Multitarget Phytocomplex: Focus on Antibacterial Profiles of Grape Pomace and Sambucus ebulus L. Lyophilisates Against Extensively Drug-Resistant (XDR) Bacteria and In Vitro Antioxidative Power
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemicals Compounds in Lyophilisates Obtained from GP and DE
2.2. Bacterial Contamination (Microbiological Load) in Samples of Various Plant Lyophilisates
2.3. AB Effects
2.4. Drug Resistance Pattern of the Two Clinical Bacterial Isolates
2.5. Total Phenolic Content and Antioxidative Capacity
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Lyophilisation Procedure
3.4. Preparation of Extracts from Lyophilisates of Plant Raw Materials (GP, DE)
3.5. Chromatographic Analysis
3.6. Determining the Presence of Bacterial Contamination and Subsequent Isolation
3.7. AB Activity
3.8. Examination of Clinical Samples from Patients with Suspected Healthcare-Associated Infections: Isolation, Identification and Characterisation of Bacterial Isolates
3.9. Antimicrobial Susceptibility Testing (AST) of Characterised Clinical Bacterial Isolates by Performing the DD and Microdilution Method (Determination of MIC)
3.10. Determining the Total Phenolic Content and Antioxidative Activity at the Level of DPPH Radicals
3.10.1. Determining the Total Phenolic Content
3.10.2. DPPH Method
- AB is the absorbance of DPPH solution without extracts;
- AA is the absorbance of DPPH solution with extracts.
3.11. Determining the Activity of Water (aw) of Plant Raw Material Lyophilisates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Micucci, M.; Bolchi, C.; Budriesi, R.; Cevenini, M.; Maroni, L.; Capozza, S.; Chiarini, A.; Pallavicini, M.; Angeletti, A. Antihypertensive phytocomplexes of proven efficacy and well-established use: Mode of action and individual characterization of the active constituents. Phytochemistry 2020, 170, 112222. [Google Scholar] [CrossRef]
- Hayek, S.A.; Gyawali, R.; Ibrahim, S.A. Antimicrobial Natural Products. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Centre: Norristown, PA, USA, 2013; Volume 1, pp. 910–921. [Google Scholar]
- Nguyen, T.L.; Ora, A.; Häkkinen, S.T.; Ritala, A.; Räisänen, R.; Kallioinen-Mänttäri, M.; Melin, K. Innovative extraction technologies of bioactive compounds from plant by-products for textile colorants and antimicrobial agents. Biomass Convers. Biorefinery 2023, 14, 24973–25002. [Google Scholar] [CrossRef]
- Ryu, Y.A.; Lee, J.S. Clean label guideline for entry into UK and EU agro-food markets. Food Ind. Nutr. 2018, 23, 20–26. [Google Scholar]
- Câmara, A.K.F.I.; Vidal, V.A.S.; Santos, M.; Bernardinelli, O.D.; Sabadini, E.; Pollonio, M.A.R. Reducing phosphate in emulsified meat products by adding chia (Salvia hispanica L.) mucilage in powder or gel format: A clean label technological strategy. Meat Sci. 2020, 163, 108085. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Perra, M.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Castangia, I.; Rajha, H.N.; Manca, M.L.; Manconi, M. An outlook on modern and sustainable approaches to the management of grape pomace by integrating green processes, biotechnologies and advanced biomedical approaches. J. Funct. Foods 2022, 98, 105276. [Google Scholar] [CrossRef]
- Machado, T.O.X.; Portugal, I.; Kodel, H.d.A.C.; Fathi, A.; Fathi, F.; Oliveira, M.B.P.P.; Dariva, C.; Souto, E.B. Pressurized liquid extraction as an innovative high-yield greener technique for phenolic compounds recovery from grape pomace. Sustain. Chem. Pharm. 2024, 40, 101635. [Google Scholar] [CrossRef]
- Daniela, T.-R.; del Socorro, L.-C.M.; Fortunata, S.-T.; Patricia, R.-M.; Felipe, G.-O.; Teresa, H.-B.M.; de la Paz, S.-C.M. Optimization of the Extraction of Bioactive Compounds from Cabernet Sauvignon Grape Pomace from Querétaro, Mexico, Using MSPD. Separations 2024, 11, 13. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of phenolic compounds from grape pomace using ohmic heating: Chemical composition, bioactivity and bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef]
- Atta, S.; Waseem, D.; Fatima, H.; Naz, I.; Rasheed, F.; Kanwal, N. Antibacterial potential and synergistic interaction between natural polyphenolic extracts and synthetic antibiotic on clinical isolates. Saudi J. Biol. Sci. 2023, 30, 103576. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Liaudanskas, M.; Ramanauskienė, K.; Janulis, V. Biopharmaceutical Evaluation of Capsules with Lyophilized Apple Powder. Molecules 2021, 26, 1095. [Google Scholar] [CrossRef]
- Gaidhani, A.K.; Harwalkar, M.; Bhambere, D.; Nirgude, S.P. Lyophilization / freeze drying—A review. World J. Pharm. Res. 2015, 4, 516–543. [Google Scholar]
- Kittibunchakul, S.; Temviriyanukul, P.; Chaikham, P.; Kemsawasd, V. Effects of freeze drying and convective hot-air drying on predominant bioactive compounds, antioxidant potential and safe consumption of maoberry fruits. LWT 2023, 184, 114992. [Google Scholar] [CrossRef]
- Tang, X.; Pikal, M.J. Design of Freeze-Drying Processes for Pharmaceuticals: Practical Advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef]
- Abascal, K.; Ganora, L.; Yarnell, E. The Effect of Freeze-drying and its Implications for Botanical Medicine: A Review. Phytother. Res. 2005, 19, 655–660. [Google Scholar] [CrossRef]
- Chang, S.H.; Chen, C.H.; Tsai, G.J. Effects of chitosan on Clostridium perfringens and application in the preservation of pork sausage. Mar. Drugs 2020, 18, 70. [Google Scholar] [CrossRef]
- Antal, T.; Kerekes, B. Investigation of Hot Air- And Infrared-Assisted Freeze-Drying of Apple. J. Food Process. Pres. 2015, 40, 257–269. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Lenart, A. Freeze-Drying–Application in Food Processing and Biotechnology—A Review. Pol. J. Food Nutr. Sci. 2011, 61, 165–171. [Google Scholar] [CrossRef]
- Gurak, P.D.; Cabral, L.M.C.; Rocha-Leão, M.H. Production of Grape Juice Powder Obtained by Freezedrying after Concentration by Reverse Osmosis. Braz. Arch. Biol. Technol. 2013, 56, 1011–1017. [Google Scholar] [CrossRef]
- Kim, H.-J.; Sujiwo, J.; Kim, H.-J.; Jang, A. Effects of Dipping Chicken Breast Meat Inoculated with Listeria monocytogenes in Lyophilized Scallion, Garlic, and Kiwi Extracts on Its Physicochemical Quality. Food Sci. Anim. Resour. 2019, 39, 418–429. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of Carrier Agents on the Physicochemical and Technofunctional Properties and Antioxidant Capacity of Freeze-Dried Pomegranate Juice (Punica granatum) Powder. Foods 2020, 9, 1388. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef]
- Hazarika, U.; Gosztola, B. Lyophilization and its effects on the essential oil content and composition of herbs and spices—A review. Acta Sci. Pol. Technol. Aliment. 2020, 19, 467–473. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Pawłowicz, K.; Ludowicz, D.; Karaźniewicz-Łada, M.; Wdowiak, K.; Cielecka-Piontek, J. Analysis of the Composition of Lyophilisates Obtained from Aloe arborescens Gel of Leaves of Different Ages from Controlled Crops. Molecules 2021, 26, 3204. [Google Scholar] [CrossRef]
- Pawłowicz, K.; Paczkowska-Walendowska, M.; Osmałek, T.; Cielecka-Piontek, J. Towards the Preparation of a Hydrogel from Lyophilisates of the Aloe arborescens Aqueous Extract. Pharmaceutics 2022, 14, 1489. [Google Scholar] [CrossRef]
- Jude, J.; Adu, E.A.; Maiyanga, I.E.; Kamaldeen, O.S. Application of Freeze-Drying in Food Processing and Storage–A Review. BADEGGI J. Agric. Res. Environ. 2023, 5, 21–35. [Google Scholar] [CrossRef]
- Maisl, C.; Doppler, M.; Seidl, B.; Bueschl, C.; Schuhmacher, R. Untargeted Plant Metabolomics: Evaluation of Lyophilization as a Sample Preparation Technique. Metabolites 2023, 13, 686. [Google Scholar] [CrossRef]
- Giancaterino, M.; Werl, C.; Jaeger, H. Evaluation of the quality and stability of freeze-dried fruits and vegetables pre-treated by pulsed electric fields (PEF). LWT 2024, 191, 115651. [Google Scholar] [CrossRef]
- Jurčević Šangut, I.; Pavličević, L.; Šamec, D. Influence of Air Drying, Freeze Drying and Oven Drying on the Biflavone Content in Yellow Ginkgo (Ginkgo biloba L.) Leaves. Appl. Sci. 2024, 14, 2330. [Google Scholar] [CrossRef]
- ElNaker, N.A.; Daou, M.; Ochsenkühn, M.A.; Amin, S.A.; Yousef, A.F.; Yousef, L.F. A metabolomics approach to evaluate the effect of lyophilization versus oven drying on the chemical composition of plant extracts. Sci. Rep. 2021, 11, 22679. [Google Scholar] [CrossRef]
- Kaya, Y.; Haji, E.K.; Arvas, Y.E.; Aksoy, H.M. Sambucus ebulus L.: Past, present and future. AIP Conf. Proc. 2019, 2155, 020030. [Google Scholar] [CrossRef]
- Cumhur, B.; Sarialtin, S.Y.; Gumusok, S.; Coban, T.; Kilic, C.S. Antioxidant and anti-inflammatory activity of different parts of Sambucus ebulus L. J. Fac. Pharm. Ankara 2022, 46, 728–741. [Google Scholar] [CrossRef]
- Paşa, C. The use of Sambucus ebulus L. in folk medicine and chemical composition. GSC Adv. Res. Rev. 2023, 17, 081–085. [Google Scholar] [CrossRef]
- Cvetanović, A. Chapter 31—Sambucus ebulus L., antioxidants and potential in disease. In Pathology; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 321–333. ISBN 9780128159729. [Google Scholar] [CrossRef]
- Jimenez, P.; Cabrero, P.; Basterrechea, J.E.; Tejero, J.; Cordoba-Diaz, M.; Girbes, T. Effects of Short-term Heating on Total Polyphenols, Anthocyanins, Antioxidant Activity and Lectins of Different Parts of Dwarf Elder (Sambucus ebulus L.). Plant Foods Hum. Nutr. 2014, 69, 168–174. [Google Scholar] [CrossRef]
- Jabbari, M.; Daneshfard, B.; Emtiazy, M.; Khiveh, A.; Hashempur, M.H. Biological Effects and Clinical Applications of Dwarf Elder (Sambucus ebulus L.): A Review. J. Evid. Based Complementary Altern. Med. 2017, 22, 996–1001. [Google Scholar] [CrossRef]
- García-Lomillo, J.; Gonzalez-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Carnaroglio, D.; Tamburino, A.; Cravotto, G.; Grillo, G.; Ilharco, L.M.; Pagliaro, M. Eco-Friendly Extraction of Pectin and Essential Oils from Orange and Lemon Peels. ACS Sustain. Chem. Eng. 2016, 4, 2243–2251. [Google Scholar] [CrossRef]
- Scully, D.S.; Jaiswal, A.K.; Abu-Ghannam, N. An Investigation into Spent Coffee Waste as a Renewable Source of Bioactive Compounds and Industrially Important Sugars. Bioengineering 2016, 3, 33. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Reuse potential of artichoke (Cynara scolimus L.) waste for the recovery of phenolic compounds and bioenergy. J. Clean. Prod. 2016, 111, 279–284. [Google Scholar] [CrossRef]
- Cardinali, A. Phytochemicals from Artichoke by-product and Their Applications as Natural Ingredients for Cosmetic Industry. Available online: https://www.uniba.it/ateneo/editoria-stampa-e-media/linea-editoriale/fuori-collana/genppdf (accessed on 23 June 2024).
- Di Donato, P.; Taurisano, V.; Tommonaro, G.; Pasquale, V.; Jiménez, J.M.S.; de Pascual-Teresa, S.; Poli, A.; Nicolaus, B. Biological Properties of Polyphenols Extracts from Agro Industry’s Wastes. Waste Biomass Valor. 2018, 9, 1567–1578. [Google Scholar] [CrossRef]
- Jahresbericht 2018. Johann Heinrich von Thünen-Institut. Available online: https://www.thuenen.de/media/publikationen/jahresbericht/Jahresbericht_2018.pdf (accessed on 23 June 2024).
- Watson, R.R. (Ed.) Polyphenols in Plants. Isolation, Purification and Extract Preparation, 2nd ed.; Academic Press: London, UK, 2019. [Google Scholar]
- Mourtzinos, I.; Goula, A. Polyphenols in Agricultural Byproducts and Food Waste. In Polyphenols in Plants: Isolation, Purification and Extract Preparation, 2nd ed.; Watson, R.R., Ed.; Academic Press: London, UK, 2019; pp. 23–44. [Google Scholar]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Mikulic-Petkovsek, M. Harmful (cyanogenic glycoside) and beneficial (phenolic) compounds in different Sambucus species. J. Berry Res. 2019, 9, 395–409. [Google Scholar] [CrossRef]
- Burak, L.C. The use of elder marc in the food industry. New Technol. 2020, 16, 20–27. (In Russian) [Google Scholar] [CrossRef]
- FSANZ [Food Standards Australia New Zealand]. Consumer Attitudes Survey 2007: A Benchmark Survey of Consumers’ Attitudes to Food Issues; Food Standards Australia New Zealand: Wellington, New Zealand, 2008. [Google Scholar]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Kłębukowska, L.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Microbiological contamination of dried and lyophilized garlic as a potential source of food spoilage. J. Food Sci. Technol. 2013, 52, 1802–1807. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Williams, V.L.; Sooka, A.; Burger, A.; Van Der Haar, L. Microbial contamination of traditional medicinal plants sold at the Faraday muthi market, Johannesburg, South Africa. S. Afr. J Bot. 2014, 94, 95–100. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial Contamination of Fresh Produce: What, Where, and How? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Stajić, S.B.; Dmitrić, M.P.; Miletić, N.M. Food safety assessment of burger patties with added herbal plant material. Fleischwirtschaft 2022, 11, 73–78. [Google Scholar]
- Kurćubić, V.S.; Stajić, S.B.; Živković, V.; Mašković, P.Z.; Matejić, V.; Dmitrić, M.; Živković, S.; Kurćubić, L.V.; Jakovljević, V. Leftover and weed, joint efforts to preserve health: Joke or reality? Future Postharvest Food 2024, 1–17. [Google Scholar] [CrossRef]
- Kurćubić, V.; Stajić, S.; Miletić, N.; Stanišić, N. Healthier meat products are Fashionable—Consumers Love Fashion. Appl. Sci. 2022, 12, 10129. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Mašković, P.Z.; Karan, D.; Vesković-Moračanin, S.M.; Okanović, Đ.G.; Lilić, S.V.; Džinić, N.P. Sensory properties of sausage fortified by Kitaibelia vitifolia extract. Agro Food Ind. Hi. Tech. 2014, 25, 16–19. [Google Scholar]
- Kurćubić, V.S.; Mašković, P.Z.; Vujić, J.M.; Vranić, D.V.; Vesković-Moračanin, S.M.; Okanović, Đ.G.; Lilić, S.V. Antioxidant and antimicrobial activity of Kitaibelia vitifolia extract as alternative to the added nitrite in fermented dry sausage. Meat Sci. 2014, 97, 459–467. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Raketić, S.V.; Mašković, J.M.; Mašković, P.Z.; Kurćubić, L.V.; Heinz, V.; Tomasevic, I.B. Evaluation of antimicrobial activity of Kitaibelia vitifolia extract against proven Antibiotic Susceptible and Multidrug-resistant (MDR) strains of bacteria of clinical origin. Plants 2023, 12, 3236. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Stajić, S.B.; Miletić, N.M.; Petković, M.M.; Dmitrić, M.P.; Ðurović, V.M.; Heinz, V.; Tomasevic, I.B. Technofunctional properties of Burgers fortified by Wild Garlic extract: A Reconsideration. Foods 2023, 12, 2100. [Google Scholar] [CrossRef]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef]
- Moldovan, M.L.; Iurian, S.; Puscas, C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Bogdan, C.; Vlase, L.; Oniga, I.; Benedec, D. A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction. Biomolecules 2019, 9, 529. [Google Scholar] [CrossRef]
- Anđelković, M.; Radovanović, B.; Milenković Anđelković, A.; Radovanović, V.; Zarubica, A.; Stojković, N.; Nikolić, V. The Determination of Bioactive Ingredients of Grape Pomace (Vranac Variety) for Potential Use in Food and Pharmaceutical Industries. Adv. Technol. 2015, 4, 32–36. [Google Scholar] [CrossRef]
- Seymenska, D.; Shishkova, K.; Hinkov, A.; Benbassat, N.; Teneva, D.; Denev, P. Comparative Study on Phytochemical Composition, Antioxidant, and Anti-HSV-2 Activities of Sambucus nigra L. and Sambucus ebulus L. Extracts. Appl. Sci. 2023, 13, 12593. [Google Scholar] [CrossRef]
- Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stressand Inflammation-Induced Health Disorders. Molecules 2023, 28, 3148. [Google Scholar] [CrossRef]
- Păvăloiu, R.-D.; Sháat, F.; Bubueanu, C.; Deaconu, M.; Neagu, G.; Sháat, M.; Anastasescu, M.; Mihailescu, M.; Matei, C.; Nechifor, G.; et al. Polyphenolic Extract from Sambucus ebulus L. Leaves Free and Loaded into Lipid Vesicles. Nanomaterials 2020, 10, 56. [Google Scholar] [CrossRef]
- Schwarz, K.; Bertelsen, G.; Nissen, L.R.; Gardner, P.T.; Heinonen, M.I.; Huynh-Ba, A.H.; Lambelet, P.; McPhail, D.L.H.; Tijburg, L. Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assays based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur. Food Res. Technol. 2001, 212, 319–328. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; Du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- SRPS CEN ISO/TS 13136:2014; Microbiology of Food and Animal Feed–Real-Time Polymerase Chain Reaction (PCR)-Based Method for the Detection of Food-Borne Pathogens–Horizontal Method for the Detection of Shiga Toxin-Producing Escherichia coli (STEC) and the Determination of O157, O111, O26, O103 and O145 Serogroups. ISO: Geneva, Switzerland, 2014.
- SRPS EN ISO 11290-1:2017; Microbiology of the Food Chain–Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. ISO: Geneva, Switzerland, 2017.
- SRPS EN ISO 21528-2:2017; Microbiology of the Food Chain–Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. ISO: Geneva, Switzerland, 2017.
- SRPS EN ISO 4833-1:2014+/A1:2022; Microbiology of Food and Animal Feeding Stuffs–Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C and Amendment 1: Clarification of Scope. ISO: Geneva, Switzerland, 2022.
- SRPS EN ISO 6579-1:2017; Microbiology of the Food Chain–Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- SRPS EN ISO 6888-1:2021; Microbiology of the Food Chain–Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Method Using Baird-Parker Agar Medium. ISO: Geneva, Switzerland, 2021.
- SRPS EN ISO 7932:2009; Microbiology of Food and Animal Feeding Stuffs–Horizontal Method for the Enumeration of Presumptive Bacillus cereus—Colony-Count Technique at 30 °C. ISO: Geneva, Switzerland, 2009.
- SRPS ISO 15213:2011; Microbiology of Food and Animal Feeding Stuffs–Horizontal Method for the Enumeration of Sulfite-reducing Bacteria Growing under Anaerobic Conditions. ISO: Geneva, Switzerland, 2011.
- SRPS ISO 16649-2:2008; Microbiology of Food and Animal Feeding Stuffs–Horizontal Method for the Enumeration of Beta-glucuronidase-Positive Escherichia coli—Part 2: Colony–Count Technique at 44 °C Using 5-Bromo-4-chloro-3-indolyl beta-D-Glucuronide. ISO: Geneva, Switzerland, 2008.
- SRPS ISO 21527-2:2011; Microbiology of Food and Animal Feeding Stuffs–Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0.95. ISO: Geneva, Switzerland, 2011.
- SRPS ISO 4832:2014; Microbiology of Food and Animal Feeding Stuffs–Horizontal Method for the Enumeration of Coliforms–Colony-Count Technique. ISO: Geneva, Switzerland, 2014.
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape pomace as a promising antimicrobial alternative in feed: A critical review. J. Agric. Food Chem. 2019, 67, 9705–9718. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.; Opirs, O.; Cristea, E.; Sturza, R. Chemometric optimization of biologically active compounds extraction from grape marc: Composition and antimicrobial activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef]
- Da Silva, D.J.; de Oliveira, M.M.; Wang, S.H.; Carastan, D.J.; Rosa, D.S. Designing antimicrobial polypropylene films with grapepomace extract for food packaging. Food Packag. Shelf Life 2022, 34, 100929. [Google Scholar] [CrossRef]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of grape pomace polyphenols and in vitro gastrointestinal digestion on antimicrobial activity: Recovery of bioactive compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef]
- Machado, T.O.X.; Portugal, I.; Kodel, H.d.A.C.; Droppa-Almeida, D.; Lima, M.D.S.; Fathi, F.; Oliveira, M.B.P.P.; de Albuquerque-Júnior, R.L.C.; Dariva, C.; Souto, E.B. Therapeutic potential of grape pomace extracts: A review of scientific evidence. Food Biosci. 2024, 60, 104210. [Google Scholar] [CrossRef]
- Sinrod, A.J.G.; Shah, I.M.; Surek, E.; Barile, D. Uncovering the promising role of grape pomace as a modulator of the gut microbiome: An in-depth review. Heliyon 2023, 9, e20499. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Berenbaum, M.C. What is synergy? Pharmacol. Rev. 1989, 41, 93–141, Erratum in Pharmacol. Rev. 1990, 41, 93–141. [Google Scholar]
- Pemovska, T.; Bigenzahn, J.W.; Superti-Furga, G. Recent advances in combinatorial drug screening and synergy scoring. Curr. Opin. Pharmacol. 2018, 42, 102–110. [Google Scholar] [CrossRef]
- Caesar, L.K.; Nadja, B.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869. [Google Scholar] [CrossRef]
- Gyawali, R.; Salam Ibrahim, A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Pop, A.; Bogdan, C.; Fizesan, I.; Iurian, S.; Carpa, R.; Bacali, C.; Vlase, L.; Benedec, D.; Moldovan, M.L. In Vitro Evaluation of Biological Activities of Canes and Pomace Extracts from Several Varieties of Vitis vinifera L. for Inclusion in Freeze-Drying Mouthwashes. Antioxidants 2022, 11, 218. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef]
- Silva, J.T.; López-Medrano, F. Cefiderocol, a new antibiotic against multidrug-resistant Gram-negative bacteria. Rev. Esp. Quimioter. 2021, 34 (Suppl. S1), 41–43. [Google Scholar] [CrossRef]
- El-Lababidi, R.M.; Rizk, J.G. Cefiderocol: A Siderophore Cephalosporin. Ann Pharmacother. 2020, 54, 1215–1231. [Google Scholar] [CrossRef]
- Parsels, K.A.; Mastro, K.A.; Steele, J.M.; Thomas, S.J.; Kufel, W.D. Cefiderocol: A novel siderophore cephalosporin for multidrug-resistant Gram-negative bacterial infections. J. Antimicrob. Chemother. 2021, 76, 1379–1391. [Google Scholar] [CrossRef]
- Mondal, A.H.; Khare, K.; Saxena, P.; Debnath, P.; Mukhopadhyay, K.; Yadav, D. A Review on Colistin Resistance: An Antibiotic of Last Resort. Microorganisms 2024, 12, 772. [Google Scholar] [CrossRef]
- Romo-Castillo, M.; Flores-Bautista, V.A.; Guzmán-Gutiérrez, S.L.; Reyes-Chilpa, R.; León-Santiago, M.; Luna-Pineda, V.M. Synergy of Plant Essential Oils in Antibiotic Therapy to Combat Klebsiella pneumoniae Infections. Pharmaceuticals 2023, 16, 839. [Google Scholar] [CrossRef]
- Kalan, L.; Wright, G.D. Antibiotic adjuvants: Multicomponent anti-infective strategies. Expert Rev. Mol. Med. 2011, 13, e5. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Hossain, M.E.; Mannan Mithi, F.; Ahmed, M.; Saldías, M.; Akkol, E.K.; Sobarzo-Sánchez, E. Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties. Antibiotics 2021, 10, 1076. [Google Scholar] [CrossRef]
- Si, Z.; Pethe, K.; Chan-Park, M.B. Chemical Basis of Combination Therapy to Combat Antibiotic Resistance. JACS Au 2023, 3, 276–292. [Google Scholar] [CrossRef]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A. Biotransformed grape pomace as a potential source of anti-inflammatory polyphenolics: Effects in Caco-2 cells. Food Biosci. 2020, 35, 100607. [Google Scholar] [CrossRef]
- Tasinov, O.; Kiselova-Kaneva, Y.; Ivanova, D. Antioxidant activity, total polyphenol content and anthocyanins content of Sambucus ebulus L. aqueous and aqueous-ethanolic extracts depend on the type and concentration of extragent. Sci. Technol. 2012, 2, 37–41. [Google Scholar]
- Tasinov, O.; Kiselova-Kaneva, Y.; Ivanova, D. Sambucus Ebulus–From Traditional Medicine to Recent Studies. Scr. Sci. Med. 2013, 45, 36–42. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Ehsanifar, S.; Eslami, B. Sambucus ebulus elburensis fruits: A good source for antioxidants. Phcog. Mag. 2009, 4, 213–218. [Google Scholar]
- Hosseinimehr, S.J.; Pourmorad, F.; Shahabimajd, N.; Shahrbandy, K.; Hosseinzadeh, R. In vitro antioxidant activity of Polygonium hyrcanicum, Centaurea depressa, Sambucus ebulus, Mentha spicata and Phytolacca americana. Pak. J. Biol. Sci. 2007, 10, 637–640. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.F.; Nabavi, S.M. Antioxidant Activities of Methanol Extract of Sambucus ebulus L. Flower. Pak. J. Biol. Sci. 2009, 12, 447–450. [Google Scholar] [CrossRef]
- Topuzović, M.D.; Stanković, M.S.; Jakovljević, D.Z.; Bojović, B.M. Plant part variability of Sambucus ebulus L. secondary metabolites content and antioxidant activity. Agro. Food Industry Hi Tech. 2016, 27, 60–63. [Google Scholar]
- Lak, E.; Ranjbar, R.; Najafzadeh, H.; Morovvati, H.; Khaksary, M. Protective effect of Sambucus elbus extract on teratogenicity of albendazole. Middle-East J. Sci. Res. 2011, 8, 606–610. [Google Scholar]
- Wu, H.; Johnson, M.C.; Lu, C.-H.; Fritsche, K.L.; Thomas, A.L.; Cai, Z.; Greenlief, C.M. Determination of Anthocyanins and Total Polyphenols in a Variety of Elderberry Juices by UPLC-MS/MS and other Methods. Acta Hort. 2015, 1061, 43–51. [Google Scholar] [CrossRef]
- Laurutis, A.; Liobikas, J.; Stanciauskaite, M.; Marksa, M.; Ramanauskiene, K.; Majiene, D. Comparison of the Formulation, Stability and Biological Effects of Hydrophilic Extracts from Black Elder Flowers (Sambucus nigra L.). Pharmaceutics 2022, 14, 2831. [Google Scholar] [CrossRef]
- Gudrun, R.; Müller, M.; Guttenberger, H.; Bucar, F.J. Influence of Altitudinal Variation on the Content of Phenolic Compounds in Wild Populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. Agric. Food Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef]
- Frum, A.; Dobrea, C.M.; Rus, L.L.; Virchea, L.-I.; Morgovan, C.; Chis, A.A.; Arseniu, A.M.; Butuca, A.; Gligor, F.G.; Vicas, L.G.; et al. Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing. Nutrients 2022, 14, 3065. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. Physicochemical Characterization and Evaluation of Gastrointestinal In Vitro Behavior of Alginate-Based Microbeads with Encapsulated Grape Pomace Extracts. Pharmaceutics 2023, 15, 980. [Google Scholar] [CrossRef]
- Mansour, G.; Ghanem, C.; Mercenaro, L.; Nassif, N.; Hassoun, G.; Del Caro, A. Effects of altitude on the chemical composition of grapes and wine: A review. OENO One 2022, 56, 227–239. [Google Scholar] [CrossRef]
- Sharafan, M.; Malinowska, M.A.; Ekiert, H.; Kwásniak, B.; Sikora, E.; Szopa, A. Vitis vinifera (Vine Grape) as a Valuable Cosmetic Raw Material. Pharmaceutics 2023, 15, 1372. [Google Scholar] [CrossRef]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; CastroLópez, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martínez-Ávila, G.C.G. Grape Pomace—Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef]
- Đurović, V.; Mandić, L.; Igrošanac, M.; Radovanović, M.; Pešaković, M.; Mladenović, J.; Đukić, D. Celery (Apium graveolens L.) as a source of phytochemicals with antioxidant and antibacterial effects. In Proceedings of the 1st International Symposium on Biotechnology, University of Kragujevac, Faculty of Agronomy Čačak, Kragujevac, Serbia, 17–18 March 2023; pp. 315–322, ISBN 978-86-87611-88-7. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Đurović, V.; Radovanović, M.; Mandić, L.; Knežević, D.; Zornić, V.; Đukić, D. Chemical and microbial evaluation of biscuits made from wheat flour substituted with wheat sprout. Food Sci. Technol. Int. 2021, 27, 172–183. [Google Scholar] [CrossRef]
- Mašković, P.; Veličković, V.; Mitić, M.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc- Gajić, J.; Vujić, J. Summer savory extracts prepared by novel extraction methods resulted in enhanced biological activity. Ind. Crops Prod. 2017, 109, 875–881. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 12 August 2024).
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolic Compounds with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- ISO 18787:2017; Foodstuffs–Determination of Water Activity. ISO: Geneva, Switzerland, 2017.
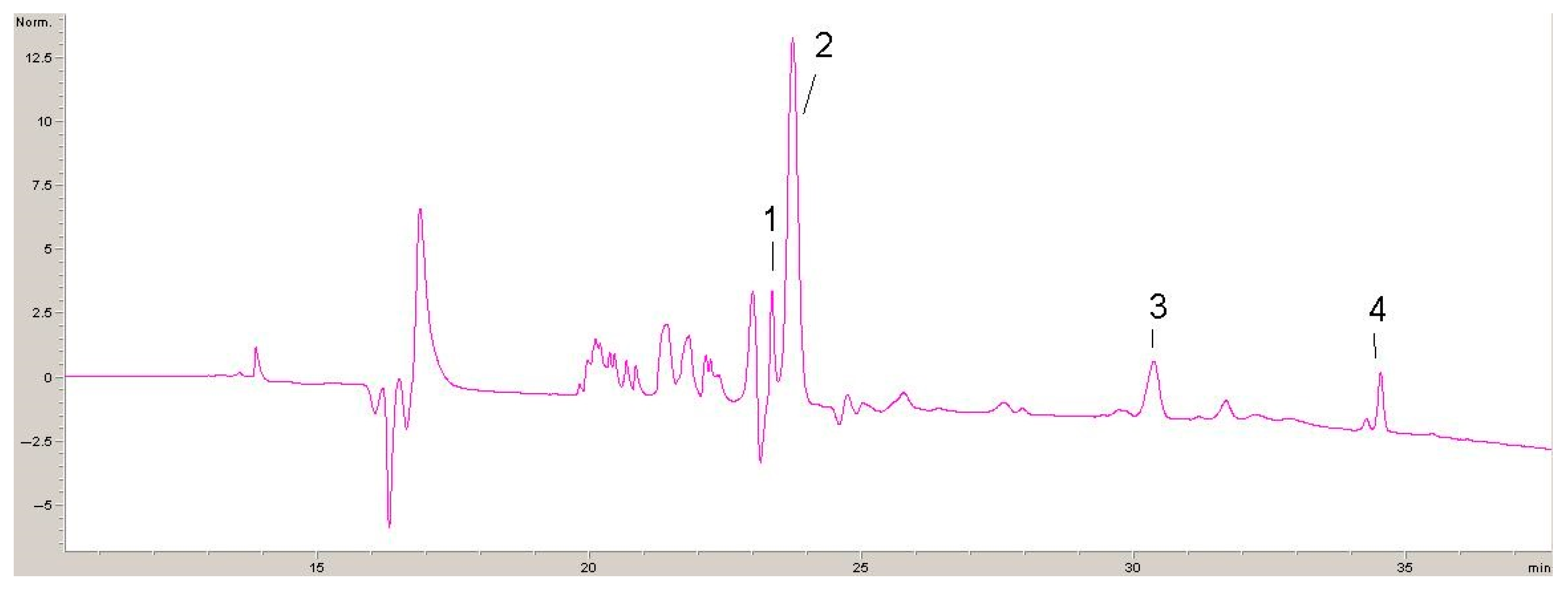
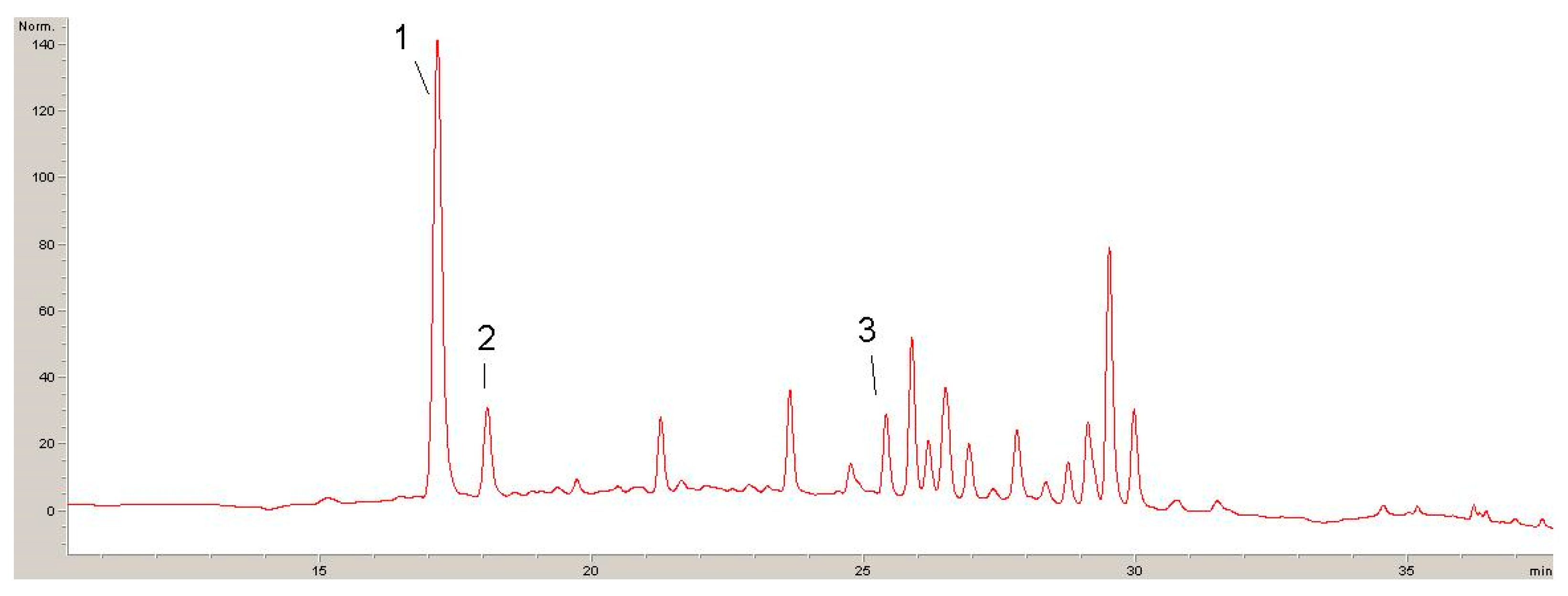
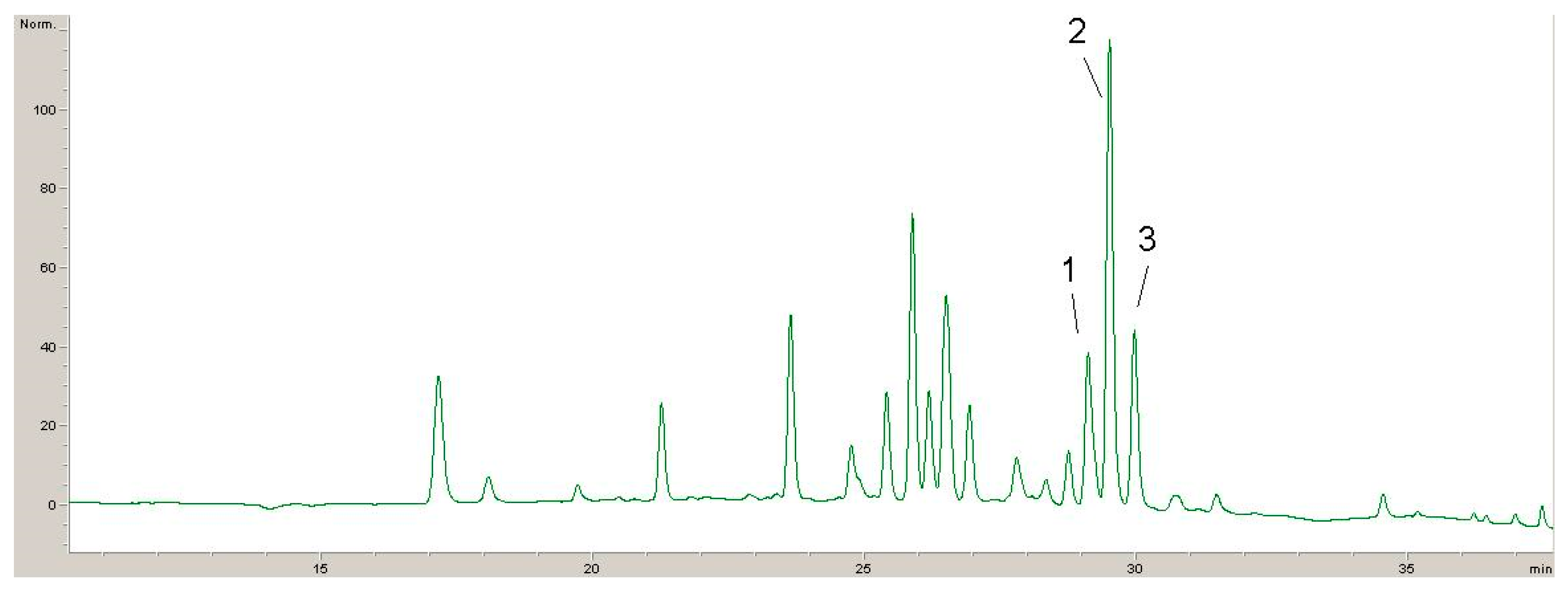
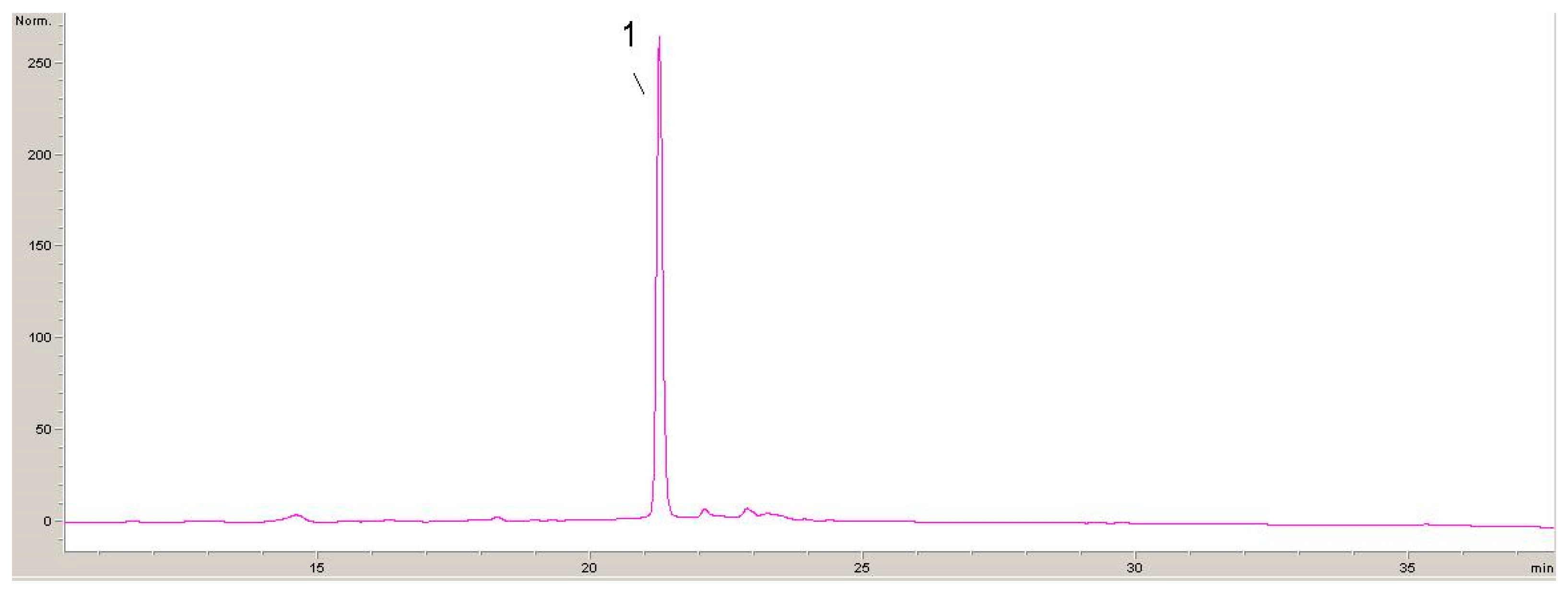
| Compound | Calibration Curve | (R2) | LOD µg/mL | LOQ µg/mL |
|---|---|---|---|---|
| Caffeic acid | 0.9998 | 0.08 | 0.26 | |
| Chlorogenic acid | 0.9992 | 0.01 | 0.04 | |
| p-Coumaric acid | 0.9997 | 0.04 | 0.14 | |
| Rutin | 0.9995 | 0.05 | 0.17 | |
| Hyperoside | 0.9990 | 0.03 | 0.10 | |
| Isoquercetin | 0.9996 | 0.03 | 0.10 | |
| Cyanidin-glucoside | 0.9992 | 0.03 | 0.10 | |
| Petunidin-glucoside | 0.9992 | 0.02 | 0.07 | |
| Malvidin-glucoside | 0.9995 | 0.02 | 0.07 |
| Compound | Content (mg/g) |
|---|---|
| Malvidin-3-glucoside | 2.388 |
| Malvidin-acetyl-glucoside | 0.359 |
| Petunidin-3-glucoside | 0.268 |
| Malvidin-coumaroyl-glucoside | 0.174 |
| Summary | 3.189 |
| Compound | Content (mg/g) |
|---|---|
| Cyanidin derivative | 3.730 |
| Hyperoside (que-galactoside) | 2.319 |
| Rutin | 0.944 |
| Isoquercetin (que-glucoside) | 0.909 |
| Caffeic acid | 0.468 |
| Chlorogenic acid | 0.235 |
| Summary | 8.605 |
| Phytocomplex GP + DE | Content (mg/g) |
|---|---|
| Cyanidin-3 glucoside | 4.134 |
| Hyperoside (que-galactoside) | 2.454 |
| Petunidin-3-glucoside | 2.348 |
| Rutin | 0.996 |
| Isoquercetin (que-glucoside) | 0.991 |
| Caffeic acid | 0.497 |
| Chlorogenic acid | 0.241 |
| Malvidin-coumaroyl-glucoside | 0.192 |
| p-Coumaric acid | 0.144 |
| Cyanidin derivative | 0.059 |
| Cyanidin derivative | 0.049 |
| Malvidin-acetyl-glucoside | 0.045 |
| Samples (Lyophilised Plants) | Parameters | Unit of Measure | Results | Method | Found Contaminants |
|---|---|---|---|---|---|
| Grape Pomace (GP) | Aerobic colony count | cfu/g | 170 | SRPS EN ISO 4833-1:2014 [75] | None |
| Enterobacteriaceae | cfu/g | <10 | SRPS EN ISO 21528-2:2017 [76] | ||
| Coagulase-positive staphylococci (CNS) | cfu/g | <10 | SRPS EN ISO 6888-1:2021 [77] | ||
| Salmonella spp. | in 25 g | Not detected | SRPS EN ISO 6579-1:2017 [78] | ||
| Total coliforms | cfu/g | <10 | SRPS ISO 4832:2014 [79] | ||
| Listeria monocytogenes | in 25 g | Not detected | SRPS EN ISO 11290-1:2017 [80] | ||
| Escherichia coli | cfu/g | <10 | SRPS ISO 16649-2:2008 [81] | ||
| Yeasts | cfu/g | <10 | SRPS ISO 21527-2:2011 [82] | ||
| Moulds | cfu/g | <10 | SRPS ISO 21527-2:2011 [83] | ||
| Bacillus cereus | cfu/g | <10 | SRPS EN ISO 7932:2009 [84] | ||
| Sulphite-reducing clostridia | cfu/g | <10 | SRPS ISO 15213:2011 [85] | ||
| Shiga toxin-producing Escherichia coli (STEC) | in 25 g | Not detected | SRPS CEN ISO/TS 13136:2014 [86] | ||
| Danewort, Dwarf Elder (Sambucus ebulus L.) (DE) | Aerobic colony count | cfu/g | 1700 | SRPS EN ISO 4833-1:2014 [75] | None |
| Enterobacteriaceae | cfu/g | <10 | SRPS EN ISO 21528-2:2017 [76] | ||
| Coagulase-positive staphylococci (CNS) | cfu/g | <10 | SRPS EN ISO 6888-1:2021 [77] | ||
| Salmonella spp. | in 25 g | Not detected | SRPS EN ISO 6579-1:2017 [78] | ||
| Total coliforms | cfu/g | <10 | SRPS ISO 4832:2014 [79] | ||
| Listeria monocytogenes | in 25 g | Not detected | SRPS EN ISO 11290-1:2017 [80] | ||
| Escherichia coli | cfu/g | <10 | SRPS ISO 16649-2:2008 [81] | ||
| Yeasts | cfu/g | 60 | SRPS ISO 21527-2:2011 [82] | ||
| Moulds | cfu/g | <10 | SRPS ISO 21527-2:2011 [83] | ||
| Bacillus cereus | cfu/g | <10 | SRPS EN ISO 7932:2009 [84] | ||
| Sulphite-reducing clostridia | cfu/g | <10 | SRPS ISO 15213:2011 [85] | ||
| Shiga toxin-producing Escherichia coli (STEC) | in 25 g | Not detected | SRPS CEN ISO/TS 13136:2014 [86] |
| Tested Samples (Lyophilised Plants) | GP | DE |
|---|---|---|
| aw values | 0.310 ± 0.002 a | 0.257 ± 0.005 b |
| Tested Bacterial Strains (Conventional, Standard Strains) | GP | DE | COCKTAIL (GP + DE) | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| Listeria monocytogenes ATCC 13932 | 3.125 | >6.250 | 6.250 | >12.500 | 3.125 | >12.500 |
| Bacillus subtilis ATCC 6633 | 6.250 | >12.500 | 6.250 | >12.500 | 3.125 | >12.500 |
| Pseudomonas aeruginosa ATCC 27853 | 12.500 | >25.000 | 12.500 | >25.000 | 6.250 | 25.000 |
| Proteus mirabilis ATCC 35659 | 12.500 | >25.000 | 12.500 | >25.000 | 6.250 | 25.000 |
| Salmonella enteritidis ATCC 13076 | 6.250 | >12.500 | 6.250 | >12.500 | 6.250 | >12.500 |
| Enterococcus faecalis WDCM 00009 VT 000096 vitroides | 0.780 | >1.560 | 3.125 | >6.250 | 3.125 | >12.500 |
| Staphylococcus aureus ATCC 25923 | 3.125 | >6.250 | 3.125 | >6.250 | 3.125 | >12.500 |
| Escherichia coli WDCM 00012 vitroides | 6.250 | >12.500 | 6.250 | >12.500 | 3.125 | >12.500 |
| XDR bacteria | ||||||
| Klebsiella spp. | / | / | / | / | 12.500 | >50.000 |
| Acinetobacter baumannii complex | / | / | / | / | 6.250 | 50.500 |
| Strain Designation | AMA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gen | Ami | Tob | Pip | Ert | Ced | Imi | Mer | Chl | Cer | Cef | Cep | Cip | Lev | Tri | Amp | Acl | Col | |
| 7840 | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| Strain Designation | AMA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gen | Ami | Tob | Imi | Mer | Cip | Lev | Tri | Col | |
| 7849 | R | R | R | R | R | R | R | R | S |
| Plant Material (Lyophilisates) | Total Phenolic Content (mg GAE/g) | DPPH (Inhibition, %) |
|---|---|---|
| Grape Pomace | 11.26 ± 0.98 b | 92.14 ± 1.13 a |
| Danewort, Dwarf Elder (Sambucus ebulus L.) | 22.93 ± 0.21 a | 66.71 ± 1.17 b |
| Phytocomplex (cocktail of Grape Pomace and Dwarf Elder) | 21.22 ± 1.20 a | 69.15 ± 1.39 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurćubić, V.S.; Đurović, V.; Stajić, S.B.; Dmitrić, M.; Živković, S.; Kurćubić, L.V.; Mašković, P.Z.; Mašković, J.; Mitić, M.; Živković, V.; et al. Multitarget Phytocomplex: Focus on Antibacterial Profiles of Grape Pomace and Sambucus ebulus L. Lyophilisates Against Extensively Drug-Resistant (XDR) Bacteria and In Vitro Antioxidative Power. Antibiotics 2024, 13, 980. https://doi.org/10.3390/antibiotics13100980
Kurćubić VS, Đurović V, Stajić SB, Dmitrić M, Živković S, Kurćubić LV, Mašković PZ, Mašković J, Mitić M, Živković V, et al. Multitarget Phytocomplex: Focus on Antibacterial Profiles of Grape Pomace and Sambucus ebulus L. Lyophilisates Against Extensively Drug-Resistant (XDR) Bacteria and In Vitro Antioxidative Power. Antibiotics. 2024; 13(10):980. https://doi.org/10.3390/antibiotics13100980
Chicago/Turabian StyleKurćubić, Vladimir S., Vesna Đurović, Slaviša B. Stajić, Marko Dmitrić, Saša Živković, Luka V. Kurćubić, Pavle Z. Mašković, Jelena Mašković, Milan Mitić, Vladimir Živković, and et al. 2024. "Multitarget Phytocomplex: Focus on Antibacterial Profiles of Grape Pomace and Sambucus ebulus L. Lyophilisates Against Extensively Drug-Resistant (XDR) Bacteria and In Vitro Antioxidative Power" Antibiotics 13, no. 10: 980. https://doi.org/10.3390/antibiotics13100980
APA StyleKurćubić, V. S., Đurović, V., Stajić, S. B., Dmitrić, M., Živković, S., Kurćubić, L. V., Mašković, P. Z., Mašković, J., Mitić, M., Živković, V., & Jakovljević, V. (2024). Multitarget Phytocomplex: Focus on Antibacterial Profiles of Grape Pomace and Sambucus ebulus L. Lyophilisates Against Extensively Drug-Resistant (XDR) Bacteria and In Vitro Antioxidative Power. Antibiotics, 13(10), 980. https://doi.org/10.3390/antibiotics13100980










