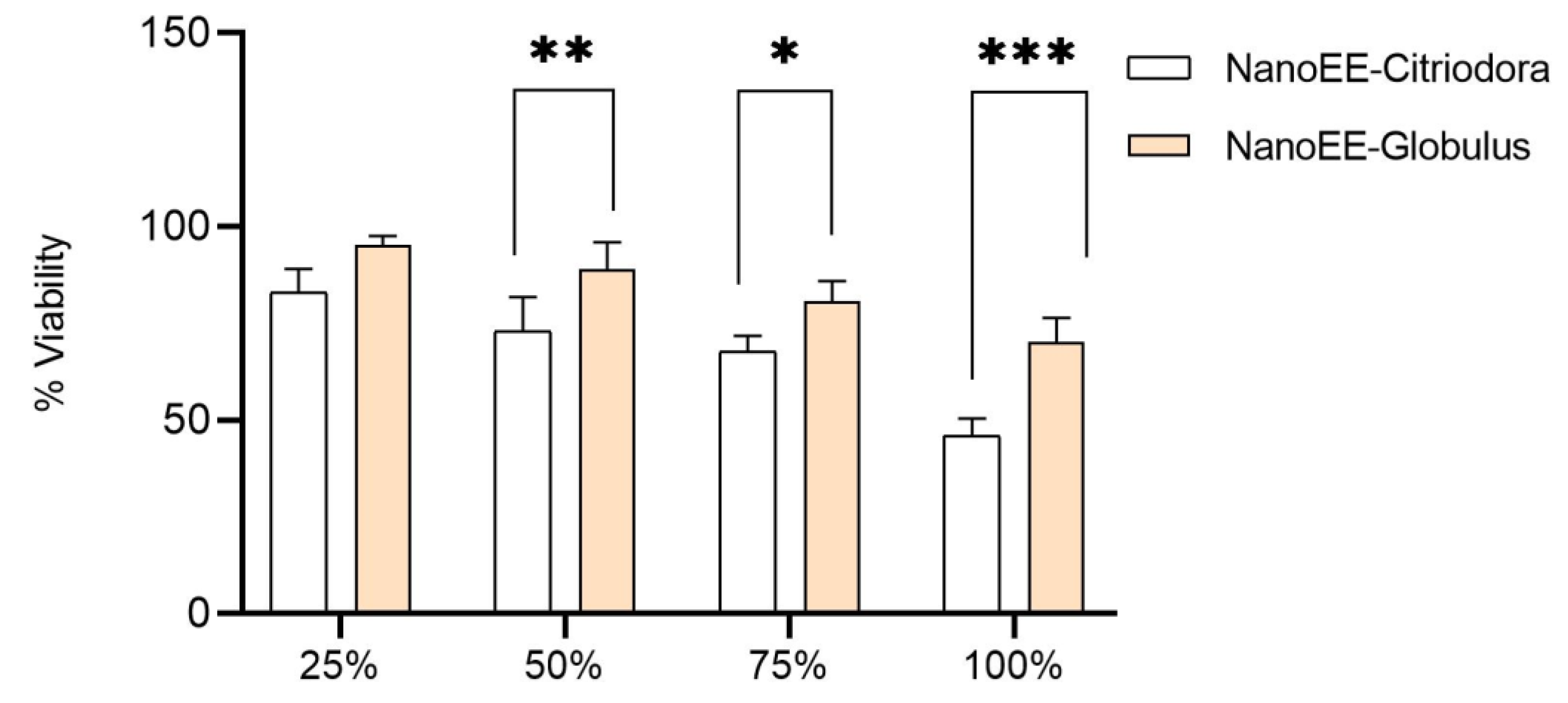
2.2.5. Nanoemulsion Cytotoxicity and Cell Viability Assays
2.2.6. Antimicrobial Activity Assays: Inhibitory Concentration of Mouthwashes Functionalized with Nanoemulsions
3. Materials and Methods
3.1. Extraction and Characterization of Essential Oils
3.2. Production of Nanoemulsions
3.3. Physicochemical and Morphological Characterization of Eucalyptus Essential Oil Nanoemulsions
3.4. Antimicrobial Evaluation of Nanoemulsions
3.5. Cytotoxicity and Cell Viability Test
3.6. Application of Nanoemulsions in Mouthwashes
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of Periodontal Disease, Its Association with Systemic Diseases and Prevention. Int. J. Health Sci. 2017, 11, 72. [Google Scholar]
- Kassebaum, N.J.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, J.; Carter, A.; Casey, D.C.; Charlson, F.J.; Coates, M.M.; Coggeshall, M.; et al. Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 315 Diseases and Injuries and Healthy Life Expectancy (HALE), 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of Microbial Biofilms in the Maintenance of Oral Health and in the Development of Dental Caries and Periodontal Diseases. Consensus Report of Group 1 of the Joint EFP/ORCA Workshop on the Boundaries between Caries and Periodontal Disease. J. Clin. Periodontol. 2017, 44, S5–S11. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Dental Biofilms: Difficult Therapeutic Targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef]
- Araujo, M.W.B.; Charles, C.A.; Weinstein, R.B.; McGuire, J.A.; Parikh-Das, A.M.; Du, Q.; Zhang, J.; Berlin, J.A.; Gunsolley, J.C. Meta-Analysis of the Effect of an Essential Oil–Containing Mouthrinse on Gingivitis and Plaque. J. Am. Dent. Assoc. 2015, 146, 610–622. [Google Scholar] [CrossRef]
- Takenaka, S.; Sotozono, M.; Ohkura, N.; Noiri, Y. Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects. Antibiotics 2022, 11, 727. [Google Scholar] [CrossRef]
- Sreenivasan, P.; Gaffar, A. Antiplaque Biocides and Bacterial Resistance: A Review. J. Clin. Periodontol. 2002, 29, 965–974. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, Medicinal and Toxicological Significance of Eucalyptus Leaf Essential Oil: A Review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Mahyari, S.; Mahyari, B.; Emami, S.A.; Malaekeh-Nikouei, B.; Jahanbakhsh, S.P.; Sahebkar, A.; Mohammadpour, A.H. Evaluation of the Efficacy of a Polyherbal Mouthwash Containing Zingiber Officinale, Rosmarinus officinalis and Calendula Officinalis Extracts in Patients with Gingivitis: A Randomized Double-Blind Placebo-Controlled Trial. Complement. Ther. Clin. Pract. 2016, 22, 93–98. [Google Scholar] [CrossRef]
- Stoeken, J.E.; Paraskevas, S.; Van Der Weijden, G.A. The Long-Term Effect of a Mouthrinse Containing Essential Oils on Dental Plaque and Gingivitis: A Systematic Review. J. Periodontol. 2007, 78, 1218–1228. [Google Scholar] [CrossRef]
- Nehavarshini, V.; Unnikrishnan, S.; Ramalingam, K. Exploring the Potential of a Herbal Nanoemulsion as an Antimicrobial Mouthwash. Appl. Biochem. Biotechnol. 2023, 195, 5777–5791. [Google Scholar] [CrossRef]
- Mostafa, N.M. Antibacterial Activity of Ginger (Zingiber Officinale) Leaves Essential Oil Nanoemulsion against the Cariogenic Streptococcus Mutans. J. App Pharm. Sci. 2018, 8, 34–41. [Google Scholar] [CrossRef]
- Horváth, B.; Balázs, V.L.; Varga, A.; Böszörményi, A.; Kocsis, B.; Horváth, G.; Széchenyi, A. Preparation, Characterisation and Microbiological Examination of Pickering Nano-Emulsions Containing Essential Oils, and Their Effect on Streptococcus mutans Biofilm Treatment. Sci. Rep. 2019, 9, 16611. [Google Scholar] [CrossRef]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-Energy Formation of Edible Nanoemulsions: Factors Influencing Droplet Size Produced by Emulsion Phase Inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An Advanced Mode of Drug Delivery System. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Linstrom, P. NIST Chemistry WebBook, NIST Standard Reference Database 69. 1997. Available online: https://webbook.nist.gov/chemistry/ (accessed on 28 September 2024).
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Quatrin, P.M.; Verdi, C.M.; De Souza, M.E.; De Godoi, S.N.; Klein, B.; Gundel, A.; Wagner, R.; De Almeida Vaucher, R.; Ourique, A.F.; Santos, R.C.V. Antimicrobial and Antibiofilm Activities of Nanoemulsions Containing Eucalyptus globulus Oil against Pseudomonas aeruginosa and Candida Spp. Microb. Pathog. 2017, 112, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 9th ed.; Cockerill, F.R., Clinical and Laboratory Standards Institute, Eds.; Clinical and Laboratory Standards Institute, CLSI: Wayne, PA, USA, 2012; ISBN 978-1-56238-784-6. [Google Scholar]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves da Rosa, C.; Zapelini de Melo, A.P.; Sganzerla, W.G.; Machado, M.H.; Nunes, M.R.; Vinicius de Oliveira Brisola Maciel, M.; Bertoldi, F.C.; Manique Barreto, P.L. Application in Situ of Zein Nanocapsules Loaded with Origanum Vulgare Linneus and Thymus Vulgaris as a Preservative in Bread. Food Hydrocoll. 2020, 99, 105339. [Google Scholar] [CrossRef]
- Rosso, A.; Lollo, G.; Chevalier, Y.; Troung, N.; Bordes, C.; Bourgeois, S.; Maniti, O.; Granjon, T.; Dugas, P.-Y.; Urbaniak, S.; et al. Development and Structural Characterization of a Novel Nanoemulsion for Oral Drug Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124614. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Nunes, M.R.; Da Rosa, C.G.; De Borba, J.R.; Dos Santos, G.M.; Ferreira, A.L.; Barreto, P.L.M. Zein Nanoparticles: Bioactive Compounds and Controlled Delivery. In Nanoengineering of Biomaterials; Jana, S., Jana, S., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 411–436. ISBN 978-3-527-34904-3. [Google Scholar]
- De Melo, A.P.Z.; Da Rosa, C.G.; Sganzerla, W.G.; Nunes, M.R.; Noronha, C.M.; Brisola Maciel, M.V.D.O.; Villetti, M.A.; Bertoldi, F.C.; Barreto, P.L.M. Syntesis and Characterization of Zein Nanoparticles Loaded with Essential Oil of Ocimum gratissimum and Pimenta racemosa. Mater. Res. Express 2019, 6, 095084. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Castro, L.E.N.; Da Rosa, C.G.; Almeida, A.D.R.; Maciel-Silva, F.W.; Kempe, P.R.G.; De Oliveira, A.L.R.; Forster-Carneiro, T.; Bertoldi, F.C.; Barreto, P.L.M.; et al. Production of Nanocomposite Films Functionalized with Silver Nanoparticles Bioreduced with Rosemary (Rosmarinus officinalis L.) Essential Oil. J. Agric. Food Res. 2023, 11, 100479. [Google Scholar] [CrossRef]
- Jummes, B.; Sganzerla, W.G.; Da Rosa, C.G.; Noronha, C.M.; Nunes, M.R.; Bertoldi, F.C.; Barreto, P.L.M. Antioxidant and Antimicrobial Poly-ε-Caprolactone Nanoparticles Loaded with Cymbopogon martinii Essential Oil. Biocatal. Agric. Biotechnol. 2020, 23, 101499. [Google Scholar] [CrossRef]
- Maciel, M.V.D.O.B.; Da Rosa, C.G.; Almeida, A.D.R.; Nunes, M.R.; Noronha, C.M.; Jummes, B.; Martelli, S.M.; Bertoldi, F.C.; Barreto, P.L.M. Thymol Loaded Zein Microparticles Obtained by Spray-Drying: Physical-Chemical Characterization. Biocatal. Agric. Biotechnol. 2021, 37, 102177. [Google Scholar] [CrossRef]
- De Melo, A.P.Z.; Da Rosa, C.G.; Noronha, C.M.; Machado, M.H.; Sganzerla, W.G.; Bellinati, N.V.D.C.; Nunes, M.R.; Verruck, S.; Prudêncio, E.S.; Barreto, P.L.M. Nanoencapsulation of Vitamin D3 and Fortification in an Experimental Jelly Model of Acca Sellowiana: Bioaccessibility in a Simulated Gastrointestinal System. LWT 2021, 145, 111287. [Google Scholar] [CrossRef]
- Noronha, C.M.; De Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of Antioxidant Methylcellulose Film Incorporated with α-Tocopherol Nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Lino, R.C.; De Carvalho, S.M.; Noronha, C.M.; Sganzerla, W.G.; Da Rosa, C.G.; Nunes, M.R.; D’Avila, R.F.; Zambiazi, R.C.; Barreto, P.L.M. Production of Methylcellulose Films Functionalized with Poly-ε-Caprolactone Nanocapsules Entrapped β-Carotene for Food Packaging Application. Food Res. Int. 2022, 160, 111750. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; Agostinetto, L.; Da Rosa, C.G.; Sganzerla, W.G.; Pires, M.F.; Munaretto, G.A.; Rosar, C.R.; Bertoldi, F.C.; Barreto, P.L.M.; Veeck, A.P.D.L.; et al. Application of Nanoparticles Entrapped Orange Essential Oil to Inhibit the Incidence of Phytopathogenic Fungi during Storage of Agroecological Maize Seeds. Food Res. Int. 2024, 175, 113738. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S.; Mudgil, P. Antibacterial Properties of Eucalyptus Globulus Essential Oil against MRSA: A Systematic Review. Antibiotics 2023, 12, 474. [Google Scholar] [CrossRef]
- Santos, B.; Farias, J.H.A.; Simões, M.M.; Medeiros, M.A.A.; Alves, M.S.; Diniz, A.F.; Soares, A.P.O.; Cavalcante, A.P.T.M.; Silva, B.J.N.; Almeida, J.C.S.; et al. Evaluation of the Antimicrobial Activity of Eucalyptus radiata Essential Oil against Escherichia coli Strains Isolated from Meat Products. Braz. J. Biol. 2024, 84, e281361. [Google Scholar] [CrossRef]
- Harkat-Madouri, L.; Asma, B.; Madani, K.; Bey-Ould Si Said, Z.; Rigou, P.; Grenier, D.; Allalou, H.; Remini, H.; Adjaoud, A.; Boulekbache-Makhlouf, L. Chemical Composition, Antibacterial and Antioxidant Activities of Essential Oil of Eucalyptus Globulus from Algeria. Ind. Crops Prod. 2015, 78, 148–153. [Google Scholar] [CrossRef]
- Palmas, L.; Aroffu, M.; Petretto, G.L.; Escribano-Ferrer, E.; Díez-Sales, O.; Usach, I.; Peris, J.-E.; Marongiu, F.; Ghavam, M.; Fais, S.; et al. Entrapment of Citrus Limon Var. Pompia Essential Oil or Pure Citral in Liposomes Tailored as Mouthwash for the Treatment of Oral Cavity Diseases. Pharmaceuticals 2020, 13, 216. [Google Scholar] [CrossRef]
- Santana Neto, M.C.; Costa, M.L.V.D.A.; Fialho, P.H.D.S.; Lopes, G.L.N.; Figueiredo, K.A.; Pinheiro, I.M.; De Lima, S.G.; Nunes, R.D.S.; Quelemes, P.V.; Carvalho, A.L.M. Development of Chlorhexidine Digluconate and Lippia Sidoides Essential Oil Loaded in Microemulsion for Disinfection of Dental Root Canals: Substantivity Profile and Antimicrobial Activity. AAPS PharmSciTech 2020, 21, 302. [Google Scholar] [CrossRef]
- Horstmann Risso, N.; Stopiglia, C.D.; Oliveira, M.T.; Haas, S.E.; Ramos Maciel, T.; Reginatto Lazzari, N.; Kelmer, E.L.; Pinto Vilela, J.A.; Beckmann, D.V. Chlorhexidine Nanoemulsion: A New Antiseptic Formulation. Int. J. Nanomed. 2020, 15, 6935–6944. [Google Scholar] [CrossRef]
- Karnjana, K.; Jewboonchu, J.; Niyomtham, N.; Tangngamsakul, P.; Bunluepuech, K.; Goodla, L.; Mordmuang, A. The Potency of Herbal Extracts and Its Green Synthesized Nanoparticle Formulation as Antibacterial Agents against Streptococcus mutans Associated Biofilms. Biotechnol. Rep. 2023, 37, e00777. [Google Scholar] [CrossRef] [PubMed]
- Toumba, K.J.; Twetman, S.; Splieth, C.; Parnell, C.; Van Loveren, C.; Lygidakis, N.A. Guidelines on the Use of Fluoride for Caries Prevention in Children: An Updated EAPD Policy Document. Eur. Arch. Paediatr. Dent. 2019, 20, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.B. Prevention and Reversal of Dental Caries: Role of Low Level Fluoride. Comm. Dent. Oral Epid. 1999, 27, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Marquis, R.E. Antimicrobial Actions of Fluoride for Oral Bacteria. Can. J. Microbiol. 1995, 41, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Goldbeck, J.C.; Do Nascimento, J.E.; Jacob, R.G.; Fiorentini, Â.M.; Da Silva, W.P. Bioactivity of Essential Oils from Eucalyptus globulus and Eucalyptus urograndis against Planktonic Cells and Biofilms of Streptococcus mutans. Ind. Crops Prod. 2014, 60, 304–309. [Google Scholar] [CrossRef]
- Salem, N.; Kefi, S.; Tabben, O.; Ayed, A.; Jallouli, S.; Feres, N.; Hammami, M.; Khammassi, S.; Hrigua, I.; Nefisi, S.; et al. Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crops Prod 2018, 124, 115–125. [Google Scholar] [CrossRef]
- Choi, J.E.; Waddell, J.N.; Lyons, K.M.; Kieser, J.A. Intraoral pH and Temperature during Sleep with and without Mouth Breathing. J. Oral. Rehabil. 2016, 43, 356–363. [Google Scholar] [CrossRef]

| Compound | RIC | RIL | Eucalyptus globulus | Eucalyptus citriodora |
|---|---|---|---|---|
| Composition (%) | ||||
| α-pinene | 934 | 933 | 1.69 | - |
| β-pinene | 979 | 978 | 0.43 | - |
| β-myrcene | 990 | 989 | 0.50 | - |
| p-cymene | 1026 | 1025 | 3.79 | - |
| Limonene | 1030 | 1028 | 5.46 | - |
| 1,8-cineol | 1034 | 1033 | 87.21 | - |
| γ-terpinene | 1060 | 1058 | 0.92 | - |
| Isopulegol | 1151 | 1150 | - | 4.90 |
| Citronelal | 1154 | 1153 | - | 90.26 |
| Citronelol | 1228 | 1228 | - | 4.84 |
| Total identified | - | - | 100 | 100 |
| Sample | Mean Size (nm) | PDI | Span | Zeta Potential (mV) | pH |
|---|---|---|---|---|---|
| NanoEE-Globulus | 117.2 ± 0.9 b | 0.214 ± 0.03 a | 2.17± 0.30 | −19.2 ± 0.5 a | 6.6 ± 0.17 a |
| NanoEE-Citriodora | 168.8 ± 1.2 a | 0.209 ± 0.001 a | 1.77 ± 0.21 | −33.8 ± 1.2 b | 6.8 ± 0.02 a |
| Bactericidal | Bacteriostatic | |
|---|---|---|
| NanoEE-Globulus | ||
| 10% | - | - |
| 8% | - | - |
| 6% | - | - |
| 4% | - | × |
| NanoEE-Citriodora | ||
| 10% | - | × |
| 8% | - | × |
| 6% | - | × |
| 4% | - | - |
| Chlorhexidine | ||
| 0.12% | × | × |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, D.G.; Sganzerla, W.G.; da Silva, L.R.; Vieira, Y.G.S.; Almeida, A.R.; Dominguini, D.; Ceretta, L.; Pinheiro, A.C.; Bertoldi, F.C.; Becker, D.; et al. Antimicrobial and Cytotoxic Potential of Eucalyptus Essential Oil-Based Nanoemulsions for Mouthwashes Application. Antibiotics 2024, 13, 942. https://doi.org/10.3390/antibiotics13100942
Batista DG, Sganzerla WG, da Silva LR, Vieira YGS, Almeida AR, Dominguini D, Ceretta L, Pinheiro AC, Bertoldi FC, Becker D, et al. Antimicrobial and Cytotoxic Potential of Eucalyptus Essential Oil-Based Nanoemulsions for Mouthwashes Application. Antibiotics. 2024; 13(10):942. https://doi.org/10.3390/antibiotics13100942
Chicago/Turabian StyleBatista, Dione Glauco, William Gustavo Sganzerla, Lysa Ribeiro da Silva, Yasmin Gabriele Schmitt Vieira, Aline R. Almeida, Diogo Dominguini, Luciane Ceretta, Adriana Castro Pinheiro, Fabiano Cleber Bertoldi, Daniela Becker, and et al. 2024. "Antimicrobial and Cytotoxic Potential of Eucalyptus Essential Oil-Based Nanoemulsions for Mouthwashes Application" Antibiotics 13, no. 10: 942. https://doi.org/10.3390/antibiotics13100942
APA StyleBatista, D. G., Sganzerla, W. G., da Silva, L. R., Vieira, Y. G. S., Almeida, A. R., Dominguini, D., Ceretta, L., Pinheiro, A. C., Bertoldi, F. C., Becker, D., Hotza, D., Nunes, M. R., da Rosa, C. G., & Masiero, A. V. (2024). Antimicrobial and Cytotoxic Potential of Eucalyptus Essential Oil-Based Nanoemulsions for Mouthwashes Application. Antibiotics, 13(10), 942. https://doi.org/10.3390/antibiotics13100942









