Antibiotic Residues in Milk and Milk-Based Products Served in Kuwait Hospitals: Multi-Hazard Risk Assessment
Abstract
:1. Introduction
2. Results
2.1. Frequency of Antibiotic Residues in Milk and Milk Product Samples
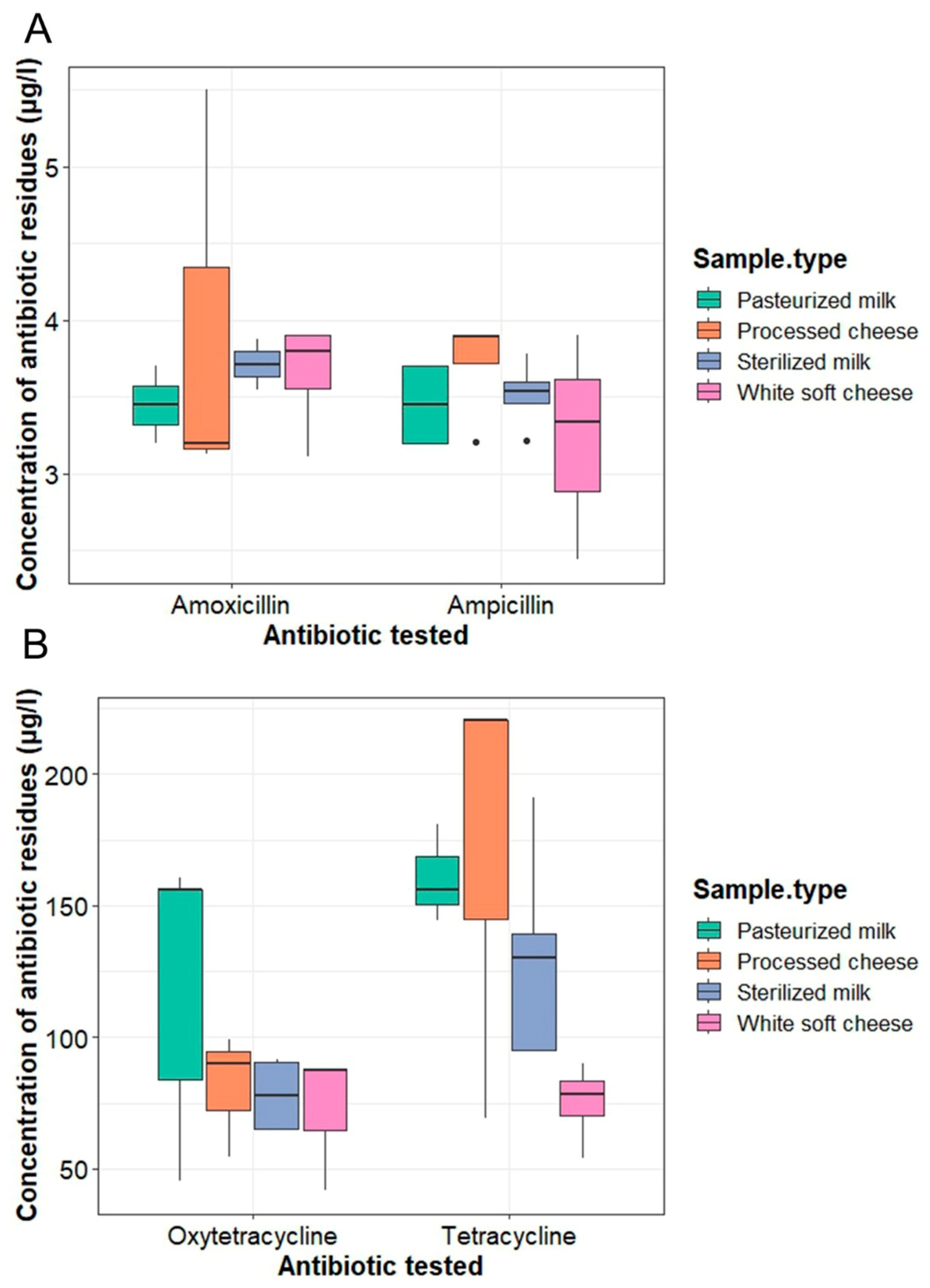
2.2. Concentrations of Antibiotic Residues in Milk and Milk Product Samples
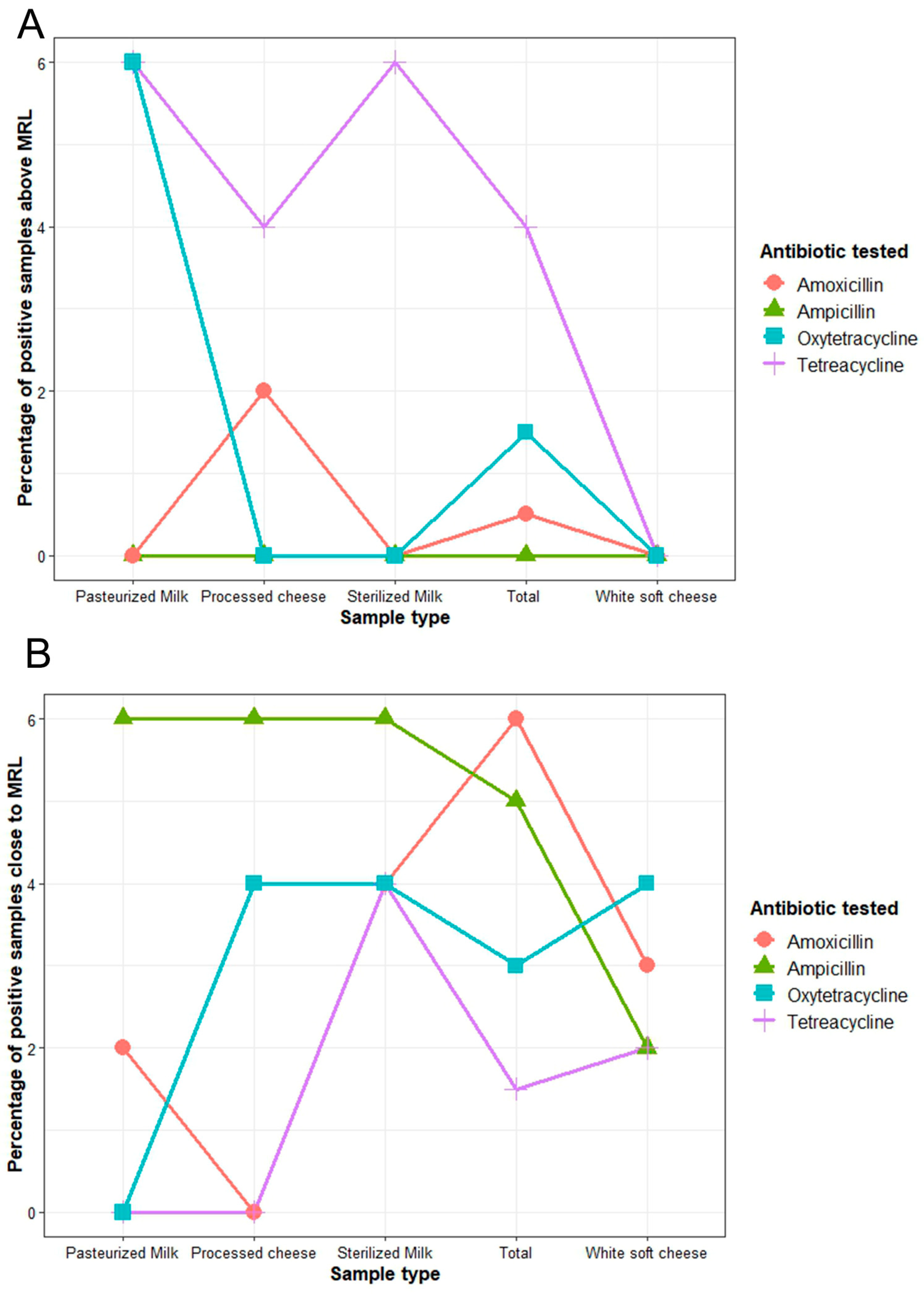
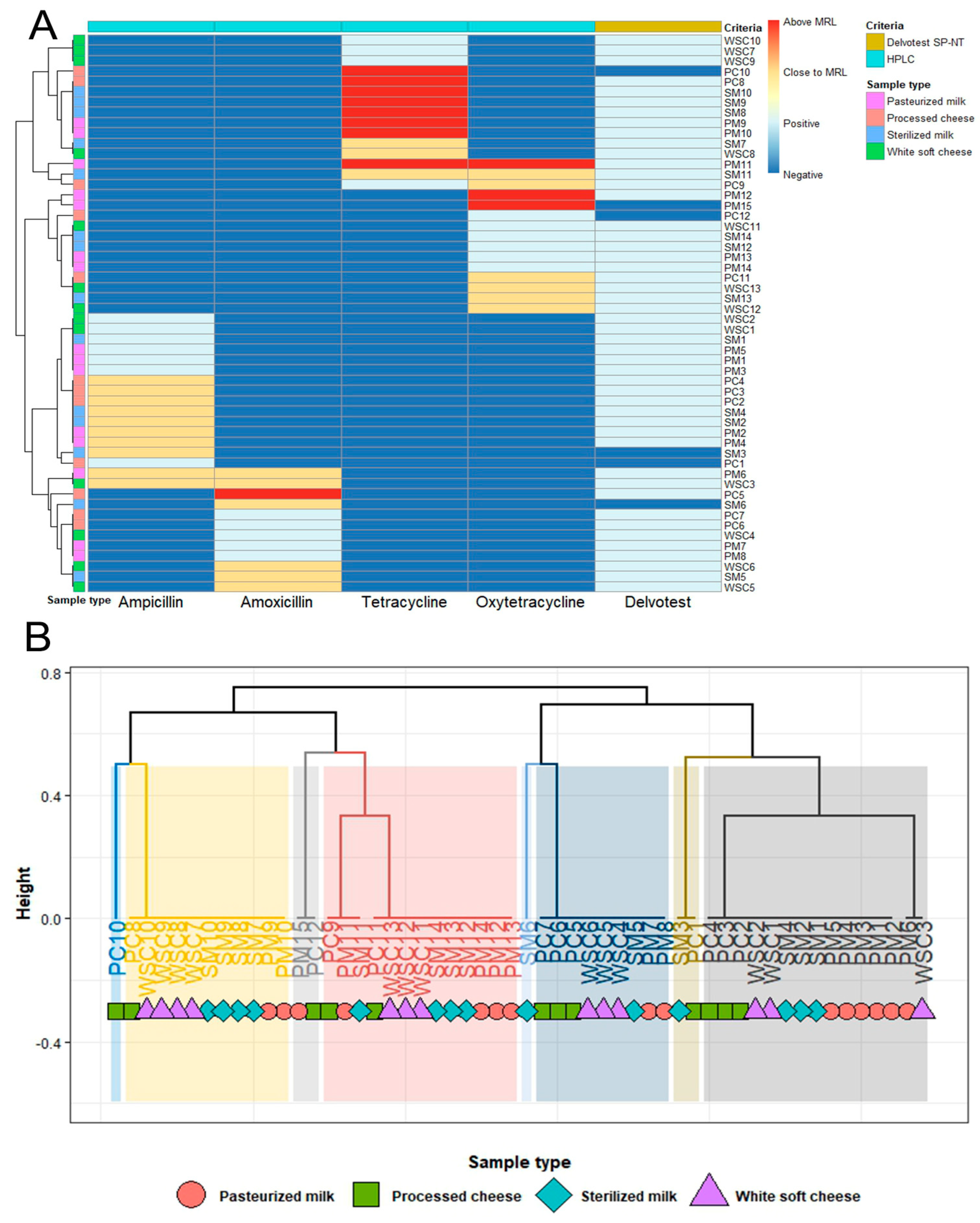
2.3. Maximum Residue Limits and Risk of Antibiotic Residues in Milk and Cheese Samples
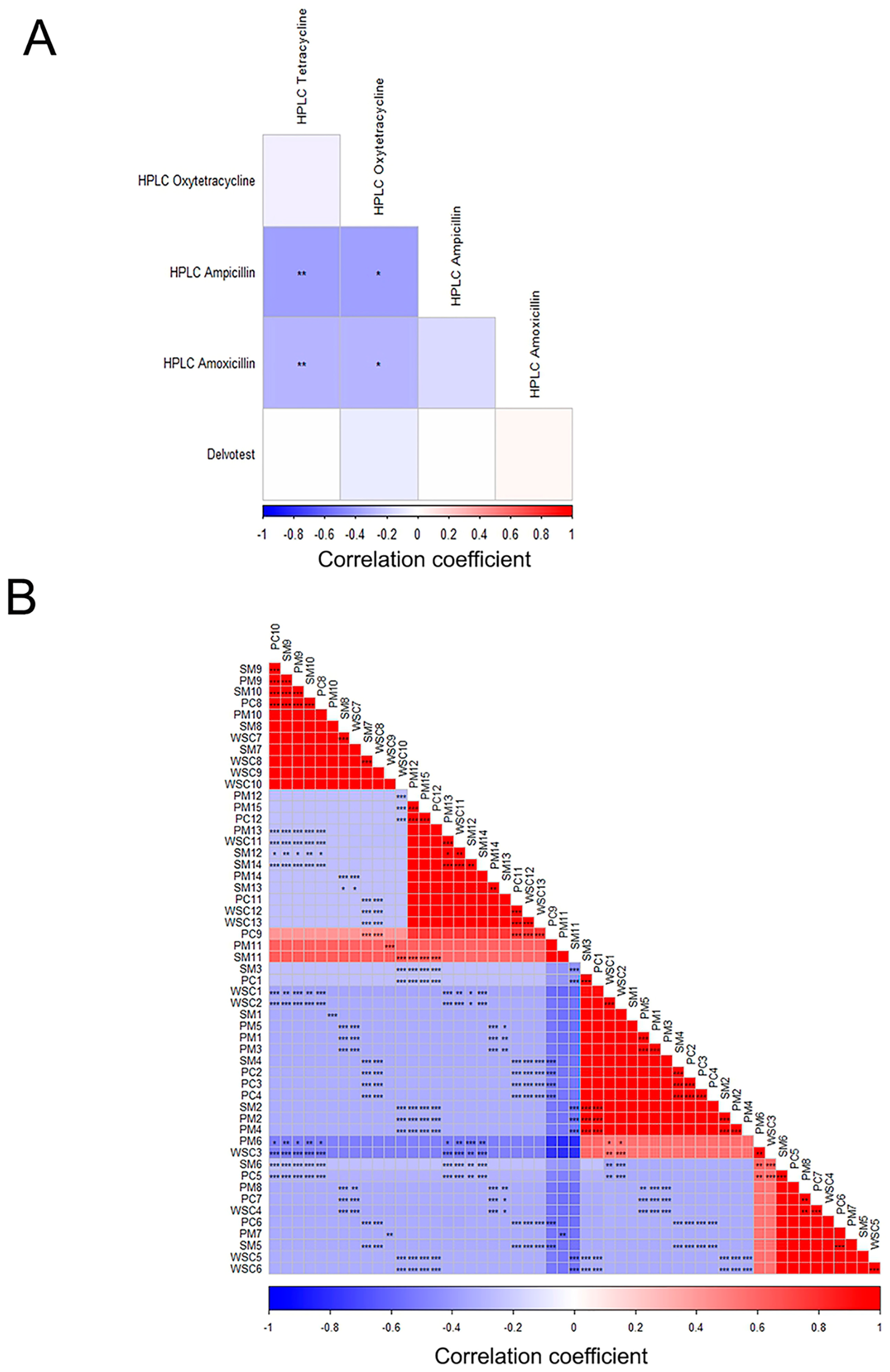
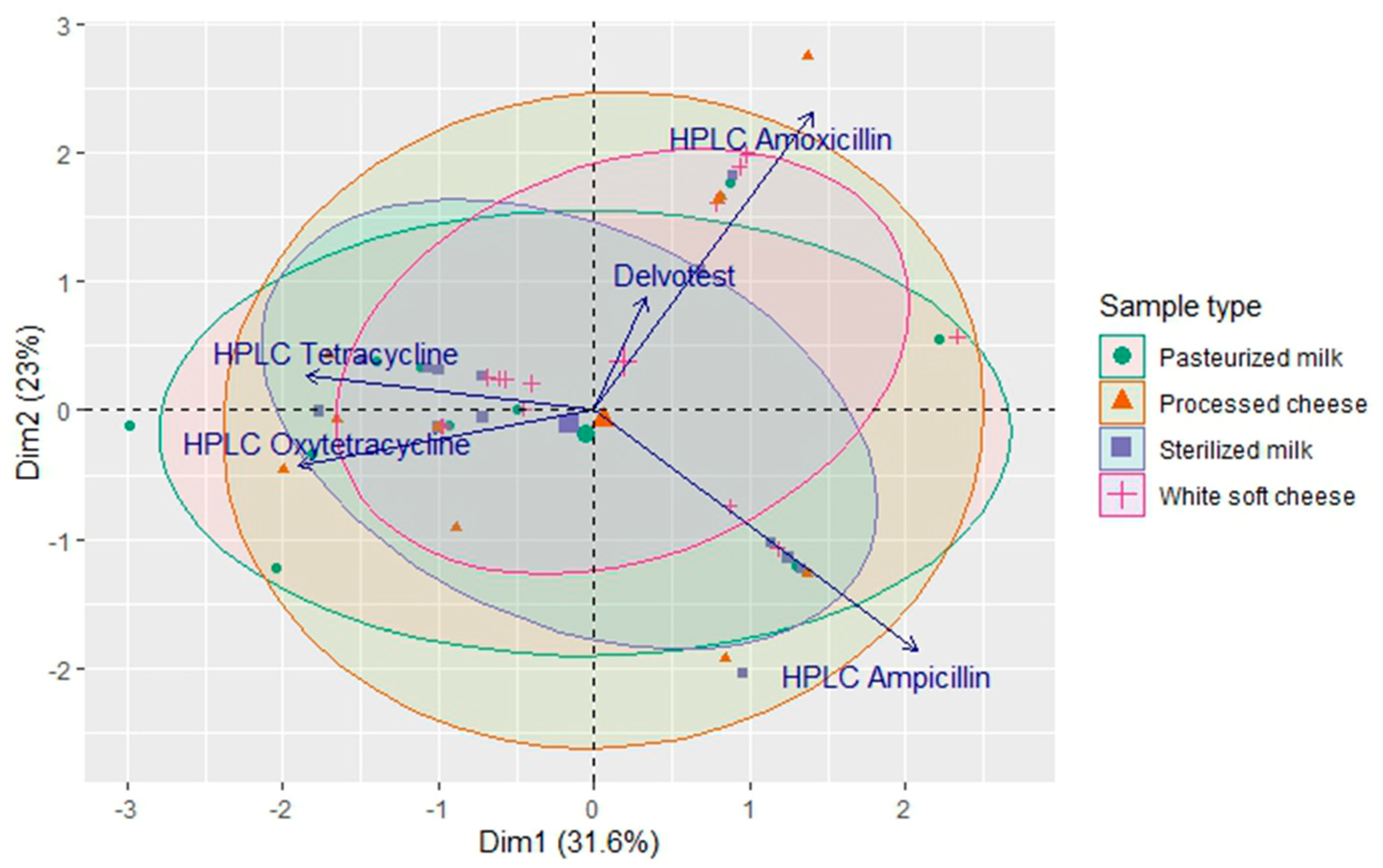
2.4. Correlation Between Analytical Methods
2.5. Validation Parameters for HPLC Method
2.6. Potential Health Risk Associated with Antibiotic Residues
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sampling
5.2. Chemicals and Reagents
5.3. Screening Method Analysis
5.4. HPLC Analysis for Quantitative Detection of β-Lactam and Tetracycline Residues
5.4.1. Preparation of Samples for Quantification of β-Lactam Antibiotic Residues
5.4.2. Samples Preparation for Quantification of Tetracyclines Residues
5.4.3. Chromatographic Analysis of Antibiotic Residues
5.4.4. Preparation of Standards
5.4.5. HPLC Validation
5.5. Determination of the Potential Risk Associated with the Antibiotic Residues
5.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bacanlı, M.G. The two faces of antibiotics: An overview of the effects of antibiotic residues in foodstuffs. Arch. Toxicol. 2024, 98, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. What Is Antimicrobial Resistance and How Can We Tackle It? 2024. Available online: https://www.weforum.org/agenda/2024/08/antimicrobial-resistance-superbugs-antibiotics (accessed on 1 August 2024).
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 1 August 2024).
- World Economic Forum. This Is How Many People Antibiotic Resistance Could Kill Every Year by 2050 If Nothing Is Done. 2016. Available online: https://www.weforum.org/agenda/2016/09/this-is-how-many-people-will-die-from-antimicrobial-resistance-every-year-by-2050-if-nothing-is-done/ (accessed on 1 August 2024).
- Drug-Resistant Infections: A Threat to Our Economic Future (March 2024). Available online: https://www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economic-future (accessed on 1 August 2024).
- EMA; EFSA; EMA Committee for Medicinal Products for Veterinary Use (CVMP) and EFSA Panel on Biological Hazards (BIOHAZ). EMA and EFSA joint scientific opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J. 2017, 15, e04666. [Google Scholar]
- Han, R.; Zheng, N.; Yu, Z.; Xu, X.; Qu, X.; Li, S.; Zhang, Y.; Wang, J. Simultaneous determination of 38 veterinary antibiotic residues in raw milk by UPLC–MS/MS. Food Chem. 2015, 181, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.R.; Sarker, M.; Das, R.; Azad, A.K.; Hasan, M. A review on antibiotic residue in foodstuffs from animal source: Global health risk and alternatives. Int. J. Environ. Anal. Chem. 2021, 103, 3704–3721. [Google Scholar] [CrossRef]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef]
- Arsène, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D.M. The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet. World 2022, 15, 662–671. [Google Scholar] [CrossRef]
- Sadighara, P.; Rostami, S.; Shafaroodi, H.; Sarshogi, A.; Mazaheri, Y.; Sadighara, M. The e ect of residual antibiotics in food on intestinal microbiota: A systematic review. Front. Sustain. Food Syst. 2023, 7, 1163885. [Google Scholar] [CrossRef]
- Treiber, F.M.; Beranek-Knauer, H. Antimicrobial Residues in Food from Animal Origin—A Review of the Literature Focusing on Products Collected in Stores and Markets Worldwide. Antibiotics 2021, 10, 534. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Vercelli, C.; Amadori, M.; Gambino, G.; Re, G. A review on the most frequently used methods to detect antibiotic residues in bovine raw milk. Int. Dairy J. 2023, 144, 105695. [Google Scholar] [CrossRef]
- Chiesa, L.M.; DeCastelli, L.; Nobile, M.; Martucci, F.; Mosconi, G.; Fontana, M.; Castrica, M.; Arioli, F.; Panseri, S. Analysis of antibiotic residues in raw bovine milk and their impact toward food safety and on milk starter cultures in cheese-making process. LWT Food Sci. Technol. 2020, 131, 109783. [Google Scholar] [CrossRef]
- Butovskaya, E.; Gambi, L.; Giovanetti, A.; Fedrizzi, G. Screening of antibiotic residues in raw bovine milk in Lombardy, Italy: Microbial growth inhibition assay and LC-HRMS technique integration for an accurate monitoring. Heliyon 2023, 9, e15395. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Hassan, M.M.; Chowdhury, S. Determination of antibiotic residues in milk and assessment of human health risk in Bangladesh. Heliyon 2021, 7, e07739. [Google Scholar] [CrossRef] [PubMed]
- Climova, N.; Nejeschlebová, H.; Hasoňová, L.; Hanuš, O.; Čítek, J.; Reindl, K.; Honesová, S.J.; Vorlová, L.; Samková, E. The presence of antibiotic residues in raw milk samples obtained after the withdrawal period and other quality parameters in relation to selected factors. Food Control. 2024, 161, 110374. [Google Scholar] [CrossRef]
- Kantiani, L.; Farré, M.; Barceló, D. Analytical methodologies for the detection of β-lactam antibiotics in milk and feed samples. TrAC Trends Anal. Chem. 2009, 28, 729–744. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Regulation (EC), N. Laying Down Specific Hygiene Rules for the Hygiene of Hygiene Rules for Food of Animal Origin. OJEU; L139. 2004. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32004R0853 (accessed on 1 August 2024).
- European Union. Regulation (EU) no. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Commun. 2010, L15, 1–72. [Google Scholar]
- European Union. Regulation (EU) 2019/6 of the European parliament and of the council of 11 December 2018 on veterinary medicinal products and repealing directive 2001/82/ec. Off. J. 2019, L4, 43–167. [Google Scholar]
- Evans, R.M.; Scholze, M.; Kortenkamp, A. Examining the feasibility of mixture risk assessment: A case study using a tiered approach with data of 67 pesticides from the Joint FAO/WHO Meeting on Pesticide Residues (JMPR). Food Chem. Toxicol. 2015, 84, 260–269. [Google Scholar] [CrossRef]
- Yu, R.; Liu, Q.; Liu, J.; Wang, Q.; Wang, Y. Concentrations of organophosphorus pesticides in fresh vegetables and related human health risk assessment in Changchun, Northeast China. Food Control. 2016, 60, 353–360. [Google Scholar] [CrossRef]
- Doménech, E.; Martorell, S. Assessment of safety margins of exposure to non-genotoxic chemical substances in food. Food Control. 2017, 79, 1–9. [Google Scholar] [CrossRef]
- Quintanilla, P.; Doménech, E.; Escriche, I.; Beltrán, M.C.; Molina, M.P. Food Safety Margin Assessment of Antibiotics: Pasteurized Goat’s Milk and Fresh Cheese. J. Food Prot. 2019, 82, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission Standards (CAC). Maximum Residue Limits (MRLs) and Risk Management Recommendations (RMRs) for Residues of Veterinary Drugs in Foods; CXM 2-2023; FAO-WHO: Rome, Italy, 2023. [Google Scholar]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Ali, M.; Muzammil, S.; Aslam, B.; Shahid, M.; Saqalein, M.; Akash, M.; Almatroudi, A.; Allemailem, K.; Khurshid, M. Antibiotic residues in food chains; impact on the environment and human health: A review. Appl. Ecol. Environ. Res. 2021, 19, 3959–3977. [Google Scholar] [CrossRef]
- Nutrinews. Addressing Antibiotic Residues in Milk for Health and Sustainability. 2023. Available online: https://nutrinews.com/en/addressing-antibiotic-residues-in-milk-for-health-and-sustainability (accessed on 1 August 2024).
- European Union. Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Commun. 2002, L221, 8–36. [Google Scholar]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S.; Kumar, A. Validation of HPLC Multi-residue Method for Determination of Fluoroquinolones, Tetracycline, Sulphonamides and Chloramphenicol Residues in Bovine Milk. Food Anal. Methods 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.; Shabbir, M.A.B.; Cheng, G.; Yuan, Z. Current advances in immunoassays for the detection of antibiotics residues: A review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Ferrini, A.M.; Agrimi, U.; Appicciafuoco, B.; Borroni, R.; Ciccaglioni, G.; Galati, F.; Massaro, M.R.; Patriarca, M. PT as a tool to point out criticalities in the strategy for control of antibiotic residues in milk: The Italian experience. Accredit. Qual. Assur. 2015, 20, 267–272. [Google Scholar] [CrossRef]
- Brocca, D.; Salvatore, S. Report for 2021 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. Publ. 2023, 20, 7886E. [Google Scholar] [CrossRef]
- Rama, A.; Lucatello, L.; Benetti, C.; Galina, G.; Bajraktari, D. Assessment of antibacterial drug residues in milk for consumption in Kosovo. J. Food Drug Anal. 2017, 25, 525–532. [Google Scholar] [CrossRef]
- Layada, S.; Benouareth, D.-E.; Coucke, W.; Andjelkovic, M. Assessment of antibiotic residues in commercial and farm milk collected in the region of Guelma (Algeria). Int. J. Food Contam. 2016, 3, 19. [Google Scholar] [CrossRef]
- Santamarina-García, G.; Amores, G.; Gandarias, N.; Hernández, I.; Virto, M. Cross-sectional, commercial testing, and chromatographic study of the occurrence of antibiotic residues throughout an artisanal raw milk cheese production chain. Food Chem. 2024, 442, 138445. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Muraoka, A.; Bedenbaugh, M.; Childress, B.; Pernot, L.; Wiencek, M.; Peterson, Y.K. The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition. Front. Microbiol. 2022, 13, 807955. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, S.; Zanardi, E.; Varisco, G.; Chizzolini, R. Residues of β-lactam antibiotics in bovine milk: Confirmatory analysis by liquid chromatography tandem mass spectrometry after microbial assay screening residues of b-lactam antibiotics in bovine milk: Confirmatory analysis by liquid chromatography tandem mass spectrometry after microbial assay screening. Food Addit. Contam. 2010, 20, 528–534. [Google Scholar] [CrossRef]
- Casseri, E.; Bulut, E.; Soto, S.L.; Wemette, M.; Stout, A.; Safi, A.G.; Lynch, R.; Moroni, P.; Ivanek, R. Understanding Antibiotic Resistance as a Perceived Threat towards Dairy Cattle through Beliefs and Practices: A Survey-Based Study of Dairy Farmers. Antibiotics 2022, 11, 997. [Google Scholar] [CrossRef]
- Abebew, D.; Belihu, K.; Zewde, G.; Zeit, D. Detection and determination of oxytetracycline and penicillin g antibiotic residue levels in bovine bulk milk from nazareth dairy farms, ethiopia. Ethiop. Vet. J. 2014, 8, 1–15. [Google Scholar]
- Widiastuti, R.; Martindah, E.; Anastasia, Y. Tetracycline residues in fresh dairy milk from three districts in Indonesia: Occurrence and dietary exposure assessment. Vet. World 2023, 16, 2230–2235. [Google Scholar] [CrossRef]
- Chauhan, S.L.; Garg, P.S.; Jadhav, V.J. Dietary exposure assessment of tetracycline residues in milk in Haryana. Int. J. Chem. Sci. 2019, 7, 1862–1865. [Google Scholar]
- Omairi, R.; Krayem, M.; Khaled, S.; Salla, M.; El Khatib, S. Antibiotic residues in milk and milk products: A momentous challenge for the pharmaceutical industry and medicine. World J. Pharmacol. 2022, 11, 48–55. [Google Scholar] [CrossRef]
- Kellnerová, E.; Navrátilová, P.; Borkovcová, I. Effect of pasteurization on the residues of tetracyclines in milk. Acta Vet. Brno 2014, 83, S21–S26. [Google Scholar] [CrossRef]
- Anika, T.; Noman, Z.; Ferdous, M.; Khan, S.; Mukta, M.; Islam, S.; Hossain, T.; Rafiq, K. Time dependent screening of antibiotic residues in milk of antibiotics treated cows. J. Adv. Vet. Anim. Res. 2019, 6, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Virto, M.; Santamarina-García, G.; Amores, G.; Hernández, I. Antibiotics in Dairy Production: Where Is the Problem? Dairy 2022, 3, 541–564. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.F.; Paula, G.H.; Silva, R.C.F.; Leão, P.V.T.; Leão, K.M.; Nicolau, E.S.; Silva, M.A. Residues of antibiotics in milk: Persistence and quality interference. Can. J. Anim. Sci. 2020, 100, 93–101. [Google Scholar] [CrossRef]
- Burmańczuk, A.; Tomasz, G.; Gbylik-Sikorska, M.; Gajda, A.; Kowalski, C. Withdrawal of amoxicillin and penicillin G procaine from milk after intramammary administration in dairy cows with mastitis. J. Vet. Res. 2017, 61, 37–43. [Google Scholar] [CrossRef]
- Alomirah, H.; Al-Mazeedi, H.; Al-Zenki, S.; Al-Aati, T.; Al-Otaibi, J.; Al-Batel, M.; Sidhu, J. Prevalence of antimicrobial residues in milk and dairy products in the state of kuwait. J. Food Qual. 2007, 30, 745–763. [Google Scholar] [CrossRef]
- Cinquina, A.L.; Longo, F.; Anastasi, G.; Giannetti, L.; Cozzani, R. Validation of a high-performance liquid chromatography method for the determination of oxytetracycline, tetracycline, chlortetracycline and doxycycline in bovine milk and muscle. J. Chromatogr. A 2003, 987, 227–233. [Google Scholar] [CrossRef]
- Mamani, M.C.V.; Reyes, F.G.R.; Rath, S. Multiresidue determination of tetracyclines, sulphonamides and chloramphenicol in bovine milk using HPLC-DAD. Food Chem. 2009, 117, 545–552. [Google Scholar] [CrossRef]
- Juan, C.; Moltó, J.C.; Mañes, J.; Font, G. Determination of macrolide and lincosamide antibiotics by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry in meat and milk. Food Control. 2010, 21, 1703–1709. [Google Scholar] [CrossRef]
- European Dairy Association (EDA). Daily Dairy Recommendations: Are We Eating Enough Dairy? EDA: Brussels, Belgium, 2021; Available online: https://eda.euromilk.org/fileadmin/user_upload/Public_Documents/EDA_Position_papers-FactSheets/Fact-sheets/EDA-Factsheet-Daily-Dairy_Recommendations (accessed on 1 August 2024).
- Food and Agriculture Organization of The United Nations. Milk and Dairy Products in Human Nutrition; FAO: Rome, Italy, 2013; Available online: https://www.fao.org/4/i3396e/i3396e (accessed on 1 August 2024).

| HPLC | ||||
|---|---|---|---|---|
| HPLC Non-Conform | HPLC Conform | Total | ||
| Delvotest SP-NT | Delvotest negative | 146 | 6 | 152 |
| Delvotest positive | 0 | 48 | 48 | |
| Total | 146 | 54 | 200 | |
| Antibiotic | Sample Type | No. of Results, Frequency (%) | |
|---|---|---|---|
| HPLC Conform and Delvotest Negative | HPLC Conform and Delvotest Positive | ||
| Ampicillin | Pasteurized milk | 0 | 6 (12) |
| Processed cheese | 1 (2) | 3 (6) | |
| Sterilized milk | 1 (2) | 3 (6) | |
| White soft cheese | 0 | 3 (6) | |
| Total | 2 (1) | 15 (7.5) | |
| Amoxicillin | Pasteurized milk | 0 | 3 (6) |
| Processed cheese | 0 | 3 (6) | |
| Sterilized milk | 1 (2) | 1 (2) | |
| White soft cheese | 0 | 4 (8) | |
| Total | 1 (0.5) | 11 (5.5) | |
| Tetracycline | Pasteurized milk | 0 | 3 (6) |
| Processed cheese | 1 (2) | 2 (4) | |
| Sterilized milk | 0 | 5 (10) | |
| White soft cheese | 0 | 4 (8) | |
| Total | 1 (0.5) | 14 (7) | |
| Oxytetracycline | Pasteurized milk | 1 (2) | 4 (8) |
| Processed cheese | 1 (2) | 2 (2) | |
| Sterilized milk | 0 | 4 (8) | |
| White soft cheese | 0 | 3 (6) | |
| Total | 2 (1) | 13 (6.5) | |
| Antibiotic Tested | ADI a (μg/Day) | Sample Type | EDI b (μg/Day) | Hazard Quotient |
|---|---|---|---|---|
| Ampicillin | 210 | Pasteurized Milk | 12.32 ± 0.399 a | 0.059 ± 0.002 a |
| Sterilized Milk | 12.57 ± 0.41 a | 0.059 ± 0.002 a | ||
| White soft cheese | 2.77 ± 0.364 b | 0.013 ± 0.002 b | ||
| Processed cheese | 3.19 ± 0.14 b | 0.015 ± 0.001 b | ||
| p-value | <0.0001 | <0.0001 | ||
| Total | 8.55 ± 1.71 | 0.041 ± 0.006 | ||
| Amoxicillin | 140 | Pasteurized Milk | 12.32 ± 0.515 a | 0.088 ± 0.004 a |
| Sterilized Milk | 13.27 ± 0.589 a | 0.095 ± 0.004 a | ||
| White soft cheese | 3.13 ± 0.16 b | 0.022 ± 0.001 b | ||
| Processed cheese | 3.38 ± 0.667 b | 0.024 ± 0.005 b | ||
| p-value | <0.0001 | <0.0001 | ||
| Total | 7.18 ± 1.42 | 0.051 ± 0.01 | ||
| Tetracycline | 2100 | Pasteurized Milk | 573.09 ± 38.09 a | 0.273 ± 0.018 a |
| Sterilized Milk | 464.71 ± 63.22 a | 0.221 ± 0.03 a | ||
| White soft cheese | 64.38 ± 6.55 b | 0.031 ± 0.003 b | ||
| Processed cheese | 145.63 ± 43.19 b | 0.069 ± 0.021 b | ||
| p-value | <0.0001 | <0.0001 | ||
| Total | 315.82 ± 59.74 | 0.15 ± 0.028 | ||
| Oxytetracycline | 2100 | Pasteurized Milk | 430.29 ± 84.17 a | 0.205 ± 0.04 a |
| Sterilized Milk | 277.86 ± 26.52 ab | 0.132 ± 0.013 ab | ||
| White soft cheese | 61.99 ± 13.19 b | 0.029 ± 0.006 b | ||
| Processed cheese | 69.59 ± 11.67 b | 0.033 ± 0.006 b | ||
| p-value | 0.003 | 0.003 | ||
| Total | 243.84 ± 49.79 | 0.116 ± 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenezi, M.S.; Tartor, Y.H.; El-Sherbini, M.; Pet, E.; Ahmadi, M.; Abdelkhalek, A. Antibiotic Residues in Milk and Milk-Based Products Served in Kuwait Hospitals: Multi-Hazard Risk Assessment. Antibiotics 2024, 13, 1073. https://doi.org/10.3390/antibiotics13111073
Alenezi MS, Tartor YH, El-Sherbini M, Pet E, Ahmadi M, Abdelkhalek A. Antibiotic Residues in Milk and Milk-Based Products Served in Kuwait Hospitals: Multi-Hazard Risk Assessment. Antibiotics. 2024; 13(11):1073. https://doi.org/10.3390/antibiotics13111073
Chicago/Turabian StyleAlenezi, Maha S., Yasmine H. Tartor, Mohammed El-Sherbini, Elena Pet, Mirela Ahmadi, and Adel Abdelkhalek. 2024. "Antibiotic Residues in Milk and Milk-Based Products Served in Kuwait Hospitals: Multi-Hazard Risk Assessment" Antibiotics 13, no. 11: 1073. https://doi.org/10.3390/antibiotics13111073
APA StyleAlenezi, M. S., Tartor, Y. H., El-Sherbini, M., Pet, E., Ahmadi, M., & Abdelkhalek, A. (2024). Antibiotic Residues in Milk and Milk-Based Products Served in Kuwait Hospitals: Multi-Hazard Risk Assessment. Antibiotics, 13(11), 1073. https://doi.org/10.3390/antibiotics13111073








