Assessing the Reliability and Validity of a Questionnaire Evaluating Medical Students’ Attitudes, Knowledge, and Perceptions of Antibiotic Education and Antimicrobial Resistance in University Training
Abstract
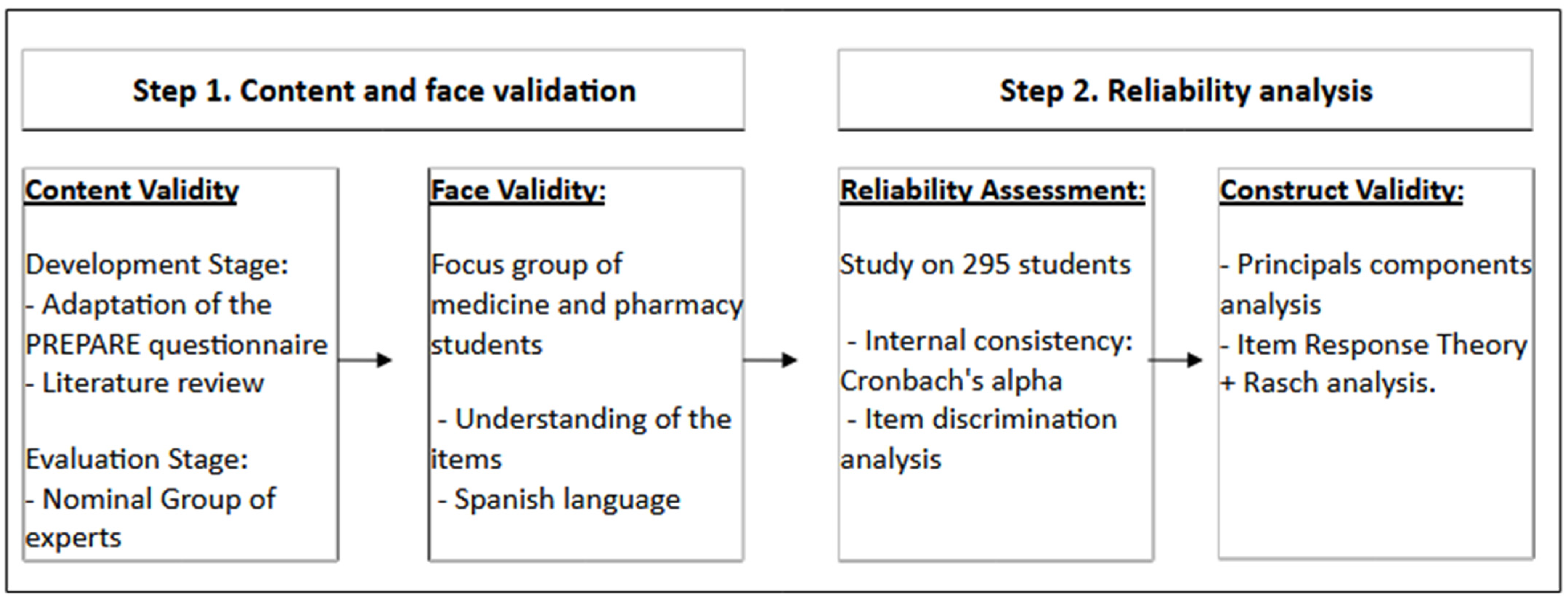
:1. Introduction
2. Results
2.1. Demographic Characteristics of Participants
2.2. Validation and Reliability of the Questionnaire
2.2.1. Internal Consistency and Item Discrimination Analysis
2.2.2. Construct Validity
2.3. Exploratory Findings of Questionnaire Responses
2.3.1. Students’ Perceptions of Their Preparedness in the Skills Required for Effective Infection Diagnosis and Treatment
2.3.2. Teaching Strategies and Antibiotic Education at the Faculty Level
3. Discussion
3.1. Questionnaire Development
3.2. Strengths and Limitations
4. Materials and Methods
4.1. Questionnaire Dissemination
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machowska, A.; Stålsby Lundborg, C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Lubwama, M.; Onyuka, J.; Ayazika, K.T.; Ssetaba, L.J.; Siboko, J.; Daniel, O.; Mushi, M.F. Knowledge, attitudes, and perceptions about antibiotic use and antimicrobial resistance among final year undergraduate medical and pharmacy students at three universities in East Africa. PLoS ONE 2021, 16, e0251301. [Google Scholar] [CrossRef] [PubMed]
- Yuste, J.R.; Matteo, A.B.; Gruber, F. Impact of Infectious Diseases training in the perception of antibiotic resistance and rational use of antibiotics among Spanish medical students—A cross-sectional study. BMC Med. Educ. 2022, 22, 550. [Google Scholar] [CrossRef] [PubMed]
- Dopelt, K.; Amar, A.; Yonatan, N.; Davidovitch, N. Knowledge, Attitudes, and Practices Regarding Antibiotic Use and Resistance: A Cross-Sectional Study among Students in Israel. Antibiotics 2023, 12, 1028. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- IHME Pathogen Core Group. Global burden associated with 85 pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Infect. Dis. 2024, 24, 868–895. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Pan American Health Organization PAHO/WHO Collaborating Centers. Document CD59/9. In Proceedings of the 59th Directing Council, 73rd Session of the Regional Committee of WHO for the Americas, Virtual, 20–24 July 2021; PAHO: Washington, DC, USA, 2021. Available online: https://www.paho.org/en/documents/cd599-one-health-comprehensive-approach-addressing-health-threats-human-animal (accessed on 22 November 2024).
- World Health Organization. 118th Session, 11 May 2006, Provisional Agenda Item 5.3, Rational Use of Medicines: Progress in Implementing the WHO Medicines Strategy; WHO: Geneva, Switzerland, 2006; Available online: https://apps.who.int/gb/ebwha/pdf_files/EB118/B118_6-en.pdf (accessed on 13 June 2024).
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 13 June 2024).
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 14 June 2024).
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Sánchez-Fabra, D.; Dyar, O.J.; Del Pozo, J.L.; Amiguet, J.A.; Colmenero, J.D.; Fariñas, M.D.C.; López-Medrano, F.; Portilla, J.; Praena, J.; Torre-Cisneros, J.; et al. Perspective of Spanish medical students regarding undergraduate education in infectious diseases, bacterial resistance and antibiotic use. Enferm. Infecc. Microbiol. Clin. 2019, 37, 25–30. [Google Scholar] [CrossRef]
- De Vries, E.; Johnson, Y.; Willems, B.; Bedeker, W.; Ras, T.; Coetzee, R.; Tembo, Y.; Brink, A. Improving primary care antimicrobial stewardship by implementing a peer audit and feedback intervention in Cape Town community healthcare centres. S. Afr. Med. J. 2022, 112, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Knowles, R.; Chandler, C.; O’Neill, S. A systematic review of national interventions and policies to optimize antibiotic use in healthcare settings in England. J. Antimicrob. Chemother. 2024, 79, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Dyar, O. Final results of the Student-PREPARE survey among European medical students: Knowledge and skills for improved antimicrobial prescribing. [abstract E069]. In Proceedings of the Program and Abstracts of the 26th European Conference on Clinical Microbiology and Infectious Diseases–ECCMID, Amsterdam, The Netherland, 9–12 April 2016; Available online: https://2016.eccmid.org/ (accessed on 2 October 2024).
- Slekovec, C.; Lepiller, Q.; Anxionnat, R.; Mouillet, S.; Ferreira, D.; Guillaume, A.; Kubicki, A.; Le Bourvellec, L.; Maitre, D.; Meurisse, A.; et al. French healthcare students and antibiotics: Are they ready to promote their appropriate use? JAC Antimicrob. Resist. 2023, 6, dlad147. [Google Scholar] [CrossRef]
- Bonna, A.S.; Mazumder, S.; Manna, R.M.; Pavel, S.R.; Nahin, S.; Ahmad, I.; Nabilah, N.; Ali, M.; Amin, M.A. Knowledge attitude and practice of antibiotic use among medical students in Bangladesh: A cross-sectional study. Health Sci. Rep. 2024, 7, e70030. [Google Scholar] [CrossRef] [PubMed]
- Boletín Oficial del Estado. Resolución de 17 de Febrero de 2011, de la Universidad de Santiago de Compostela, por la que se Publica el PLAN de Estudios de Graduado en Medicina; Boletín Oficial del Estado: Madrid, Spain, 2011; pp. 24404–24409. Available online: https://www.boe.es/diario_boe/txt.php?id=BOE-A-2011-4077 (accessed on 4 October 2024).
- Agencia Española de Medicamentos y Productos Sanitarios. Programa de Optimización del uso de Antibióticos (PROA); AEMPS: Madrid, Spain, 2017; 28p, Available online: https://www.resistenciaantibioticos.es/sites/default/files/documentos/prorgramas_de_optimizacion_de_uso_de_antibioticos_proa.pdf (accessed on 4 October 2024).
- Beier, M.E.; Ackerman, P.L. Determinants of Health Knowledge: An Investigation of Age, Gender, Abilities, Personality, and Interests. J. Pers. Soc. Psychol. 2003, 84, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Elsworth, G.; Osborne, R.H. Validity Evidence Based on Relations to Other Variables of the eHealth Literacy Questionnaire (eHLQ): Bayesian Approach to Test for Known-Groups Validity. J. Med. Internet Res. 2021, 23, e30243. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report 2022; ECDC: Stockholm, Sweden, 2023; 27p, Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER-antimicrobial-consumption.pdf (accessed on 18 July 2024).
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2023—2021 Data; ECDC: Stockholm, Sweden; WHO: Geneva, Switzerland, 2023; 186p, Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial%20resistance%20surveillance%20in%20Europe%202023%20-%202021%20data.pdf (accessed on 18 July 2024).
- Sun, H.; Gao, Y.; Liu, W.; Zhang, J.; Wu, I.X. Measurement of medical students’ knowledge, attitude and practice towards antibiotic use and resistance: A scoping review. J. Eval. Clin. Pract. 2024, 30, 1182–1195. [Google Scholar] [CrossRef]
- Vázquez-Lago, J.M.; Montes-Villalba, R.A.; Vázquez-Cancela, O.; Otero-Santiago, M.; López-Durán, A.; Figueiras, A. Knowledge, Perceptions, and Perspectives of Medical Students Regarding the Use of Antibiotics and Antibiotic Resistance: A Qualitative Research in Galicia, Spain. Antibiotics 2023, 12, 558. [Google Scholar] [CrossRef]
- Molina-Romera, G.; Vazquez-Cancela, O.; Vazquez-Lago, J.M.; Montes-Villalba, R.A.; Roque, F.; Herdeiro, M.T.; Figueiras, A. Knowledge, Attitudes and Practice Regarding Antibiotic Prescription by Medical Interns: A Qualitative Study in Spain. Antibiotics 2023, 12, 457. [Google Scholar] [CrossRef]
- Carretero-Dios, H.; Pérez, C. Normas para el desarrollo y revisión de estudios instrumentales: Consideraciones sobre la selección de tests en la investigación psicológica. Int. Clin. Health Psychol. 2007, 7, 863–882. [Google Scholar]
- University of Santiago de Compostela. Santiago de Compostela: University of Santiago de Compostela. Medicine Degree. Available online: https://www.usc.gal/en/studies/degrees/health-sciences/medicine-degree (accessed on 4 October 2024).
- Dyar, O.J.; Nathwani, D.; Monnet, D.L.; Gyssens, I.C.; Stålsby Lundborg, C.; Pulcini, C.; ESGAP Student-PREPARE Working Group. Do medical students feel prepared to prescribe antibiotics responsibly? Results from a cross-sectional survey in 29 European countries. J. Antimicrob. Chemother. 2018, 73, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Rusic, D.; Bozic, J.; Vilovic, M.; Bukic, J.; Zivkovic, P.M.; Leskur, D.; Seselja Perisin, A.; Tomic, S.; Modun, D. Attitudes and Knowledge Regarding Antimicrobial Use and Resistance Among Pharmacy and Medical Students at the University of Split, Croatia. Microb. Drug Resist. 2018, 24, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.J.; Tichelaar, J.; Graaf, S.; Otten, R.H.J.; Richir, M.C.; van Agtmael, M.A. Do final-year medical students have sufficient prescribing competencies? A systematic literature review. Br. J. Clin. Pharmacol. 2018, 84, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.A.A.; Singh, K.; Hilaire, M.G.S.; Rahman, S.; Sa, B.; Haque, M. Tackling antimicrobial resistance by promoting antimicrobial stewardship in medical and allied health professional curricula. Expert Rev. Anti-Infect. Ther. 2020, 18, 1245–1258. [Google Scholar] [CrossRef]
- Tangcharoensathien, V.; Chanvatik, S.; Sommanustweechai, A. Complex determinants of inappropriate use of antibiotics. Bull World Health Organ. 2018, 96, 141–144. [Google Scholar] [CrossRef]
- Pulcini, C.; Wencker, F.; Frimodt-Møller, N.; Kern, W.V.; Nathwani, D.; Rodríguez-Baño, J.; Simonsen, G.S.; Vlahović-Palčevski, V.; Gyssens, I.C.; ESGAP Curriculum Working Group. European survey on principles of prudent antibiotic prescribing teaching in undergraduate students. Clin. Microbiol. Infect. 2015, 21, 354–361. [Google Scholar] [CrossRef]
- Epstein, J.; Santo, M.R.; Guillemin, F. Cross-cultural adaptation of questionnaires: Review of concepts and current guidelines. J. Clin. Epidemiol. 2015, 68, 435–441. [Google Scholar] [CrossRef]
- Escoffery, C.; Lebow-Skelley, E.; Haardoerfer, R.; Boing, E.; Udelson, H.; Wood, R.; Hartman, M.; Fernandez, M.E.; Mullen, P.D. A systematic review of adaptations of evidence-based public health interventions globally. Implement. Sci. 2018, 13, 125. [Google Scholar] [CrossRef]
- Moore, G.; Campbell, M.; Copeland, L.; Craig, P.; Movsisyan, A.; Hoddinott, P.; Littlecott, H.; O’Cathain, A.; Pfadenhauer, L.; Rehfuess, E. Adapting interventions to new contexts—The ADAPT guidance. BMJ 2021, 374, n1679. [Google Scholar] [CrossRef]
- Bernal, G.; Adames, C. Cultural Adaptations: Conceptual, Ethical, Contextual, and Methodological Issues for Working with Ethnocultural and Majority-World Populations. Prev. Sci. 2017, 18, 681–688. [Google Scholar] [CrossRef]
- Wilson, C.J.; Bowden, S.C.; Byrne, L.K.; Joshua, N.R.; Marx, W.; Weiss, L.G. The cross-cultural generalizability of cognitive ability measures: A systematic literature review. Intelligence 2023, 98, 101751. [Google Scholar] [CrossRef]
- Chen, E.K.; Reid, M.C.; Parker, S.J.; Pillemer, P.K. Tailoring Evidence-Based Interventions for New Populations: A Method for Program Adaptation Through Community Engagement. Eval. Health Prof. 2013, 36, 73–92. [Google Scholar] [CrossRef] [PubMed]
- van Alphen, A.; Halfens, R.; Hasman, A.; Imbos, T. Likert or Rasch? Nothing is more applicable than good theory. J. Adv. Nurs. 1994, 20, 196–201. [Google Scholar] [CrossRef]
- Derrick, B.; White, P. Comparing Two Samples from an Individual Likert Question. Int. J. Math. Stat. 2017, 18, 1–13. [Google Scholar]
- Norman, G. Likert scales, levels of measurement and the “laws” of statistics. Adv. Health Sci. Educ. Theory Pract. 2010, 15, 625–632. [Google Scholar] [CrossRef]
- De Von, H.A.; Block, M.E.; Moyle-Wright, P.; Ernst, D.M.; Hayden, S.J.; Lazzara, D.J.; Savoy, S.M.; Kostas-Polston, E. A psychometric toolbox for testing validity and reliability. J. Nurs. Scholarsh. 2007, 39, 155–164. [Google Scholar] [CrossRef]
- Roco Videla, A.; Hernández Orellana, M.; Silva González, O. ¿Cuál es el tamaño muestral adecuado para validar un cuestionario? Nutr. Hosp. 2021, 38, 877–878. [Google Scholar] [CrossRef]
- Johanson, G.A.; Brooks, G.P. Initial Scale Development: Sample Size for Pilot Studies. Educ. Psychol. Meas. 2009, 70, 394–400. [Google Scholar] [CrossRef]
- Alabi, A.T.; Jelili, M.O. Clarifying likert scale misconceptions for improved application in urban studies. Qual. Quant. 2023, 57, 1337–1350. [Google Scholar] [CrossRef]
- Celenza, A.; Rogers, I.R. Comparison of visual analogue and Likert scales in evaluation of an emergency department bedside teaching programme. Emerg. Med. Australas. 2011, 23, 68–75. [Google Scholar] [CrossRef]
- Foley, D.K. Development of a visual analogue scale to measure curriculum outcomes. J. Nurs. Educ. 2008, 47, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Krosnick, J.A.; Presser, S. Question and Questionnaire Design. In Handbook of Survey Research, 2nd ed.; Marsden, P.V., Wright, J.D., Eds.; Emerald Group Publishing: Bingley, UK, 2010; pp. 263–314. [Google Scholar]
- Shrestha, N. Factor Analysis as a Tool for Survey Analysis. Am. J. Appl. Math. Stat. 2021, 9, 4–11. [Google Scholar] [CrossRef]
- Goretzko, D.; Pham, H.; Buehner, M. Exploratory factor analysis: Current use, methodological developments and recommendations for good practice. Curr. Psychol. 2021, 40, 3510–3521. [Google Scholar] [CrossRef]
- Bond, T.; Yan, Z.; Heene, M. Applying the Rasch Model; Routledge: London, UK, 2020. [Google Scholar] [CrossRef]
- Hays, R.D.; Morales, L.S.; Reise, S.P. Item Response Theory and Health Outcomes Measurement in the 21st Century. Med. Care 2000, 38, II28–II42. [Google Scholar] [CrossRef]
- DeVellis, R.F. Scale Development: Theory and Applications, 4th ed.; SAGE Publications: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- Esteba-Castillo, S.; Torrents-Rodas, D.; García-Alba, J.; Ribas-Vidal, N.; Novell-Alsina, R. Translation and validation of the Spanish version of the Health of the Nation Outcome Scales for People with Learning Disabilities (HoNOS-LD). Rev. Psiquiatr. Salud Ment. 2018, 11, 141–150. [Google Scholar] [CrossRef]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C.W. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef]
- Yaghmaie, F. Content validity and its estimation. J. Med. Educ. 2003, 3, 25–27. [Google Scholar]
- Alumran, A.; Hou, X.Y.; Hurst, C. Validity and reliability of instruments designed to measure factors influencing the overuse of antibiotics. J. Infect. Public Health 2012, 5, 221–232. [Google Scholar] [CrossRef]
- Nunnally, J.C.; Bernstein, I.H. Psychometric Theory, 3rd ed.; McGraw-Hill: New York, NY, USA, 1994. [Google Scholar]
- García de Yébenes Prous, M.J.; Rodríguez Salvanés, F.; Carmona Ortells, L. Validation of questionnaires. Reumatol. Clin. 2009, 5, 171–177. [Google Scholar] [CrossRef]
- Guilford, J.P. Psychometric Methods, 2nd ed.; McGraw-Hill: New York, NY, USA, 1954. [Google Scholar]
- Zijlmans, E.A.O.; Tijmstra, J.; van der Ark, L.A.; Sijtsma, K. Item-score reliability in empirical-data sets and its relationship with other item indices. Educ. Psychol. Meas. 2018, 78, 998–1020. [Google Scholar] [CrossRef]
| Item (Variable) | Cronbach’s Alpha if Item Deleted | Homogeneity Index |
|---|---|---|
| Item 7: I feel able to recognize the clinical signs of infection | 0.921 | 0.442 |
| Item 8: I feel able to assess the clinical severity of infection (e.g., using criteria, such as the septic shock criteria) | 0.921 | 0.463 |
| Item 9: I feel able to use point-of-care tests (e.g., urine dipstick, rapid diagnostic tests for streptococcal pharyngitis) | 0.922 | 0.390 |
| Item 10: I feel able to interpret biochemical markers of inflammation (e.g., CRP) | 0.921 | 0.459 |
| Item 11: I feel able to decide when it is important to take microbiological samples before starting antibiotic therapy | 0.921 | 0.487 |
| Item 12: I feel able to interpret basic microbiological investigations (e.g., blood cultures, antibiotic susceptibility reporting) | 0.920 | 0.547 |
| Item 13: I feel able to identify clinical situations when not to prescribe an antibiotic | 0.921 | 0.482 |
| Item 14: I feel able to differentiate between bacterial colonization and infection (e.g., asymptomatic bacteriuria) | 0.921 | 0.487 |
| Item 15: I feel able to differentiate between bacterial and viral upper respiratory tract infections | 0.920 | 0.545 |
| Item 16: I feel able to select initial empirical therapy based on the most likely pathogen(s) and antibiotic resistance patterns, without using guidelines | 0.919 | 0.616 |
| Item 17: I feel able to decide the urgency of antibiotic administration in different situations (e.g., <1 h for severe sepsis, non-urgent for chronic bone infections) | 0.919 | 0.614 |
| Item 18: I feel able to prescribe antibiotic therapy according to national/local guidelines | 0.920 | 0.575 |
| Item 19: I feel able to assess antibiotic allergies (e.g., differentiating between anaphylaxis and hypersensitivity) | 0.919 | 0.624 |
| Item 20: I feel able to decide the shortest possible adequate duration of antibiotic therapy for a specific infection | 0.919 | 0.634 |
| Item 21: I feel able to prescribe using principles of surgical antibiotic prophylaxis | 0.920 | 0.564 |
| Item 22: I feel able to review the need to continue or change antibiotic therapy after 48–72 h, based on clinical evolution and laboratory results | 0.919 | 0.693 |
| Item 23: I feel able to assess clinical outcomes and possible reasons for failure of antibiotic treatment | 0.918 | 0.685 |
| Item 24: I feel able to decide when to switch from intravenous (IV) to oral antibiotic therapy | 0.919 | 0.646 |
| Item 25: I feel able to measure/audit antibiotic use in a clinical setting, and to interpret the results of such studies | 0.920 | 0.567 |
| Item 26: I feel able to work within the multidisciplinary team in managing antibiotic use in hospitals | 0.919 | 0.623 |
| Item 27: I feel to explain the importance of appropriate antibiotic use to patients and their families | 0.919 | 0.628 |
| Item 28: I feel to discuss antibiotic use and resistance issues effectively with healthcare professionals and team members | 0.919 | 0.608 |
| Item 29: I feel able to use knowledge of the common mechanisms of antibiotic resistance in pathogens | 0.919 | 0.629 |
| Item 30: I feel able to use knowledge of the epidemiology of bacterial resistance, including local/regional variations | 0.919 | 0.597 |
| Item 31: I feel able to practice effective Infection control and hygiene (to prevent spread of bacteria) | 0.919 | 0.603 |
| Item 32: I feel able to use knowledge of the negative consequences of antibiotic use (bacterial resistance, toxic/adverse effects, cost, Clostridium difficile infections) | 0.919 | 0.601 |
| Item 33: Faculty methodology: lectures with >15 people | 0.924 | 0.120 |
| Item 34: Faculty methodology: small group teaching with <15 people | 0.923 | 0.234 |
| Item 35: Faculty methodology: discussion of clinical cases and vignettes | 0.922 | 0.397 |
| Item 36: Faculty methodology: active learning assignments | 0.922 | 0.371 |
| Item 37: e-learning | 0.923 | 0.284 |
| Item 38: Faculty methodology: role-playing | 0.923 | 0.253 |
| Item 39: Faculty methodology: infectious diseases clinical placement | 0.923 | 0.313 |
| Item 40: Faculty methodology: microbiology clinical placement | 0.923 | 0.224 |
| Item 41: Faculty methodology: peer or near-peer teaching | 0.923 | 0.237 |
| Item 42: Overall, do you feel you have received sufficient teaching at medical school in antibiotic use for your future practice as a junior doctor? | 0.926 | −0.299 |
| Item 43: Have any of your medical school examinations included questions on antibiotic treatment? | 0.924 | −0.141 |
| Item (Variable) | Correlation Coefficient | p-Value |
|---|---|---|
| Item 7: I feel able to recognize the clinical signs of infection | 0.477 | <0.01 |
| Item 8: I feel able to assess the clinical severity of infection (e.g., using criteria, such as the septic shock criteria) | 0.500 | <0.01 |
| Item 9: I feel able to use point-of-care tests (e.g., urine dipstick, rapid diagnostic tests for streptococcal pharyngitis) | 0.446 | <0.01 |
| Item 10: I feel able to interpret biochemical markers of inflammation (e.g., CRP) | 0.498 | <0.01 |
| Item 11: I feel able to decide when it is important to take microbiological samples before starting antibiotic therapy | 0.530 | <0.01 |
| Item 12: I feel able to interpret basic microbiological investigations (e.g., blood cultures, antibiotic susceptibility reporting) | 0.587 | <0.01 |
| Item 13: I feel able to identify clinical situations when not to prescribe an antibiotic | 0.522 | <0.01 |
| Item 14: I feel able to differentiate between bacterial colonization and infection (e.g., asymptomatic bacteriuria) | 0.526 | <0.01 |
| Item 15: I feel able to differentiate between bacterial and viral upper respiratory tract infections | 0.579 | <0.01 |
| Item 16: I feel able to select initial empirical therapy based on the most likely pathogen(s) and antibiotic resistance patterns, without using guidelines | 0.646 | <0.01 |
| Item 17: I feel able to decide the urgency of antibiotic administration in different situations (e.g., <1 h for severe sepsis, non-urgent for chronic bone infections) | 0.645 | <0.01 |
| Item 18: I feel able to prescribe antibiotic therapy according to national/local guidelines | 0.613 | <0.01 |
| Item 19: I feel able to assess antibiotic allergies (e.g., differentiating between anaphylaxis and hypersensitivity) | 0.656 | <0.01 |
| Item 20: I feel able to decide the shortest possible adequate duration of antibiotic therapy for a specific infection | 0.664 | <0.01 |
| Item 21: I feel able to prescribe using principles of surgical antibiotic prophylaxis | 0.599 | <0.01 |
| Item 22: I feel able to review the need to continue or change antibiotic therapy after 48–72 h, based on clinical evolution and laboratory results | 0.718 | <0.01 |
| Item 23: I feel able to assess clinical outcomes and possible reasons for failure of antibiotic treatment | 0.712 | <0.01 |
| Item 24: I feel able to decide when to switch from intravenous (IV) to oral antibiotic therapy | 0.676 | <0.01 |
| Item 25: I feel able to measure/audit antibiotic use in a clinical setting, and to interpret the results of such studies | 0.614 | <0.01 |
| Item 26: I feel able to work within the multidisciplinary team in managing antibiotic use in hospitals | 0.664 | <0.01 |
| Item 27: I feel to explain the importance of appropriate antibiotic use to patients and their families | 0.667 | <0.01 |
| Item 28: I feel to discuss antibiotic use and resistance issues effectively with healthcare professionals and team members | 0.650 | <0.01 |
| Item 29: I feel able to use knowledge of the common mechanisms of antibiotic resistance in pathogens | 0.659 | <0.01 |
| Item 30: I feel able to use knowledge of the epidemiology of bacterial resistance, including local/regional variations | 0.630 | <0.01 |
| Item 31: I feel able to practice effective Infection control and hygiene (to prevent spread of bacteria) | 0.638 | <0.01 |
| Item 32: I feel able to use knowledge of the negative consequences of antibiotic use (bacterial resistance, toxic/adverse effects, cost, Clostridium difficile infections) | 0.637 | <0.01 |
| Item 33: Faculty methodology: lectures with >15 people | 0.164 | <0.01 |
| Item 34: Faculty methodology: small group teaching with <15 people | 0.283 | <0.01 |
| Item 35: Faculty methodology: discussion of clinical cases and vignettes | 0.436 | <0.01 |
| Item 36: Faculty methodology: active learning assignments | 0.414 | <0.01 |
| Item 37: e-learning | 0.326 | <0.01 |
| Item 38: Faculty methodology: role-playing | 0.296 | <0.01 |
| Item 39: Faculty methodology: infectious diseases clinical placement | 0.365 | <0.01 |
| Item 40: Faculty methodology: microbiology clinical placement | 0.261 | <0.01 |
| Item 41: Faculty methodology: peer or near-peer teaching | 0.284 | <0.01 |
| Item 42: Overall, do you feel you have received sufficient teaching at medical school in antibiotic use for your future practice as a junior doctor? | −0.275 | <0.01 |
| Item 43: Have any of your medical school examinations included questions on antibiotic treatment? | −0.136 | 0.03 |
| Item (Variable) | Component | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Item 20: I feel able to decide the shortest possible adequate duration of antibiotic therapy for a specific infection | 0.757 | |||||||
| Item 21: I feel able to prescribe using principles of surgical antibiotic prophylaxis | 0.718 | |||||||
| Item 17: I feel able to decide the urgency of antibiotic administration in different situations (e.g., <1 h for severe sepsis, non-urgent for chronic bone infections) | 0.696 | |||||||
| Item 16: I feel able to select initial empirical therapy based on the most likely pathogen(s) and antibiotic resistance patterns, without using guidelines | 0.675 | |||||||
| Item 24: I feel able to decide when to switch from intravenous (IV) to oral antibiotic therapy | 0.585 | |||||||
| Item 18: I feel able to prescribe antibiotic therapy according to national/local guidelines | 0.573 | |||||||
| Item 42: Overall, do you feel you have received sufficient teaching at medical school in antibiotic use for your future practice as a junior doctor? | −0.521 | |||||||
| Item 22: I feel able to review the need to continue or change antibiotic therapy after 48–72 h, based on clinical evolution and laboratory results | 0.491 | |||||||
| Item 19: I feel able to assess antibiotic allergies (e.g., differentiating between anaphylaxis and hypersensitivity) | 0.457 | |||||||
| Item 23: I feel able to assess clinical outcomes and possible reasons for failure of antibiotic treatment | 0.434 | 0.430 | ||||||
| Item 8: I feel able to assess the clinical severity of infection (e.g., using criteria, such as the septic shock criteria) | 0.750 | |||||||
| Item 7: I feel able to recognize the clinical signs of infection | 0.733 | |||||||
| Item 10: I feel able to interpret biochemical markers of inflammation (e.g., CRP) | 0.722 | |||||||
| Item 11: I feel able to decide when it is important to take microbiological samples before starting antibiotic therapy | 0.666 | |||||||
| Item 12: I feel able to interpret basic microbiological investigations (e.g., blood cultures, antibiotic susceptibility reporting) | 0.567 | |||||||
| Item 9: I feel able to use point-of-care tests (e.g., urine dipstick, rapid diagnostic tests for streptococcal pharyngitis) | 0.537 | |||||||
| Item 28: I feel to discuss antibiotic use and resistance issues effectively with healthcare professionals and team members | 0.852 | |||||||
| Item 26: I feel able to work within the multidisciplinary team in managing antibiotic use in hospitals | 0.827 | |||||||
| Item 27: I feel to explain the importance of appropriate antibiotic use to patients and their families | 0.794 | |||||||
| Item 25: I feel able to measure/audit antibiotic use in a clinical setting, and to interpret the results of such studies | 0.632 | |||||||
| Item 31: I feel able to practice effective Infection control and hygiene (to prevent spread of bacteria) | 0.803 | |||||||
| Item 32: I feel able to use knowledge of the negative consequences of antibiotic use (bacterial resistance, toxic/adverse effects, cost, Clostridium difficile infections) | 0.739 | |||||||
| Item 29: I feel able to use knowledge of the common mechanisms of antibiotic resistance in pathogens | 0.441 | 0.611 | ||||||
| Item 30: I feel able to use knowledge of the epidemiology of bacterial resistance, including local/regional variations | 0.520 | |||||||
| Item 37: e-learning | 0.661 | |||||||
| Item 40: Faculty methodology: microbiology clinical placement | 0.652 | |||||||
| Item 39: Faculty methodology: infectious diseases clinical placement | 0.645 | |||||||
| Item 38: Faculty methodology: role-playing | 0.638 | |||||||
| Item 36: Faculty methodology: active learning assignments | 0.614 | |||||||
| Item 41: Faculty methodology: peer or near-peer teaching | 0.470 | |||||||
| Item 13: I feel able to identify clinical situations when not to prescribe an antibiotic | 0.708 | |||||||
| Item 14: I feel able to differentiate between bacterial colonization and infection (e.g., asymptomatic bacteriuria) | 0.658 | |||||||
| Item 15: I feel able to differentiate between bacterial and viral upper respiratory tract infections | 0.472 | 0.528 | ||||||
| Item 33: Faculty methodology: lectures with >15 people | 0.729 | |||||||
| Item 34: Faculty methodology: small group teaching with <15 people | 0.701 | |||||||
| Item 43: Have any of your medical school examinations included questions on antibiotic treatment? | 0.708 | |||||||
| Item 35: Faculty methodology: discussion of clinical cases and vignettes | −0.410 | |||||||
| Cronbach’s Alpha | 0.814 | 0.784 | 0.852 | 0.828 | 0.723 | 0.848 | 0.844 | 0.726 |
| Cronbach’s Alpha of the total scale | 0.923 | |||||||
| Item (Variable) | IRT | Rasch Analysis | |
|---|---|---|---|
| Difficulty Index * | Discrimination Index ** | Difficulty Index | |
| Item 7: I feel able to recognize the clinical signs of infection | 0.427 | 0.245 | 0.638 |
| Item 8: I feel able to assess the clinical severity of infection (e.g., using criteria, such as the septic shock criteria) | 0.315 | 0.216 | 0.735 |
| Item 9: I feel able to use point-of-care tests (e.g., urine dipstick, rapid diagnostic tests for streptococcal pharyngitis) | 0.359 | 0.230 | 0.799 |
| Item 10: I feel able to interpret biochemical markers of inflammation (e.g., CRP) | 0.288 | 0.205 | 0.288 |
| Item 11: I feel able to decide when it is important to take microbiological samples before starting antibiotic therapy | 0.454 | 0.248 | 0.853 |
| Item 12: I feel able to interpret basic microbiological investigations (e.g., blood cultures, antibiotic susceptibility reporting) | 0.386 | 0.237 | 0.691 |
| Item 13: I feel able to identify clinical situations when not to prescribe an antibiotic | 0.349 | 0.227 | 0.477 |
| Item 14: I feel able to differentiate between bacterial colonization and infection (e.g., asymptomatic bacteriuria) | 0.336 | 0.224 | 0.496 |
| Item 15: I feel able to differentiate between bacterial and viral upper respiratory tract infections | 0.431 | 0.246 | 0.431 |
| Item 16: I feel able to select initial empirical therapy based on the most likely pathogen(s) and antibiotic resistance patterns, without using guidelines | 0.498 | 0.250 | 0.584 |
| Item 17: I feel able to decide the urgency of antibiotic administration in different situations (e.g., <1 h for severe sepsis, non-urgent for chronic bone infections) | 0.499 | 0.250 | 0.594 |
| Item 18: I feel able to prescribe antibiotic therapy according to national/local guidelines | 0.453 | 0.248 | 0.565 |
| Item 19: I feel able to assess antibiotic allergies (e.g., differentiating between anaphylaxis and hypersensitivity) | 0.387 | 0.237 | 0.597 |
| Item 20: I feel able to decide the shortest possible adequate duration of antibiotic therapy for a specific infection | 0.476 | 0.249 | 0.636 |
| Item 21: I feel able to prescribe using principles of surgical antibiotic prophylaxis | 0.454 | 0.248 | 0.565 |
| Item 22: I feel able to review the need to continue or change antibiotic therapy after 48–72 h, based on clinical evolution and laboratory results | 0.512 | 0.250 | 0.693 |
| Item 23: I feel able to assess clinical outcomes and possible reasons for failure of antibiotic treatment | 0.491 | 0.250 | 0.691 |
| Item 24: I feel able to decide when to switch from intravenous (IV) to oral antibiotic therapy | 0.465 | 0.248 | 0.621 |
| Item 25: I feel able to measure/audit antibiotic use in a clinical setting, and to interpret the results of such studies | 0.401 | 0.240 | 0.569 |
| Item 26: I feel able to work within the multidisciplinary team in managing antibiotic use in hospitals | 0.440 | 0.246 | 0.604 |
| Item 27: I feel to explain the importance of appropriate antibiotic use to patients and their families | 0.489 | 0.250 | 0.616 |
| Item 28: I feel to discuss antibiotic use and resistance issues effectively with healthcare professionals and team members | 0.466 | 0.248 | 0.582 |
| Item 29: I feel able to use knowledge of the common mechanisms of antibiotic resistance in pathogens | 0.476 | 0.249 | 0.632 |
| Item 30: I feel able to use knowledge of the epidemiology of bacterial resistance, including local/regional variations | 0.412 | 0.242 | 0.596 |
| Item 31: I feel able to practice effective Infection control and hygiene (to prevent spread of bacteria) | 0.452 | 0.248 | 0.598 |
| Item 32: I feel able to use knowledge of the negative consequences of antibiotic use (bacterial resistance, toxic/adverse effects, cost, Clostridium difficile infections) | 0.437 | 0.246 | 0.589 |
| Item 33: Faculty methodology: lectures with >15 people | 0.324 | 0.220 | 0.115 |
| Item 34: Faculty methodology: small group teaching with <15 people | 0.189 | 0.154 | 0.223 |
| Item 35: Faculty methodology: discussion of clinical cases and vignettes | 0.369 | 0.233 | 0.369 |
| Item 36: Faculty methodology: active learning assignments | 0.353 | 0.228 | 0.353 |
| Item 37: e-learning | 0.403 | 0.241 | 0.403 |
| Item 38: Faculty methodology: role-playing | 0.273 | 0.198 | 0.232 |
| Item 39: Faculty methodology: infectious diseases clinical placement | 0.375 | 0.200 | 0.274 |
| Item 40: Faculty methodology: microbiology clinical placement | 0.237 | 0.181 | 0.205 |
| Item 41: Faculty methodology: peer or near-peer teaching | 0.396 | 0.239 | 0.216 |
| Item 42: Overall, do you feel you have received sufficient teaching at medical school in antibiotic use for your future practice as a junior doctor? | 0.044 | 0.042 | −0.262 |
| Item 43: Have any of your medical school examinations included questions on antibiotic treatment? | 0.003 | 0.003 | −0.134 |
| Dimension | Item (Variable) | M | SD | Agreement (%) | Neutral (%) | Disagreement (%) |
|---|---|---|---|---|---|---|
| Students’ perception of their preparedness for infection diagnosis | Item 7: I feel able to recognize the clinical signs of infection | 5.15 | 1.33 | 42.3 | 56.3 | 1.4 |
| Item 8: I feel able to assess the clinical severity of infection (e.g., using criteria, such as the septic shock criteria) | 4.70 | 1.42 | 31.2 | 64.0 | 4.8 | |
| Item 9: I feel able to use point-of-care tests (e.g., urine dipstick, rapid diagnostic tests for streptococcal pharyngitis) | 3.96 | 1.99 | 26.6 | 56.6 | 16.8 | |
| Item 10: I feel able to interpret biochemical markers of inflammation (e.g., CRP) | 4.63 | 1.47 | 28.7 | 64.0 | 7.3 | |
| Item 11: I feel able to decide when it is important to take microbiological samples before starting antibiotic therapy | 4.36 | 1.71 | 29.0 | 56.9 | 14.1 | |
| Item 12: I feel able to interpret basic microbiological investigations (e.g., blood cultures, antibiotic susceptibility reporting) | 4.08 | 1.69 | 21.5 | 63.0 | 15.6 | |
| Indications for avoiding antibiotic prescriptions | Item 13: I feel able to identify clinical situations when not to prescribe an antibiotic | 4.17 | 1.54 | 20.6 | 68.0 | 11.3 |
| Item 14: I feel able to differentiate between bacterial colonization and infection (e.g., asymptomatic bacteriuria) | 4.12 | 1.50 | 19.6 | 71.1 | 9.3 | |
| Item 15: I feel able to differentiate between bacterial and viral upper respiratory tract infections | 4.47 | 1.43 | 25.2 | 67.6 | 7.2 | |
| Understanding of empirical antibiotic therapy | Item 16: I feel able to select initial empirical therapy based on the most likely pathogen(s) and antibiotic resistance patterns, without using guidelines | 2.71 | 1.51 | 5.9 | 44.6 | 49.5 |
| Item 17: I feel able to decide the urgency of antibiotic administration in different situations (e.g., <1 h for severe sepsis, non-urgent for chronic bone infections) | 2.75 | 1.46 | 3.5 | 51.2 | 45.3 | |
| Item 18: I feel able to prescribe antibiotic therapy according to national/local guidelines | 3.44 | 1.69 | 12.5 | 58.5 | 28.9 | |
| Item 19: I feel able to assess antibiotic allergies (e.g., differentiating between anaphylaxis and hypersensitivity) | 3.85 | 1.59 | 14.6 | 69.1 | 16.3 | |
| Item 20: I feel able to decide the shortest possible adequate duration of antibiotic therapy for a specific infection | 2.77 | 1.51 | 5.3 | 50.7 | 44.0 | |
| Item 21: I feel able to prescribe using principles of surgical antibiotic prophylaxis | 3.10 | 1.54 | 5.7 | 56.9 | 37.5 | |
| Re-evaluation of antibiotic therapy | Item 22: I feel able to review the need to continue or change antibiotic therapy after 48–72 h, based on clinical evolution and laboratory results | 3.33 | 1.45 | 7.2 | 64.4 | 28.4 |
| Item 23: I feel able to assess clinical outcomes and possible reasons for failure of antibiotic treatment | 3.49 | 1.48 | 8.6 | 66.1 | 25.3 | |
| Item 24: I feel able to decide when to switch from intravenous (IV) to oral antibiotic therapy | 2.98 | 1.53 | 5.3 | 58.9 | 35.8 | |
| Perceived quality of care | Item 25: I feel able to measure/audit antibiotic use in a clinical setting, and to interpret the results of such studies | 3.09 | 2.03 | 7.3 | 56.5 | 36.2 |
| Item 26: I feel able to work within the multidisciplinary team in managing antibiotic use in hospitals | 2.62 | 1.95 | 7.6 | 45.4 | 47.0 | |
| Perceived preparedness in communication skills | Item 27: I feel to explain the importance of appropriate antibiotic use to patients and their families | 3.06 | 1.93 | 6.6 | 58.4 | 35.0 |
| Item 28: I feel to discuss antibiotic use and resistance issues effectively with healthcare professionals and team members | 2.76 | 2.02 | 8.2 | 50.2 | 41.6 | |
| Knowledge regarding antibiotic resistance | Item 29: I feel able to use knowledge of the common mechanisms of antibiotic resistance in pathogens | 3.38 | 1.46 | 8.3 | 64.7 | 27.0 |
| Item 30: I feel able to use knowledge of the epidemiology of bacterial resistance, including local/regional variations | 2.94 | 1.51 | 7.3 | 52.4 | 40.2 | |
| Item 31: I feel able to practice effective Infection control and hygiene (to prevent spread of bacteria) | 4.37 | 1.62 | 28.3 | 57.0 | 14.7 | |
| Item 32: I feel able to use knowledge of the negative consequences of antibiotic use (bacterial resistance, toxic/adverse effects, cost, Clostridium difficile infections) | 4.68 | 1.75 | 37.7 | 50.9 | 11.4 |
| Dimension | Item (Variable) | M | SD | Agreement (%) | Neutral (%) | Disagreement (%) |
|---|---|---|---|---|---|---|
| Opinions on teaching methodologies | Item 33: Faculty methodology: lectures with >15 people | 0.73 | 1.34 | 11.4 | 11.4 | 77.2 |
| Item 34: Faculty methodology: small group teaching with <15 people | 1.43 | 1.52 | 31.7 | 17.1 | 51.2 | |
| Item 35: Faculty methodology: discussion of clinical cases and vignettes | 2.41 | 1.35 | 58.8 | 21.1 | 20.1 | |
| Item 36: Faculty methodology: active learning assignments | 1.37 | 1.47 | 27.1 | 19.9 | 52.9 | |
| Item 37: e-learning | 1.00 | 1.34 | 15.1 | 18.9 | 66.0 | |
| Item 38: Faculty methodology: role-playing | 0.74 | 1.38 | 16.2 | 6.6 | 77.2 | |
| Item 39: Faculty methodology: infectious diseases clinical placement | 1.53 | 1.71 | 37.6 | 7.9 | 54.5 | |
| Item 40: Faculty methodology: microbiology clinical placement | 0.61 | 1.18 | 12.5 | 8.3 | 79.2 | |
| Item 41: Faculty methodology: peer or near-peer teaching | 1.15 | 1.47 | 30.5 | 8.9 | 60.6 | |
| Item 42: Overall, do you feel you have received sufficient teaching at medical school in antibiotic use for your future practice as a junior doctor? | 2.42 | 0.78 | 13.9 | 4.4 | 81.6 | |
| Item 43: Have any of your medical school examinations included questions on antibiotic treatment? | 1.01 | 0.18 | 99.7 | 0.3 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Cancela, O.; Lens-Perol, G.; Mascareñas-Garcia, M.; Santana-Armas, M.; Vazquez-Lago, J.M. Assessing the Reliability and Validity of a Questionnaire Evaluating Medical Students’ Attitudes, Knowledge, and Perceptions of Antibiotic Education and Antimicrobial Resistance in University Training. Antibiotics 2024, 13, 1126. https://doi.org/10.3390/antibiotics13121126
Vázquez-Cancela O, Lens-Perol G, Mascareñas-Garcia M, Santana-Armas M, Vazquez-Lago JM. Assessing the Reliability and Validity of a Questionnaire Evaluating Medical Students’ Attitudes, Knowledge, and Perceptions of Antibiotic Education and Antimicrobial Resistance in University Training. Antibiotics. 2024; 13(12):1126. https://doi.org/10.3390/antibiotics13121126
Chicago/Turabian StyleVázquez-Cancela, Olalla, Guillermo Lens-Perol, Marta Mascareñas-Garcia, Magdalena Santana-Armas, and Juan Manuel Vazquez-Lago. 2024. "Assessing the Reliability and Validity of a Questionnaire Evaluating Medical Students’ Attitudes, Knowledge, and Perceptions of Antibiotic Education and Antimicrobial Resistance in University Training" Antibiotics 13, no. 12: 1126. https://doi.org/10.3390/antibiotics13121126
APA StyleVázquez-Cancela, O., Lens-Perol, G., Mascareñas-Garcia, M., Santana-Armas, M., & Vazquez-Lago, J. M. (2024). Assessing the Reliability and Validity of a Questionnaire Evaluating Medical Students’ Attitudes, Knowledge, and Perceptions of Antibiotic Education and Antimicrobial Resistance in University Training. Antibiotics, 13(12), 1126. https://doi.org/10.3390/antibiotics13121126






