Development of Novel Biocomposites with Antimicrobial-Activity-Based Magnesium-Doped Hydroxyapatite with Amoxicillin
Abstract
:1. Introduction
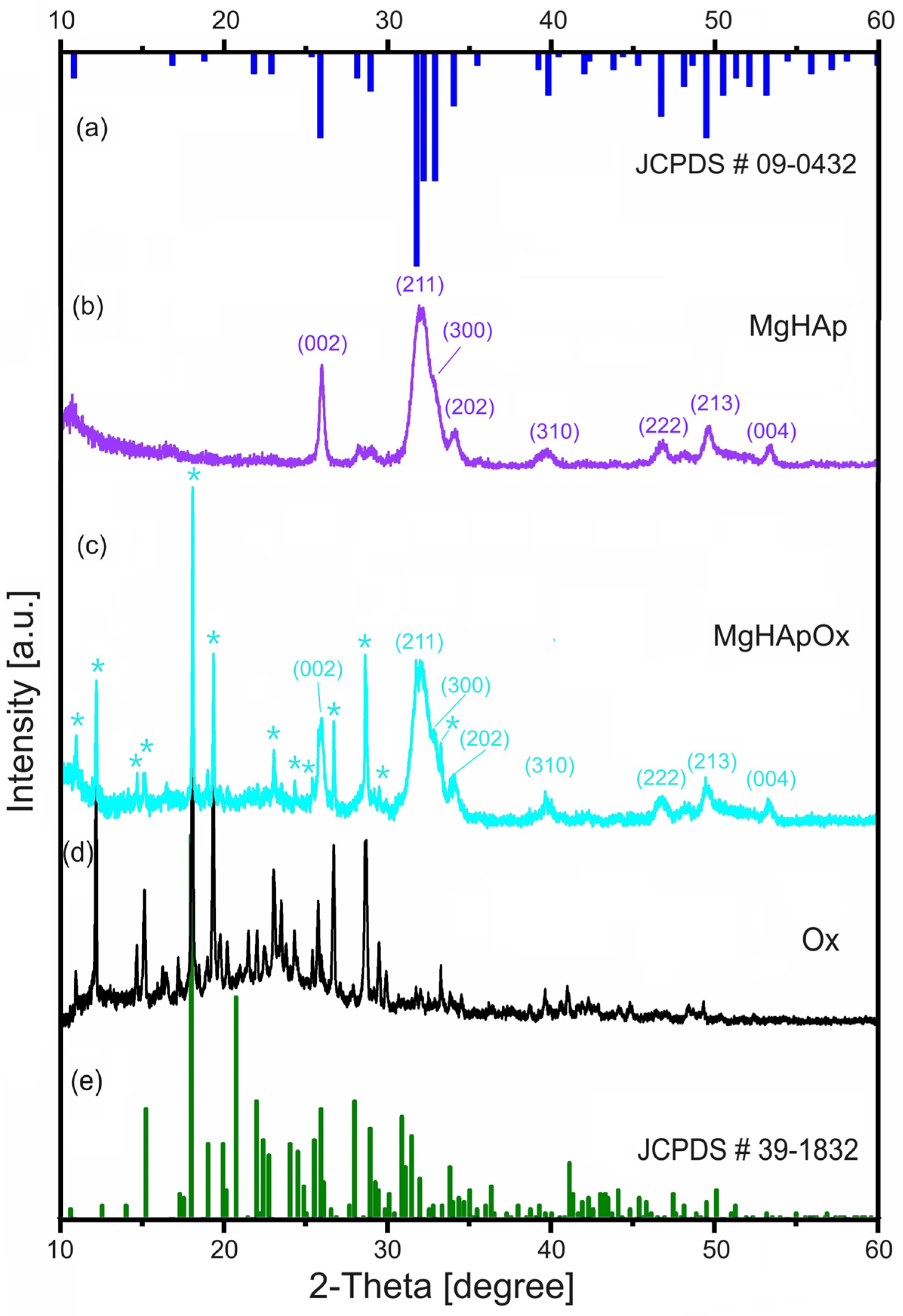
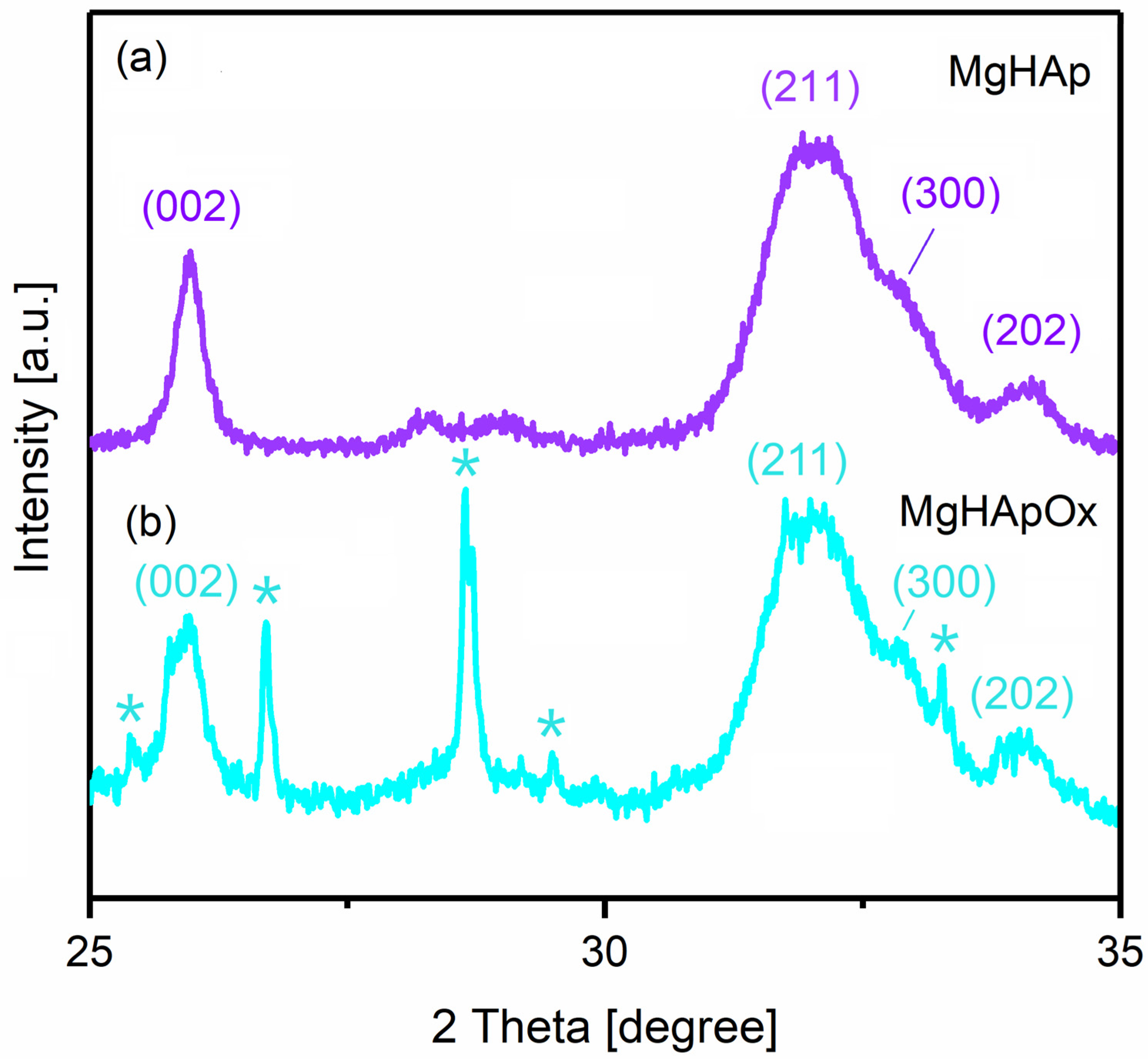
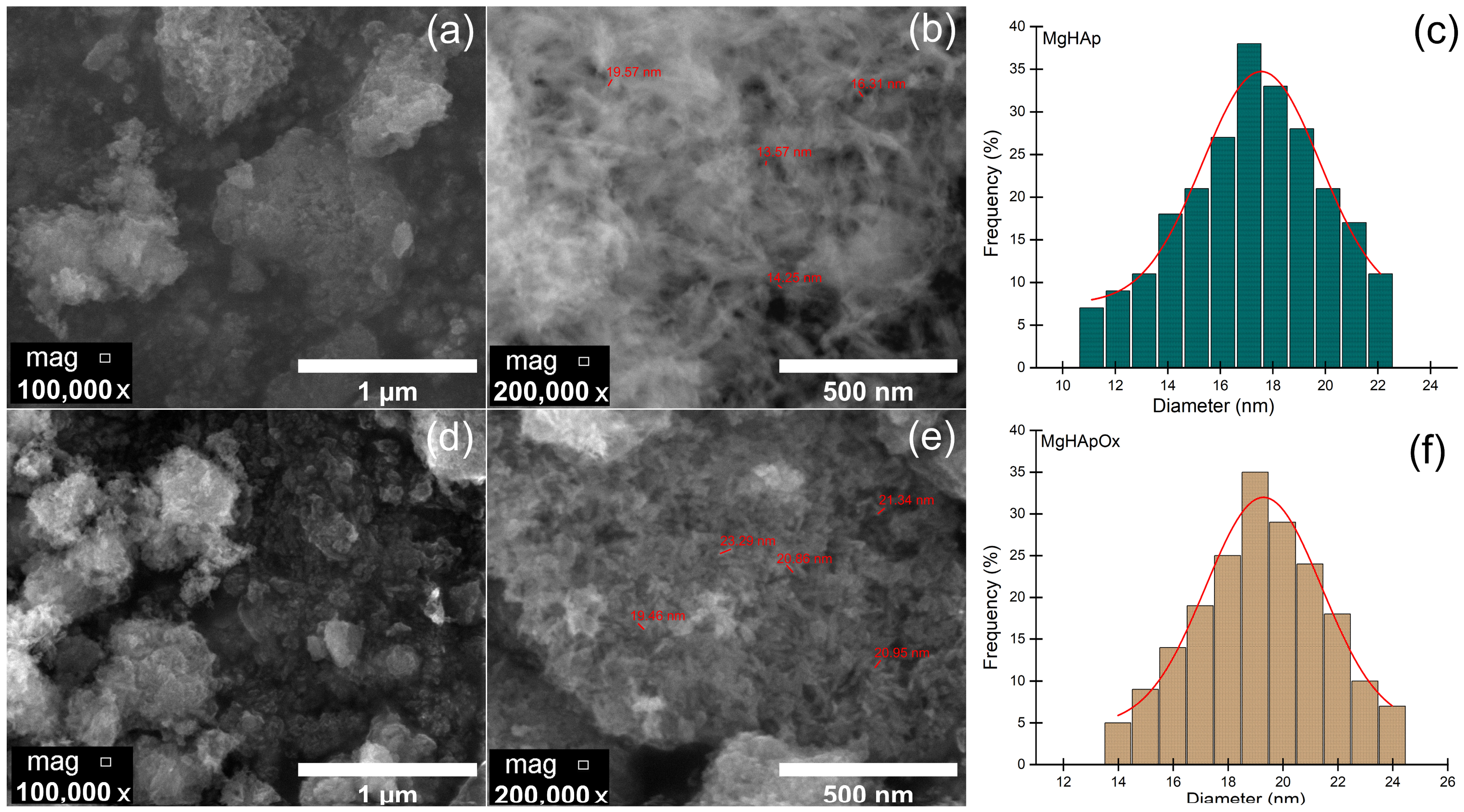
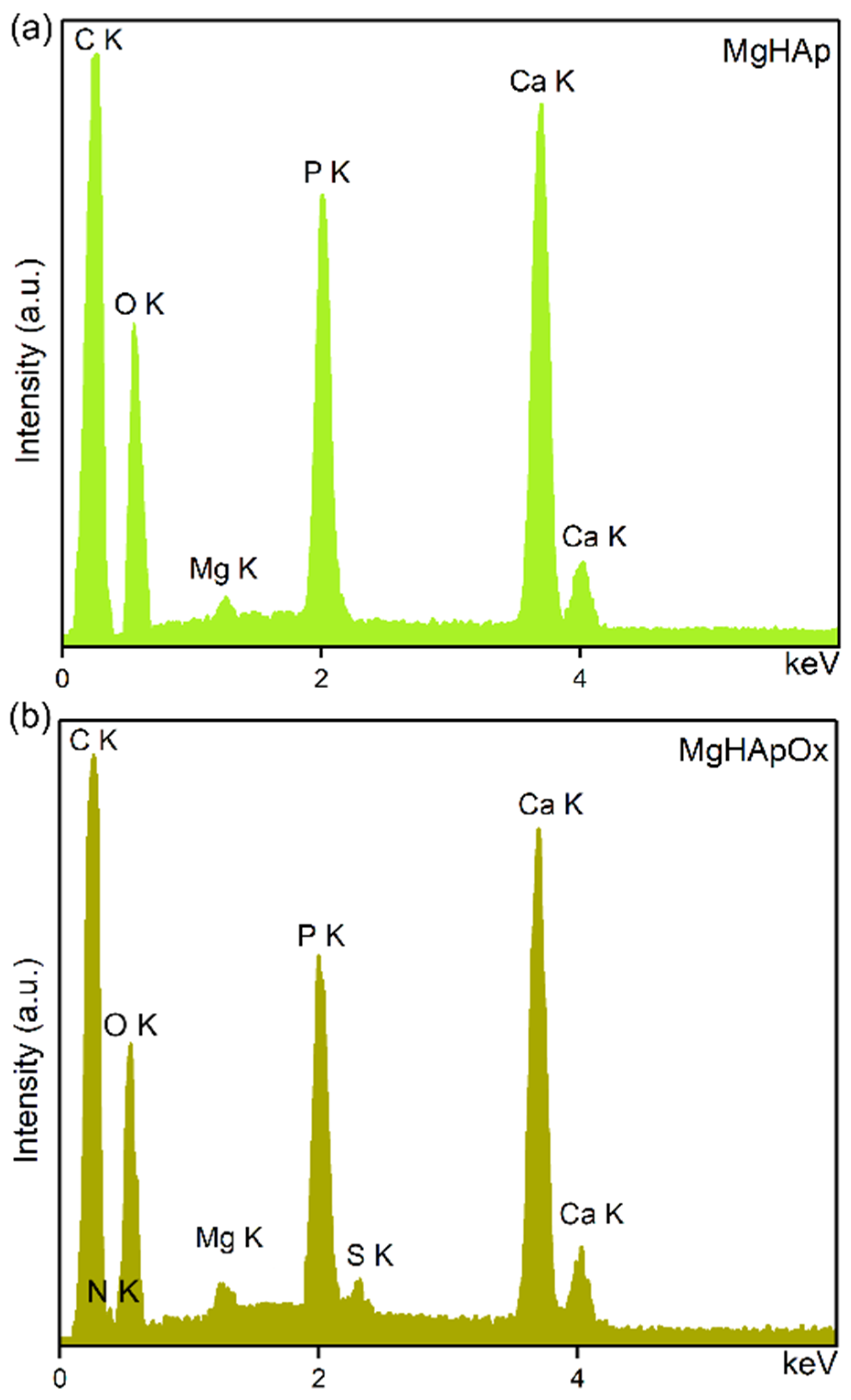
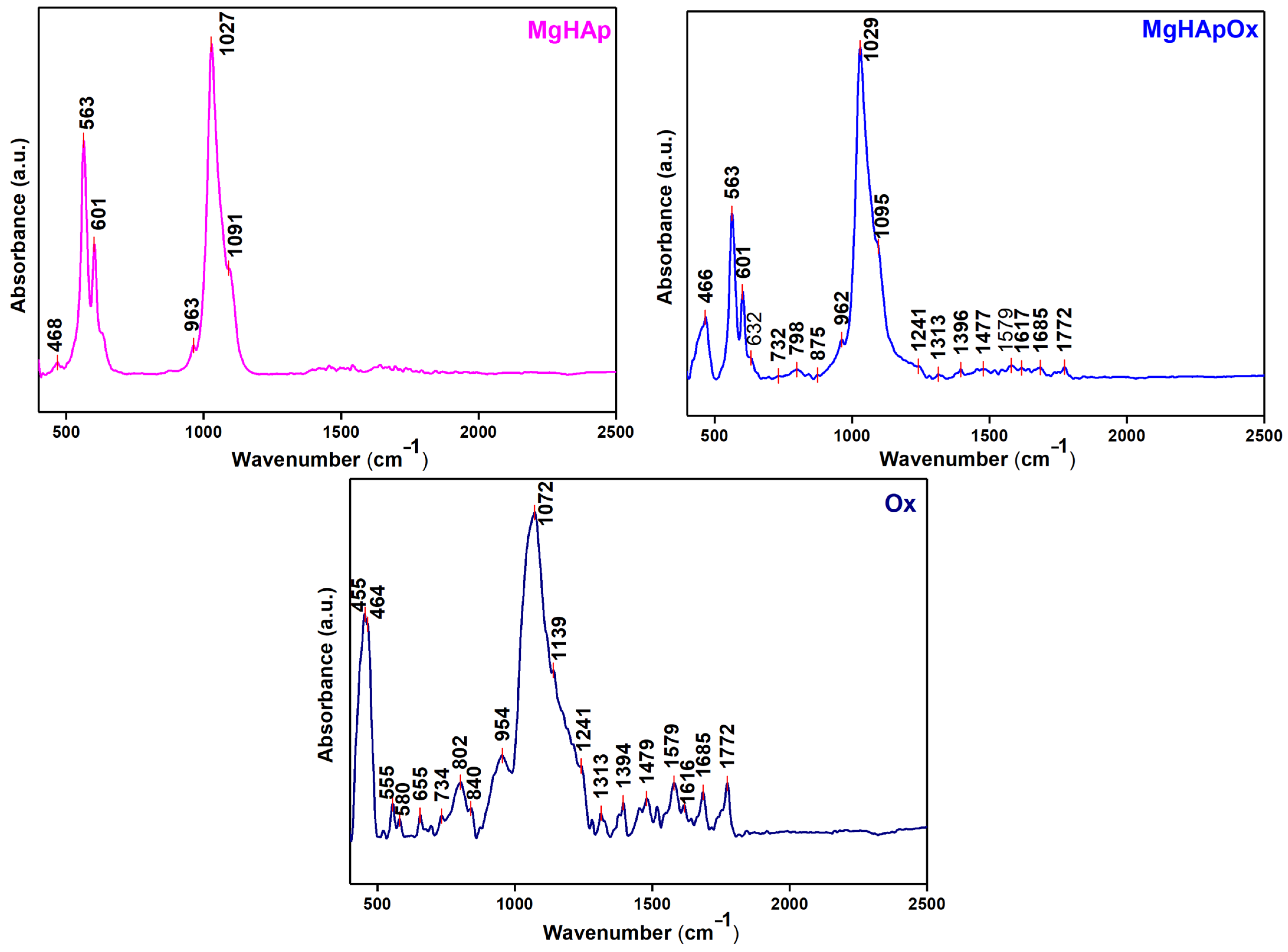
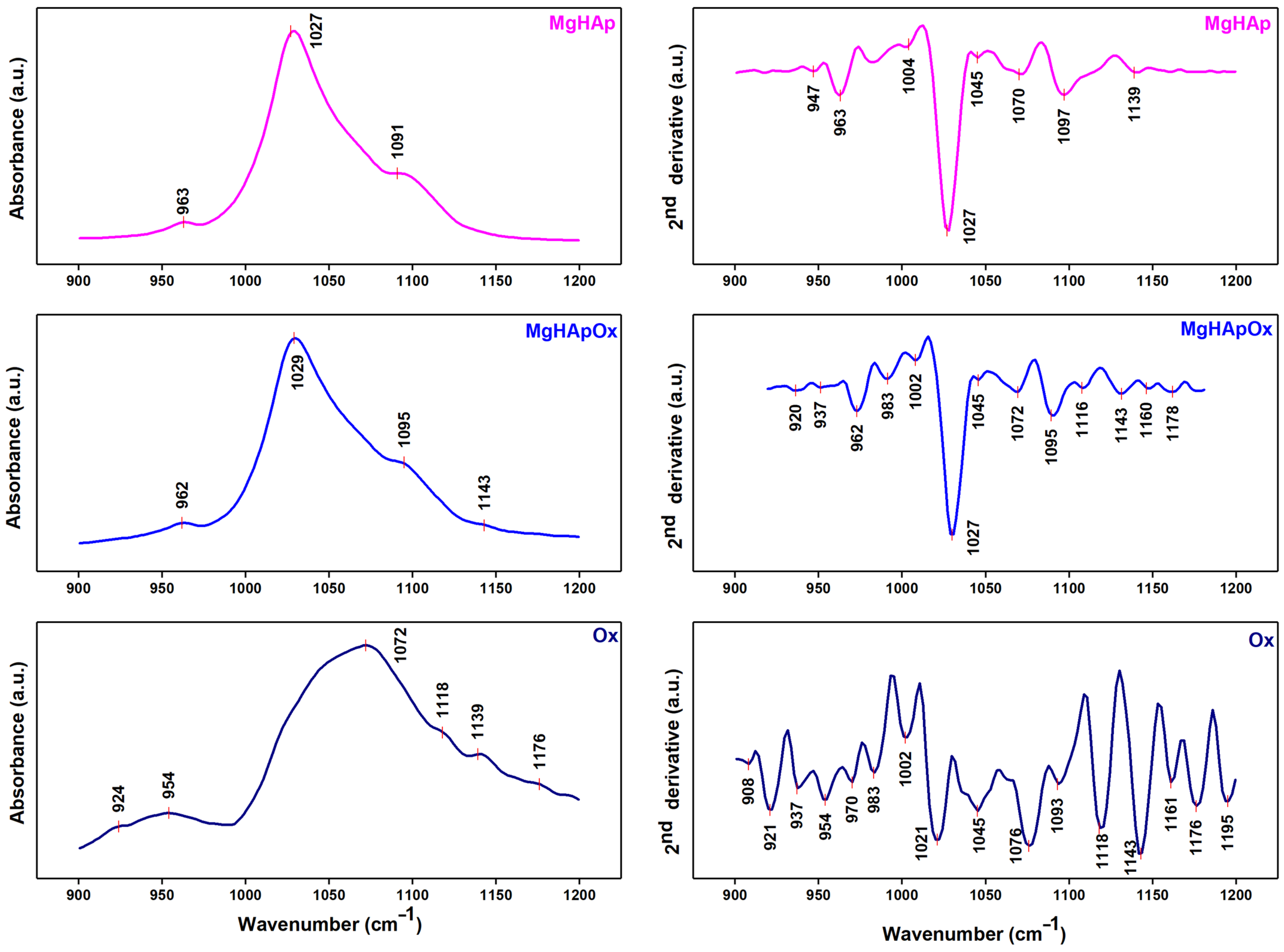
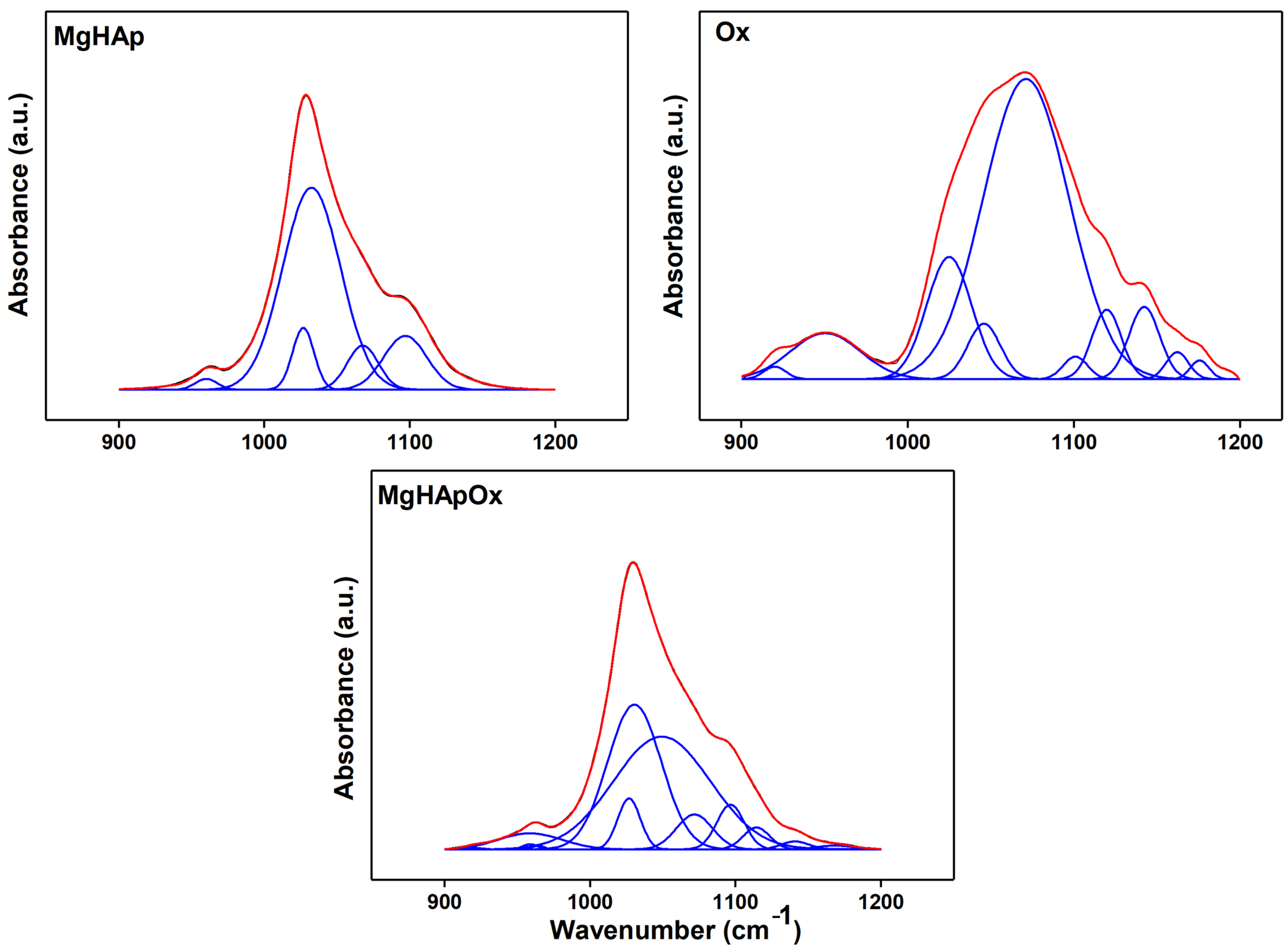
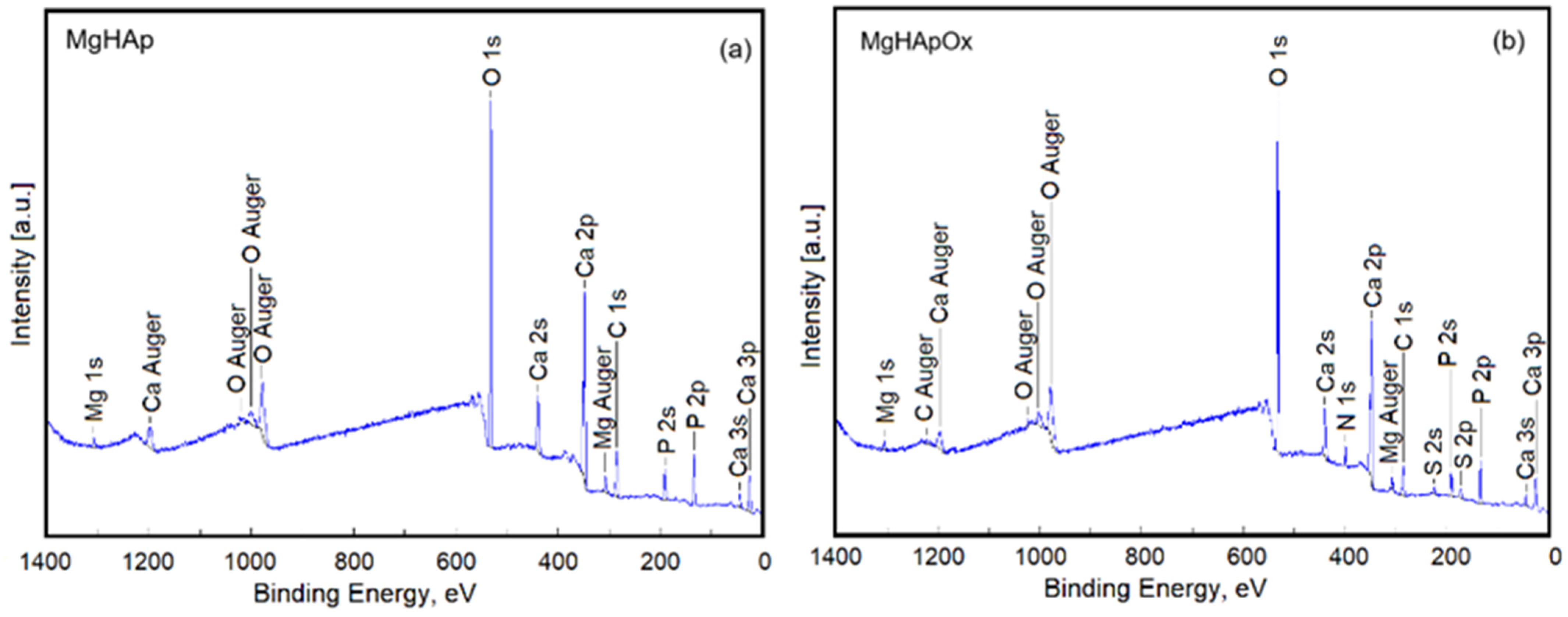
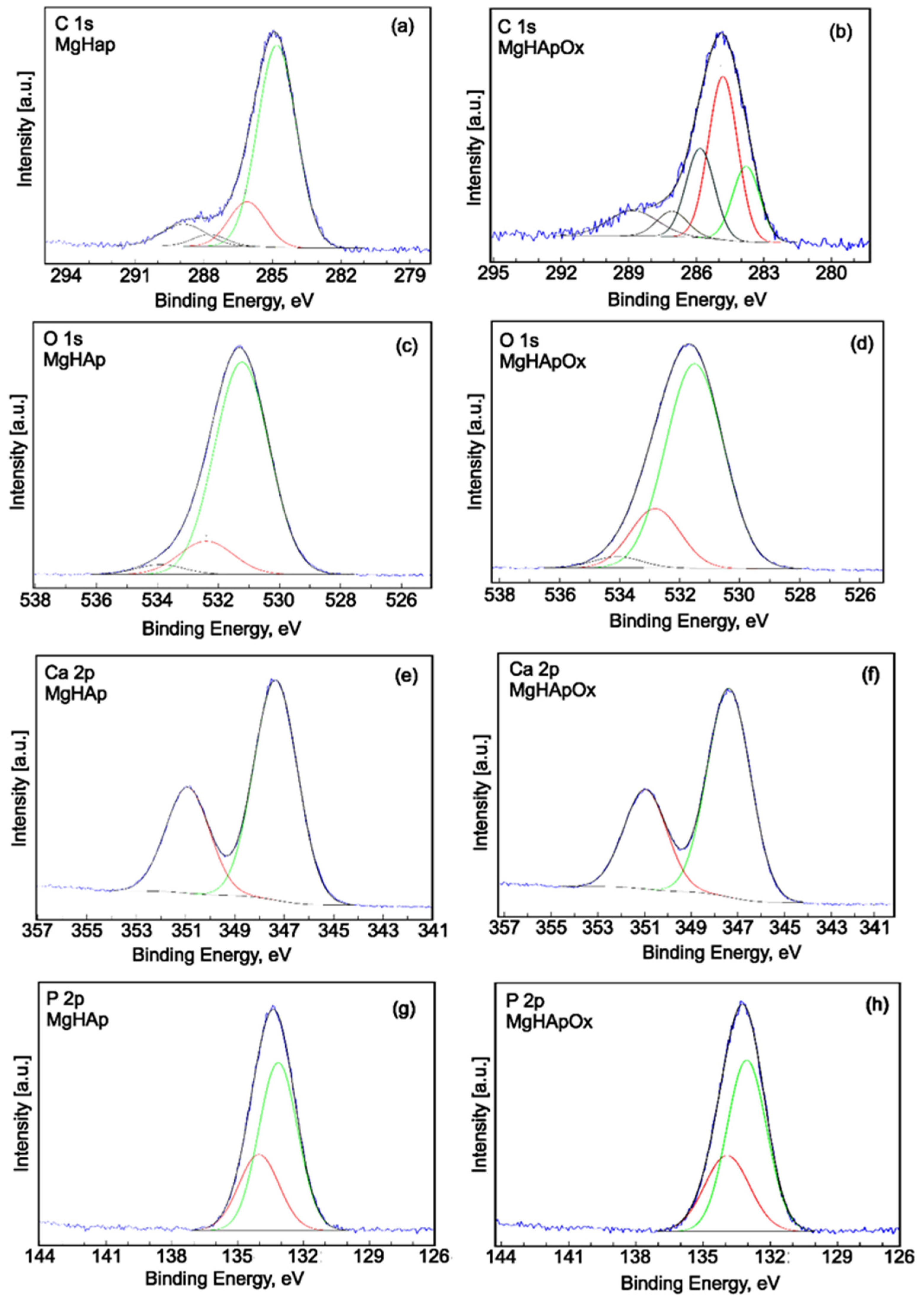
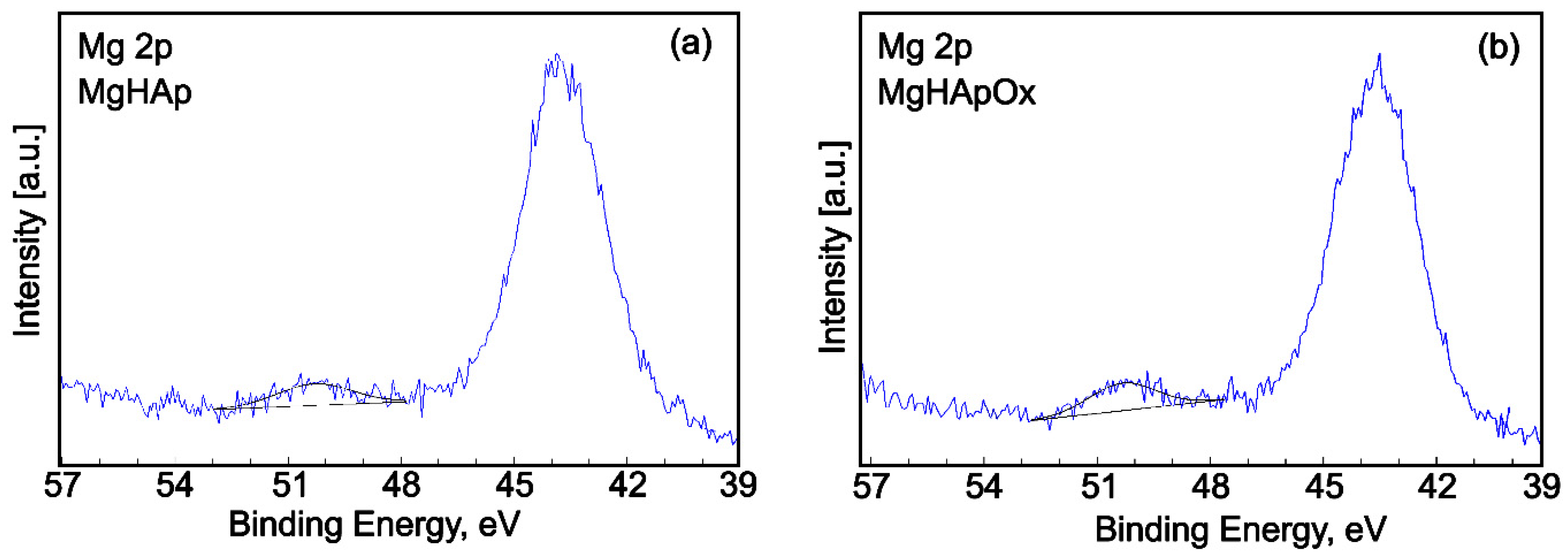
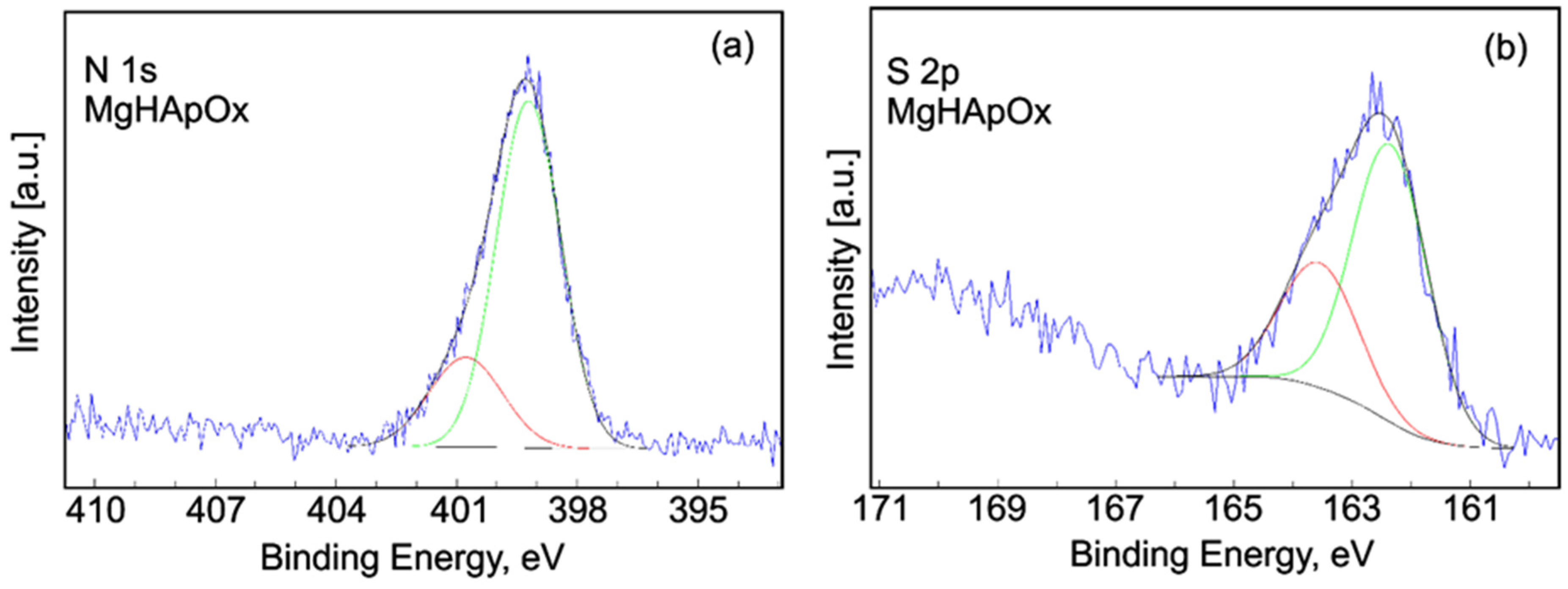
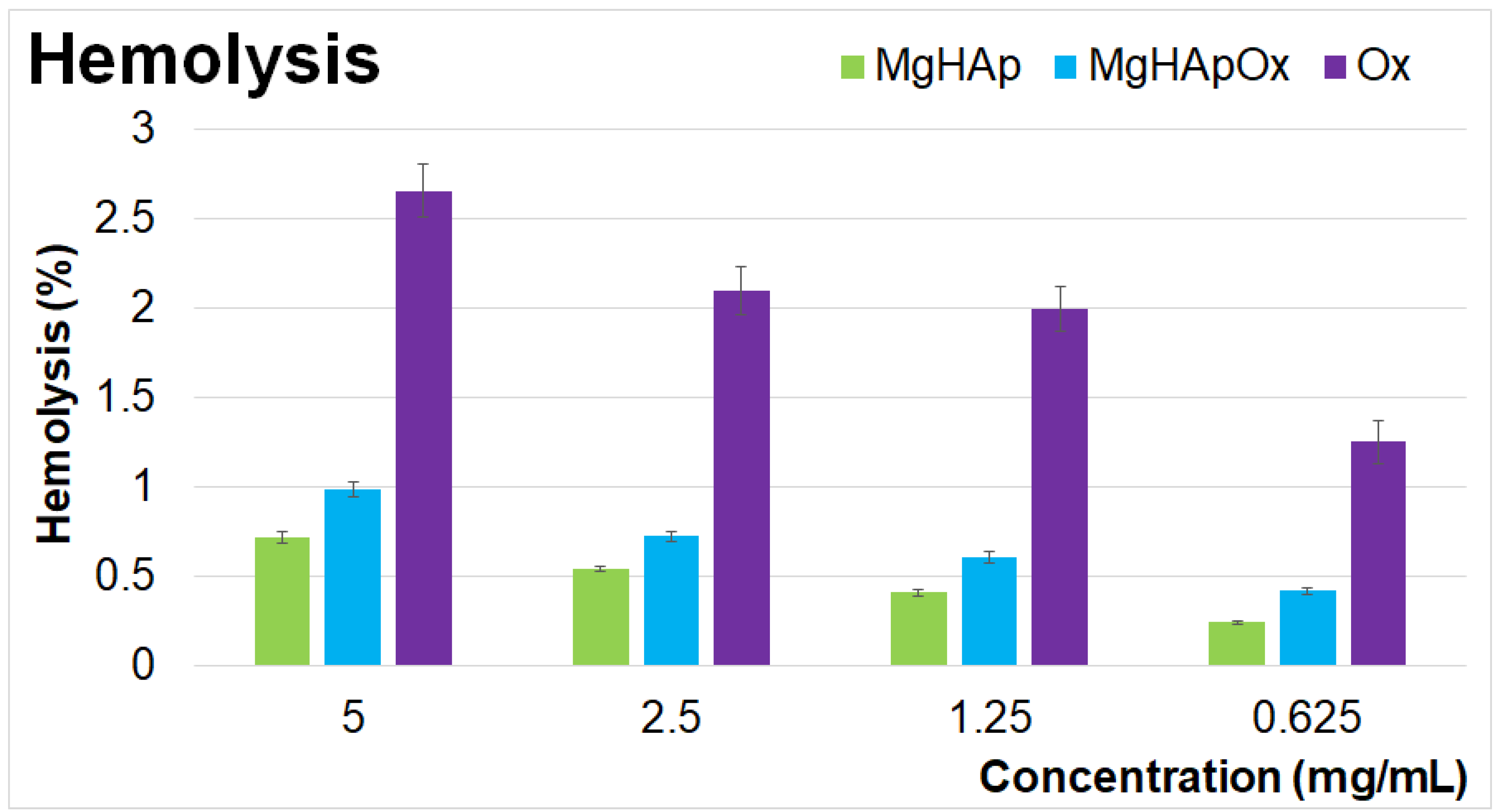
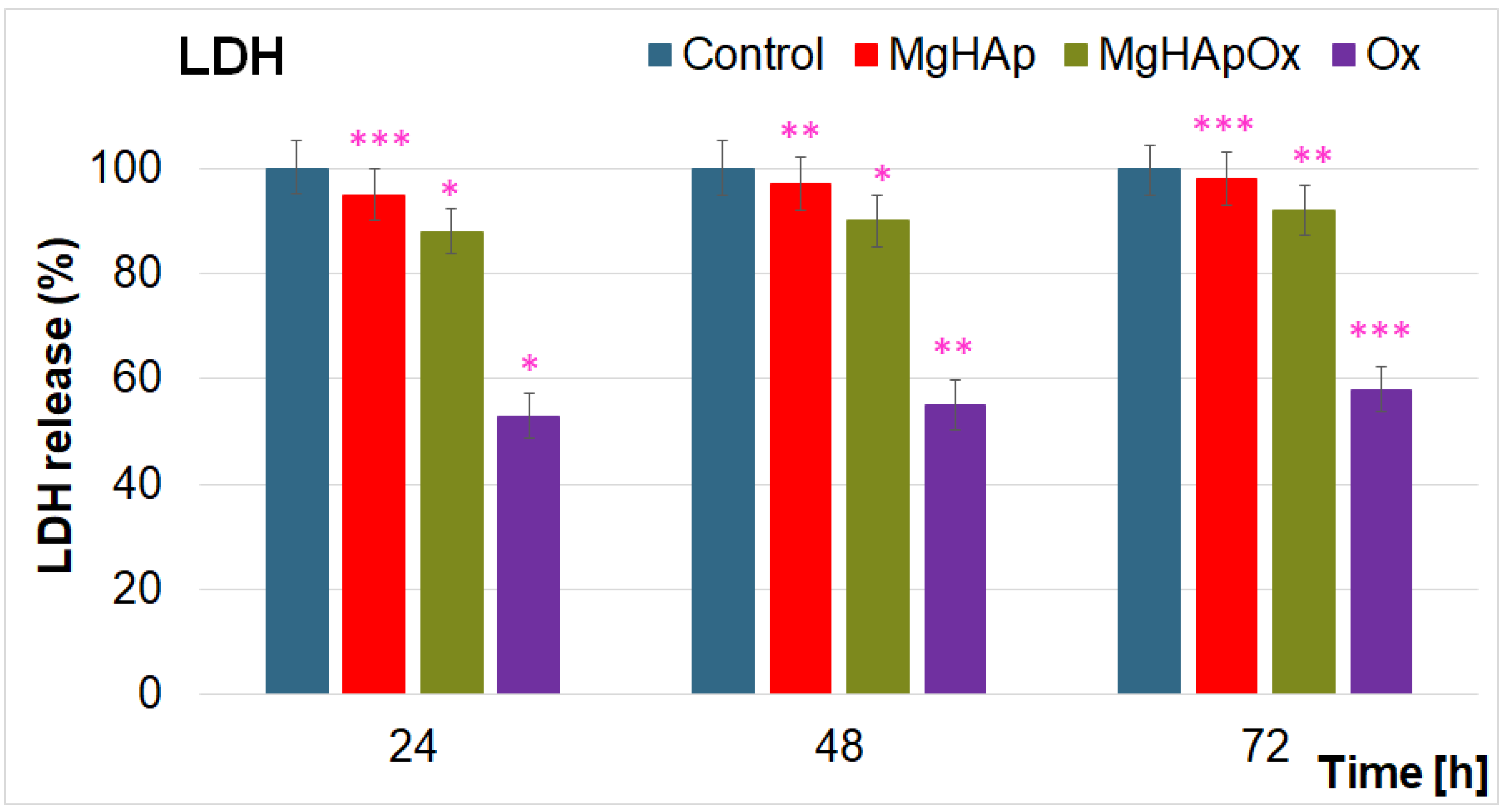
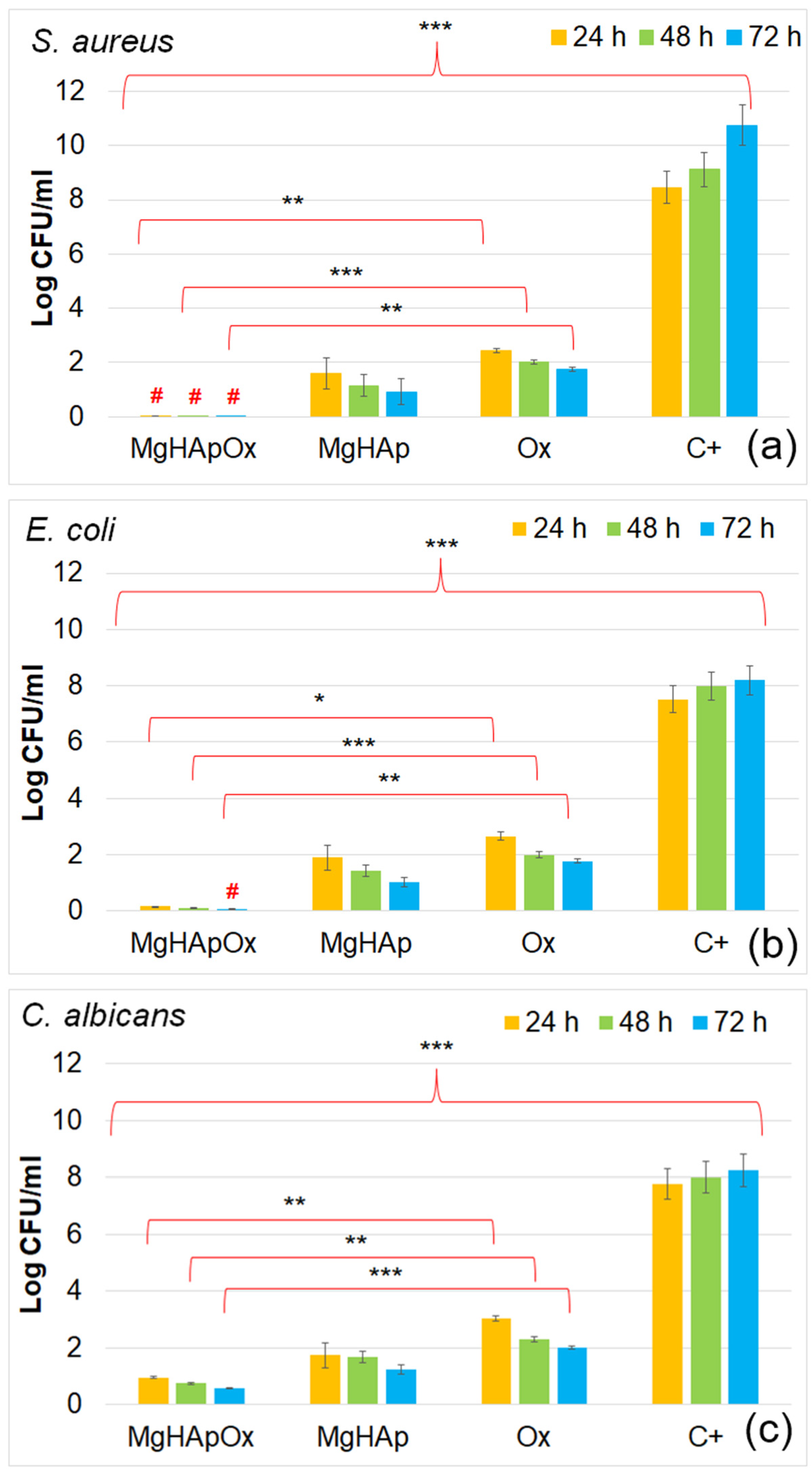
2. Results and Discussions
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Magnesium-Doped Hydroxyapatite Enriched with Amoxicillin Suspensions
3.3. Phisycochemical Characterization of Samples
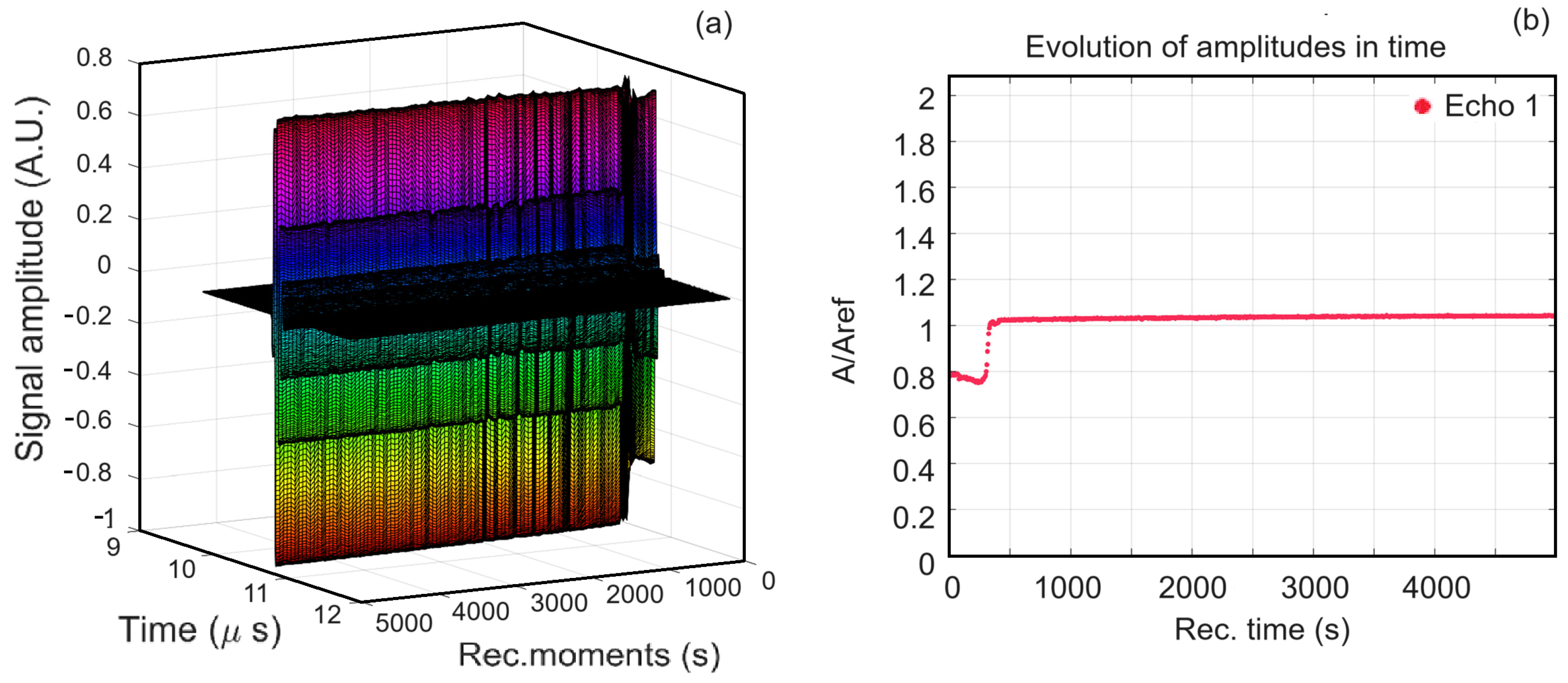
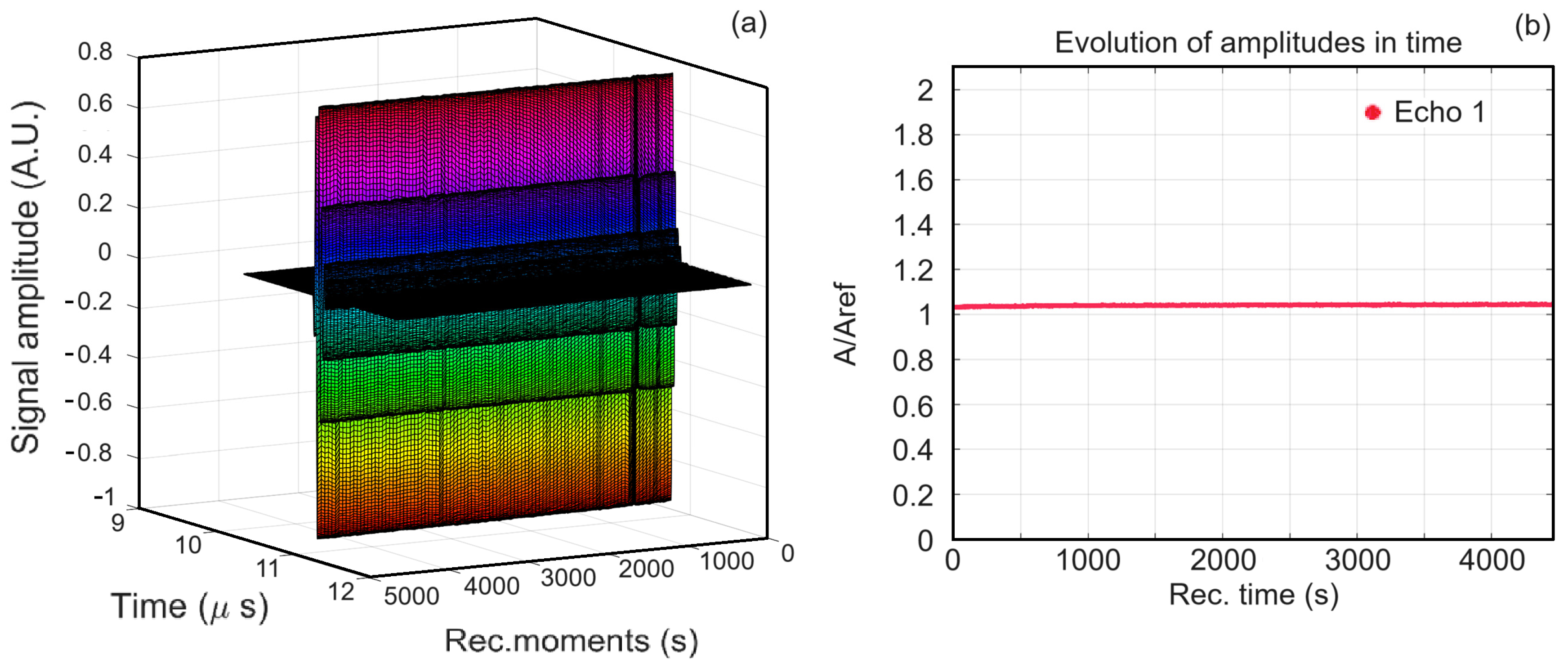
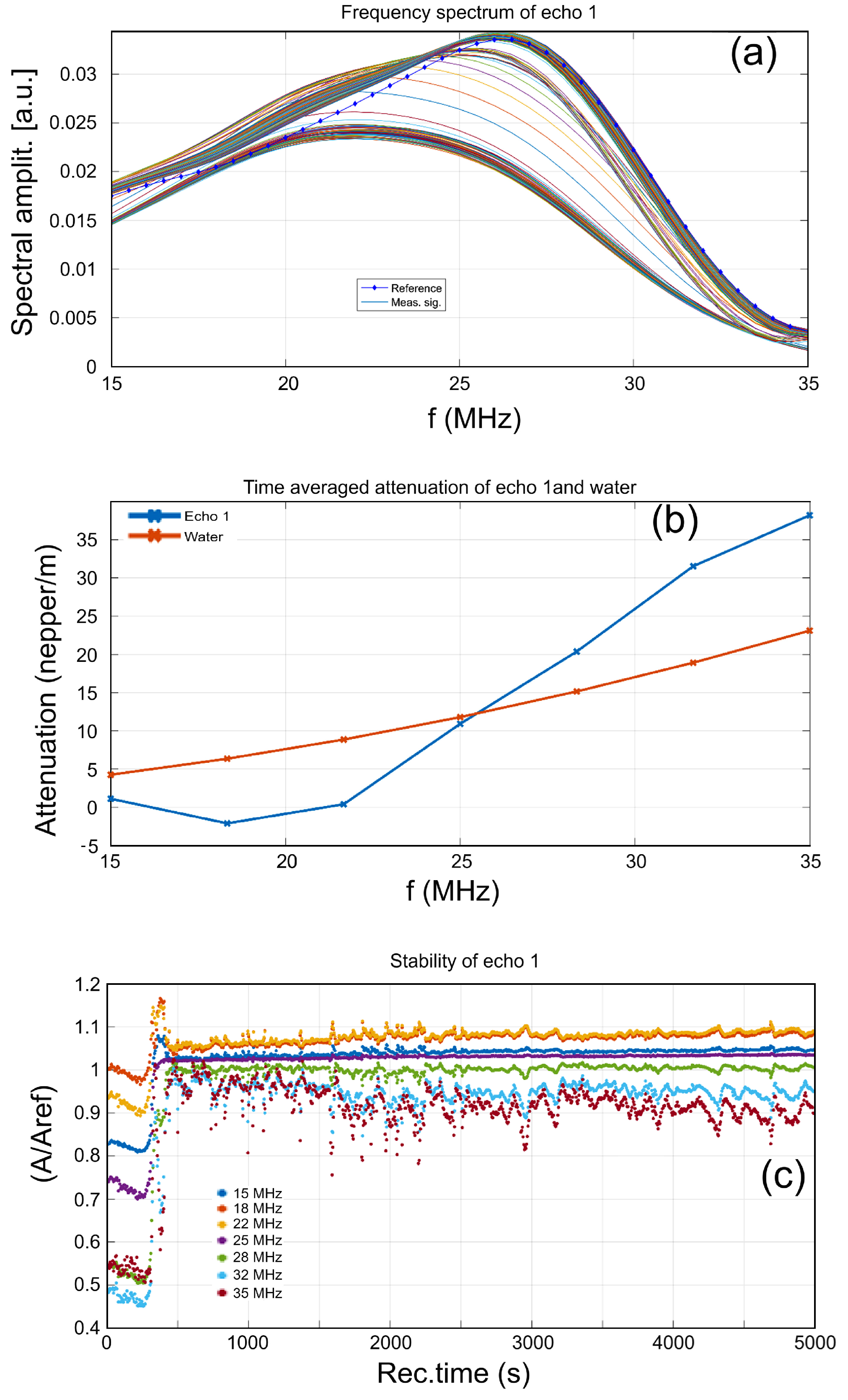
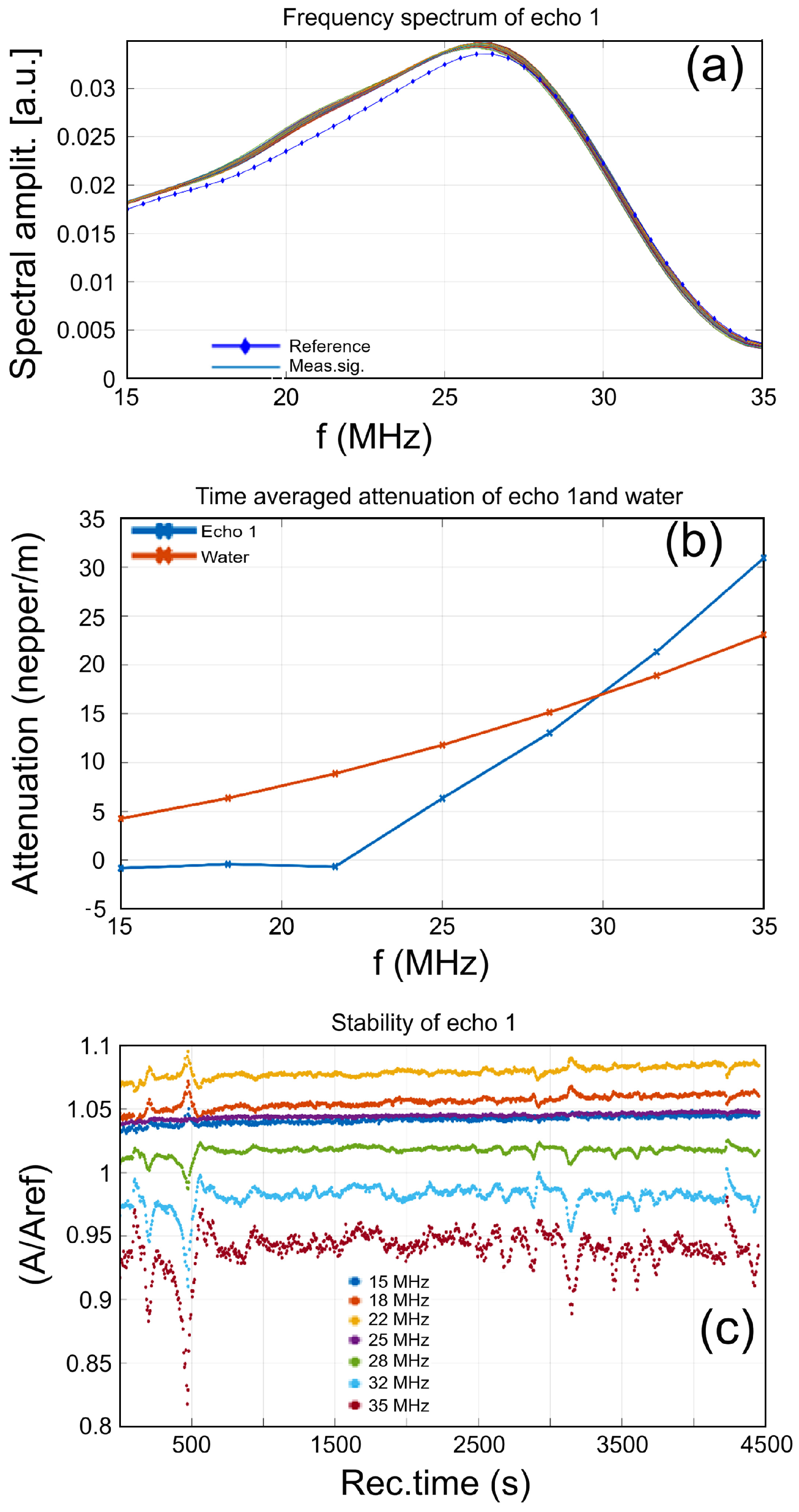
3.3.1. Ultrasound Studies
3.3.2. Zeta (ζ)-Potential Measurements
3.3.3. X-ray Diffraction
3.3.4. Scanning Electron Microscopy
3.3.5. Fourier Transform Infrared Spectroscopy
3.3.6. X-ray Photoelectron Spectroscopy
3.4. In Vitro Biological Assays
3.4.1. Hemolysis Assay
3.4.2. Colorimetric Test Assay 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
3.4.3. Lactate Dehydrogenase (LDH) Release Measurement
3.4.4. In Vitro Antimicrobial Assay
3.4.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehrabani, M.G.; Karimian, R.; Mehramouz, B.; Rahimi, M.; Kafil, H.S. Preparation of biocompatible and biodegradable silk fibroin/chitin/silver nanoparticles 3D scaffolds as a bandage for antimicrobial wound dressing. Int. J. Biol. Macromol. 2018, 114, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Leong, K. Scaffolding in tissue engineering: General approaches and tissue specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.M.; Zhang, C.; Chou, L. Effect of Magnesium on Dentinogenesis of Human Dental Pulp Cells. Int. J. Biomater. 2021, 2021, 6567455. [Google Scholar] [CrossRef] [PubMed]
- Cross, L.M.; Thakur, A.; Jalili, N.A.; Detamore, M.; Gaharwar, A.K. Nanoengineered biomaterials for repair and regeneration of orthopedic tissue interfaces. Acta Biomater. 2016, 42, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.; Kamaraj, Y.; Kumaresan, V.; Kannaiyan, M.; David, E.; Ranganathan, B.; Selvaraj, V.; Balupillai, A. Green synthesized zinc oxide nanoparticles induce apoptosis by suppressing PI3K/Akt/mTOR signaling pathway in osteosarcoma MG63 cells. Glob. Transl. Med. 2022, 1, 34. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Siddiqui, N.; Madala, S.; Parcha, S.R.; Mallick, S.P. Osteogenic differentiation ability of human mesenchymal stem cells on Chitosan/Poly(Caprolactone)/nano beta Tricalcium Phosphate composite scaffolds. Biomed. Phys. Eng. Express 2020, 6, 015018. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Yahia, I.S. Novel and facile microwave-assisted synthesis of Mo-doped hydroxyapatite nanorods: Characterization, gamma absorption coefficient, and bioactivity. Mater. Sci. Eng. C 2017, 78, 1093–1100. [Google Scholar] [CrossRef]
- Demurtas, M.; Perry, C.C. Facile one-pot synthesis of amoxicillin-coated gold nanoparticles and their antimicrobial activity. Gold Bull 2014, 47, 103–107. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Orlov, A.V.; Znoyko, S.L.; Bragina, V.A.; Gorshkov, B.G.; Ksenevich, T.I.; Cherkasov, V.R.; Nikitin, P.I. Multiplex biosensing with highly sensitive magnetic nanoparticle quantification method. J. Magn. Magn. Mater 2018, 459, 260–264. [Google Scholar] [CrossRef]
- Li, Z.; Chu, D.; Gao, Y.; Jin, L.; Zhang, X.; Cui, W.; Li, J. Biomimicry, biomineralization, and bioregeneration of bone using advanced three-dimensional fi-brous hydroxyapatite scaffold. Mater. Today Adv. 2019, 3, 100014. [Google Scholar] [CrossRef]
- Ozawa, T.; Mickle, D.A.; Weisel, R.D.; Koyama, N.; Ozawa, S.; Li, R.-K. Optimal biomaterial for creation of autologous cardiac grafts. Circulation 2002, 106, I-176–I-182. [Google Scholar] [CrossRef]
- Zafar, M.S.; Alnazzawi, A.A.; Alrahabi, M.; Fareed, M.A.; Najeeb, S.; Khurshid, Z. Nanotechnology and nanomaterials in dentistry. In Advanced Dental Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 477–505. [Google Scholar] [CrossRef]
- Nesseri, E.; Boyatzis, S.C.; Boukos, N.; Panagiaris, G. Optimizing the biomimetic synthesis of hydroxyapatite for the consolidation of bone using diammonium phosphate, simulated body fluid, and gelatin. SN Appl. Sci. 2020, 2, 1892. [Google Scholar] [CrossRef]
- Morris, H.F.; Ochi, S. Hydroxyapatite-coated implants: A case for their use. J. Oral Maxillofac. Surg. 1998, 56, 1303–1311. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, M.V. Influence of the Biological Medium on the Properties of Magnesium Doped Hydroxyapatite Composite Coatings. Coatings 2023, 13, 409. [Google Scholar] [CrossRef]
- Luo, J.; Mamat, B.; Yue, Z.; Zhang, N.; Xu, X.; Li, Y.; Su, Z.; Ma, C.; Zang, F.; Wang, Y. Multi-metal ions doped hydroxyapatite coatings via electrochemical methods for antibacterial and osteogenesis. Colloids Interface Sci. Commun. 2021, 43, 100435. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Fernández-Calderón, M.C.; Pacha-Olivenza, M.A.; Perez-Giraldo, C.; Gallardo-Moreno, A.M.; González-Martín, M.L. The role of magnesium in biomaterials related infections. Colloids Surf. B 2020, 191, 110996. [Google Scholar] [CrossRef]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a detailed understanding of magnesium ions on hydroxyapatite crystallization inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, Q.; Peel, S.; Wang, X.; He, F. Effects of magnesium-substituted nanohydroxyapatite coating on implant osseointegration. Clin. Oral Implant. Res. 2013, 24, 34–41. [Google Scholar] [CrossRef]
- Li, F.-Y.; Chaigne-Delalande, B.; Kanellopoulou, C. Second messenger role for Mg2+ revealed by human T cell immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef]
- Jenifer, A.; Senthilarasan, K.; Arumugam, S.; Sivaprakash, P.; Sagadevan, S.; Sakthivel, P. Investigation on antibacterial and hemolytic properties of magnesium-doped hydroxyapatite nanocomposite. Chem. Phys. Lett. 2021, 771, 138539. [Google Scholar] [CrossRef]
- Khan, F.; Bai, Z.; Kelly, S.; Skidmore, B.; Dickson, C.; Nunn, A.; Rutledge-Taylor, K.; Wells, G. Effectiveness and Safety of Antibiotic Prophylaxis for Persons Exposed to Cases of Invasive Group A Streptococcal Disease: A Systematic Review. Open Forum Infect. Dis. 2022, 9, ofac244. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, U.; Al-Moraissi, E.; Alabed, S. Systemic antibiotic prophylaxis for preventing infectious complications in maxillofacial trauma surgery. Cochrane Database Syst Rev. 2017, 2017, CD012603. [Google Scholar] [CrossRef]
- Kaur, S.; Rao, R.; Nanda, S. Amoxicillin: A Broad Spectrum Antibiotic. Int. J. Pharm. Sci. 2011, 3, 3. [Google Scholar]
- Greenwood, R.; Kendall, K. Selection of suitable dispersants for aqueous suspensions of zirconia and titania powders using acoustophoresis. J. Eur. Ceram. Soc. 1999, 19, 479–488. [Google Scholar] [CrossRef]
- O’Brien, R.W.; Midmore, B.R.; Lamb, A.; Hunter, R.J. Electroacoustic studies of moderately concentrated colloidal suspensions. Faraday Discuss. Chem. Soc. 1990, 90, 301–312. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Fairhurst, D. An Overview of the Zeta Potential Part 3: Uses and Applications, American Pharmaceutical Review. 2013. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/139288-An-Overview-of-the-Zeta-Potential-Part-3-Uses-and-Applications/ (accessed on 3 July 2024).
- Freitas, C.; Müller, R.H. Effect of Light and Temperature on Zeta Potential and Physical Stability in Solid Lipid Nanoparticle (SLN®) Dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, S.A.; Ţălu, Ş. Hydroxyapatite: Innovations in Synthesis, Properties, Nanocomposites, Biomedical Applications, and Technological Developments; Napoca Star Publishing House: Cluj-Napoca, Romania, 2024; ISBN 978-606-062-901-6. [Google Scholar]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Ţălu, Ş.; Predoi, S.A.; Buton, N.; Ramos, G.Q.; da Fonseca Filho, H.D.; Matos, R.S. Synthesis, characterization, and antifungal properties of chrome-doped hydroxyapatite thin films. Mater. Chem. Phys. 2024, 324, 129690. [Google Scholar] [CrossRef]
- Ciobanu, S.C.; Iconaru, S.L.; Predoi, M.V.; Ghegoiu, L.; Badea, M.L.; Predoi, D.; Jiga, G. Physico-Chemical and Antimicrobial Features of Magnesium Doped Hydroxyapatite Nanoparticles in Polymer Matrix. Macromol. Symp. 2024, 413, 2400022. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Suganthi, K.S.; Rajan, K.S. Temperature induced changes in ZnO–water nanofluid: Zeta potential, size distribution and viscosity profiles. Int. J. Heat Mass Transf. 2012, 55, 7969. [Google Scholar] [CrossRef]
- Mirzaee, M.; Vaezi, M.; Palizdar, Y. Synthesis and characterization of silver doped hydroxyapatite nanocomposite coatings and evaluation of their antibacterial and corrosion resistance properties in simulated body fluid. Mater. Sci. Eng. C 2016, 69, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.H.; Lee, E.J.; Yoon, B.H.; Shin, D.S.; Kim, H.E.; Oh, J.S. Chitosan/nanohydroxyapatite composite membranes via dynamic filtration for guided bone regeneration. J. Biomed. Mater. Res. 2009, 88, 569–580. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Motelica-Heino, M.; Predoi, D. Study on europium-doped hydroxyapatite nanoparticles by Fourier transform infrared spectroscopy and their antimicrobial properties. J. Spectrosc. 2013, 2013, 284285. [Google Scholar] [CrossRef]
- Palanikumar, L.; Ramasamy, S.; Hariharan, G.; Balachandran, C. Influence of particle size of nano zinc oxide on the controlled delivery of Amoxicillin. Appl. Nanosci. 2013, 3, 441–451. [Google Scholar] [CrossRef]
- Prasanna, A.P.S.; Venkatasubbu, G.D. Sustained release of amoxicillin from hydroxyapatite nanocomposite for bone infections. Prog. Biomater. 2018, 7, 289–296. [Google Scholar] [CrossRef]
- Lemnaru, G.-M.; Truşcă, R.D.; Ilie, C.-I.; Țiplea, R.E.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Dițu, L.-M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069. [Google Scholar] [CrossRef]
- Paul, B.; Adimoolam, S.; Qureshi, M.J. Development and Evaluation of Amoxicillin Loaded Carbopol 934P Mucoadhesive Microcapsules for Sustained Drug Release for H. pylori Treatment: Amoxicillin mucoadhesive microcapsules. Iran. J. Pharm. Res. 2019, 15, 61–80. [Google Scholar] [CrossRef]
- Bisson-Boutelliez, C.; Fontanay, S.; Finance, C.; Kedzierewicz, F. Preparation and physicochemical characterization of amoxicillin β-cyclodextrin complexes. Aaps. Pharmscitech. 2010, 11, 574–581. [Google Scholar] [CrossRef]
- Teoh Si Min, N.; Khan, N.H. Comparative Purity Study by UV Spectrophotometric and Fourier-Transform Infrared Spectroscopic (FTIR) Techniques for the Simultaneous Determination of Amoxicillin Tri-hydrate Capsules. Biomed. J. Sci. Tech. Res 2020, 31, 24219–24235. [Google Scholar] [CrossRef]
- Song, F.; Zhang, H.; Wang, S.; Liu, L.; Tan, X.; Liu, S. Atomic Level Design of CoOH+−Hydroxyapatite@C Catalysts for Superfast Degradation of Organics via Peroxymonosulfate Activation. Chem. Commun. 2018, 54, 4919–4922. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.S.; Ayyoob, M. O2- and O1- types of oxygen species on Ni and barium-dosed Ni and Cu surfaces. Surf. Sci. 1986, 173, L635–L640. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Vijayakrishnan, V.; Kulkarni, G.U.; Rajumon, M.K. A comparative study of the interaction of oxygen with clusters and single-crystal surfaces of nickel. Appl. Surf. Sci. 1995, 84, 285–289. [Google Scholar] [CrossRef]
- Kulkarni, G.U.; Rao, C.N.R.; Roberts, M.W. Coadsorption of Dioxygen and Water on the Ni(110) Surface: Role of O1--Type Species in the Dissociation of Wate. Langmuir 1995, 11, 2572–2575. [Google Scholar] [CrossRef]
- Guo, J.; Yu, H.; Dong, F.; Zhu, B.; Huang, W.; Zhang, S. High efficiency and stability of Au-Cu/hydroxyapatite catalyst for the oxidation of carbon monoxide. RSC Adv. 2017, 7, 45420–45431. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- Bee, S.-L.; Bustami, Y.; Ul-Hamid, A.; Lim, K.; Abdul Hamid, Z.A. Synthesis of Silver Nanoparticle-Decorated Hydroxyapatite Nanocomposite with Combined Bioactivity and Antibacterial Properties. J. Mater. Sci. Mater. Med. 2021, 32, 106. [Google Scholar] [CrossRef]
- Gomes, G.C.; Borghi, F.F.; Ospina, R.O.; L´opez, E.O.; Borges, F.O.; Mello, A. Nd:YAG (532 nm) pulsed laser deposition produces crystalline hydroxyapatite thin coatings at room temperature. Surf. Coat. Technol. 2017, 329, 174–183. [Google Scholar] [CrossRef]
- Sinulingga, K.; Sirait, M.; Siregar, N.; Abdullah, H. Synthesis and characterizations of natural limestone-derived nano-hydroxyapatite (HAp): A comparison study of different metals doped Haps on antibacterial activity. RSC Adv. 2021, 11, 15896–15904. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, L.; Fadoni, M.; Vercelli, B. Magnesium salts and oxide: An XPS overview. Appl. Surf. Sci. 1997, 119, 253–259. [Google Scholar] [CrossRef]
- Ley, L.; McFeely, F.R.; Kowalczyk, S.P.; Jenkin, J.G.; Shirley, D.A. Many-body e ects in X-ray photoemission from magnesium. Phys. Rev. B 1975, 11, 600e12. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Elsener, B.; Atzei, D.; Rigoldi, A.; Rossi, A. Exploiting XPS for the identification of sulfides and polysulfides. RSC Adv. 2015, 5, 75953–75963. [Google Scholar] [CrossRef]
- Predoi, S.A.; Ciobanu, S.C.; Chifiriuc, C.M.; Iconaru, S.L.; Predoi, D.; Negrila, C.C.; Marinas, I.C.; Raaen, S.; Rokosz, K.; Motelica-Heino, M. Sodium bicarbonate-hydroxyapatite used for removal of lead ions from aqueous solution. Ceram. Int. 2024, 50, 1742–1755. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Predoi, S.A.; Chifiriuc, M.C.; Raaen, S.; Badea, M.L.; Rokosz, K. Impact of Gamma Irradiation on the Properties of Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Materials 2022, 15, 5372. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Yoshida, Y.; Okazaki, M.; Shimazu, A.; Kubo, T.; Akagawa, Y.; Uchida, T. Action of FGMgCO3Ap–collagen composite in promoting bone formation. Biomaterials 2003, 24, 4913–4920. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Yoshida, Y.; Okazaki, M.; Shimazu, A.; Uchida, T.; Kubo, T.; Akagawa, Y.; Hamada, Y.; Takahashi, J.; Matsuura, N. Synthesis of functionally graded MgCO3 apatite accelerating osteoblast adhesion. J. Biomed. Mater. Res. 2002, 62, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-substituted hydroxyapatite: From synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Ciobanu, C.S.; Predoi, G.; Rokosz, K.; Chifiriuc, M.C.; Bleotu, C.; Stanciu, G.; Hristu, R.; Raaen, S.; Raita, S.M.; et al. Biological and Physico-Chemical Properties of Composite Layers Based on Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Micromachines 2022, 13, 1574. [Google Scholar] [CrossRef]
- Fox, C.; Ramsoomair, D.; Carter, C. Magnesium: Its proven and potential clinical significance. South Med. J. 2001, 94, 1195–1201. [Google Scholar] [CrossRef]
- Geng, F.; Tan, L.L.; Jin, X.X.; Yang, J.Y.; Yang, K. The preparation, cytocompatibility, and in vitro biodegradation study of pure β-TCP on magnesium. J. Mater. Sci. Mater. Med. 2009, 20, 1149–1157. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices–Part 1: Evaluation and Testing within a Risk Management Process. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/68936.html (accessed on 3 May 2024).
- Wątroba, M.; Bednarczyk, W.; Szewczyk, P.K.; Kawałko, J.; Mech, K.; Grünewald, A.; Unalan, I.; Taccardi, N.; Boelter, G.; Banzhaf, M.; et al. In vitro cytocompatibility and antibacterial studies on biodegradable Zn alloys supplemented by a critical assessment of direct contact cytotoxicity assay. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 241–260. [Google Scholar] [CrossRef]
- Sukumaran, A.; Sweety, V.K.; Vikas, B.; Joseph, B. Cytotoxicity and Cell Viability Assessment of Biomaterials. In Cytotoxicity-Understanding Cellular Damage and Response; Sukumaran, A., Mahmoud, A.M., Eds.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Akram, M.; Goh, Y.F.; Tariq, U.; Butt, F.K.; Abdolahi, A.; Hussain, R. Synthesis, characterization, in vitro bioactivity and antimicrobial activity of magnesium and nickel doped silicate hydroxyapatite. Ceram. Int. 2015, 41, 11886–11898. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Raaen, S.; Badea, M.L.; Rokosz, K. Physicochemical and Biological Evaluation of Chitosan-Coated Magnesium-Doped Hydroxyapatite Composite Layers Obtained by Vacuum Deposition. Coatings 2022, 12, 702. [Google Scholar] [CrossRef]
- Veljovic, D.; Matic, T.; Stamenic, T.; Kojic, V.; Dimitrijevic-Brankovic, S.; Lukic, M.J.; Jevtic, S.; Radovanovic, Z.; Petrovic, R.; Janackovic, D. Mg/Cu co-substituted hydroxyapatite–Biocompatibility, mechanical properties and antimicrobial activity. Ceram. Int. 2019, 45, 22029–22039. [Google Scholar] [CrossRef]
- Demishtein, K.; Reifen, R.; Shemesh, M. Antimicrobial Properties of Magnesium Open Opportunities to Develop Healthier Food. Nutrients 2019, 11, 2363. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Kumar, S.; Thakur, P. Essential oil-mediated biocompatible magnesium nanoparticles with enhanced antibacterial, antifungal, and photocatalytic efficacies. Sci. Rep. 2022, 12, 11431. [Google Scholar] [CrossRef]
- Hans, S.; Fatima, Z.; Ahmad, A.; Hameed, S. Magnesium impairs Candida albicans immune evasion by reduced hyphal damage, enhanced -glucan exposure and altered vacuole homeostasis. PLoS ONE 2022, 17, e0270676. [Google Scholar] [CrossRef]
- de Marco, B.A.; Natori, J.S.H.; Fanelli, S.; Tótoli, E.G.; Salgado, H.R.N. Characteristics, Properties and Analytical Methods of Amoxicillin: A Review with Green Approach. Crit. Rev. Anal. Chem. 2017, 47, 267–277. [Google Scholar] [CrossRef]
- Todd, P.A.; Benfield, P. Amoxicillin/Clavulanic Acid. Drugs 1990, 39, 264–307. [Google Scholar] [CrossRef]
- Bankole, O.M.; Ojubola, K.I.; Adanlawo, O.S.; Adesina, A.O.; Lawal, I.O.; Ogunlaja, A.S.; Achadu, O.J. Amoxicillin Encapsulation on Alginate/Magnetite Composite and Its Antimicrobial Properties Against Gram-Negative and Positive Microbes. BioNanoScience 2022, 12, 1136–1149. [Google Scholar] [CrossRef]
- Weber, D.J.; Tolkoff-Rubin, N.E.; Rubin, R.H. Amoxicillin and Potassium Clavulanate: An Antibiotic Combination Mechanism of Action, Pharmacokinetics, Antimicrobial Spectrum, Clinical Efficacy and Adverse Effects. Pharmacotherapy 1984, 4, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Güncüm, E.; Işıklan, N.; Anlaş, C.; Ünal, N.; Bulut, E.; Bakırel, T. Development and characterization of polymeric-based nanoparticles for sustained release of amoxicillin—An antimicrobial drug. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 964–973. [Google Scholar] [CrossRef]
- Amin, A.S.; El-Ansary, A.L.; Issa, Y.M. Colorimetric determination of amoxicillin in pure form and in pharmaceutical preparations. Talanta 1994, 41, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, G.S.; Ribeiro, F.M.; Vicente, F.L.; Struchiner, C.J. Development and validation of limited-sampling strategies for predicting amoxicillin pharmacokinetic and pharmacodynamics parameters. Antimicrob. Agents Chemother. 2001, 45, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C. Antimicrobial activity and human pharmacology of amoxicillin. J. Infect. Diseases 1974, 129, 123–131. [Google Scholar] [CrossRef]
- Enan, E.T.; Ashour, A.A.; Basha, S.; Felemban, N.H.; El-Rab, S.M.G. Antimicrobial activity of biosynthesized silver nanoparticles, amoxicillin, and glass-ionomer cement against Streptococcus mutans and Staphylococcus aureus. Nanotechnology 2021, 32, 5101. [Google Scholar] [CrossRef]
- Bigi, A.; Foresti, E.; Gregorini, R.; Ripamonti, A.; Roveri, N.; Shah, J.S. The role of magnesium on the structure of biological apatites. Calcif. Tissue Intern. 1992, 50, 439–444. [Google Scholar] [CrossRef]
- Ballardini, A.; Montesi, M.; Panseri, S.; Vandini, A.; Balboni, P.G.; Tampieri, A.; Sprio, S. New hydroxyapatite nanophases with enhanced osteogenic and anti-bacterial activity. J. Biomed. Mater. Res. A 2018, 106, 521–530. [Google Scholar] [CrossRef]
- Cazalbou, S.; Eichert, D.; Ranz, X.; Drouet, C.; Combes, C.; Harmand, M.F.; Rey, C. Ion exchanges in apatites for biomedical application. J. Mater. Sci. Mater. Med. 2005, 16, 405–409. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Xiong, G.; Nie, Y.; Ji, D.; Li, J.; Li, C.; Li, W.; Zhu, Y.; Luo, H.; Wan, Y. Characterization of biomedical hydroxyapatite/magnesium composites prepared by powder metallurgy assisted with microwave sintering. Curr. Appl. Phys. 2016, 16, 830–836. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Heimann, R.B. Osseoconductive and Corrosion-Inhibiting Plasma-Sprayed Calcium Phosphate Coatings for Metallic Medical Implants. Metals 2017, 7, 468. [Google Scholar] [CrossRef]
- Heimann, R.B.; Lehmann, H.D. Recent Research and Patents on Controlling Corrosion of Bioresorbable Mg Alloy Implants: Towards Next Generation Biomaterials. Recent Pat. Mate Sci. 2017, 10, 2–19. [Google Scholar] [CrossRef]
- Cizek, J.; Matejicek, J. Medicine Meets Thermal Spray Technology: A Review of Patents. J. Therm. Spray Technol. 2018, 27, 1251–1279. [Google Scholar] [CrossRef]
- Costescu, A.; Ciobanu, C.S.; Iconaru, S.L.; Ghita, R.V.; Chifiriuc, C.M.; Marutescu, L.G.; Predoi, D. Fabrication, characterization, and antimicrobial activity, evaluation of low silver concentrations in silver-doped hydroxyapatite nanoparticles. J. Nanomater. 2013, 2013, 194854. [Google Scholar] [CrossRef]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In vivo evaluation of biodegradable magnesium alloy bone implants in the first 6 months implantation. J. Biomed. Mater. Res. A 2009, 90, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, S.-A.; Buton, N.; Megier, C.; Beuran, M. Development of Iron-Doped Hydroxyapatite Coatings. Coatings 2021, 11, 186. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Rokosz, K.; Raaen, S.; Negrila, C.C.; Buton, N.; Ghegoiu, L.; Badea, M.L. Physico-Chemical and Biological Features of Fluorine-Substituted Hydroxyapatite Suspensions. Materials 2024, 17, 3404. [Google Scholar] [CrossRef]
- Predoi, S.A.; Ciobanu, S.C.; Chifiriuc, M.C.; Motelica-Heino, M.; Predoi, D.; Iconaru, S.L. Hydroxyapatite Nanopowders for Effective Removal of Strontium Ions from Aqueous Solutions. Materials 2023, 16, 229. [Google Scholar] [CrossRef]
- Predoi, S.-A.; Ciobanu, C.S.; Motelica-Heino, M.; Chifiriuc, M.C.; Badea, M.L.; Iconaru, S.L. Preparation of Porous Hydroxyapatite Using Cetyl Trimethyl Ammonium Bromide as Surfactant for the Removal of Lead Ions from Aquatic Solutions. Polymers 2021, 13, 1617. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Komarovska, L.; Viksna, A. Efficient zinc incorporation in hydroxyapatite through crystallization of an amorphous phase could extend the properties of zinc apatites. J. Australas. Ceram. Soc. 2013, 49, 129–135. [Google Scholar]
- Rusu, V.M.; Ng, C.H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size- controlled hydroxyapatite nanoparticles as self-organized organic–inorganic composite materials. Biomater. 2005, 26, 5414–5424. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, F.; Kono, Y.; Suyama, Y. Formation and structure of zinc-substituted calcium hydroxyapatite. Mater. Res. Bull. 2005, 40, 209–220. [Google Scholar] [CrossRef]
- Miyaji, F.; Kono, Y.; Suyama, Y. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf. B Biointerfaces 2013, 111, 556–560. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef]


| Sample | Average Crystal Size (nm) | d-Spacing (nm) | Lattice Parameters (Å) | Unit Cell Volume (Å3) | |
|---|---|---|---|---|---|
| c-Axis | a-Axis | ||||
| MgHAp | 15.31 | 3.40 | 6.85 | 9.39 | 605 |
| MgHApOx | 17.79 | 3.43 | 6.87 | 9.41 | 608 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimpeanu, C.; Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Rokosz, K.; Predoi, M.V.; Raaen, S.; Badea, M.L. Development of Novel Biocomposites with Antimicrobial-Activity-Based Magnesium-Doped Hydroxyapatite with Amoxicillin. Antibiotics 2024, 13, 963. https://doi.org/10.3390/antibiotics13100963
Cimpeanu C, Predoi D, Ciobanu CS, Iconaru SL, Rokosz K, Predoi MV, Raaen S, Badea ML. Development of Novel Biocomposites with Antimicrobial-Activity-Based Magnesium-Doped Hydroxyapatite with Amoxicillin. Antibiotics. 2024; 13(10):963. https://doi.org/10.3390/antibiotics13100963
Chicago/Turabian StyleCimpeanu, Carmen, Daniela Predoi, Carmen Steluta Ciobanu, Simona Liliana Iconaru, Krzysztof Rokosz, Mihai Valentin Predoi, Steinar Raaen, and Monica Luminita Badea. 2024. "Development of Novel Biocomposites with Antimicrobial-Activity-Based Magnesium-Doped Hydroxyapatite with Amoxicillin" Antibiotics 13, no. 10: 963. https://doi.org/10.3390/antibiotics13100963
APA StyleCimpeanu, C., Predoi, D., Ciobanu, C. S., Iconaru, S. L., Rokosz, K., Predoi, M. V., Raaen, S., & Badea, M. L. (2024). Development of Novel Biocomposites with Antimicrobial-Activity-Based Magnesium-Doped Hydroxyapatite with Amoxicillin. Antibiotics, 13(10), 963. https://doi.org/10.3390/antibiotics13100963










