Novel Fosfomycin Resistance Mechanism in Pseudomonas entomophila Due to Atypical Pho Regulon Control of GlpT
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fosfomycin Resistance in P. entomophila Involves Mechanisms Beyond glpT Inactivating Mutations
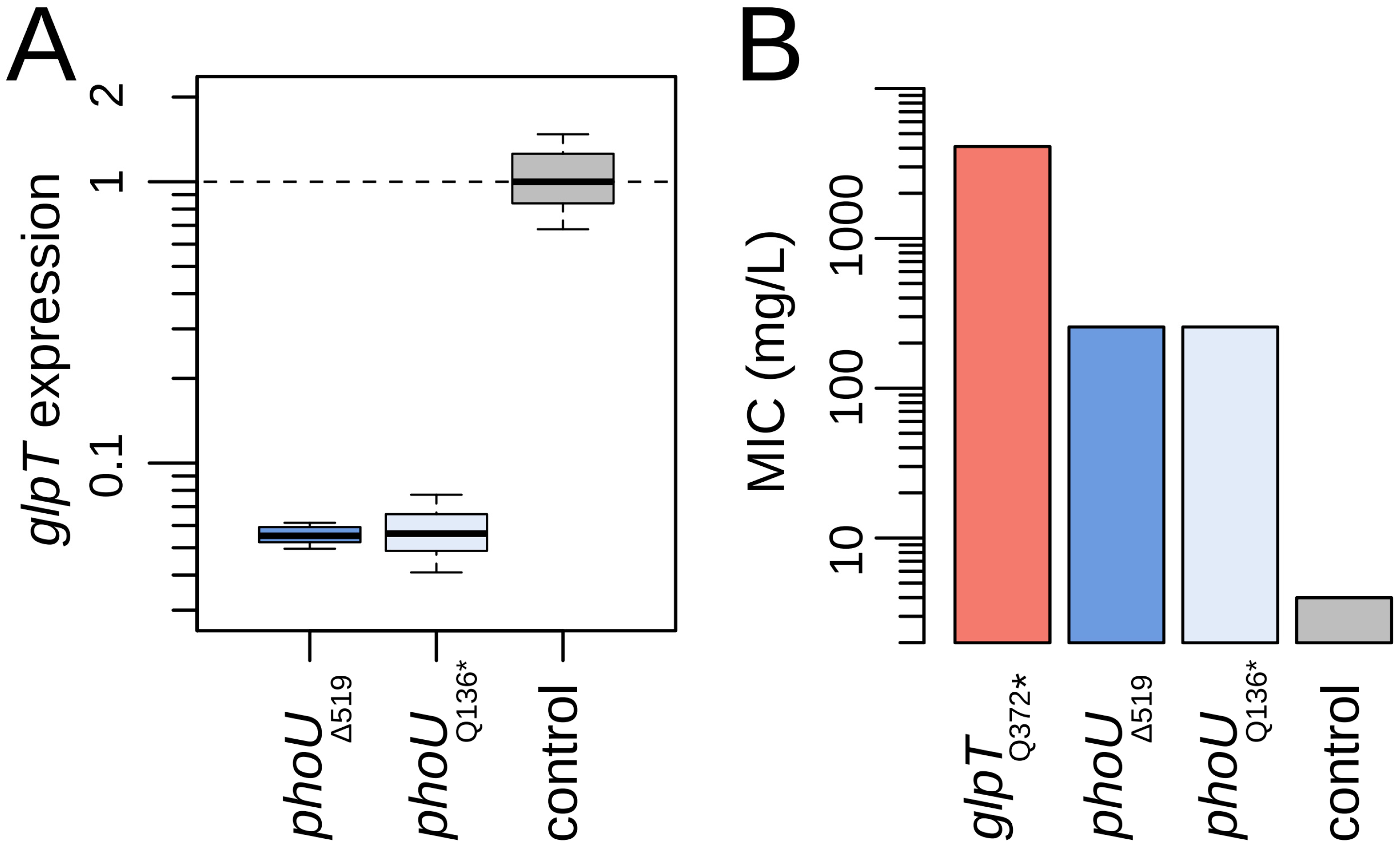
2.2. Mutations in phoU Confer Fosfomycin Resistance in P. entomophila by Reducing GlpT Expression
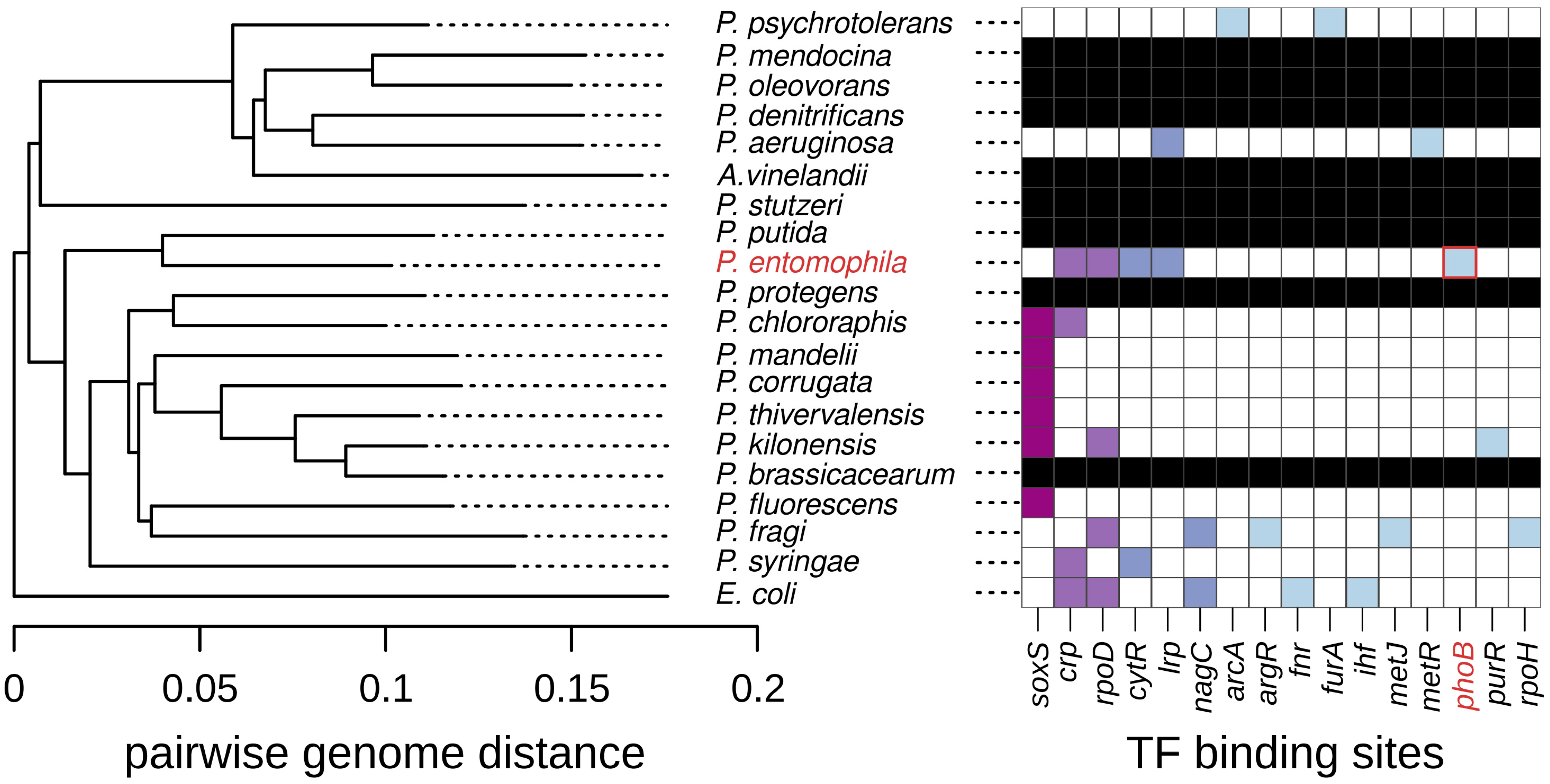
2.3. Control of GlpT by the Pho Regulon Seems Peculiar to P. entomophila
3. Materials and Methods
3.1. Isolation of Spontaneous Fosfomycin Resistant Mutants
3.2. Antimicrobial Susceptibility Testing
3.3. Sanger and Whole-Genome Sequencing
3.4. Quantitative Real-Time PCR
3.5. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vodovar, N.; Vallenet, D.; Cruveiller, S.; Rouy, Z.; Barbe, V.; Acosta, C.; Cattolico, L.; Jubin, C.; Lajus, A.; Segurens, B.; et al. Complete Genome Sequence of the Entomopathogenic and Metabolically Versatile Soil Bacterium Pseudomonas entomophila. Nat. Biotechnol. 2006, 24, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Vodovar, N.; Vinals, M.; Liehl, P.; Basset, A.; Degrouard, J.; Spellman, P.; Boccard, F.; Lemaitre, B. Drosophila Host Defense after Oral Infection by an Entomopathogenic Pseudomonas Species. Proc. Natl. Acad. Sci. USA 2005, 102, 11414–11419. [Google Scholar] [CrossRef] [PubMed]
- Dieppois, G.; Opota, O.; Lalucat, J.; Lemaitre, B. Pseudomonas entomophila: A Versatile Bacterium with Entomopathogenic Properties. In Pseudomonas: Volume 7: New Aspects of Pseudomonas Biology; Ramos, J.-L., Goldberg, J.B., Filloux, A., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 25–49. ISBN 978-94-017-9555-5. [Google Scholar]
- Takishita, Y.; Charron, J.-B.; Smith, D.L. Biocontrol Rhizobacterium Pseudomonas sp. 23S Induces Systemic Resistance in Tomato (Solanum lycopersicum L.) Against Bacterial Canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2018, 9, 909. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Gely, I.; Opota, O.; Boniface, A.; Novikov, A.; Lemaitre, B. A Secondary Metabolite Acting as a Signalling Molecule Controls Pseudomonas entomophila Virulence. Cell. Microbiol. 2010, 12, 1666–1679. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.E.; Faria, V.G.; Teixeira, L.; Magalhães, S.; Sucena, É. Host Adaptation Is Contingent upon the Infection Route Taken by Pathogens. PLoS Pathog. 2013, 9, e1003601. [Google Scholar] [CrossRef]
- Ragheb, R.; Chuyen, A.; Torres, M.; Defaye, A.; Seyres, D.; Kremmer, L.; Fernandez-Nunez, N.; Tricoire, H.; Rihet, P.; Nguyen, C.; et al. Interplay between Trauma and Pseudomonas entomophila Infection in Flies: A Central Role of the JNK Pathway and of CrebA. Sci. Rep. 2017, 7, 16222. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Liehl, P.; Buchon, N.; Lemaitre, B. Infection-Induced Host Translational Blockage Inhibits Immune Responses and Epithelial Renewal in the Drosophila Gut. Cell Host Microbe 2012, 12, 60–70. [Google Scholar] [CrossRef]
- Opota, O.; Vallet-Gély, I.; Vincentelli, R.; Kellenberger, C.; Iacovache, I.; Gonzalez, M.R.; Roussel, A.; van der Goot, F.-G.; Lemaitre, B. Monalysin, a Novel ß-Pore-Forming Toxin from the Drosophila Pathogen Pseudomonas entomophila, Contributes to Host Intestinal Damage and Lethality. PLoS Pathog. 2011, 7, e1002259. [Google Scholar] [CrossRef]
- Acken, K.A.; Li, B. Pseudomonas Virulence Factor Controls Expression of Virulence Genes in Pseudomonas entomophila. PLoS ONE 2023, 18, e0284907. [Google Scholar] [CrossRef]
- Ahlawat, N.; Geeta Arun, M.; Maggu, K.; Prasad, N.G. Enemies Make You Stronger: Coevolution between Fruit Fly Host and Bacterial Pathogen Increases Postinfection Survivorship in the Host. Ecol. Evol. 2021, 11, 9563–9574. [Google Scholar] [CrossRef]
- Patel, S.S.; Balfour, J.A.; Bryson, H.M. Fosfomycin Tromethamine. A Review of Its Antibacterial Activity, Pharmacokinetic Properties and Therapeutic Efficacy as a Single-Dose Oral Treatment for Acute Uncomplicated Lower Urinary Tract Infections. Drugs 1997, 53, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Giannopoulou, K.P.; Kokolakis, G.N.; Rafailidis, P.I. Fosfomycin: Use beyond Urinary Tract and Gastrointestinal Infections. Clin. Infect. Dis. 2008, 46, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Mirakhur, A.; Gallagher, M.J.; Ledson, M.J.; Hart, C.A.; Walshaw, M.J. Fosfomycin Therapy for Multiresistant Pseudomonas Aeruginosa in Cystic Fibrosis. J. Cyst. Fibros. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.I.; Berg, O.G.; Aspevall, O.; Kahlmeter, G.; Andersson, D.I. Biological Costs and Mechanisms of Fosfomycin Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2003, 47, 2850–2858. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, T.; Mistry, A.; Wonacott, A.; Hutchinson, S.E.; Kelly, V.A.; Duncan, K. Structure of UDP-N-Acetylglucosamine Enolpyruvyl Transferase, an Enzyme Essential for the Synthesis of Bacterial Peptidoglycan, Complexed with Substrate UDP-N-Acetylglucosamine and the Drug Fosfomycin. Structure 1996, 4, 1465–1474. [Google Scholar] [CrossRef]
- Couce, A.; Briales, A.; Rodríguez-Rojas, A.; Costas, C.; Pascual, A.; Blázquez, J. Genomewide Overexpression Screen for Fosfomycin Resistance in Escherichia coli: MurA Confers Clinical Resistance at Low Fitness Cost. Antimicrob. Agents Chemother. 2012, 56, 2767–2769. [Google Scholar] [CrossRef]
- Takahata, S.; Ida, T.; Hiraishi, T.; Sakakibara, S.; Maebashi, K.; Terada, S.; Muratani, T.; Matsumoto, T.; Nakahama, C.; Tomono, K. Molecular Mechanisms of Fosfomycin Resistance in Clinical Isolates of Escherichia coli. Int. J. Antimicrob. Agents 2010, 35, 333–337. [Google Scholar] [CrossRef]
- Silver, L.L. Fosfomycin: Mechanism and Resistance. Cold Spring Harb. Perspect. Med. 2017, 7, a025262. [Google Scholar] [CrossRef]
- Winkler, H.H. Distribution of an Inducible Hexose-Phosphate Transport System among Various Bacteria. J. Bacteriol. 1973, 116, 1079–1081. [Google Scholar] [CrossRef]
- Rojo, F. Carbon Catabolite Repression in Pseudomonas: Optimizing Metabolic Versatility and Interactions with the Environment. FEMS Microbiol. Rev. 2010, 34, 658–684. [Google Scholar] [CrossRef]
- Castañeda-García, A.; Rodríguez-Rojas, A.; Guelfo, J.R.; Blázquez, J. The Glycerol-3-Phosphate Permease GlpT Is the Only Fosfomycin Transporter in Pseudomonas Aeruginosa. J. Bacteriol. 2009, 191, 6968–6974. [Google Scholar] [CrossRef] [PubMed]
- Couce, A.; Rodríguez-Rojas, A.; Blázquez, J. Determinants of Genetic Diversity of Spontaneous Drug Resistance in Bacteria. Genetics 2016, 203, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rojas, A.; Blázquez, J. The Pseudomonas Aeruginosa pfpI Gene Plays an Antimutator Role and Provides General Stress Protection. J. Bacteriol. 2009, 191, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.R.; Morero, N.R.; Miguel, V.; Argaraña, C.E. nfxB as a Novel Target for Analysis of Mutation Spectra in Pseudomonas aeruginosa. PLoS ONE 2013, 8, e66236. [Google Scholar] [CrossRef]
- Reyrat, J.-M.; Pelicic, V.; Gicquel, B.; Rappuoli, R. Counterselectable Markers: Untapped Tools for Bacterial Genetics and Pathogenesis. Infect. Immun. 1998, 66, 4011–4017. [Google Scholar] [CrossRef]
- Penn, W.D.; McKee, A.G.; Kuntz, C.P.; Woods, H.; Nash, V.; Gruenhagen, T.C.; Roushar, F.J.; Chandak, M.; Hemmerich, C.; Rusch, D.B.; et al. Probing Biophysical Sequence Constraints within the Transmembrane Domains of Rhodopsin by Deep Mutational Scanning. Sci. Adv. 2020, 6, eaay7505. [Google Scholar] [CrossRef]
- Schweizer, H.; Boos, W.; Larson, T.J. Repressor for the Sn-Glycerol-3-Phosphate Regulon of Escherichia coli K-12: Cloning of the glpR Gene and Identification of Its Product. J. Bacteriol. 1985, 161, 563–566. [Google Scholar] [CrossRef]
- Yang, B.; Gerhardt, S.G.; Larson, T.J. Action at a Distance for Glp Repressor Control of glpTQ Transcription in Escherichia coli K-12. Mol. Microbiol. 1997, 24, 511–521. [Google Scholar] [CrossRef]
- Lin, E.C. Glycerol Dissimilation and Its Regulation in Bacteria. Annu. Rev. Microbiol. 1976, 30, 535–578. [Google Scholar] [CrossRef]
- Santos-Beneit, F. The Pho Regulon: A Huge Regulatory Network in Bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef]
- Wanner, B.L. Gene Regulation by Phosphate in Enteric Bacteria. J. Cell. Biochem. 1993, 51, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Lee, E.-J. PhoU: A Multifaceted Regulator in Microbial Signaling and Homeostasis. Curr. Opin. Microbiol. 2024, 77, 102401. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Stock, A.M. Temporal Hierarchy of Gene Expression Mediated by Transcription Factor Binding Affinity and Activation Dynamics. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jeong, G.; Choi, E.; Lee, E.-J. A Dual Regulatory Role of the PhoU Protein in Salmonella Typhimurium. mBio 2022, 13, e00811-22. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, T.-W.; Wen, S.-Y.; Chang, C.-Y.; Tsai, S.-F.; Wu, W.-F.; Chang, C.-H. Genome-Wide PhoB Binding and Gene Expression Profiles Reveal the Hierarchical Gene Regulatory Network of Phosphate Starvation in Escherichia coli. PLoS ONE 2012, 7, e47314. [Google Scholar] [CrossRef]
- Golla, V.K.; Sans-Serramitjana, E.; Pothula, K.R.; Benier, L.; Bafna, J.A.; Winterhalter, M.; Kleinekathöfer, U. Fosfomycin Permeation through the Outer Membrane Porin OmpF. Biophys. J. 2019, 116, 258–269. [Google Scholar] [CrossRef]
- Bourret, R.B. Learning from Adversity? J. Bacteriol. 2017, 199, e00420-17. [Google Scholar] [CrossRef]
- Özen, A.I.; Ussery, D.W. Defining the Pseudomonas Genus: Where Do We Draw the Line with Azotobacter? Microb. Ecol. 2012, 63, 239–248. [Google Scholar] [CrossRef]
- Vallenet, D.; Labarre, L.; Rouy, Z.; Barbe, V.; Bocs, S.; Cruveiller, S.; Lajus, A.; Pascal, G.; Scarpelli, C.; Médigue, C. MaGe: A Microbial Genome Annotation System Supported by Synteny Results. Nucleic Acids Res. 2006, 34, 53–65. [Google Scholar] [CrossRef]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative Stress in Bacteria and Protein Damage by Reactive Oxygen Species. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2000, 3, 3–8. [Google Scholar]
- Solovyev, V.; Salamov, A.; Seledtsov, I.; Vorobyev, D.; Bachinsky, A. Automatic annotation of bacterial community sequences and application to infections diagnostic. In Proceedings of the International Conference on Bioinformatics Models, Methods, and Algorithms, Rome, Italy, 26–29 January 2011; Pellegrini, M., Fred, A.L.N., Filipe, J., Gamboa, H., Eds.; SciTePress: Setúbal, Portugal, 2011; pp. 346–353. [Google Scholar]
- Candy, D.J.; Kilby, B.A. Insect Biochemistry and Function; Chapman and Hall; Wiley: London, UK; New York, NY, USA, 1975; ISBN 978-0-470-13347-7. [Google Scholar]
- Miyagi, A.; Kawai-Yamada, M.; Uchimiya, M.; Ojima, N.; Suzuki, K.; Uchimiya, H. Metabolome Analysis of Food-Chain between Plants and Insects. Metabolomics 2013, 9, 1254–1261. [Google Scholar] [CrossRef]
- Kato, A.; Mitrophanov, A.Y.; Groisman, E.A. A Connector of Two-Component Regulatory Systems Promotes Signal Amplification and Persistence of Expression. Proc. Natl. Acad. Sci. USA 2007, 104, 12063–12068. [Google Scholar] [CrossRef] [PubMed]
- Winfield, M.D.; Groisman, E.A. Phenotypic Differences between Salmonella and Escherichia coli Resulting from the Disparate Regulation of Homologous Genes. Proc. Natl. Acad. Sci. USA 2004, 101, 17162–17167. [Google Scholar] [CrossRef] [PubMed]
- Winfield, M.D.; Latifi, T.; Groisman, E.A. Transcriptional Regulation of the 4-Amino-4-Deoxy-L-Arabinose Biosynthetic Genes in Yersinia Pestis*. J. Biol. Chem. 2005, 280, 14765–14772. [Google Scholar] [CrossRef] [PubMed]
- Wray, G.A. The Evolutionary Significance of Cis-Regulatory Mutations. Nat. Rev. Genet. 2007, 8, 206–216. [Google Scholar] [CrossRef]
- Perez, J.C.; Groisman, E.A. Evolution of Transcriptional Regulatory Circuits in Bacteria. Cell 2009, 138, 233–244. [Google Scholar] [CrossRef]
- Oren, Y.; Smith, M.B.; Johns, N.I.; Kaplan Zeevi, M.; Biran, D.; Ron, E.Z.; Corander, J.; Wang, H.H.; Alm, E.J.; Pupko, T. Transfer of Noncoding DNA Drives Regulatory Rewiring in Bacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 16112–16117. [Google Scholar] [CrossRef]
- Trouillon, J.; Sentausa, E.; Ragno, M.; Robert-Genthon, M.; Lory, S.; Attrée, I.; Elsen, S. Species-Specific Recruitment of Transcription Factors Dictates Toxin Expression. Nucleic Acids Res. 2020, 48, 2388–2400. [Google Scholar] [CrossRef]
- Keshavarz, M.; Zanchi, C.; Rolff, J. The Effect of Combined Knockdowns of Attacins on Survival and Bacterial Load in Tenebrio Molitor. Front. Immunol. 2023, 14, 1140627. [Google Scholar] [CrossRef]
- M07 Ed12|Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 12th Edition. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 9 May 2024).
- Hombach, M.; Maurer, F.P.; Pfiffner, T.; Böttger, E.C.; Furrer, R. Standardization of Operator-Dependent Variables Affecting Precision and Accuracy of the Disk Diffusion Method for Antibiotic Susceptibility Testing. J. Clin. Microbiol. 2015, 53, 3864–3869. [Google Scholar] [CrossRef]
- Deatherage, D.E.; Barrick, J.E. Identification of Mutations in Laboratory-Evolved Microbes from next-Generation Sequencing Data Using Breseq. Methods Mol. Biol. 2014, 1151, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, B.; Colley, B.; Klebensberger, J.; McDougald, D.; Rice, S.A. Expression Stability of 13 Housekeeping Genes during Carbon Starvation of Pseudomonas Aeruginosa. J. Microbiol. Methods 2016, 127, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast Genome and Metagenome Distance Estimation Using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef]
- Simonsen, M.; Mailund, T.; Pedersen, C.N.S. Rapid Neighbour-Joining. In Algorithms in Bioinformatics; Crandall, K.A., Lagergren, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 113–122. [Google Scholar]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R Language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]

| Position | Base Change | Codon | glpT Mutations | Effect | Domain |
|---|---|---|---|---|---|
| 196 | Δ14 bp | 65 | Q65 Δ14 bp | frameshift | transmembrane |
| 305 | t → g | 103 | L102R | missense | transmembrane |
| 399 | g → a | 133 | W133* | nonsense | transmembrane |
| 511 | g → a | 171 | G171D | missense | transmembrane |
| 549 | g → t | 183 | W183C | missense | transmembrane |
| 808 | c → a | 270 | R270S | missense | transmembrane |
| 961 | +4 bp | 321 | N321 + 4 bp | frameshift | Intracellular loop |
| 1114 | c → t | 372 | Q372* | nonsense | transmembrane |
| 1221 | c → a | 407 | Y407* | nonsense | transmembrane |
| 1268 | +4 bp | 423 | S423 + 4 bp | frameshift | transmembrane |
| 1268 | Δ4 bp | 423 | S423 Δ4 bp | frameshift | transmembrane |
| 1268 | Δ4 bp | 423 | S423 Δ4 bp | frameshift | transmembrane |
| 1268 | Δ4 bp | 423 | S423 Δ4 bp | frameshift | transmembrane |
| 1268 | Δ4 bp | 423 | S423 Δ4 bp | frameshift | transmembrane |
| - | - | - | none | - | - |
| - | - | - | none | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Maroto, L.; Gella, P.; Couce, A. Novel Fosfomycin Resistance Mechanism in Pseudomonas entomophila Due to Atypical Pho Regulon Control of GlpT. Antibiotics 2024, 13, 1008. https://doi.org/10.3390/antibiotics13111008
Sánchez-Maroto L, Gella P, Couce A. Novel Fosfomycin Resistance Mechanism in Pseudomonas entomophila Due to Atypical Pho Regulon Control of GlpT. Antibiotics. 2024; 13(11):1008. https://doi.org/10.3390/antibiotics13111008
Chicago/Turabian StyleSánchez-Maroto, Laura, Pablo Gella, and Alejandro Couce. 2024. "Novel Fosfomycin Resistance Mechanism in Pseudomonas entomophila Due to Atypical Pho Regulon Control of GlpT" Antibiotics 13, no. 11: 1008. https://doi.org/10.3390/antibiotics13111008
APA StyleSánchez-Maroto, L., Gella, P., & Couce, A. (2024). Novel Fosfomycin Resistance Mechanism in Pseudomonas entomophila Due to Atypical Pho Regulon Control of GlpT. Antibiotics, 13(11), 1008. https://doi.org/10.3390/antibiotics13111008






