A Screen of Traditional Chinese Medicinal Plant Extracts Reveals 17 Species with Antimicrobial Properties
Abstract
1. Introduction
2. Results
2.1. Experimental Overview and Identification of TCM Plant Extracts with Antimicrobial Properties
2.2. Extract Screening Reveals 17 TCM Plant Species with Antimicrobial Properties
| * Family. | Genus | Species | Common Name | Chinese Name | Tested for Antimicrobial Activity [Citation] |
|---|---|---|---|---|---|
| Compositae | Asarum | heterotropoides | Wild Ginger | 細辛 | Ralstonia solanacearum, Xanthomonas oryzae, Pseudomonas syringae, Xanthomonas axonopodis [27]; Fusobacterium nucleatum, Prevotella intermedia, Porphyromonas gingivalis [28]. Clostridioides difficile, Clostridium paraputrificum, Clostridium perfringens, Staphylococcus aureus, Bacteroides fragilis, Escherichia coli, Salmonella enterica serovar Typhimurium [29]; Listeria monocytogenes [30]; Staphylococcus epidermidis, Micrococcus luteus, Corynebacterium jeikeium, Corynebacterium xerosis, Propionibacterium freudenreichii [31] |
| Boraginaceae | Lithospermum | erythrorhizon | Purple Gromwell | 紫草 | Bacillus subtilis, Bacillus thuringiensis, Clavibacter michiganensis, Agrobacterium radiobacter, Agrobacterium rhizogenes, Agrobacterium tumefaciens, Bulkholderia cepacian, Erwinia herbicola, Ralstonia solanacearum [32]; Staphylococcus aureus, Micrococcus roseus, Micrococcus luteus, Bacillus subtilis [33] |
| Compositae | Tussilago | farfara | Coltsfoot | 款冬花 | Bacillus cereus, Staphylococcus aureus [34]; Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus [35];. Escherichia coli, Klebsiella pneumoniae, Salmonella enterica, Shigella sonnei, Yersinia enterocolitia, Bacillus thuringiensis, Clostridium perfringens, Haemophilus influenzae, Listeria monocytogenes, Staphylococcus aureus [36]; Escherichia coli, Serratia rubidaea, Staphylococcus epidermis, Lactobacillus rhamnosus, Pseudomonas aeruginosa, Enterococcus raffinosus [37] |
| Compositae | Echinops | latifolius | Globe Thistle | 驴欺口 | None |
| Compositae | Artemisia | annua | Sweet Wormwood | 青蒿 | Haemophilus inflenzae, Enterococcus faecalis, Streptococcus pneumoniae, Micrococcus luteus [38]; Bacillus cereus, Staphylococcus aureus, Micrococcus luteus, Escherichia coli, Klebsiella pneumoniae, Salmonella enteritidis, Shigella sp. [39]; Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Bacillus thuringiensis [40]; Enterococcus hirae [41]; Staphylococcus aureus, Escherichia coli [42]; Staphylococcus aureus, Escherichia coli, Bacillus cereus, Enterococcus faecalis, Pseudomonas aeruginosa [43]; Staphylococcus aureus, Bacillus subtilis, Bacillus pumilus, Bacillus cereus, Micrococcus luteus, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa [44] |
| Compositae | Artemisia | argyi | Mugwort | 艾草 | Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Listeria monocytogenes, Pseudomonas aeruginosa, Streptococcus pneumoniae, Proteus mirabilis, Enterococcus faecalis, Streptococcus agalactiae [45]; Staphylococcus aureus, Bacillus subtilis, Listeria monocytogenes, Escherichia coli, Proteus vulgaris, Salmonella enteritidis [46] |
| Compositae | Sigesbeckia | orientalis | St. Paul’s Wort | 豨莶 | Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli [47]; Bacillus subtilis, Staphylococcus epidermidis, Staphylococcus aureus, Streptococcus oralis, Acinetobacter baumannii, Escherichia coli, Pseudomonas aeruginosa [48] |
| Dioscoreaceae | Dioscorea | nipponica | Japanese Yam | 穿龙薯蓣 | Bacillus subtilis, Staphylococcus aureus, Proteus vulgaris, Salmonella Typhimurium [49] |
| Juncaceae | Juncus | effusus | Soft Rush | 灯心草 | Staphylococcus aureus, Bacillus subtilis [50]; Micrococcus luteus, Bacillus subtilis, Staphylococcus aureus [51] |
| Lamiaceae | Pogostemon | cablin | Patchouli | 广藿香 | Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus epidermidis, Streptococcus oralis, Streptococcus pneumoniae, Streptococcus constellatus, Streptococcus pyogenes, Streptococcus mitis [52]; Staphylococcus aureus, Shigella sp. [53] |
| Labiatae | Salvia | miltiorrhiza | Red Sage | 丹参 | Agrobacterium tumefaciens, Escherichia coli, Pseudomonas syringae, Ralstonia solanacearum, Xanthomonas vesicatoria, Bacillus subtilis, Staphylococcus aureus, Staphylococcus haemolyticus [54] |
| Fabaceae | Glycyrrhiza | uralensis | Chinese Licorice | 甘草 | Streptococcus mutans [55]; Staphylococcus aureus [56] |
| Fabaceae | Sophora | flavescens | Sophora Root | 苦参 | Staphylococcus aureus, Bacillus subtilis, Salmonella Typhimurium, Proteus vulgaris, Escherichia coli [57]; Streptococcus mutans [58] |
| Compositae | Areca | catechu | Areca Palm | 檳榔 | Bacillus subtilis, Staphylococcus aureus [59] |
| Rosaceae | Agrimonia | pilosa | Chinese Agrimony | 龙芽草 | Listeria monocytogenes, Streptococcus enteritidis, Escherichia coli [60] |
| Rubiaceae | Rubia | cordifolia | Indian Madder | 茜草 | Erwinia herbicola, Agrobacterium tumefaciens, Xanthamonas campestris [61]; Bacillus cereus, Bacillus pumilus, Bacillus subtilis, Micrococcus luteus, Mycobacterium luteum, Staphylococcus aureus, P. aeruginosa [62] |
| Rutaceae | Evodia | rutaecarpa | Evodia Fruit | 吳茱萸 | Escherichia coli, Staphylococcus aureus [63]; Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis [64] |
2.3. Liquid Assay
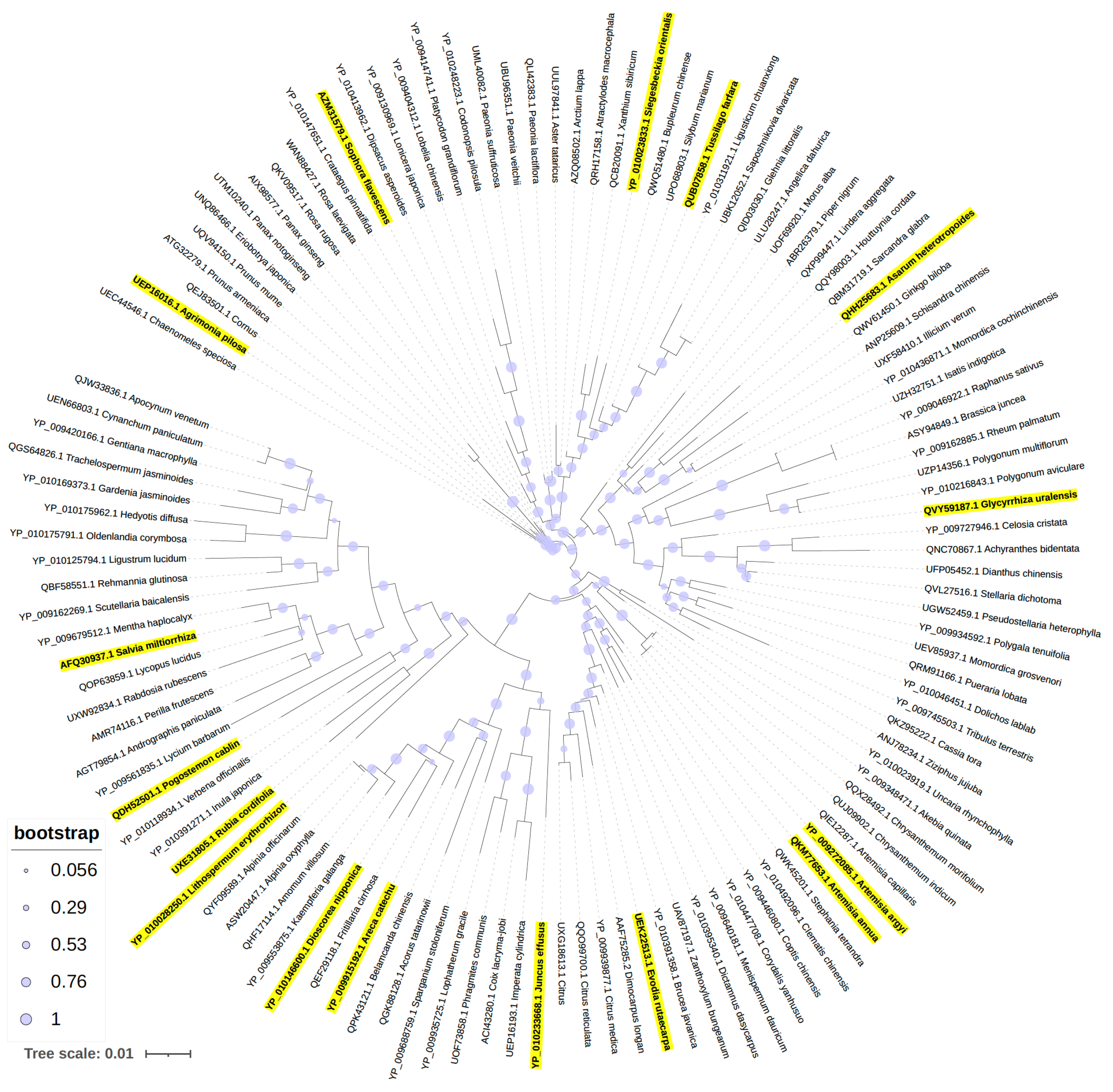
2.4. Phylogenetic Analysis
2.5. Active Components of TCM Plant Extracts
3. Discussion
3.1. TCM Plant-Derived Antimicrobials
3.2. Antimicrobial Activities of TCM Plant Extracts
3.3. Active Components of TCM Plant Extracts
4. Materials and Methods
4.1. TCM Plant Extracts
4.2. Bacteria and Culture
4.3. Disk Diffusion Assay
4.4. Plate Dilution Assay
4.5. Phylogenetic Analysis
4.6. Compound Structure Similarity
4.7. Lipinski Rule of Five
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2019.
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Jonas, O.I.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.; Marquez, P. Drug-Resistant Infections: A Threat to Our Economic Future (Vol. 2): Final Report (English); World Bank Group: Washington, DC, USA, 2017; p. 172. [Google Scholar]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; CABI: Wallingford, UK, 2016. [Google Scholar]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; WHO: Geneva, Switzerland, 2024.
- Ruekit, S.; Srijan, A.; Serichantalergs, O.; Margulieux, K.R.; Mc Gann, P.; Mills, E.G.; Stribling, W.C.; Pimsawat, T.; Kormanee, R.; Nakornchai, S.; et al. Molecular characterization of multidrug-resistant ESKAPEE pathogens from clinical samples in Chonburi, Thailand (2017–2018). BMC Infect. Dis. 2022, 22, 695. [Google Scholar] [CrossRef] [PubMed]
- Årdal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic development—Economic, regulatory and societal challenges. Nat. Rev. Microbiol. 2020, 18, 267–274. [Google Scholar] [CrossRef]
- Spellberg, B.; Bartlett, J.; Wunderink, R.; Gilbert, D.N. Novel approaches are needed to develop tomorrow’s antibacterial therapies. Am. J. Respir. Crit. Care Med. 2015, 191, 135–140. [Google Scholar] [CrossRef]
- Czyz, D.M.; Potluri, L.P.; Jain-Gupta, N.; Riley, S.P.; Martinez, J.J.; Steck, T.L.; Crosson, S.; Shuman, H.A.; Gabay, J.E. Host-directed antimicrobial drugs with broad-spectrum efficacy against intracellular bacterial pathogens. mBio 2014, 5, e01534-14. [Google Scholar] [CrossRef]
- Dove, A.S.; Dzurny, D.I.; Dees, W.R.; Qin, N.; Nunez Rodriguez, C.C.; Alt, L.A.; Ellward, G.L.; Best, J.A.; Rudawski, N.G.; Fujii, K.; et al. Silver nanoparticles enhance the efficacy of aminoglycosides against antibiotic-resistant bacteria. Front. Microbiol. 2022, 13, 1064095. [Google Scholar] [CrossRef]
- Gorski, A.; Miedzybrodzki, R.; Wegrzyn, G.; Jonczyk-Matysiak, E.; Borysowski, J.; Weber-Dabrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef]
- Ye, J.; Chen, X. Current Promising Strategies against Antibiotic-Resistant Bacterial Infections. Antibiotics 2022, 12, 67. [Google Scholar] [CrossRef]
- Eisenberg, D.M.; Harris, E.S.; Littlefield, B.A.; Cao, S.; Craycroft, J.A.; Scholten, R.; Bayliss, P.; Fu, Y.; Wang, W.; Qiao, Y.; et al. Developing a library of authenticated Traditional Chinese Medicinal (TCM) plants for systematic biological evaluation—Rationale, methods and preliminary results from a Sino-American collaboration. Fitoterapia 2011, 82, 17–33. [Google Scholar] [CrossRef]
- Chen, K.; Wu, W.; Hou, X.; Yang, Q.; Li, Z. A review: Antimicrobial properties of several medicinal plants widely used in Traditional Chinese Medicine. Food Qual. Saf. 2021, 5, fyab020. [Google Scholar] [CrossRef]
- Li, J.; Feng, S.; Liu, X.; Jia, X.; Qiao, F.; Guo, J.; Deng, S. Effects of Traditional Chinese Medicine and its Active Ingredients on Drug-Resistant Bacteria. Front. Pharmacol. 2022, 13, 837907. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, M.; Sol, C.; de Nova, P.J.G.; Puyalto, M.; Mesas, L.; Puente, H.; Mencía-Ares, Ó.; Miranda, R.; Argüello, H.; Rubio, P.; et al. Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porc. Health Manag. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Dahlem Junior, M.A.; Nguema Edzang, R.W.; Catto, A.L.; Raimundo, J.M. Quinones as an Efficient Molecular Scaffold in the Antibacterial/Antifungal or Antitumoral Arsenal. Int. J. Mol. Sci. 2022, 23, 14108. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Wong, Y.K.; Li, Y.; Liao, F.; Jiang, T.; Tu, Y. Artemisinin, the Magic Drug Discovered from Traditional Chinese Medicine. Engineering 2019, 5, 32–39. [Google Scholar] [CrossRef]
- Su, X.Z.; Miller, L.H. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Berida, T.I.; Adekunle, Y.A.; Dada-Adegbola, H.; Kdimy, A.; Roy, S.; Sarker, S.D. Plant antibacterials: The challenges and opportunities. Heliyon 2024, 10, e31145. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Miao, G. Strategies, Achievements, and Potential Challenges of Plant and Microbial Chassis in the Biosynthesis of Plant Secondary Metabolites. Molecules 2024, 29, 2106. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Kong, D.; He, S.; Chen, J.; Jiang, Y.; Ma, Z.; Feng, J.; Yan, H. Phenanthrene Derivatives from Asarum heterotropoides Showed Excellent Antibacterial Activity against Phytopathogenic Bacteria. J. Agric. Food Chem. 2021, 69, 14520–14529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, F.; Zhang, H.; Peng, L.; Zhen, Y.; Wang, L.; Xu, Y.; He, D.; Li, X. Orthogonal test design for optimization of the extraction of essential oil from Asarum heterotropoides var. Mandshuricum and evaluation of its antibacterial activity against periodontal pathogens. 3 Biotech 2018, 8, 473. [Google Scholar] [CrossRef]
- Perumalsamy, H.; Jung, M.Y.; Hong, S.M.; Ahn, Y.J. Growth-Inhibiting and morphostructural effects of constituents identified in Asarum heterotropoides root on human intestinal bacteria. BMC Complement. Altern. Med. 2013, 13, 245. [Google Scholar] [CrossRef]
- Oh, J.; Hwang, I.H.; Kim, D.C.; Kang, S.C.; Jang, T.S.; Lee, S.H.; Na, M. Anti-listerial compounds from Asari Radix. Arch. Pharmacal Res. 2010, 33, 1339–1345. [Google Scholar] [CrossRef]
- Haque, A.; Moon, J.N.; Saravana, P.S.; Tilahun, A.; Chun, B.S. Composition of Asarum heterotropoides var. mandshuricum radix oil from different extraction methods and activities against human body odor-producing bacteria. J. Food Drug Anal. 2016, 24, 813–821. [Google Scholar] [CrossRef]
- Brigham, L.A.; Michaels, P.J.; Flores, H.E. Cell-specific production and antimicrobial activity of naphthoquinones in roots of lithospermum erythrorhizon. Plant Physiol. 1999, 119, 417–428. [Google Scholar] [CrossRef]
- Honda, G.; Sakakibara, F.; Yazaki, K.; Tabata, M. Isolation of Deoxyshikonin, an Antidermatophytic Principle from Lithospermum erythrorhizon Cell Cultures. J. Nat. Prod. 1988, 51, 152–154. [Google Scholar] [CrossRef]
- Kokoska, L.; Polesny, Z.; Rada, V.; Nepovim, A.; Vanek, T. Screening of some Siberian medicinal plants for antimicrobial activity. J. Ethnopharmacol. 2002, 82, 51–53. [Google Scholar] [CrossRef]
- Lee, Y.J.; Song, K.; Cha, S.H.; Cho, S.; Kim, Y.S.; Park, Y. Sesquiterpenoids from Tussilago farfara Flower Bud Extract for the Eco-Friendly Synthesis of Silver and Gold Nanoparticles Possessing Antibacterial and Anticancer Activities. Nanomaterials 2019, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Ivanišová, E.; Kačániová, M.; Petrová, J.; Frančáková, H.; Tokár, M. The evaluation of antioxidant and antimicrobial effect of Tussilago farfara L. and Cetraria islandica L. Sci. Pap. Anim. Sci. Biotechnol. 2016, 49, 46. [Google Scholar]
- Kacaniova, M.; Hleba, L.; Petrová, J.; Felšöciová, S.; Pavelková, A.; Miklášová, K.; Bobková, A.; Čuboň, J. Antimicrobial activity of Tussilago farfara L. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 1343–1350. [Google Scholar]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Radulović, N.S.; Randjelović, P.J.; Stojanović, N.M.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Ilić, I.R.; Djordjević, V.B. Toxic essential oils. Part II: Chemical, toxicological, pharmacological and microbiological profiles of Artemisia annua L. volatiles. Food Chem. Toxicol. 2013, 58, 37–49. [Google Scholar] [CrossRef]
- Li, Y.; Hu, H.; Zheng, X.; Zhu, J.; Liu, L. Composition and antimicrobial activity of essential oil from the aerial part of Artemisia annua. J. Med. Plants Res. 2011, 5, 3629–3633. [Google Scholar]
- Juteau, F.; Masotti, V.; Bessière, J.M.; Dherbomez, M.; Viano, J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia 2002, 73, 532–535. [Google Scholar] [CrossRef]
- Verdian, R.M.; Sadat, E.E.; Haji, A.A.; Fazeli, M.; Pirali, H.M. Chemical composition and antimicrobial activity of Artemisia annua L. essential oil from Iran. J. Med. Plant 2008, 7, 58–62. [Google Scholar]
- Massiha, A.; Khoshkholgh-Pahlaviani, M.M.; Issazadeh, K.; Bidarigh, S.; Zarrabi, S. Antibacterial Activity of Essential Oils and Plant Extracts of Artemisia (Artemisia annua L.) In Vitro. Zahedan J. Res. Med. Sci. 2013, 15, e92933. [Google Scholar]
- Gupta, P.C.; Dutta, B.; Pant, D.; Joshi, P.; Lohar, D. In vitro antibacterial activity of Artemisia annua Linn. growing in India. Int. J. Green Pharm. (IJGP) 2009, 3, 255. [Google Scholar] [CrossRef]
- Li, D.; Wang, R.; You, M.; Chen, N.; Sun, L.; Chen, N. The antimicrobial effect and mechanism of the Artemisia argyi essential oil against bacteria and fungus. Braz. J. Microbiol. 2024, 55, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Ge, D.; Li, S.; Huang, K.; Liu, J.; Li, F. Chemical Composition and Antimicrobial Activities of Artemisia argyi Lévl. et Vant Essential Oils Extracted by Simultaneous Distillation-Extraction, Subcritical Extraction and Hydrodistillation. Molecules 2019, 24, 483. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, H.; Lei, J.; Yu, J. Biological activity of extracts and active compounds isolated from Siegesbeckia orientalis L. Ind. Crops Prod. 2016, 94, 288–293. [Google Scholar] [CrossRef]
- Wang, J.P.; Zhou, Y.M.; Zhang, Y.H. Kirenol production in hairy root culture of Siegesbeckea orientalis and its antimicrobial activity. Pharmacogn. Mag. 2012, 8, 149–155. [Google Scholar] [CrossRef]
- Kwon, J.-B.; Kim, M.S.; Sohn, H.Y. Evaluation of Antimicrobial, Antioxidant, and Antithrombin Activities of the Rhizome of Various Dioscorea Species. Korean J. Food Preserv. 2010, 17, 391–397. [Google Scholar]
- Hanawa, F.; Okamoto, M.; Towers, G.H.N. Antimicrobial DNA-binding Photosensitizers from the Common Rush, Juncus effusus. Photochem. Photobiol. 2002, 76, 51–56. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, L.L.; Zhang, X.; Gong, X.W.; Zhu, D.L.; Xu, X.H.; Wang, F.; Yang, X.L. Three new phenanthrenes with antimicrobial activities from the aerial parts of Juncus effusus. Fitoterapia 2018, 130, 247–250. [Google Scholar] [CrossRef]
- Karimi, A.R. Characterization and Antimicrobial Activity of Patchouli Essential Oil Extracted from Pogostemon cablin (Blanco) Benth. (lamiaceae). Adv. Environ. Biol. 2014, 8, 2301–2309. [Google Scholar]
- Aisyah, Y.; Yunita, D.; Amanda, A. Antimicrobial activity of patchouli (Pogostemon cablin Benth) citronella (Cymbopogon nardus), and nutmeg (Myristica fragrans) essential oil and their mixtures against pathogenic and food spoilage microbes. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 012020. [Google Scholar] [CrossRef]
- Zhao, J.; Lou, J.; Mou, Y.; Li, P.; Wu, J.; Zhou, L. Diterpenoid tanshinones and phenolic acids from cultured hairy roots of Salvia miltiorrhiza Bunge and their antimicrobial activities. Molecules 2011, 16, 2259–2267. [Google Scholar] [CrossRef]
- Yang, S.Y.; Choi, Y.R.; Lee, M.J.; Kang, M.K. Antimicrobial Effects against Oral Pathogens and Cytotoxicity of Glycyrrhiza uralensis Extract. Plants 2020, 9, 838. [Google Scholar] [CrossRef]
- Lee, J.W.; Ji, Y.J.; Yu, M.H.; Bo, M.H.; Seo, H.J.; Lee, S.P.; Lee, I.S. Antimicrobial effect and resistant regulation of Glycyrrhiza uralensis on methicillin-resistant Staphylococcus aureus. Nat. Prod. Res. 2009, 23, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Yang, W.Y.; Chung, S.C.; Kim, T.Y.; Oh, K.B.; Shin, J. In vitro sortase A inhibitory and antimicrobial activity of flavonoids isolated from the roots of Sophora flavescens. Arch. Pharmacal Res. 2011, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Park, S.-N.; Ahn, S.-J.; Seo, Y.-W.; Lee, Y.-J.; Lim, Y.K.; Freire, M.O.; Cho, E.; Kook, J.-K. Antimicrobial effect of sophoraflavanone G isolated from Sophora flavescens against mutans streptococci. Anaerobe 2013, 19, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Sultana, P.; Islam, M.S.; Mahmud, M.T.; Rashid, M.M.O.; Hossen, F. Comparative Antimicrobial Activity of Areca catechu Nut Extracts using Different Extracting Solvents. Bangladesh J. Microbiol. 2016, 31, 19–23. [Google Scholar] [CrossRef]
- McMurray, R.L.; Ball, M.E.E.; Tunney, M.M.; Corcionivoschi, N.; Situ, C. Antibacterial Activity of Four Plant Extracts Extracted from Traditional Chinese Medicinal Plants against Listeria monocytogenes, Escherichia coli, and Salmonella enterica subsp. enterica serovar Enteritidis. Microorganisms 2020, 8, 962. [Google Scholar] [CrossRef]
- Naidu, K.; Lalam, R.; Bobbarala, V. Antimicrobial agents from Rubia cordifolia and Glycyrrhiza glabra against phytopathogens of Gossypium. Int. J. PharmTech Res. 2009, 1, 1512–1518. [Google Scholar]
- Basu, S.; Ghosh, A.; Hazra, B. Evaluation of the antibacterial activity of Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn.: Isolation of emodin and physcion as active antibacterial agents. Phytother. Res. PTR 2005, 19, 888–894. [Google Scholar] [CrossRef]
- Liang, X.; Li, B.; Wu, F.; Li, T.; Wang, Y.; Ma, Q.; Liang, S. Bitterness and antibacterial activities of constituents from Evodia rutaecarpa. BMC Complement. Altern. Med. 2017, 17, 180. [Google Scholar] [CrossRef]
- Wang, X.-X.; Zan, K.; Shi, S.-P.; Zeng, K.-W.; Jiang, Y.; Guan, Y.; Xiao, C.-L.; Gao, H.-Y.; Wu, L.-J.; Tu, P.-F. Quinolone alkaloids with antibacterial and cytotoxic activities from the fruits of Evodia rutaecarpa. Fitoterapia 2013, 89, 1–7. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lee, D.-Y.; Kim, Y.B.; Lee, S.-W.; Cha, S.-W.; Park, H.-W.; Kim, G.-S.; Kwon, D.-Y.; Lee, M.-H.; Han, S.-H. The Mechanism Underlying the Antibacterial Activity of Shikonin against Methicillin-Resistant Staphylococcus aureus. Evid. -Based Complement. Altern. Med. 2015, 2015, 520578. [Google Scholar] [CrossRef]
- Boucher, M.-A.; Côté, H.; Pichette, A.; Ripoll, L.; Legault, J. Chemical composition and antibacterial activity of Tussilago farfara (L.) essential oil from Quebec, Canada. Nat. Prod. Res. 2020, 34, 545–548. [Google Scholar] [CrossRef]
- Zhao, J.; Evangelopoulos, D.; Bhakta, S.; Gray, A.I.; Seidel, V. Antitubercular activity of Arctium lappa and Tussilago farfara extracts and constituents. J. Ethnopharmacol. 2014, 155, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Santomauro, F.; Bilia, A.R.; Flamini, G.; Sacco, C. Antibacterial activity of Tuscan Artemisia annua essential oil and its major components against some foodborne pathogens. LWT—Food Sci. Technol. 2015, 64, 1251–1254. [Google Scholar] [CrossRef]
- In vitro Effects of Essential Oils from Aerial Parts of Artemisia annus L. Against Antibiotic-Susceptible and -Resistance Strains of Salmenella typhimurium. YAKHAK HOEJI 2007, 51, 355–360.
- Marinas, I.C.; Oprea, E.; Chifiriuc, M.C.; Badea, I.A.; Buleandra, M.; Lazar, V. Chemical Composition and Antipathogenic Activity of Artemisia annua Essential Oil from Romania. Chem. Biodivers. 2015, 12, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Ivarsen, E.; Fretté, X.C.; Christensen, K.B.; Christensen, L.P.; Engberg, R.M.; Grevsen, K.; Kjaer, A. Bioassay-Guided Chromatographic Isolation and Identification of Antibacterial Compounds from Artemisia annua L. That Inhibit Clostridium perfringens Growth. J. AOAC Int. 2014, 97, 1282–1290. [Google Scholar] [CrossRef]
- Wen, W.; Xiang, H.; Qiu, H.; Chen, J.; Ye, X.; Wu, L.; Chen, Z.; Tong, S. Screening and identification of antibacterial components in Artemisia argyi essential oil by TLC–direct bioautography combined with comprehensive 2D GC × GC-TOFMS. J. Chromatogr. B 2024, 1234, 124026. [Google Scholar] [CrossRef]
- Peng, F.; Wan, F.; Xiong, L.; Peng, C.; Dai, M.; Chen, J. In vitro and in vivo antibacterial activity of Pogostone. Chin. Med. J. 2014, 127, 4001–4005. [Google Scholar] [CrossRef]
- Lee, D.-S.; Lee, S.-H.; Noh, J.-G.; Hong, S.-D. Antibacterial Activities of Cryptotanshinone and Dihydrotanshinone I from a Medicinal Herb, Salvia miltiorrhiza Bunge. Biosci. Biotechnol. Biochem. 1999, 63, 2236–2239. [Google Scholar] [CrossRef]
- Chen, B.-C.; Ding, Z.-S.; Dai, J.-S.; Chen, N.-P.; Gong, X.-W.; Ma, L.-F.; Qian, C.-D. New Insights Into the Antibacterial Mechanism of Cryptotanshinone, a Representative Diterpenoid Quinone from Salvia miltiorrhiza Bunge. Front. Microbiol. 2021, 12, 647289. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zhang, J.; Li, M.; Chen, D.; Wu, T. Minor compounds of the high purity salvianolic acid B freeze-dried powder from Salvia miltiorrhiza and antibacterial activity assessment. Nat. Prod. Res. 2018, 32, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, L.; Heber, D.; Shi, W.; Lu, Q.-Y. Antibacterial Compounds from Glycyrrhiza uralensis. J. Nat. Prod. 2006, 69, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Villinski, J.R.; Bergeron, C.; Cannistra, J.C.; Gloer, J.B.; Coleman, C.M.; Ferreira, D.; Azelmat, J.; Grenier, D.; Gafner, S. Pyrano-isoflavans from Glycyrrhiza uralensis with Antibacterial Activity against Streptococcus mutans and Porphyromonas gingivalis. J. Nat. Prod. 2014, 77, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, Z.; Zhang, Z.; Bai, H. Antimicrobial Effects of Sophora flavescens Alkaloids on Metronidazole-Resistant Gardnerella vaginalis in Planktonic and Biofilm Conditions. Curr. Microbiol. 2023, 80, 263. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, C.; Dai, W.; Jing, H.; Du, X.; Peng, M.; Zhang, Y.; Mo, L.; Wang, L.; Chen, X.; et al. Effects of different extraction on the antibacterial and antioxidant activities of phenolic compounds of areca nut (husks and seeds). J. Food Meas. Charact. 2022, 16, 1502–1515. [Google Scholar] [CrossRef]
- Yamaki, M.; Kashihara, M.; Ishiguro, K.; Takagi, S. Antimicrobial Principles of Xian he cao (Agrimonia pilosa). Planta Medica 1989, 55, 169–170. [Google Scholar] [CrossRef]
- Kasai, S.; Watanabe, S.; Kawabata, J.; Tahara, S.; Mizutani, J. Antimicrobial catechin derivatives of Agrimonia pilosa. Phytochemistry 1992, 31, 787–789. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Chang, M.-C.; Chen, C.-S.; Lin, H.-C.; Tsai, H.-P.; Yang, C.-C.; Yang, C.-H.; Lin, C.-M. Topoisomerase I Inhibitor Evodiamine Acts As an Antibacterial Agent against Drug-Resistant Klebsiella pneumoniae. Planta Medica 2013, 79, 27–29. [Google Scholar] [CrossRef]
- Su, X.-L.; Xu, S.; Shan, Y.; Yin, M.; Chen, Y.; Feng, X.; Wang, Q.-Z. Three new quinazolines from Evodia rutaecarpa and their biological activity. Fitoterapia 2018, 127, 186–192. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Woo, S.; Marquez, L.; Crandall, W.J.; Risener, C.J.; Quave, C.L. Recent advances in the discovery of plant-derived antimicrobial natural products to combat antimicrobial resistant pathogens: Insights from 2018–2022. Nat. Prod. Rep. 2023, 40, 1271–1290. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2020, 11, 586548. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Grkovic, T.; Evans, J.R.; Thornburg, C.C.; Akee, R.K.; Thompson, J.R.; Whitt, J.A.; Harris, M.J.; Loyal, J.A.; Britt, J.R.; et al. The NCI library of traditional Chinese medicinal plant extracts—Preliminary assessment of the NCI-60 activity and chemical profiling of selected species. Fitoterapia 2019, 137, 104285. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Saitis, T.; Shareef, R.; Harb, C.; Lakhani, M.; Ahmad, Z. Shikonin and Alkannin inhibit ATP synthase and impede the cell growth in Escherichia coli. Int. J. Biol. Macromol. 2023, 253, 127049. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, X.; Zhang, P.; Zhang, M.; Kou, M.; Shi, C.; Peng, X.; Wang, X. Control of Foodborne Staphylococcus aureus by Shikonin, a Natural Extract. Foods 2021, 10, 2954. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Kong, J.; Jiang, X.; Han, Y.; Feng, L.; Sun, Y.; Chen, L.; Zhou, T. An effective antimicrobial strategy of colistin combined with the Chinese herbal medicine shikonin against colistin-resistant Escherichia coli. Microbiol. Spectr. 2023, 11, e0145923. [Google Scholar] [CrossRef]
- Li, Q.Q.; Chae, H.S.; Kang, O.H.; Kwon, D.Y. Synergistic Antibacterial Activity with Conventional Antibiotics and Mechanism of Action of Shikonin against Methicillin-Resistant Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 7551. [Google Scholar] [CrossRef]
- Yarosh, D.B.; Galvin, J.W.; Nay, S.L.; Peña, A.V.; Canning, M.T.; Brown, D.A. Anti-inflammatory activity in skin by biomimetic of Evodia rutaecarpa extract from traditional Chinese medicine. J. Dermatol. Sci. 2006, 42, 13–21. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kim, J.B.; Lee, P.; Kim, S.H. Evodiamine Inhibits Helicobacter pylori Growth and Helicobacter pylori-Induced Inflammation. Int. J. Mol. Sci. 2021, 22, 3385. [Google Scholar] [CrossRef]
- Panda, M.; Tripathi, S.K.; Zengin, G.; Biswal, B.K. Evodiamine as an anticancer agent: A comprehensive review on its therapeutic application, pharmacokinetic, toxicity, and metabolism in various cancers. Cell Biol. Toxicol. 2023, 39, 1–31. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Chen, Q.; Chen, W.; Yang, J.; Zhou, X.; Zhang, C.; Wu, A.; Lai, J.; Chen, J.; Mei, Q.; et al. A comprehensive review of Rubia cordifolia L.: Traditional uses, phytochemistry, pharmacological activities, and clinical applications. Front. Pharmacol. 2022, 13, 965390. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ezati, P.; Rhim, J.W. Alizarin: Prospects and sustainability for food safety and quality monitoring applications. Colloids Surf. B Biointerfaces 2023, 223, 113169. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, G.; Shukla, M.; Kaul, G.; K, R.; Chopra, S.; Pandey, R. Characterization and antimicrobial evaluation of anthraquinones and triterpenes from Rubia cordifolia. J. Asian Nat. Prod. Res. 2023, 25, 1110–1116. [Google Scholar] [CrossRef]
- Wada, A.; Murakami, K.; Ishikawa, Y.; Amoh, T.; Hirao, K.; Hosokawa, Y.; Hinode, D.; Miyake, Y.; Yumoto, H. Anti-Inflammatory and Protective Effects of Juncus effusus L. Water Extract on Oral Keratinocytes. BioMed Res. Int. 2022, 2022, 9770899. [Google Scholar] [CrossRef]
- Hu, H.C.; Tsai, Y.H.; Chuang, Y.C.; Lai, K.H.; Hsu, Y.M.; Hwang, T.L.; Lin, C.C.; Fülöp, F.; Wu, Y.C.; Yu, S.Y.; et al. Estrogenic and anti-neutrophilic inflammatory phenanthrenes from Juncus effusus L. Nat. Prod. Res. 2022, 36, 3043–3053. [Google Scholar] [CrossRef]
- Ma, W.; Liu, F.; Ding, Y.Y.; Zhang, Y.; Li, N. Four new phenanthrenoid dimers from Juncus effusus L. with cytotoxic and anti-inflammatory activities. Fitoterapia 2015, 105, 83–88. [Google Scholar] [CrossRef]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential Oil of Artemisia annua L.: An Extraordinary Component with Numerous Antimicrobial Properties. Evid. -Based Complement. Altern. Med. Ecam 2014, 2014, 159819. [Google Scholar] [CrossRef]
- Das, S.; Vörös-Horváth, B.; Bencsik, T.; Micalizzi, G.; Mondello, L.; Horváth, G.; Kőszegi, T.; Széchenyi, A. Antimicrobial Activity of Different Artemisia Essential Oil Formulations. Molecules 2020, 25, 2390. [Google Scholar] [CrossRef]
- Kshirsagar, S.G.; Rao, R.V. Antiviral and Immunomodulation Effects of Artemisia. Medicina 2021, 57, 217. [Google Scholar] [CrossRef]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef] [PubMed]
- Golbarg, H.; Mehdipour Moghaddam, M.J. Antibacterial Potency of Medicinal Plants including Artemisia annua and Oxalis corniculata against Multi-Drug Resistance E. coli. BioMed Res. Int. 2021, 2021, 9981915. [Google Scholar] [CrossRef] [PubMed]
- Bordean, M.E.; Ungur, R.A.; Toc, D.A.; Borda, I.M.; Marțiș, G.S.; Pop, C.R.; Filip, M.; Vlassa, M.; Nasui, B.A.; Pop, A.; et al. Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants 2023, 12, 596. [Google Scholar] [CrossRef]
- Zhang, J.J.; Qu, L.B.; Bi, Y.F.; Pan, C.X.; Yang, R.; Zeng, H.J. Antibacterial activity and mechanism of chloroform fraction from aqueous extract of mugwort leaves (Artemisia argyi L.) against Staphylococcus aureus. Lett. Appl. Microbiol. 2022, 74, 893–900. [Google Scholar] [CrossRef]
- Liu, B.; Ma, R.; Yang, Q.; Yang, Y.; Fang, Y.; Sun, Z.; Song, D. Effects of Traditional Chinese Herbal Feed Additive on Production Performance, Egg Quality, Antioxidant Capacity, Immunity and Intestinal Health of Laying Hens. Animals 2023, 13, 2510. [Google Scholar] [CrossRef]
- Zhou, X.; Li, S.; Jiang, Y.; Deng, J.; Yang, C.; Kang, L.; Zhang, H.; Chen, X. Use of fermented Chinese medicine residues as a feed additive and effects on growth performance, meat quality, and intestinal health of broilers. Front. Vet Sci. 2023, 10, 1157935. [Google Scholar] [CrossRef]
- Guo, S.; Ma, J.; Xing, Y.; Xu, Y.; Jin, X.; Yan, S.; Shi, L.; Zhang, L.; Shi, B. Effects of Artemisia annua L. Water Extract on Growth Performance and Intestinal Related Indicators in Broilers. J. Poult. Sci. 2023, 60, 2023024. [Google Scholar] [CrossRef]
- McMurray, R.L.; Ball, M.E.E.; Linton, M.; Pinkerton, L.; Kelly, C.; Lester, J.; Donaldson, C.; Balta, I.; Tunney, M.M.; Corcionivoschi, N.; et al. The Effects of Agrimonia pilosa Ledeb, Anemone chinensis Bunge, and Smilax glabra Roxb on Broiler Performance, Nutrient Digestibility, and Gastrointestinal Tract Microorganisms. Animals 2022, 12, 1110. [Google Scholar] [CrossRef]
- Ma, Q.; Tan, D.; Gong, X.; Ji, H.; Wang, K.; Lei, Q.; Zhao, G. An Extract of Artemisia argyi Leaves Rich in Organic Acids and Flavonoids Promotes Growth in BALB/c Mice by Regulating Intestinal Flora. Animals 2022, 12, 1519. [Google Scholar] [CrossRef]
- Chen, G.; Li, Z.; Liu, S.; Tang, T.; Chen, Q.; Yan, Z.; Peng, J.; Yang, Z.; Zhang, G.; Liu, Y.; et al. Fermented Chinese Herbal Medicine Promoted Growth Performance, Intestinal Health, and Regulated Bacterial Microbiota of Weaned Piglets. Animals 2023, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.P.; Feng, D.Y.; Xia, M.H.; He, X.J.; Liu, Y.H.; Tan, H.Z.; Zou, S.G.; Ou, X.H.; Zheng, T.; Cao, Y.; et al. Effects of a traditional Chinese medicine formula supplementation on growth performance, carcass characteristics, meat quality and fatty acid profiles of finishing pigs. Livest. Sci. 2017, 202, 135–142. [Google Scholar] [CrossRef]
- Song, X.; Luo, J.; Fu, D.; Zhao, X.; Bunlue, K.; Xu, Z.; Qu, M. Traditional chinese medicine prescriptions enhance growth performance of heat stressed beef cattle by relieving heat stress responses and increasing apparent nutrient digestibility. Asian-Australas J. Anim. Sci. 2014, 27, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Dadras, F.; Velisek, J.; Zuskova, E. An update about beneficial effects of medicinal plants in aquaculture: A review. Vet Med. 2023, 68, 449–463. [Google Scholar] [CrossRef]
- Gong, J.; Yin, F.; Hou, Y.; Yin, Y. Review: Chinese herbs as alternatives to antibiotics in feed for swine and poultry production: Potential and challenges in application. Can. J. Anim. Sci. 2014, 94, 223–241. [Google Scholar] [CrossRef]
- Martin, A.L.A.R.; De Menezes, I.R.A.; Sousa, A.K.; Farias, P.A.M.; dos Santos, F.A.V.; Freitas, T.S.; Figueredo, F.G.; Ribeiro-Filho, J.; Carvalho, D.T.; Coutinho, H.D.M.; et al. In vitro and in silico antibacterial evaluation of coumarin derivatives against MDR strains of Staphylococcus aureus and Escherichia coli. Microb. Pathog. 2023, 177, 106058. [Google Scholar] [CrossRef]
- Osorio, M.; Carvajal, M.; Vergara, A.; Butassi, E.; Zacchino, S.; Mascayano, C.; Montoya, M.; Mejías, S.; Martín, M.C.; Vásquez-Martínez, Y. Prenylated Flavonoids with Potential Antimicrobial Activity: Synthesis, Biological Activity, and In Silico Study. Int. J. Mol. Sci. 2021, 22, 5472. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J.; Dempsey, D.M.; Dickerman, A.; Dietrich, E.M.; Kenyon, R.W.; et al. Introducing the Bacterial and Viral Bioinformatics Resource Center (BV-BRC): A resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2023, 51, D678–D689. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Backman, T.W.; Cao, Y.; Girke, T. ChemMine tools: An online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011, 39, W486–W491. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Tanchuk, V.Y. Application of Associative Neural Networks for Prediction of Lipophilicity in ALOGPS 2.1 Program. J. Chem. Inf. Comput. Sci. 2002, 42, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory—Design and description. J. Comput. -Aided Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]



| Zone of Inhibition (mm) | ||||||
|---|---|---|---|---|---|---|
| Spot # | Genus | Species | M. luteus * | E. coli | S. aureus | S. epidermidis |
| 20 | Tussilago | farfara | 10 | --- | --- | --- |
| 27 | Asarum | heterotropoides | 10 | --- | 12.6 | --- |
| 33 | Evodia | rutaecarpa | 17.6 | --- | 11.6 | 10.8 |
| 42 | Rubia | cordifolia | 11.6 | --- | --- | --- |
| 55 | Dioscorea | nipponica | 9.7 | --- | --- | --- |
| 83 | Dioscorea | nipponica | 11.6 | --- | --- | --- |
| 95 | Echinops | latifolius | 10.9 | --- | 11.8 | --- |
| 201 | Artemisia | annua | 19.4 | --- | --- | --- |
| 245 | Glycyrrhiza | uralensis | 12.6 | --- | 11.7 | 11.6 |
| 248 | Areca | catechu | 10.6 | --- | --- | --- |
| 251 | Artemisia | argyi | 11 | --- | --- | --- |
| 275 | Juncus | effusus | 10.2 | --- | 11.2 | --- |
| 285 | Artemisia | argyi | 8.4 | --- | --- | --- |
| 395 | Juncus | effusus | 13 | --- | 13.2 | 11.8 |
| 396 | Lithospermum | erythrorhizon | 14 | 9.5 | 13.9 | 14.8 |
| 413 | Sophora | flavescens | 12.2 | --- | 11.2 | 11.4 |
| 415 | Juncus | effusus | 11.2 | --- | 11.4 | --- |
| 416 | Rubia | cordifolia | 12 | --- | --- | --- |
| 424 | Evodia | rutaecarpa | 19 | --- | 9.8 | --- |
| 456 | Agrimonia | pilosa | 11.4 | --- | 12.2 | 10.6 |
| 460 | Lithospermum | erythrorhizon | 12.6 | --- | 10.2 | 12.4 |
| 694 | Salvia | miltiorrhiza | 12.2 | --- | 9.81 | 15.6 |
| 704 | Siegesbeckia | orientalis | 9.4 | --- | --- | --- |
| 727 | Evodia | rutaecarpa | 18.4 | --- | 11 | 11.8 |
| 749 | Pogostemon | cablin | 21.4 | --- | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellward, G.L.; Binda, M.E.; Dzurny, D.I.; Bucher, M.J.; Dees, W.R.; Czyż, D.M. A Screen of Traditional Chinese Medicinal Plant Extracts Reveals 17 Species with Antimicrobial Properties. Antibiotics 2024, 13, 1220. https://doi.org/10.3390/antibiotics13121220
Ellward GL, Binda ME, Dzurny DI, Bucher MJ, Dees WR, Czyż DM. A Screen of Traditional Chinese Medicinal Plant Extracts Reveals 17 Species with Antimicrobial Properties. Antibiotics. 2024; 13(12):1220. https://doi.org/10.3390/antibiotics13121220
Chicago/Turabian StyleEllward, Garrett L., Macie E. Binda, Dominika I. Dzurny, Michael J. Bucher, Wren R. Dees, and Daniel M. Czyż. 2024. "A Screen of Traditional Chinese Medicinal Plant Extracts Reveals 17 Species with Antimicrobial Properties" Antibiotics 13, no. 12: 1220. https://doi.org/10.3390/antibiotics13121220
APA StyleEllward, G. L., Binda, M. E., Dzurny, D. I., Bucher, M. J., Dees, W. R., & Czyż, D. M. (2024). A Screen of Traditional Chinese Medicinal Plant Extracts Reveals 17 Species with Antimicrobial Properties. Antibiotics, 13(12), 1220. https://doi.org/10.3390/antibiotics13121220








