Diversity, Distribution, and Resistance Profiles of Bacterial Bloodstream Infections in Three Tertiary Referral Hospitals in Rwanda Between 2020 and 2022
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Study Sites
4.2. Data Collection and Laboratory Methods
4.3. Data Management and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartorius, B.; Gray, A.P.; Weaver, N.D.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Mestrovic, T.; Chung, E.; Wool, E.E.; Han, C.; et al. The Burden of Bacterial Antimicrobial Resistance in the WHO African Region in 2019: A Cross-Country Systematic Analysis. Lancet Glob. Health 2024, 12, e201–e216. [Google Scholar] [CrossRef] [PubMed]
- Mestrovic, T.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Weaver, N.D.; Han, C.; Wool, E.E.; Hayoon, A.G.; Hay, S.I.; et al. The Burden of Bacterial Antimicrobial Resistance in the WHO European Region in 2019: A Cross-Country Systematic Analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Roth, G.A.; Aravkin, A.Y.; Zheng, P.; Abate, K.H.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M.; et al. Global Burden and Strength of Evidence for 88 Risk Factors in 204 Countries and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar] [CrossRef] [PubMed]
- Vollset, S.E.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbastabar, H.; Magied, A.H.A.A.A.; ElHafeez, S.A.; Abdelkader, A.; et al. Burden of Disease Scenarios for 204 Countries and Territories, 2022–2050: A Forecasting Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef]
- Cuella Martin, I.; Hakizayezu, F.; Ahmed, A.; Runyambo, D.; Niyompano, H.; Keysers, J.; De Rijk, W.B.; Mulders, W.; Mitchell, E.M.; Decroo, T. High Rates of False Rifampicin Resistance in Paucibacillary Tuberculosis Patients by Xpert MTB/RIF Ultra. Preprints 2024. [Google Scholar]
- Ledesma, J.R.; Ma, J.; Zhang, M.; Basting, A.V.; Chu, H.T.; Vongpradith, A.; Novotney, A.; LeGrand, K.E.; Xu, Y.Y.; Dai, X. Global, Regional, and National Age-Specific Progress towards the 2020 Milestones of the WHO End TB Strategy: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 698–725. [Google Scholar] [CrossRef]
- Donkor, E.S.; Muhsen, K.; Johnson, S.A.M.; Kotey, F.C.N.; Dayie, N.T.K.D.; Tetteh-Quarcoo, P.B.; Tette, E.M.A.; Osei, M.-M.; Egyir, B.; Nii-Trebi, N.I.; et al. Multicenter Surveillance of Antimicrobial Resistance among Gram-Negative Bacteria Isolated from Bloodstream Infections in Ghana. Antibiotics 2023, 12, 255. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.; Dar, M.A.; Kaur, R.J.; Charan, J.; Iskandar, K.; Haque, M.; Murti, K.; Ravichandiran, V.; Dhingra, S. Menace of Antimicrobial Resistance in LMICs: Current Surveillance Practices and Control Measures to Tackle Hostility. J. Infect. Public Health 2022, 15, 172–181. [Google Scholar] [CrossRef]
- Gagliotti, C.; Balode, A.; Baquero, F.; Degener, J.; Grundmann, H.; Gür, D.; Jarlier, V.; Kahlmeter, G.; Monen, J.; Monnet, D. Escherichia Coli and Staphylococcus Aureus: Bad News and Good News from the European Antimicrobial Resistance Surveillance Network (EARS-Net, Formerly EARSS), 2002 to 2009. Eurosurveillance 2011, 16, 172–181. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, E.-J.; Kim, D.; Jeong, S.H.; Won, E.J.; Shin, J.H.; Kim, S.H.; Shin, J.H.; Shin, K.S.; Kim, Y.A. Antimicrobial Resistance of Major Clinical Pathogens in South Korea, May 2016 to April 2017: First One-Year Report from Kor-GLASS. Eurosurveillance 2018, 23, 1800047. [Google Scholar] [CrossRef]
- Hattori, H.; Maeda, M.; Nagatomo, Y.; Takuma, T.; Niki, Y.; Naito, Y.; Sasaki, T.; Ishino, K. Epidemiology and Risk Factors for Mortality in Bloodstream Infections: A Single-Center Retrospective Study in Japan. Am. J. Infect. Control 2018, 46, e75–e79. [Google Scholar] [CrossRef] [PubMed]
- Musicha, P.; Cornick, J.E.; Bar-Zeev, N.; French, N.; Masesa, C.; Denis, B.; Kennedy, N.; Mallewa, J.; Gordon, M.A.; Msefula, C.L. Trends in Antimicrobial Resistance in Bloodstream Infection Isolates at a Large Urban Hospital in Malawi (1998–2016): A Surveillance Study. Lancet Infect. Dis. 2017, 17, 1042–1052. [Google Scholar] [CrossRef]
- Kariuki, S.; Kering, K.; Wairimu, C.; Onsare, R.; Mbae, C. Antimicrobial Resistance Rates and Surveillance in Sub-Saharan Africa: Where Are We Now? Infect. Drug Resist. 2022, 3589–3609. [Google Scholar] [CrossRef]
- Igizeneza, A.; Bitunguhari, L.; Masaisa, F.; Hahirwa, I.; Uwamahoro, L.D.; Sebatunzi, O.; Umugwaneza, N.; Pauwels, I.; Versporten, A.; Vlieghe, E.; et al. Prescription Practices and Usage of Antimicrobials in a Tertiary Teaching Hospital in Rwanda: A Call for Antimicrobial Stewardship. Preprints 2024, 2024072545. [Google Scholar] [CrossRef]
- Sutherland, T.; Mpirimbanyi, C.; Nziyomaze, E.; Niyomugabo, J.-P.; Niyonsenga, Z.; Muvunyi, C.M.; Mueller, A.; Bebell, L.M.; Nkubana, T.; Musoni, E. Widespread Antimicrobial Resistance among Bacterial Infections in a Rwandan Referral Hospital. PLoS ONE 2019, 14, e0221121. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.G.; Sirota, S.B.; Swetschinski, L.R.; Dominguez, R.-M.V.; Novotney, A.; Wool, E.E.; Ikuta, K.S.; Vongpradith, A.; Rogowski, E.L.B.; Doxey, M.; et al. Global, Regional, and National Incidence and Mortality Burden of Non-COVID-19 Lower Respiratory Infections and Aetiologies, 1990–2021: A Systematic Analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 974–1002. [Google Scholar] [CrossRef] [PubMed]
- Houssaini, Z.; Harrar, N.; Zerouali, K.; Belabbes, H.; Elmdaghri, N. Prevalence of Coagulase-Negative Staphylococci in Blood Cultures at the Ibn-Rochd University Hospital in Casablanca. Pan Afr. Med. J. 2019, 33, 193. [Google Scholar] [CrossRef]
- Kern, W.; Rieg, S. Burden of Bacterial Bloodstream Infection—A Brief Update on Epidemiology and Significance of Multidrug-Resistant Pathogens. Clin. Microbiol. Infect. 2020, 26, 151–157. [Google Scholar] [CrossRef]
- Abebe, W.; Tegene, B.; Feleke, T.; Sharew, B. Bacterial Bloodstream Infections and Their Antimicrobial Susceptibility Patterns in Children and Adults in Ethiopia: A 6-Year Retrospective Study. Clin. Lab. 2021, 67, 2453–2461. [Google Scholar] [CrossRef]
- Habyarimana, T.; Murenzi, D.; Musoni, E.; Yadufashije, C.; N Niyonzima, F. Bacteriological Profile and Antimicrobial Susceptibility Patterns of Bloodstream Infection at Kigali University Teaching Hospital. Infect. Drug Resist. 2021, 14, 699–707. [Google Scholar] [CrossRef]
- Kallel, H.; Houcke, S.; Resiere, D.; Roy, M.; Mayence, C.; Mathien, C.; Mootien, J.; Demar, M.; Hommel, D.; Djossou, F. Epidemiology and Prognosis of Intensive Care Unit–Acquired Bloodstream Infection. Am. J. Trop. Med. Hyg. 2020, 103, 508. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Kilpatrick, C.; Storr, J.; Kelley, E.; Park, B.J.; Donaldson, L. Global Infection Prevention and Control Priorities 2018–22: A Call for Action. Lancet Glob. Health 2017, 5, e1178–e1180. [Google Scholar] [CrossRef] [PubMed]
- Deku, J.G.; Dakorah, M.P.; Lokpo, S.Y.; Orish, V.N.; Ussher, F.A.; Kpene, G.E.; Angmorkie Eshun, V.; Agyei, E.; Attivor, W.; Osei-Yeboah, J. The Epidemiology of Bloodstream Infections and Antimicrobial Susceptibility Patterns: A Nine-Year Retrospective Study at St. Dominic Hospital, Akwatia, Ghana. J. Trop. Med. 2019, 2019, 6750864. [Google Scholar] [CrossRef] [PubMed]
- Del Bono, V.; Giacobbe, D.R. Bloodstream Infections in Internal Medicine. Virulence 2016, 7, 353–365. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Karlowsky, J.A.; de Jonge, B.L.; Stone, G.G.; Sahm, D.F. Epidemiology of Carbapenem Resistance Determinants Identified in Meropenem-Nonsusceptible Enterobacterales Collected as Part of a Global Surveillance Program, 2012 to 2017. Antimicrob. Agents Chemother. 2021, 65, 10–1128. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Wampande, E.; Ejobi, F. A Systematic Review: The Current Status of Carbapenem Resistance in East Africa. BMC Res. Notes 2018, 11, 629. [Google Scholar] [CrossRef]
- Jousset, A.B.; Bernabeu, S.; Bonnin, R.A.; Creton, E.; Cotellon, G.; Sauvadet, A.; Naas, T.; Dortet, L. Development and Validation of a Multiplex Polymerase Chain Reaction Assay for Detection of the Five Families of Plasmid-Encoded Colistin Resistance. Int. J. Antimicrob. Agents 2019, 53, 302–309. [Google Scholar] [CrossRef]
- Leshaba, T.M.S.; Mbelle, N.M.; Osei Sekyere, J. Current and Emerging Polymyxin Resistance Diagnostics: A Systematic Review of Established and Novel Detection Methods. J. Appl. Microbiol. 2022, 132, 8–30. [Google Scholar] [CrossRef]
- Baker, S.; Thomson, N.; Weill, F.-X.; Holt, K.E. Genomic Insights into the Emergence and Spread of Antimicrobial-Resistant Bacterial Pathogens. Science 2018, 360, 733–738. [Google Scholar] [CrossRef]
- Remera, E.; Rwagasore, E.; Muvunyi, C.M.; Ahmed, A. Emergence of the First Molecularly Confirmed Outbreak of Rift Valley Fever among Humans in Rwanda, Calls for Institutionalizing the One Health Strategy. IJID One Health 2024, 4, 100035. [Google Scholar] [CrossRef]
- Zhou, N.; Cheng, Z.; Zhang, X.; Lv, C.; Guo, C.; Liu, H.; Dong, K.; Zhang, Y.; Liu, C.; Chang, Y.-F. Global Antimicrobial Resistance: A System-Wide Comprehensive Investigation Using the Global One Health Index. Infect. Dis. Poverty 2022, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Bordier, M.; Binot, A.; Pauchard, Q.; Nguyen, D.T.; Trung, T.N.; Fortané, N.; Goutard, F.L. Antibiotic Resistance in Vietnam: Moving towards a One Health Surveillance System. BMC Public Health 2018, 18, 1136. [Google Scholar] [CrossRef] [PubMed]
- Nyatanyi, T.; Wilkes, M.; McDermott, H.; Nzietchueng, S.; Gafarasi, I.; Mudakikwa, A.; Kinani, J.F.; Rukelibuga, J.; Omolo, J.; Mupfasoni, D.; et al. Implementing One Health as an Integrated Approach to Health in Rwanda. BMJ Glob. Health 2017, 2, e000121. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Alhumaid, S.; Mutair, A.A.; Garout, M.; Abulhamayel, Y.; Halwani, M.A.; Alestad, J.H.; Bshabshe, A.A.; Sulaiman, T.; AlFonaisan, M.K.; et al. Application of Artificial Intelligence in Combating High Antimicrobial Resistance Rates. Antibiotics 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level; World Health Organization, Country Office for Thailand: Nonthaburi, Thailand, 2020; ISBN 974-680-437-5. [Google Scholar]
- World Health Organization. Minimum Requirements for Infection Prevention and Control Programmes; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Ntirenganya, C.; Manzi, O.; Muvunyi, C.M.; Ogbuagu, O. High Prevalence of Antimicrobial Resistance among Common Bacterial Isolates in a Tertiary Healthcare Facility in Rwanda. Am. J. Trop. Med. Hyg. 2015, 92, 865. [Google Scholar] [CrossRef]


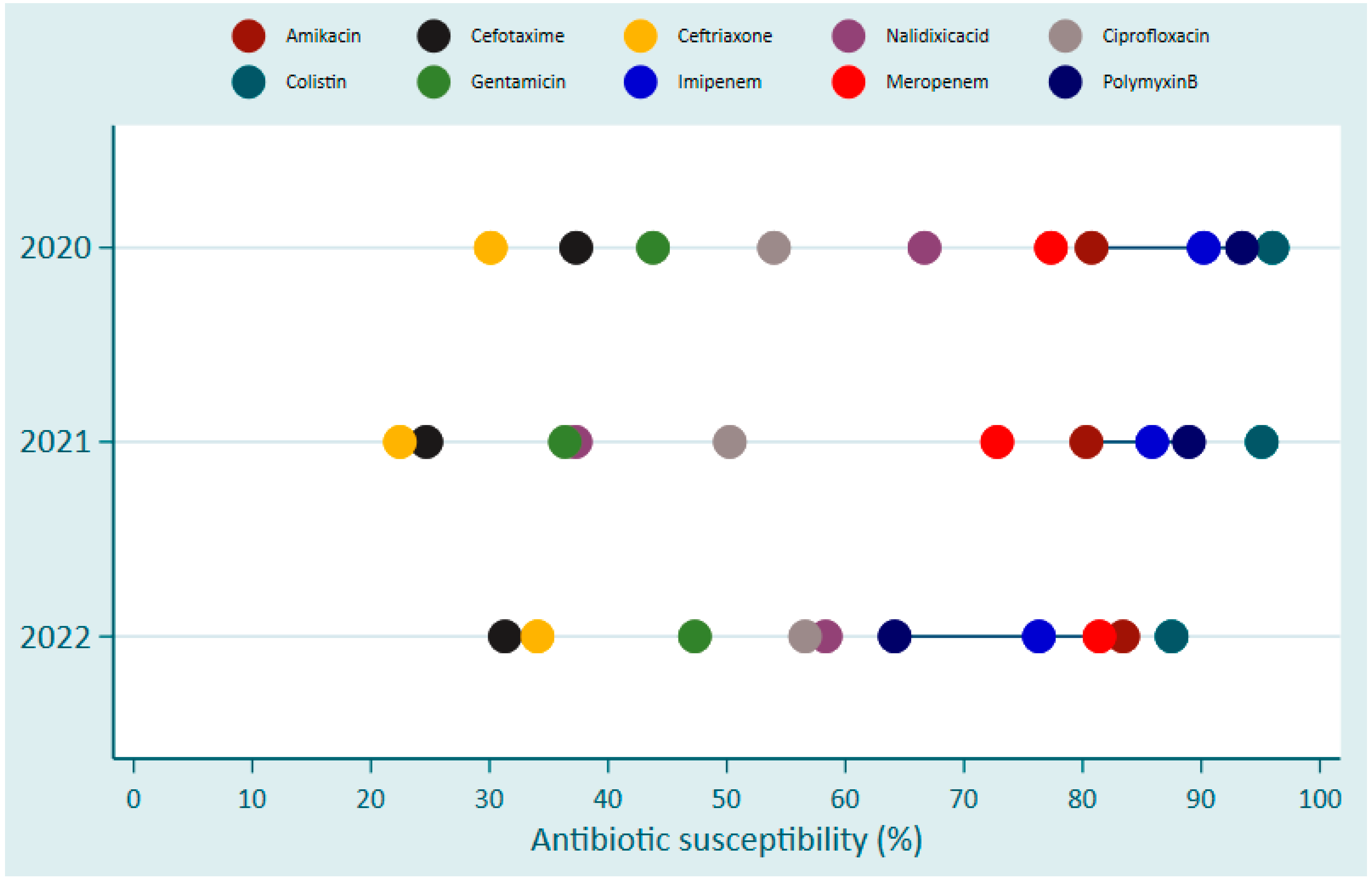
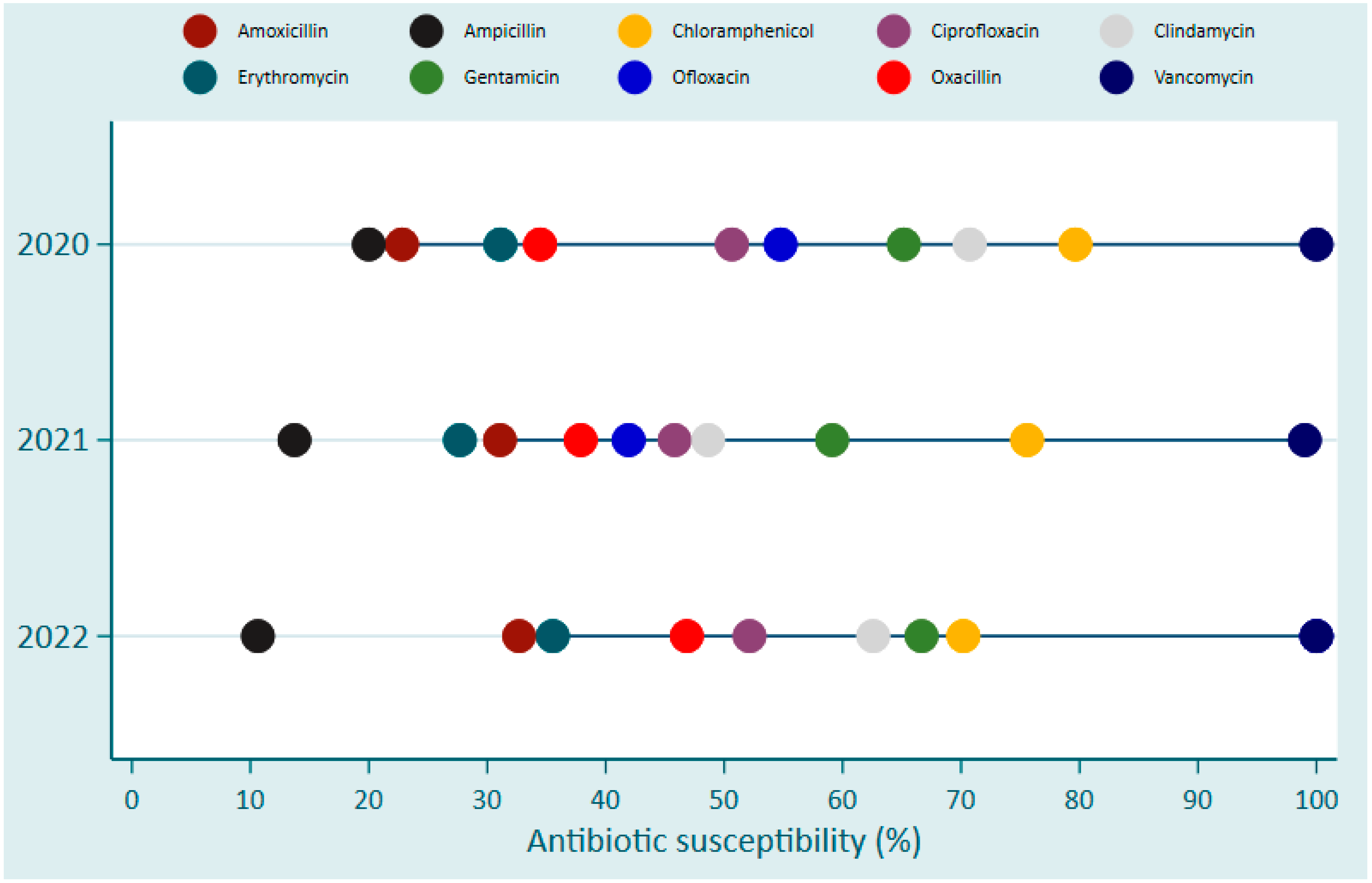
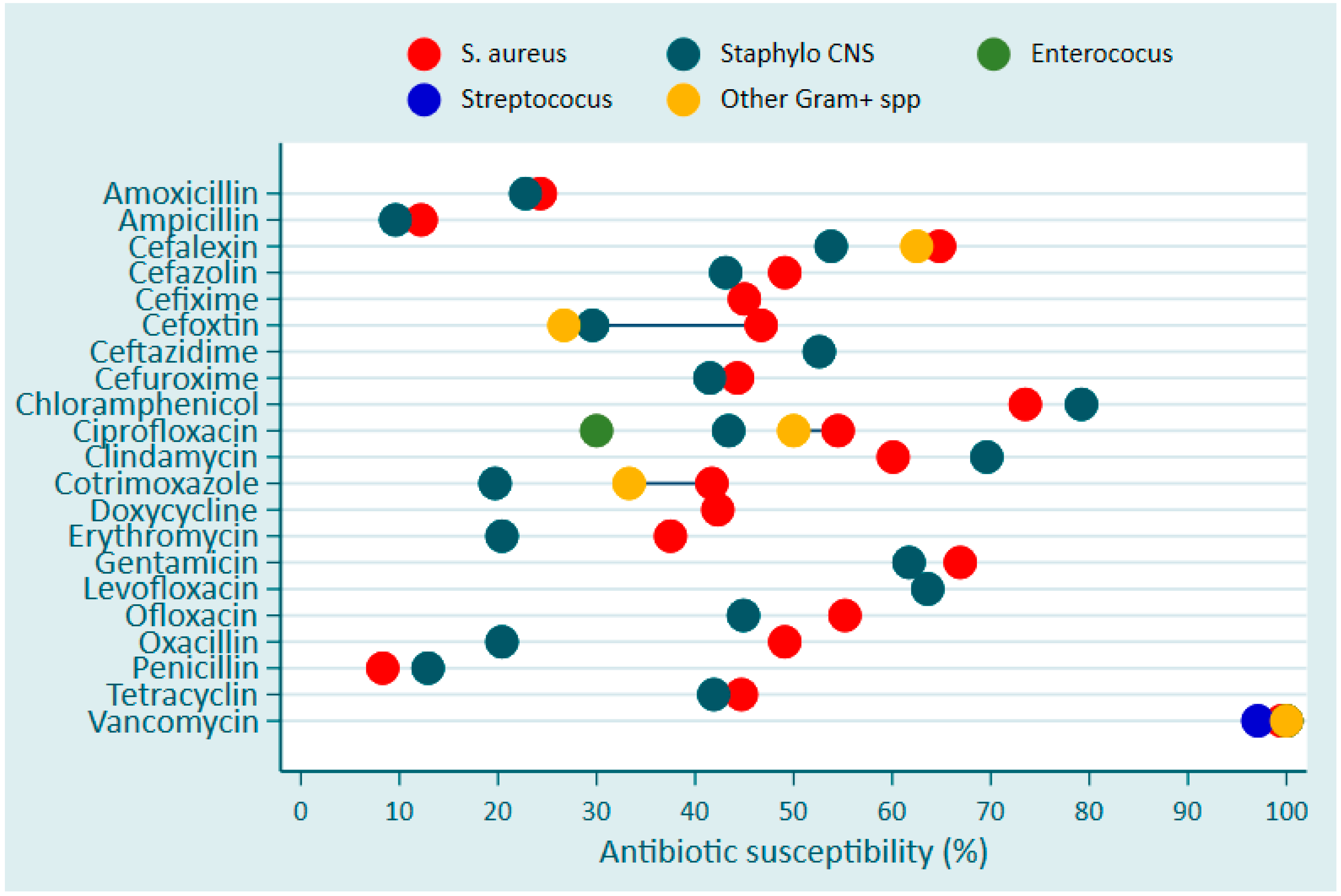
| Pathogen Species | King Faisal Hospital (KFH) | Kigali University Teaching Hospital (CHUK) | Butare University Teaching Hospital (CHUB) | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gram-negative | n | % | n | % | n | % | n | % | p-value |
| Escherichia coli | 38 | 13.7 | 78 | 23.8 | 15 | 11.4 | 131 | 17.8 | <0.001 |
| Klebsiella spp. | 89 | 32.0 | 152 | 46.3 | 59 | 44.7 | 300 | 40.7 | |
| Acinetobacter spp. | 38 | 13.7 | 51 | 15.6 | 22 | 16.7 | 111 | 15.0 | |
| Pseudomonas spp. | 28 | 10.1 | 12 | 3.7 | 8 | 6.1 | 48 | 6.5 | |
| Enterobacter spp. | 33 | 11.9 | 9 | 2.7 | 5 | 3.8 | 47 | 6.4 | |
| Salmonella spp. | 6 | 2.2 | 18 | 5.5 | 18 | 13.6 | 42 | 5.7 | |
| Serratia spp. | 17 | 6.1 | 0 | 0.0 | 0 | 0.0 | 17 | 2.3 | |
| * Other spp. | 29 | 10.4 | 8 | 2.4 | 5 | 3.8 | 42 | 5.7 | |
| Total | 278 | 100 | 328 | 100 | 132 | 100 | 738 | 100 | |
| Gram-positive | n | % | n | % | n | % | n | % | p-value |
| Staphylococcus aureus | 177 | 32.5 | 198 | 93.8 | 22 | 56.4 | 397 | 50.0 | <0.001 |
| Staphylococcus (CNS) | 269 | 49.5 | 3 | 1.4 | 11 | 28.2 | 283 | 35.6 | |
| Enterococcus spp. | 32 | 5.9 | 4 | 1.9 | 0 | 0.0 | 36 | 4.5 | |
| Streptococcus spp. | 24 | 4.4 | 6 | 2.8 | 6 | 15.4 | 36 | 4.5 | |
| ** Other spp. | 42 | 7.7 | 0 | 0.0 | 0 | 0.0 | 42 | 5.3 | |
| Total | 544 | 100 | 211 | 100 | 39 | 100 | 794 | 100 | |
| Pathogen Species | OPD | Internal Medicine | Surgery | Emergency | ICU | Pediatrics | Gynecology | Neonatology | NICU | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram − | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | p-value |
| Escherichia coli | 11 | 24.4 | 36 | 30.0 | 7 | 24.1 | 19 | 22.6 | 15 | 10.2 | 27 | 16.0 | 9 | 81.8 | 6 | 7.1 | 1 | 3.6 | 131 | 17.8 | <0.001 |
| Klebsiella spp. | 7 | 15.6 | 36 | 30.0 | 12 | 41.4 | 31 | 36.9 | 56 | 38.1 | 82 | 48.5 | 1 | 9.1 | 54 | 64.3 | 15 | 53.6 | 300 | 40.7 | |
| Acinetobacter spp. | 10 | 22.2 | 13 | 10.8 | 5 | 17.2 | 9 | 10.7 | 32 | 21.8 | 23 | 13.6 | 0 | 0.0 | 11 | 13.1 | 1 | 3.6 | 111 | 15.0 | |
| Pseudomonas spp. | 3 | 6.7 | 8 | 6.7 | 4 | 13.8 | 4 | 4.8 | 10 | 6.8 | 12 | 7.1 | 0 | 0.0 | 4 | 4.8 | 1 | 3.6 | 48 | 6.5 | |
| Enterobacter spp. | 2 | 4.4 | 13 | 10.8 | 0 | 0.0 | 4 | 4.8 | 13 | 8.8 | 6 | 3.6 | 0 | 0.0 | 4 | 4.8 | 2 | 7.1 | 47 | 6.4 | |
| Salmonella spp. | 9 | 20.0 | 8 | 6.7 | 0 | 0.0 | 9 | 10.7 | 0 | 0.0 | 15 | 8.9 | 1 | 9.1 | 0 | 0.0 | 0 | 0.0 | 42 | 5.7 | |
| Serratia spp. | 2 | 4.4 | 0 | 0.0 | 0 | 0.0 | 3 | 3.6 | 5 | 3.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 5 | 17.9 | 17 | 2.3 | |
| Other spp. | 1 | 2.2 | 6 | 5.0 | 1 | 3.5 | 5 | 6.0 | 16 | 10.9 | 4 | 2.4 | 0 | 0.0 | 5 | 6.0 | 3 | 10.7 | 42 | 5.7 | |
| Total | 45 | 100 | 120 | 100 | 29 | 100 | 84 | 100 | 147 | 100 | 169 | 100 | 11 | 100 | 84 | 100 | 28 | 100 | 738 | 100 | |
| Gram + | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | p-value |
| Staphylococcus aureus | 30 | 60.0 | 76 | 55.5 | 16 | 59.3 | 98 | 53.0 | 46 | 34.3 | 82 | 58.6 | 6 | 40.0 | 26 | 78.8 | 10 | 23.3 | 397 | 50.0 | <0.001 |
| Staphylococcus (CNS) | 19 | 38.0 | 43 | 31.4 | 4 | 14.8 | 62 | 33.5 | 64 | 47.8 | 38 | 27.1 | 8 | 53.3 | 5 | 15.2 | 19 | 44.2 | 283 | 35.6 | |
| Enterococcus spp. | 0 | 0.0 | 5 | 3.7 | 2 | 7.4 | 10 | 5.4 | 15 | 11.2 | 1 | 0.7 | 0 | 0.0 | 0 | 0.0 | 3 | 7.0 | 36 | 4.5 | |
| Streptococcus spp. | 0 | 0.0 | 6 | 4.4 | 3 | 11.1 | 6 | 3.2 | 3 | 2.2 | 11 | 7.9 | 0 | 0.0 | 1 | 3.0 | 4 | 9.3 | 36 | 4.5 | |
| Other spp. | 1 | 2.0 | 7 | 5.1 | 2 | 7.4 | 9 | 4.9 | 6 | 4.5 | 8 | 5.7 | 1 | 6.7 | 1 | 3.0 | 7 | 16.3 | 42 | 5.3 | |
| Total | 50 | 100 | 137 | 100 | 27 | 100 | 185 | 100 | 134 | 100 | 140 | 100 | 15 | 100 | 33 | 100 | 43 | 100 | 794 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gashegu, M.; Ndahindwa, V.; Rwagasore, E.; Tuyishime, A.; Musanabaganwa, C.; Gahamanyi, N.; Mukagatare, I.; Mbarushimana, D.; Green, C.A.; Dzinamarira, T.; et al. Diversity, Distribution, and Resistance Profiles of Bacterial Bloodstream Infections in Three Tertiary Referral Hospitals in Rwanda Between 2020 and 2022. Antibiotics 2024, 13, 1084. https://doi.org/10.3390/antibiotics13111084
Gashegu M, Ndahindwa V, Rwagasore E, Tuyishime A, Musanabaganwa C, Gahamanyi N, Mukagatare I, Mbarushimana D, Green CA, Dzinamarira T, et al. Diversity, Distribution, and Resistance Profiles of Bacterial Bloodstream Infections in Three Tertiary Referral Hospitals in Rwanda Between 2020 and 2022. Antibiotics. 2024; 13(11):1084. https://doi.org/10.3390/antibiotics13111084
Chicago/Turabian StyleGashegu, Misbah, Vedaste Ndahindwa, Edson Rwagasore, Albert Tuyishime, Clarisse Musanabaganwa, Noel Gahamanyi, Isabelle Mukagatare, Djibril Mbarushimana, Christopher Aird Green, Tafadzwa Dzinamarira, and et al. 2024. "Diversity, Distribution, and Resistance Profiles of Bacterial Bloodstream Infections in Three Tertiary Referral Hospitals in Rwanda Between 2020 and 2022" Antibiotics 13, no. 11: 1084. https://doi.org/10.3390/antibiotics13111084
APA StyleGashegu, M., Ndahindwa, V., Rwagasore, E., Tuyishime, A., Musanabaganwa, C., Gahamanyi, N., Mukagatare, I., Mbarushimana, D., Green, C. A., Dzinamarira, T., Ahmed, A., & Muvunyi, C. M. (2024). Diversity, Distribution, and Resistance Profiles of Bacterial Bloodstream Infections in Three Tertiary Referral Hospitals in Rwanda Between 2020 and 2022. Antibiotics, 13(11), 1084. https://doi.org/10.3390/antibiotics13111084








