Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections
Abstract
1. Introduction
2. Search Methodology
3. Principles of Nanotechnology
4. Nanotechnology in the Diagnosis of Infectious Diseases
4.1. Basic Principles in the Diagnosis of Infectious Diseases
4.2. Nanosensors Occupying Colorimetric Properties
4.3. Nanosensors Occupying Electrochemical Properties
4.4. Nanosensors Occupying Fluorescent Properties
4.5. Nanosensors Occupying SERS Properties
4.6. Nanosensors in Point-of-Care Testing
4.7. Future Perspectives in Nanotechnology and the Diagnosis of Infectious Diseases
5. Nanotechnology in the Treatment of Infectious Diseases
5.1. The Need for Non-Antibiotic Interventions in the Treatment of Infectious Diseases
5.2. Basic Principles of Nanotechnology in the Treatment of Infectious Diseases
5.3. Nanotechnology and Enhanced Drug Delivery in the Treatment of Infectious Diseases
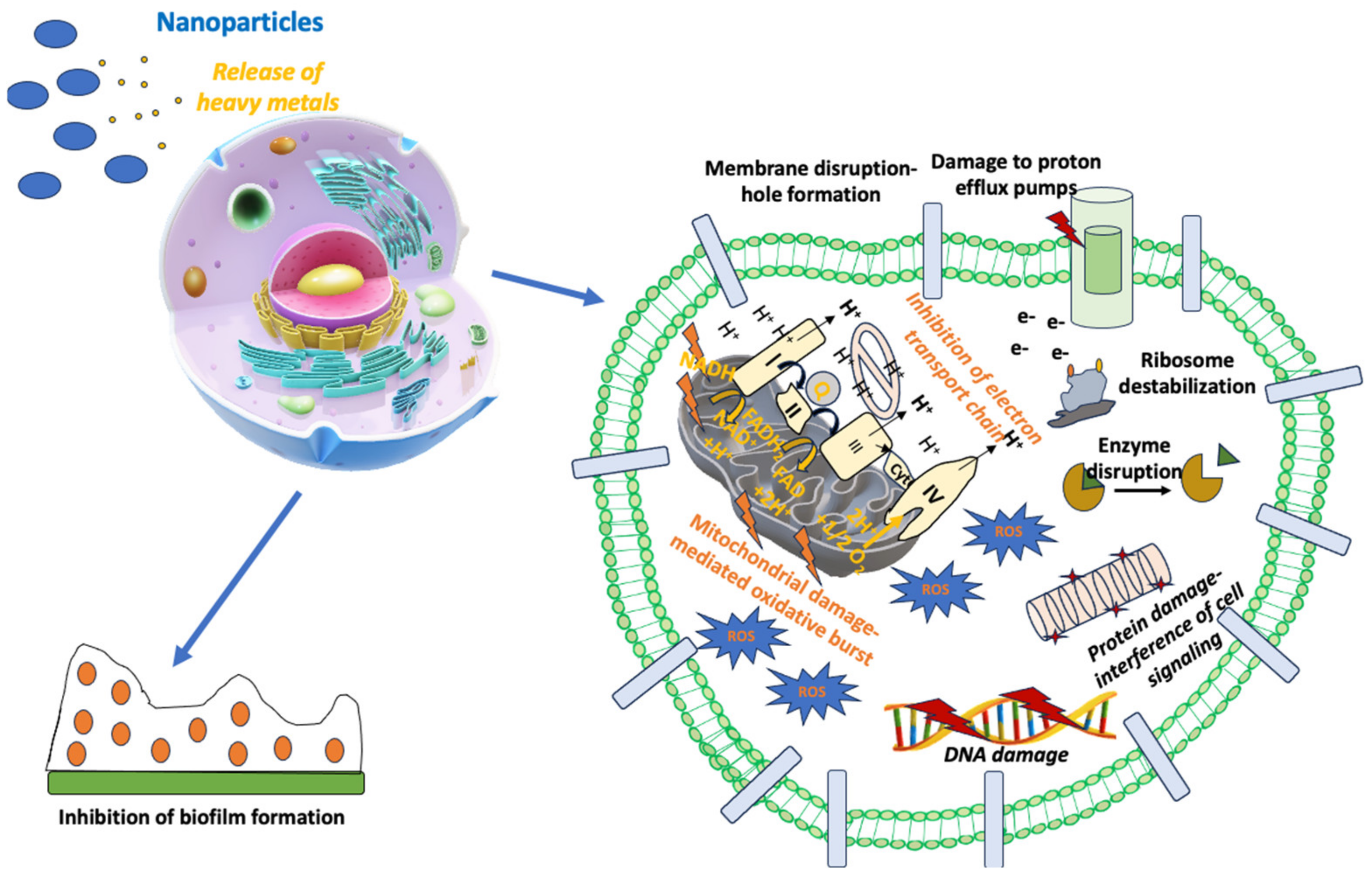
5.4. Nanoparticles as Antimicrobial Drugs
5.5. Nanoparticles and Biofilms
5.6. Combination of Nanoparticles with Antibiotics
5.7. Choosing the Right NP
6. Nanotechnology in the Prevention of Infectious Diseases
6.1. Application of Nanotechnology in Vaccine Technology
6.2. Limitations of Nanotechnology Applications in Vaccine Technology
7. Challenges in the Use of Nanotechnology in the Treatment of Infectious Diseases
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Michaud, C.M. Global Burden of Infectious Diseases. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 444–454. ISBN 978-0-12-373944-5. [Google Scholar]
- Armstrong, G.L.; Conn, L.A.; Pinner, R.W. Trends in Infectious Disease Mortality in the United States during the 20th Century. JAMA 1999, 281, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Lewis, M.; Powles, J. The Australian Mortality Decline: Cause-Specific Mortality 1907–1990. Aust. N. Z. J. Public Health 1998, 22, 37–44. [Google Scholar] [CrossRef] [PubMed]
- GBD; 2019 Child and Adolescent Communicable Disease Collaborators. The Unfinished Agenda of Communicable Diseases among Children and Adolescents before the COVID-19 Pandemic, 1990–2019: A Systematic Analysis of the Global Burden of Disease Study 2019. Lancet Lond. Engl. 2023, 402, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet Lond. Engl. 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, W.Y.; Bisht, A.; Gopinath, A.; Cheema, A.H.; Chaludiya, K.; Khalid, M.; Nwosu, M.; Konka, S.; Khan, S. A Systematic Review of Antibiotic Resistance Trends and Treatment Options for Hospital-Acquired Multidrug-Resistant Infections. Cureus 2022, 14, e29956. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed. Antimicrob. Agents Chemother. 2022, 66, e0199121. [Google Scholar] [CrossRef] [PubMed]
- Ala-Jaakkola, R.; Laitila, A.; Ouwehand, A.C.; Lehtoranta, L. Role of D-Mannose in Urinary Tract Infections—A Narrative Review. Nutr. J. 2022, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Lenger, S.M.; Bradley, M.S.; Thomas, D.A.; Bertolet, M.H.; Lowder, J.L.; Sutcliffe, S. D-Mannose vs Other Agents for Recurrent Urinary Tract Infection Prevention in Adult Women: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2020, 223, 265.e1–265.e13. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Samonis, G. Bacteriophages in Infectious Diseases and Beyond—A Narrative Review. Antibiotics 2023, 12, 1012. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Kofteridis, D.P. Antimicrobial Peptides in Infectious Diseases and Beyond—A Narrative Review. Life Basel Switz. 2023, 13, 1651. [Google Scholar] [CrossRef]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.-T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.K. Nanobiotics against Antimicrobial Resistance: Harnessing the Power of Nanoscale Materials and Technologies. J. Nanobiotechnol. 2022, 20, 375. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Wong, N. Nanotechnology and Its Use in Imaging and Drug Delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Gregoriadis, G. Enzyme Entrapment in Liposomes. Methods Enzymol. 1976, 44, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D. Lipid Bilayers and Biomembranes. Annu. Rev. Biochem. 1972, 41, 753–776. [Google Scholar] [CrossRef] [PubMed]
- Ringsdorf, H. Structure and Properties of Pharmacologically Active Polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Cornu, G.; Michaux, J.L.; Sokal, G.; Trouet, A. Daunorubicin-DNA: Further Clinical Trials in Acute Non-Lymphoblastic Leukemia. Eur. J. Cancer 1974, 10, 695–700. [Google Scholar] [CrossRef]
- Kreuter, J.; Speiser, P.P. In Vitro Studies of Poly(Methyl Methacrylate) Adjuvants. J. Pharm. Sci. 1976, 65, 1624–1627. [Google Scholar] [CrossRef]
- Couvreur, P.; Tulkens, P.; Roland, M.; Trouet, A.; Speiser, P. Nanocapsules: A New Type of Lysosomotropic Carrier. FEBS Lett. 1977, 84, 323–326. [Google Scholar] [CrossRef]
- Hurwitz, E.; Levy, R.; Maron, R.; Wilchek, M.; Arnon, R.; Sela, M. The Covalent Binding of Daunomycin and Adriamycin to Antibodies, with Retention of Both Drug and Antibody Activities. Cancer Res. 1975, 35, 1175–1181. [Google Scholar]
- Trouet, A.; Masquelier, M.; Baurain, R.; Deprez-De Campeneere, D. A Covalent Linkage between Daunorubicin and Proteins That Is Stable in Serum and Reversible by Lysosomal Hydrolases, as Required for a Lysosomotropic Drug-Carrier Conjugate: In Vitro and in Vivo Studies. Proc. Natl. Acad. Sci. USA 1982, 79, 626–629. [Google Scholar] [CrossRef]
- Davis, F.F. The Origin of Pegnology. Adv. Drug Deliv. Rev. 2002, 54, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D.; Hugo, W.B. Antimicrobial Activity and Action of Silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [CrossRef]
- Dequeker, J.; Verdickt, W.; Gevers, G.; Vanschoubroek, K. Longterm Experience with Oral Gold in Rheumatoid Arthritis and Psoriatic Arthritis. Clin. Rheumatol. 1984, 3 (Suppl. S1), 67–74. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Introduction to Nanomedicine. Molecules 2015, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Sheng, J. Introduction to Nanomedicine. In Nanomedicine; Gu, N., Ed.; Micro/Nano Technologies; Springer Nature: Singapore, 2023; pp. 3–16. ISBN 9789811689833. [Google Scholar]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of Silver Nanoparticles by Chemical and Biological Methods and Their Antimicrobial Properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Khan, S.; Mansoor, S.; Rafi, Z.; Kumari, B.; Shoaib, A.; Saeed, M.; Alshehri, S.; Ghoneim, M.M.; Rahamathulla, M.; Hani, U.; et al. A Review on Nanotechnology: Properties, Applications, and Mechanistic Insights of Cellular Uptake Mechanisms. J. Mol. Liq. 2022, 348, 118008. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.-Y.; Forrest, M.L.; Berkland, C.J. Nanoparticles for Biomedical Imaging. Expert Opin. Drug Deliv. 2009, 6, 1175–1194. [Google Scholar] [CrossRef]
- Pantarotto, D.; Singh, R.; McCarthy, D.; Erhardt, M.; Briand, J.-P.; Prato, M.; Kostarelos, K.; Bianco, A. Functionalized Carbon Nanotubes for Plasmid DNA Gene Delivery. Angew. Chem. Int. Ed Engl. 2004, 43, 5242–5246. [Google Scholar] [CrossRef]
- Shi Kam, N.W.; Jessop, T.C.; Wender, P.A.; Dai, H. Nanotube Molecular Transporters: Internalization of Carbon Nanotube-Protein Conjugates into Mammalian Cells. J. Am. Chem. Soc. 2004, 126, 6850–6851. [Google Scholar] [CrossRef]
- Pantarotto, D.; Briand, J.-P.; Prato, M.; Bianco, A. Translocation of Bioactive Peptides across Cell Membranes by Carbon Nanotubes. Chem. Commun. Camb. Engl. 2004, 16–17. [Google Scholar] [CrossRef]
- Dai, H.; Hafner, J.H.; Rinzler, A.G.; Colbert, D.T.; Smalley, R.E. Nanotubes as Nanoprobes in Scanning Probe Microscopy. Nature 1996, 384, 147–150. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon Nanotube Biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon Nanotubes: Properties, Synthesis, Purification, and Medical Applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Carnahan, M.A.; Finkelstein, S.; Prata, C.A.H.; Degoricija, L.; Lee, S.J.; Grinstaff, M.W. Dendritic Supramolecular Assemblies for Drug Delivery. Chem. Commun. Camb. Engl. 2005, 4309–4311. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Tiriveedhi, V.; Kitchens, K.M.; Nevels, K.J.; Ghandehari, H.; Butko, P. Kinetic Analysis of the Interaction between Poly(Amidoamine) Dendrimers and Model Lipid Membranes. Biochim. Biophys. Acta 2011, 1808, 209–218. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-Based Drug Delivery Systems: History, Challenges, and Latest Developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of Liposomes in Medicine and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic Nanoparticles: Technology Overview & Drug Delivery Applications in Oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Kishore, M.; Panat, S.; Upadhyay, N.; Agarwal, N.; Aggarwal, A. Nanotechnology: A Boon in Oral Cancer Diagnosis and Therapeutics. SRM J. Res. Dent. Sci. 2013, 4, 154. [Google Scholar] [CrossRef]
- Amjad, S.; Shaukat, S.; Rahman, H.M.A.U.; Usman, M.; Farooqi, Z.H.; Nazar, M.F. Application of Anionic-Nonionic Mixed Micellar System for Solubilization of Methylene Blue Dye. J. Mol. Liq. 2023, 369, 120958. [Google Scholar] [CrossRef]
- Katsuki, S.; Matoba, T.; Koga, J.-I.; Nakano, K.; Egashira, K. Anti-Inflammatory Nanomedicine for Cardiovascular Disease. Front. Cardiovasc. Med. 2017, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xie, J.; Zhang, X.; Sun, W.; Zhao, H.; Li, Y.; Wang, C. An Overview of Polymeric Nanomicelles in Clinical Trials and on the Market. Chin. Chem. Lett. 2021, 32, 243–257. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef]
- Reddy, B.P.K.; Yadav, H.K.S.; Nagesha, D.K.; Raizaday, A.; Karim, A. Polymeric Micelles as Novel Carriers for Poorly Soluble Drugs—A Review. J. Nanosci. Nanotechnol. 2015, 15, 4009–4018. [Google Scholar] [CrossRef]
- Mir, M.; Ishtiaq, S.; Rabia, S.; Khatoon, M.; Zeb, A.; Khan, G.M.; Ur Rehman, A.; Ud Din, F. Nanotechnology: From In Vivo Imaging System to Controlled Drug Delivery. Nanoscale Res. Lett. 2017, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Santos-Martinez, M.J.; Radomski, A.; Corrigan, O.I.; Radomski, M.W. Nanoparticles: Pharmacological and Toxicological Significance. Br. J. Pharmacol. 2007, 150, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum Dots as a Platform for Nanoparticle Drug Delivery Vehicle Design. Adv. Drug Deliv. Rev. 2013, 65, 703–718. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Younis, M.A.; Alsharidah, M.; Al Rugaie, O.; Tawfeek, H.M. Biomedical Applications of Quantum Dots: Overview, Challenges, and Clinical Potential. Int. J. Nanomed. 2022, 17, 1951–1970. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. Dendrimers and Dendritic Polymers in Drug Delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-Based Drug and Imaging Conjugates: Design Considerations for Nanomedical Applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Kaurav, M.; Ruhi, S.; Al-Goshae, H.A.; Jeppu, A.K.; Ramachandran, D.; Sahu, R.K.; Sarkar, A.K.; Khan, J.; Ashif Ikbal, A.M. Dendrimer: An Update on Recent Developments and Future Opportunities for the Brain Tumors Diagnosis and Treatment. Front. Pharmacol. 2023, 14, 1159131. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibody-Drug Conjugates: Targeted Drug Delivery for Cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef]
- Burgio, S.; Noori, L.; Marino Gammazza, A.; Campanella, C.; Logozzi, M.; Fais, S.; Bucchieri, F.; Cappello, F.; Caruso Bavisotto, C. Extracellular Vesicles-Based Drug Delivery Systems: A New Challenge and the Exemplum of Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2020, 21, 5432. [Google Scholar] [CrossRef]
- Martincic, M.; Tobias, G. Filled Carbon Nanotubes in Biomedical Imaging and Drug Delivery. Expert Opin. Drug Deliv. 2015, 12, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-X.; Zhu, B.-J. The Research and Applications of Quantum Dots as Nano-Carriers for Targeted Drug Delivery and Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 207. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Messarah, M.; Klibet, F.; Boumendjel, A.; Abdennour, C.; Bouzerna, N.; Boulakoud, M.S.; El Feki, A. Hepatoprotective Role and Antioxidant Capacity of Selenium on Arsenic-Induced Liver Injury in Rats. Exp. Toxicol. Pathol. Off. J. Ges. Toxikol. Pathol. 2012, 64, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and Their Role in Phytopathogens Management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, B.K.; Yadav, S.M.; Gupta, A.K. Applications of Nanotechnology in Agricultural and Their Role in Disease Management. Res. J. Nanosci. Nanotechnol. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of Nanomaterials in Agricultural Production and Crop Protection: A Review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Pan, W.; Zheng, X.; Chen, G.; Su, L.; Luo, S.; Wang, W.; Ye, S.; Weng, J.; Min, Y. Nanotechnology’s Application in Type 1 Diabetes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1645. [Google Scholar] [CrossRef] [PubMed]
- Bahman, F.; Greish, K.; Taurin, S. Nanotechnology in Insulin Delivery for Management of Diabetes. Pharm. Nanotechnol. 2019, 7, 113–128. [Google Scholar] [CrossRef]
- Wang, Z.; Gonzalez, K.M.; Cordova, L.E.; Lu, J. Nanotechnology-Empowered Therapeutics Targeting Neurodegenerative Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1907. [Google Scholar] [CrossRef] [PubMed]
- Prajnamitra, R.P.; Chen, H.-C.; Lin, C.-J.; Chen, L.-L.; Hsieh, P.C.-H. Nanotechnology Approaches in Tackling Cardiovascular Diseases. Molecules 2019, 24, 2017. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Woodrow, K.A. Nanotechnology Approaches to Eradicating HIV Reservoirs. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgem. Pharm. Verfahrenstech. EV 2019, 138, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-C.; Lin, J.-C.; Tsai, H.-H.; Hsu, C.-Y.; Shih, V.; Hu, C.-M.J. Nanotechnology Advances in Pathogen- and Host-Targeted Antiviral Delivery: Multipronged Therapeutic Intervention for Pandemic Control. Drug Deliv. Transl. Res. 2021, 11, 1420–1437. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, M. Combating Malaria with Nanotechnology-Based Targeted and Combinatorial Drug Delivery Strategies. Drug Deliv. Transl. Res. 2016, 6, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, D.C.D.S.; Cavalcanti, I.D.L.; Medeiros, S.M.d.F.R.D.S.; Souza, J.B.d.; Lira Nogueira, M.C.d.B.; Cavalcanti, I.M.F. Nanotechnology and Tuberculosis: An Old Disease with New Treatment Strategies. Tuberc. Edinb. Scotl. 2022, 135, 102208. [Google Scholar] [CrossRef]
- Herrmann, I.K. How Nanotechnology-Enabled Concepts Could Contribute to the Prevention, Diagnosis and Therapy of Bacterial Infections. Crit. Care Lond. Engl. 2015, 19, 239. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, B.; Lin, N.; Gao, J. Recent Nanotechnology-Based Strategies for Interfering with the Life Cycle of Bacterial Biofilms. Biomater. Sci. 2023, 11, 1648–1664. [Google Scholar] [CrossRef]
- Tobin, E.; Brenner, S. Nanotechnology Fundamentals Applied to Clinical Infectious Diseases and Public Health. Open Forum Infect. Dis. 2021, 8, ofab583. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef]
- Kaittanis, C.; Santra, S.; Perez, J.M. Emerging Nanotechnology-Based Strategies for the Identification of Microbial Pathogenesis. Adv. Drug Deliv. Rev. 2010, 62, 408–423. [Google Scholar] [CrossRef]
- Hauck, T.S.; Giri, S.; Gao, Y.; Chan, W.C.W. Nanotechnology Diagnostics for Infectious Diseases Prevalent in Developing Countries. Adv. Drug Deliv. Rev. 2010, 62, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better Tests, Better Care: Improved Diagnostics for Infectious Diseases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57 (Suppl. S3), S139–S170. [Google Scholar] [CrossRef] [PubMed]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral Flow (Immuno)Assay: Its Strengths, Weaknesses, Opportunities and Threats. A Literature Survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, S.; Liu, Y.; Liu, C.; Sun, J. Nanosensors for Diagnosis of Infectious Diseases. ACS Appl. Bio Mater. 2021, 4, 3863–3879. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Wan Abas, W.A.B.; Pingguan-Murphy, B.; et al. An Integrated Paper-Based Sample-to-Answer Biosensor for Nucleic Acid Testing at the Point of Care. Lab Chip 2016, 16, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.-P.; Ma, W.; Long, Y.-T. Alcohol Dehydrogenase-Catalyzed Gold Nanoparticle Seed-Mediated Growth Allows Reliable Detection of Disease Biomarkers with the Naked Eye. Anal. Chem. 2015, 87, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- de la Rica, R.; Stevens, M.M. Plasmonic ELISA for the Ultrasensitive Detection of Disease Biomarkers with the Naked Eye. Nat. Nanotechnol. 2012, 7, 821–824. [Google Scholar] [CrossRef]
- Qu, W.; Liu, Y.; Liu, D.; Wang, Z.; Jiang, X. Copper-Mediated Amplification Allows Readout of Immunoassays by the Naked Eye. Angew. Chem. Int. Ed. Engl. 2011, 50, 3442–3445. [Google Scholar] [CrossRef]
- Singh, P.; Kakkar, S.; Bharti; Kumar, R.; Bhalla, V. Rapid and Sensitive Colorimetric Detection of Pathogens Based on Silver-Urease Interactions. Chem. Commun. Camb. Engl. 2019, 55, 4765–4768. [Google Scholar] [CrossRef]
- Yen, C.-W.; de Puig, H.; Tam, J.O.; Gómez-Márquez, J.; Bosch, I.; Hamad-Schifferli, K.; Gehrke, L. Multicolored Silver Nanoparticles for Multiplexed Disease Diagnostics: Distinguishing Dengue, Yellow Fever, and Ebola Viruses. Lab Chip 2015, 15, 1638–1641. [Google Scholar] [CrossRef]
- Mancuso, M.; Jiang, L.; Cesarman, E.; Erickson, D. Multiplexed Colorimetric Detection of Kaposi’s Sarcoma Associated Herpesvirus and Bartonella DNA Using Gold and Silver Nanoparticles. Nanoscale 2013, 5, 1678–1686. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, W.; Yang, L.; Tan, Y.; Xie, Q.; Yao, S. Copper-Based Metal-Organic Framework Nanoparticles with Peroxidase-Like Activity for Sensitive Colorimetric Detection of Staphylococcus Aureus. ACS Appl. Mater. Interfaces 2017, 9, 24440–24445. [Google Scholar] [CrossRef]
- Brandão, D.; Liébana, S.; Campoy, S.; Cortés, M.P.; Alegret, S.; Pividori, M.I. Simultaneous Electrochemical Magneto Genosensing of Foodborne Bacteria Based on Triple-Tagging Multiplex Amplification. Biosens. Bioelectron. 2015, 74, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, C.H.; Mayuri, P.; Sankaran, K.; Kumar, A.S. An Electrochemical Immunosensor for Efficient Detection of Uropathogenic E. coli Based on Thionine Dye Immobilized Chitosan/Functionalized-MWCNT Modified Electrode. Biosens. Bioelectron. 2016, 82, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bai, W.; Dong, C.; Guo, R.; Liu, Z. An Ultrasensitive Electrochemical DNA Biosensor Based on Graphene/Au Nanorod/Polythionine for Human Papillomavirus DNA Detection. Biosens. Bioelectron. 2015, 68, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Vallés, C.; Benito, A.M.; Maser, W.K.; Rius, F.X.; Riu, J. Graphene-Based Potentiometric Biosensor for the Immediate Detection of Living Bacteria. Biosens. Bioelectron. 2014, 54, 553–557. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Probing the Interactions of Chitosan Capped CdS Quantum Dots with Pathogenic Bacteria and Their Biosensing Application. J. Mater. Chem. B 2013, 1, 6094–6106. [Google Scholar] [CrossRef]
- Morales-Narváez, E.; Naghdi, T.; Zor, E.; Merkoçi, A. Photoluminescent Lateral-Flow Immunoassay Revealed by Graphene Oxide: Highly Sensitive Paper-Based Pathogen Detection. Anal. Chem. 2015, 87, 8573–8577. [Google Scholar] [CrossRef]
- Zhong, D.; Zhuo, Y.; Feng, Y.; Yang, X. Employing Carbon Dots Modified with Vancomycin for Assaying Gram-Positive Bacteria like Staphylococcus Aureus. Biosens. Bioelectron. 2015, 74, 546–553. [Google Scholar] [CrossRef]
- Lai, I.P.-J.; Harroun, S.G.; Chen, S.-Y.; Unnikrishnan, B.; Li, Y.-J.; Huang, C.-C. Solid-State Synthesis of Self-Functional Carbon Quantum Dots for Detection of Bacteria and Tumor Cells. Sens. Actuators B Chem. 2016, 228, 465–470. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Aizen, R.; Yehezkeli, O.; Willner, I. Graphene Oxide/Nucleic-Acid-Stabilized Silver Nanoclusters: Functional Hybrid Materials for Optical Aptamer Sensing and Multiplexed Analysis of Pathogenic DNAs. J. Am. Chem. Soc. 2013, 135, 11832–11839. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Liu, Y.; Duan, N.; Wu, S.; Wang, Z.; Xu, B. Gold Nanoparticles Enhanced SERS Aptasensor for the Simultaneous Detection of Salmonella Typhimurium and Staphylococcus Aureus. Biosens. Bioelectron. 2015, 74, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, H.; Hasan, D.; Ruoff, R.S.; Wang, A.X.; Fan, D.L. Near-Field Enhanced Plasmonic-Magnetic Bifunctional Nanotubes for Single Cell Bioanalysis. Adv. Funct. Mater. 2013, 23, 4332–4338. [Google Scholar] [CrossRef]
- Blackie, E.J.; Le Ru, E.C.; Etchegoin, P.G. Single-Molecule Surface-Enhanced Raman Spectroscopy of Nonresonant Molecules. J. Am. Chem. Soc. 2009, 131, 14466–14472. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, Z.; Yu, J.; Choo, P.; Chen, L.; Choo, J. A SERS-Based Lateral Flow Assay Biosensor for Highly Sensitive Detection of HIV-1 DNA. Biosens. Bioelectron. 2016, 78, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Choi, N.; Cheng, Z.; Ko, J.; Chen, L.; Choo, J. Simultaneous Detection of Dual Nucleic Acids Using a SERS-Based Lateral Flow Assay Biosensor. Anal. Chem. 2017, 89, 1163–1169. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, Y. Using Commercially Available Personal Glucose Meters for Portable Quantification of DNA. Anal. Chem. 2012, 84, 1975–1980. [Google Scholar] [CrossRef]
- Lin, Q.; Wen, D.; Wu, J.; Liu, L.; Wu, W.; Fang, X.; Kong, J. Microfluidic Immunoassays for Sensitive and Simultaneous Detection of IgG/IgM/Antigen of SARS-CoV-2 within 15 Min. Anal. Chem. 2020, 92, 9454–9458. [Google Scholar] [CrossRef]
- Qin, P.; Park, M.; Alfson, K.J.; Tamhankar, M.; Carrion, R.; Patterson, J.L.; Griffiths, A.; He, Q.; Yildiz, A.; Mathies, R.; et al. Rapid and Fully Microfluidic Ebola Virus Detection with CRISPR-Cas13a. ACS Sens. 2019, 4, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Scherr, T.F.; Gupta, S.; Wright, D.W.; Haselton, F.R. An Embedded Barcode for “Connected” Malaria Rapid Diagnostic Tests. Lab Chip 2017, 17, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; Cortazar, B.; Tseng, D.; Ozkan, H.; Feng, S.; Wei, Q.; Chan, R.Y.-L.; Burbano, J.; Farooqui, Q.; Lewinski, M.; et al. Cellphone-Based Hand-Held Microplate Reader for Point-of-Care Testing of Enzyme-Linked Immunosorbent Assays. ACS Nano 2015, 9, 7857–7866. [Google Scholar] [CrossRef] [PubMed]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Vilaivan, T.; Chailapakul, O.; Henry, C.S. Multiplex Paper-Based Colorimetric DNA Sensor Using Pyrrolidinyl Peptide Nucleic Acid-Induced AgNPs Aggregation for Detecting MERS-CoV, MTB, and HPV Oligonucleotides. Anal. Chem. 2017, 89, 5428–5435. [Google Scholar] [CrossRef] [PubMed]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef]
- Jin, B.; Wang, S.; Lin, M.; Jin, Y.; Zhang, S.; Cui, X.; Gong, Y.; Li, A.; Xu, F.; Lu, T.J. Upconversion Nanoparticles Based FRET Aptasensor for Rapid and Ultrasenstive Bacteria Detection. Biosens. Bioelectron. 2017, 90, 525–533. [Google Scholar] [CrossRef]
- Cho, I.-H.; Bhandari, P.; Patel, P.; Irudayaraj, J. Membrane Filter-Assisted Surface Enhanced Raman Spectroscopy for the Rapid Detection of E. coli O157:H7 in Ground Beef. Biosens. Bioelectron. 2015, 64, 171–176. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, J.; Yu, B.; Liu, Y.; You, T. A Novel Electrochemiluminescence Immunosensor for the Analysis of HIV-1 P24 Antigen Based on P-RGO@Au@Ru-SiO₂ Composite. ACS Appl. Mater. Interfaces 2015, 7, 24438–24445. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Xiao, R.; Tang, L.; Huang, J.; Wu, D.; Liu, S.; Wang, Y.; Zhang, D.; Wang, S.; et al. Sensitive and Specific Detection of Clinical Bacteria via Vancomycin-Modified Fe3O4@Au Nanoparticles and Aptamer-Functionalized SERS Tags. J. Mater. Chem. B 2018, 6, 3751–3761. [Google Scholar] [CrossRef]
- Thwala, L.N.; Ndlovu, S.C.; Mpofu, K.T.; Lugongolo, M.Y.; Mthunzi-Kufa, P. Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests. Nanomaterials 2023, 13, 1247. [Google Scholar] [CrossRef]
- Singh, J.A. Artificial Intelligence and Global Health: Opportunities and Challenges. Emerg. Top. Life Sci. 2019, 3, 741–746. [Google Scholar] [CrossRef]
- Ramalingam, M.; Jaisankar, A.; Cheng, L.; Krishnan, S.; Lan, L.; Hassan, A.; Sasmazel, H.T.; Kaji, H.; Deigner, H.-P.; Pedraz, J.L.; et al. Impact of Nanotechnology on Conventional and Artificial Intelligence-Based Biosensing Strategies for the Detection of Viruses. Discov. Nano 2023, 18, 58. [Google Scholar] [CrossRef]
- Martinez, J.L. General Principles of Antibiotic Resistance in Bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Pandrug-Resistant Gram-Negative Bacteria: A Systematic Review of Current Epidemiology, Prognosis and Treatment Options. J. Antimicrob. Chemother. 2020, 75, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Luepke, K.H.; Suda, K.J.; Boucher, H.; Russo, R.L.; Bonney, M.W.; Hunt, T.D.; Mohr, J.F. Past, Present, and Future of Antibacterial Economics: Increasing Bacterial Resistance, Limited Antibiotic Pipeline, and Societal Implications. Pharmacotherapy 2017, 37, 71–84. [Google Scholar] [CrossRef]
- Kadouri, D.E.; To, K.; Shanks, R.M.Q.; Doi, Y. Predatory Bacteria: A Potential Ally against Multidrug-Resistant Gram-Negative Pathogens. PLoS ONE 2013, 8, e63397. [Google Scholar] [CrossRef] [PubMed]
- Wiegers, C.; van de Burgwal, L.H.M.; Larsen, O.F.A. Probiotics for the Management of Infectious Diseases: Reviewing the State of the Art. Front. Microbiol. 2022, 13, 877142. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Ricci, E.; Fedele, F.; Chiaffarino, F.; Esposito, G.; Cipriani, S. Systematic Review of the Effect of D-Mannose with or without Other Drugs in the Treatment of Symptoms of Urinary Tract Infections/Cystitis (Review). Biomed. Rep. 2022, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Manohar, A.; Ahuja, J.; Crane, J.K. Immunotherapy for Infectious Diseases: Past, Present, and Future. Immunol. Investig. 2015, 44, 731–737. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The Role of Vaccines in Combatting Antimicrobial Resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, A.; Silletti, M.F.; Faraone, I.; Milella, L. Nanoparticulate Antibiotic Systems as Antibacterial Agents and Antibiotic Delivery Platforms to Fight Infections. J. Nanomater. 2020, 2020, 6905631. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Stalikas, C.D. Qualitative Alterations of Bacterial Metabolome after Exposure to Metal Nanoparticles with Bactericidal Properties: A Comprehensive Workflow Based on (1)H NMR, UHPLC-HRMS, and Metabolic Databases. J. Proteome Res. 2016, 15, 3322–3330. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Liew, K.B.; Janakiraman, A.K.; Sundarapandian, R.; Khalid, S.H.; Razzaq, F.A.; Ming, L.C.; Khan, A.; Kalusalingam, A.; Ng, P.W. A Review and Revisit of Nanoparticles for Antimicrobial Drug Delivery. J. Med. Life 2022, 15, 328–335. [Google Scholar] [CrossRef]
- Skipper, C.P.; Atukunda, M.; Stadelman, A.; Engen, N.W.; Bangdiwala, A.S.; Hullsiek, K.H.; Abassi, M.; Rhein, J.; Nicol, M.R.; Laker, E.; et al. Phase I EnACT Trial of the Safety and Tolerability of a Novel Oral Formulation of Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, e00838-20. [Google Scholar] [CrossRef]
- Aigner, M.; Lass-Flörl, C. Encochleated Amphotericin B: Is the Oral Availability of Amphotericin B Finally Reached? J. Fungi 2020, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D. Surrogate Cells or Trojan Horses. The Discovery of Liposomes. BioEssays News Rev. Mol. Cell. Dev. Biol. 1995, 17, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Ranghar, S.; Sirohi, P.; Verma, P.; Agarwal, V. Nanoparticle-Based Drug Delivery Systems: Promising Approaches against Infections. Braz. Arch. Biol. Technol. 2013, 57, 209–222. [Google Scholar] [CrossRef]
- Kinman, L.; Brodie, S.J.; Tsai, C.C.; Bui, T.; Larsen, K.; Schmidt, A.; Anderson, D.; Morton, W.R.; Hu, S.-L.; Ho, R.J.Y. Lipid-Drug Association Enhanced HIV-1 Protease Inhibitor Indinavir Localization in Lymphoid Tissues and Viral Load Reduction: A Proof of Concept Study in HIV-2287-Infected Macaques. J. Acquir. Immune Defic. Syndr. 2003, 34, 387–397. [Google Scholar] [CrossRef]
- Pereira de Oliveira, M.; Garcion, E.; Venisse, N.; Benoit, J.-P.; Couet, W.; Olivier, J.-C. Tissue Distribution of Indinavir Administered as Solid Lipid Nanocapsule Formulation in Mdr1a (+/+) and Mdr1a (−/−) CF-1 Mice. Pharm. Res. 2005, 22, 1898–1905. [Google Scholar] [CrossRef]
- Jin, S.X.; Bi, D.Z.; Wang, J.; Wang, Y.Z.; Hu, H.G.; Deng, Y.H. Pharmacokinetics and Tissue Distribution of Zidovudine in Rats Following Intravenous Administration of Zidovudine Myristate Loaded Liposomes. Pharmazie 2005, 60, 840–843. [Google Scholar]
- Qasim, M.; Lim, D.-J.; Park, H.; Na, D. Nanotechnology for Diagnosis and Treatment of Infectious Diseases. J. Nanosci. Nanotechnol. 2014, 14, 7374–7387. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of Ketoconazole and PAMAM Dendrimers: Formulation and Antifungal Activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Agashe, H.B.; Garg, M.; Balakrishnan, P.; Kabra, M.; Jain, N.K. Poly (Propyleneimine) Dendrimer Based Nanocontainers for Targeting of Efavirenz to Human Monocytes/Macrophages in Vitro. J. Drug Target. 2007, 15, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P.; Ren, G. Potential Impact of Nanotechnology on the Control of Infectious Diseases. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1–2. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Nino, N.; Sanchez, E.M.; Ruiz, F.; Bach, H.; Av-Gay, Y. Synthesis, Characterization, and Evaluation of Antimicrobial and Cytotoxic Effect of Silver and Titanium Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 681–688. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Sioss, J.A.; Stoermer, R.L.; Sha, M.Y.; Keating, C.D. Silica-Coated, Au/Ag Striped Nanowires for Bioanalysis. Langmuir ACS J. Surf. Colloids 2007, 23, 11334–11341. [Google Scholar] [CrossRef]
- Brandenberger, C.; Rothen-Rutishauser, B.; Mühlfeld, C.; Schmid, O.; Ferron, G.A.; Maier, K.L.; Gehr, P.; Lenz, A.-G. Effects and Uptake of Gold Nanoparticles Deposited at the Air-Liquid Interface of a Human Epithelial Airway Model. Toxicol. Appl. Pharmacol. 2010, 242, 56–65. [Google Scholar] [CrossRef]
- Mihu, M.R.; Sandkovsky, U.; Han, G.; Friedman, J.M.; Nosanchuk, J.D.; Martinez, L.R. The Use of Nitric Oxide Releasing Nanoparticles as a Treatment against Acinetobacter Baumannii in Wound Infections. Virulence 2010, 1, 62–67. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release Off. J. Control. Release Soc. 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper Nanoparticle/Polymer Composites with Antifungal and Bacteriostatic Properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- Losasso, C.; Belluco, S.; Cibin, V.; Zavagnin, P.; Mičetić, I.; Gallocchio, F.; Zanella, M.; Bregoli, L.; Biancotto, G.; Ricci, A. Antibacterial Activity of Silver Nanoparticles: Sensitivity of Different Salmonella Serovars. Front. Microbiol. 2014, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Vig Slenters, T.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver Coordination Polymers for Prevention of Implant Infection: Thiol Interaction, Impact on Respiratory Chain Enzymes, and Hydroxyl Radical Induction. Antimicrob. Agents Chemother. 2010, 54, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Pei, X.; Xie, W.; Chen, J.; Li, Y.; Wang, J.; Gao, H.; Wan, Q. pH-Triggered Size-Tunable Silver Nanoparticles: Targeted Aggregation for Effective Bacterial Infection Therapy. Small Weinh. Bergstr. Ger. 2022, 18, e2200915. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-Y.; Hoon Byeon, J.; Park, J.-H.; Hwang, J. Susceptibility Constants of Escherichia Coli and Bacillus Subtilis to Silver and Copper Nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Moniri Javadhesari, S.; Alipour, S.; Mohammadnejad, S.; Akbarpour, M.R. Antibacterial Activity of Ultra-Small Copper Oxide (II) Nanoparticles Synthesized by Mechanochemical Processing against S. Aureus and E. Coli. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110011. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhang, B.; Xing, X.; Zhao, Y.; Cai, R.; Wang, W.; Gu, Q. Biosynthesis of Copper Nanoparticles Using Shewanella Loihica PV-4 with Antibacterial Activity: Novel Approach and Mechanisms Investigation. J. Hazard. Mater. 2018, 347, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kareem, M.M.; Zohri, A.A. Extracellular Mycosynthesis of Gold Nanoparticles Using Trichoderma Hamatum: Optimization, Characterization and Antimicrobial Activity. Lett. Appl. Microbiol. 2018, 67, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, M.; Kalpana, V.N.; Devi Rajeswari, V.; Panneerselvam, A. Biosynthesis, Characterization, and Evaluation of Bioactivities of Leaf Extract-Mediated Biocompatible Gold Nanoparticles from Alternanthera Bettzickiana. Biotechnol. Rep. Amst. Neth. 2018, 19, e00268. [Google Scholar] [CrossRef]
- Arockiya Aarthi Rajathi, F.; Parthiban, C.; Ganesh Kumar, V.; Anantharaman, P. Biosynthesis of Antibacterial Gold Nanoparticles Using Brown Alga, Stoechospermum Marginatum (Kützing). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 166–173. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Krishnakumar, C.; Arulmozhi, P.; Mahadevan, S.; Parameswari, N. Biosynthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from Leaf Extract of Glycosmis Pentaphylla (Retz.) DC. Microb. Pathog. 2018, 116, 44–48. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S.; Sheba, R.D.; Lakshmi, T.; Deepak Nallaswamy, V. Phyto-Assisted Synthesis of Zinc Oxide Nanoparticles Using Cassia Alata and Its Antibacterial Activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Saha, R.K.; Debanath, M.K.; Paul, B.; Medhi, S.; Saikia, E. Antibacterial and Nonlinear Dynamical Analysis of Flower and Hexagon-Shaped ZnO Microstructures. Sci. Rep. 2020, 10, 2598. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial Activities of Zinc Oxide Nanoparticles against Escherichia Coli O157:H7: Antibacterial ZnO Nanoparticles. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V.R. Studies on Antibacterial Activity of ZnO Nanoparticles by ROS Induced Lipid Peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.R.; Naghiha, R.; Kheirizadeh, M.; Sadatfaraji, H.; Mirzaei, A.; Alvand, Z.M. Microwave Assisted Extraction as an Efficient Approach for Biosynthesis of Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biological Properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1109–1118. [Google Scholar] [CrossRef]
- Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670. [Google Scholar] [CrossRef]
- Courtney, C.M.; Goodman, S.M.; McDaniel, J.A.; Madinger, N.E.; Chatterjee, A.; Nagpal, P. Photoexcited Quantum Dots for Killing Multidrug-Resistant Bacteria. Nat. Mater. 2016, 15, 529–534. [Google Scholar] [CrossRef]
- Hao, X.; Huang, L.; Zhao, C.; Chen, S.; Lin, W.; Lin, Y.; Zhang, L.; Sun, A.; Miao, C.; Lin, X.; et al. Antibacterial Activity of Positively Charged Carbon Quantum Dots without Detectable Resistance for Wound Healing with Mixed Bacteria Infection. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111971. [Google Scholar] [CrossRef]
- Fang, F.; Li, M.; Zhang, J.; Lee, C.-S. Different Strategies for Organic Nanoparticle Preparation in Biomedicine. ACS Mater. Lett. 2020, 2, 531–549. [Google Scholar] [CrossRef]
- Wang, Y. Liposome as a Delivery System for the Treatment of Biofilm-Mediated Infections. J. Appl. Microbiol. 2021, 131, 2626–2639. [Google Scholar] [CrossRef] [PubMed]
- Smiechowicz, E.; Niekraszewicz, B.; Kulpinski, P.; Dzitko, K. Antibacterial Composite Cellulose Fibers Modified with Silver Nanoparticles and Nanosilica. Cellulose 2018, 25, 3499–3517. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H. Polymeric Nanomaterials for Efficient Delivery of Antimicrobial Agents. Pharmaceutics 2021, 13, 2108. [Google Scholar] [CrossRef]
- Lu, R.; Hollingsworth, C.; Qiu, J.; Wang, A.; Hughes, E.; Xin, X.; Konrath, K.M.; Elsegeiny, W.; Park, Y.-D.; Atakulu, L.; et al. Efficacy of Oral Encochleated Amphotericin B in a Mouse Model of Cryptococcal Meningoencephalitis. mBio 2019, 10, e00724-19. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Atukunda, M.; Kagimu, E.; Musubire, A.K.; Akampurira, A.; Tugume, L.; Ssebambulidde, K.; Kasibante, J.; Nsangi, L.; Mugabi, T.; et al. Oral Lipid Nanocrystal Amphotericin B for Cryptococcal Meningitis: A Randomized Clinical Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 77, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Bozbey, I.; Gun, Z.; Karakurt, A. Parenteral Systemic Antifungal Drugs and Their Clinical Drug Informations. Med. Sci. Int. Med. J. 2019, 8, 250–254. [Google Scholar] [CrossRef]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome(®)): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Leenders, A.C.; Daenen, S.; Jansen, R.L.; Hop, W.C.; Lowenberg, B.; Wijermans, P.W.; Cornelissen, J.; Herbrecht, R.; van der Lelie, H.; Hoogsteden, H.C.; et al. Liposomal Amphotericin B Compared with Amphotericin B Deoxycholate in the Treatment of Documented and Suspected Neutropenia-Associated Invasive Fungal Infections. Br. J. Haematol. 1998, 103, 205–212. [Google Scholar] [CrossRef]
- Hamill, R.J.; Sobel, J.D.; El-Sadr, W.; Johnson, P.C.; Graybill, J.R.; Javaly, K.; Barker, D.E. Comparison of 2 Doses of Liposomal Amphotericin B and Conventional Amphotericin B Deoxycholate for Treatment of AIDS-Associated Acute Cryptococcal Meningitis: A Randomized, Double-Blind Clinical Trial of Efficacy and Safety. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 51, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J.; Agarwal, D.; Rai, M.; Murray, H.W. Single-Dose Liposomal Amphotericin B for Visceral Leishmaniasis in India. N. Engl. J. Med. 2010, 362, 504–512. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Q.; Jiang, J.; Gao, L. Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance. Antibiotics 2022, 11, 390. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Pu, F.; Liu, Z.; Liu, X.; Dong, K.; Liu, C.; Ren, J.; Qu, X. Hydrogel-Based Artificial Enzyme for Combating Bacteria and Accelerating Wound Healing. Nano Res. 2020, 13, 496–502. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Bao, X.; Ni, Y.; Teng, Y.; Liu, J.; Sun, X.; Sun, Y.; Li, H.; Zhou, Y. Nanodiamond as Efficient Peroxidase Mimic against Periodontal Bacterial Infection. Carbon 2020, 169, 370–381. [Google Scholar] [CrossRef]
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hémadi, M. Recent Advances in Nanotechnology for Eradicating Bacterial Biofilm. Theranostics 2022, 12, 2383–2405. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Baveye, P.; Neu, T.R.; Stoodley, P.; Szewzyk, U.; Wingender, J.; Wuertz, S. Who Put the Film in Biofilm? The Migration of a Term from Wastewater Engineering to Medicine and Beyond. NPJ Biofilms Microbiomes 2021, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- van Gestel, J.; Vlamakis, H.; Kolter, R. Division of Labor in Biofilms: The Ecology of Cell Differentiation. Microbiol. Spectr. 2015, 3, MB-0002-2014. [Google Scholar] [CrossRef]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should We Stay or Should We Go: Mechanisms and Ecological Consequences for Biofilm Dispersal. Nat. Rev. Microbiol. 2011, 10, 39–50. [Google Scholar] [CrossRef]
- An, D.; Parsek, M.R. The Promise and Peril of Transcriptional Profiling in Biofilm Communities. Curr. Opin. Microbiol. 2007, 10, 292–296. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic Selenium Nanoparticles: Characterization, Antimicrobial Activity and Effects on Human Dendritic Cells and Fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies against Multidrug-Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Smitha, M.S.; Singh, S.P. The Role of Nanotechnology in Combating Multi-Drug Resistant Bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A. Nanomaterials for Alternative Antibacterial Therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as Therapeutic Options for Treating Multidrug-Resistant Bacteria: Research Progress, Challenges, and Prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of Silver Nanoparticles Using Dioscorea Bulbifera Tuber Extract and Evaluation of Its Synergistic Potential in Combination with Antimicrobial Agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar] [CrossRef]
- Mukherjee, S.; Honkala, A.; Rolnik, B. Review of the Clinical Trial Failures of Nanomedicines Targeting Cancer. J. Stud. Res. 2022, 11. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Chandrasekhar, D.; PokkaVayalil, V. Cost Minimization Analysis on IV to Oral Conversion of Antimicrobial Agent by the Clinical Pharmacist Intervention. Clin. Epidemiol. Glob. Health 2019, 7, 60–65. [Google Scholar] [CrossRef]
- McCarthy, K.; Avent, M. Oral or Intravenous Antibiotics? Aust. Prescr. 2020, 43, 45–48. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccines: Past, Present and Future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B. The Contribution of Vaccination to Global Health: Past, Present and Future. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Mascola, J.R.; Fauci, A.S. Novel Vaccine Technologies for the 21st Century. Nat. Rev. Immunol. 2020, 20, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Matić, Z.; Šantak, M. Current View on Novel Vaccine Technologies to Combat Human Infectious Diseases. Appl. Microbiol. Biotechnol. 2022, 106, 25–56. [Google Scholar] [CrossRef] [PubMed]
- Lugade, A.A.; Bharali, D.J.; Pradhan, V.; Elkin, G.; Mousa, S.A.; Thanavala, Y. Single Low-Dose Un-Adjuvanted HBsAg Nanoparticle Vaccine Elicits Robust, Durable Immunity. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 923–934. [Google Scholar] [CrossRef]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines. Front. Vet. Sci. 2021, 8, 654289. [Google Scholar] [CrossRef]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.C.; Plebanski, M. Size-Dependent Immunogenicity: Therapeutic and Protective Properties of Nano-Vaccines against Tumors. J. Immunol. Baltim. Md 1950 2004, 173, 3148–3154. [Google Scholar] [CrossRef]
- Mottram, P.L.; Leong, D.; Crimeen-Irwin, B.; Gloster, S.; Xiang, S.D.; Meanger, J.; Ghildyal, R.; Vardaxis, N.; Plebanski, M. Type 1 and 2 Immunity Following Vaccination Is Influenced by Nanoparticle Size: Formulation of a Model Vaccine for Respiratory Syncytial Virus. Mol. Pharm. 2007, 4, 73–84. [Google Scholar] [CrossRef]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles Target Distinct Dendritic Cell Populations According to Their Size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Gomes, A.C.; Cabral-Miranda, G.; Krueger, C.C.; Leoratti, F.M.; Stein, J.V.; Bachmann, M.F. Delivering Adjuvants and Antigens in Separate Nanoparticles Eliminates the Need of Physical Linkage for Effective Vaccination. J. Control. Release Off. J. Control. Release Soc. 2017, 251, 92–100. [Google Scholar] [CrossRef]
- Gomes, A.C.; Mohsen, M.; Bachmann, M.F. Harnessing Nanoparticles for Immunomodulation and Vaccines. Vaccines 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.S.; Endsley, A.N.; Huang, L. Design Considerations for Liposomal Vaccines: Influence of Formulation Parameters on Antibody and Cell-Mediated Immune Responses to Liposome Associated Antigens. Vaccine 2012, 30, 2256–2272. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Zhang, M.; Chen, R.; Niu, R.; Deng, Y. Mannose Derivative and Lipid A Dually Decorated Cationic Liposomes as an Effective Cold Chain Free Oral Mucosal Vaccine Adjuvant-Delivery System. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgem. Pharm. Verfahrenstech. EV 2014, 88, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Badiee, A.; Khamesipour, A.; Samiei, A.; Soroush, D.; Shargh, V.H.; Kheiri, M.T.; Barkhordari, F.; Robert Mc Master, W.; Mahboudi, F.; Jaafari, M.R. The Role of Liposome Size on the Type of Immune Response Induced in BALB/c Mice against Leishmaniasis: Rgp63 as a Model Antigen. Exp. Parasitol. 2012, 132, 403–409. [Google Scholar] [CrossRef]
- Alexyuk, P.G.; Bogoyavlenskiy, A.P.; Alexyuk, M.S.; Turmagambetova, A.S.; Zaitseva, I.A.; Omirtaeva, E.S.; Berezin, V.E. Adjuvant Activity of Multimolecular Complexes Based on Glycyrrhiza Glabra Saponins, Lipids, and Influenza Virus Glycoproteins. Arch. Virol. 2019, 164, 1793–1803. [Google Scholar] [CrossRef]
- Morein, B.; Hu, K.-F.; Abusugra, I. Current Status and Potential Application of ISCOMs in Veterinary Medicine. Adv. Drug Deliv. Rev. 2004, 56, 1367–1382. [Google Scholar] [CrossRef]
- Pourmand, A.; Abdollahi, M. Current Opinion on Nanotoxicology. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2012, 20, 95. [Google Scholar] [CrossRef]
- Petrarca, C.; Clemente, E.; Amato, V.; Pedata, P.; Sabbioni, E.; Bernardini, G.; Iavicoli, I.; Cortese, S.; Niu, Q.; Otsuki, T.; et al. Engineered Metal Based Nanoparticles and Innate Immunity. Clin. Mol. Allergy CMA 2015, 13, 13. [Google Scholar] [CrossRef]
- Diaz-Arévalo, D.; Zeng, M. Nanoparticle-Based Vaccines. In Nanopharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–150. ISBN 978-0-12-817778-5. [Google Scholar]
- Boyce, N. Trial Halted after Gene Shows up in Semen. Nature 2001, 414, 677. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; A Ahmed, E.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Sandhiya, S.; Dkhar, S.A.; Surendiran, A. Emerging Trends of Nanomedicine--an Overview. Fundam. Clin. Pharmacol. 2009, 23, 263–269. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Al-Daihan, S. On the Toxicity of Therapeutically Used Nanoparticles: An Overview. J. Toxicol. 2009, 2009, 754810. [Google Scholar] [CrossRef] [PubMed]
- Hagens, W.I.; Oomen, A.G.; de Jong, W.H.; Cassee, F.R.; Sips, A.J.A.M. What Do We (Need to) Know about the Kinetic Properties of Nanoparticles in the Body? Regul. Toxicol. Pharmacol. RTP 2007, 49, 217–229. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug Delivery and Nanoparticles:Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the Microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
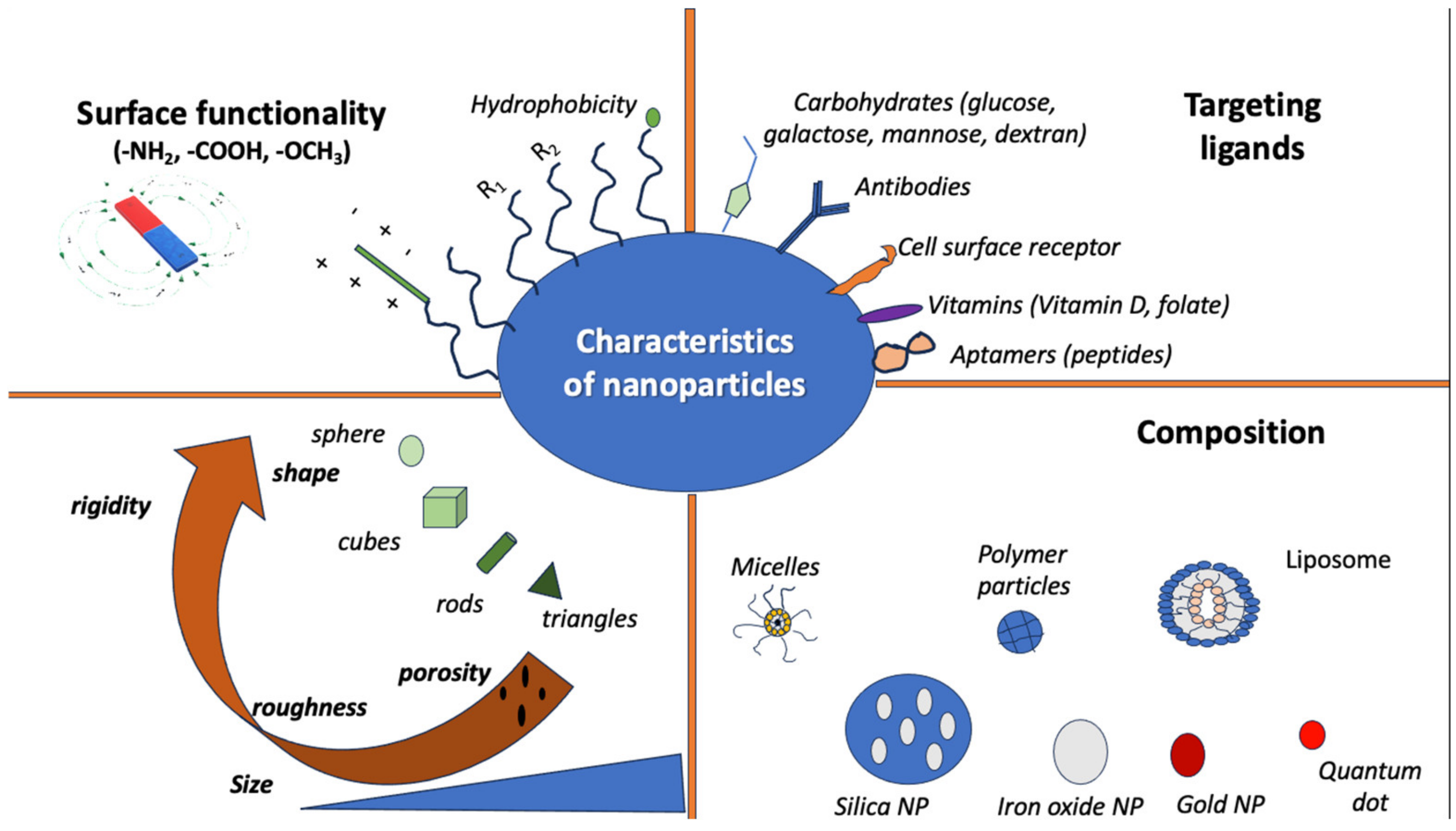
| Nanostructure | Type of Structure | Properties and Advantages | Disadvantages | References |
|---|---|---|---|---|
| Dendrimers | Polymeric | Potent activity against cancer cells | Difficult to purify and produce in large quantities, potential toxicity | [57,58,59] |
| Drug conjugates | Polymeric | Provide controlled release, increase activity and tolerability of drugs | Extensive knowledge of polymer–receptor interaction required | [60,61] |
| Liposomes | Polymeric | Lipid bilayers incorporating drugs to enhance delivery, formation of macromolecular drugs with peptides, antibodies, or polymers, biodegradable | May form crystals during prolonged storage, high production cost, low solubility, short half-life | [13,38,43,51] |
| Micelles | Polymeric | Extremely small, amphiphilic polymers, increased aqueous solubility | Moderate loading capacity | [51,52,53] |
| Carbon nanotubes | Nonpolymeric | Increased drug stability and solubility, targeted drug delivery | Limited results from clinical studies, potential toxicity, variable pharmacokinetics | [36,62] |
| Metallic nanoparticles | Nonpolymeric | Controlled and targeted delivery, potential for contrast agents | Biocompatibility, not strong optical signal | [46,47] |
| Quantum dots | Nonpolymeric | Improved bioavailability and efficacy, high quality fluorescence | Toxicity of the core, relative instability, difficult to produce massively | [47,56,63] |
| Nanoparticle | Pathogen | Detection Methodology | References |
|---|---|---|---|
| AgNPs | Dengue, Yellow Fever, and Ebola Viruses | Colorimetry | [92] |
| AgNPs | KSHV and Bartonella | Colorimetry | [93] |
| AgNPs | MERS-CoV, MTB, HPV | Colorimetry | [116] |
| AuNPs | E. coli and S. pneumoniae | Colorimetry | [87] |
| AuNPs | HBV | Colorimetry | [88] |
| AuNPs | SARS-CoV-2 | Colorimetry | [117] |
| AuNPs | E. coli ATCC 8739 | Fluorimetry | [118] |
| AuNPs | E. coli O157:H7 | SERS | [119] |
| AuNPs | HIV | SERS | [109] |
| AuNPs, AgNPs | Bartonella, KSHV | Colorimetry | [93] |
| Silica nanoparticles, AuNPs | HIV | Electrochemiluminometry | [120] |
| Fe3O4@Au nanoparticles, AuNPs | S. aureus, E. coli | SERS | [121] |
| Carbon nanotube | E.coli, HPV | Electrochemiluminometry | [97,98] |
| Copper-based metal-organic framework (Cu-MOF) NPs | S. aureus | Colorimetry | [95] |
| AuNPs | HIV-1, KSHV and BA | SERS | [109,110] |
| CdSe@ZnS quantum dots | E. coli | Fluorimetry | [101] |
| Nanoparticle Category | Nanoparticle Type | Size | Zeta Potential | In Vitro Drug Release | In Vitro Results | In Vivo Results | References |
|---|---|---|---|---|---|---|---|
| Metallic NP | Polymer thin film loading CuNPs | 3.2–5.3 nm, 80–530 nm for the nanocomposite films | NR | First-order process with an average kinetic constant of 0.014 ± 0.008/min | Clear biostatic activity on Saccharomyces cerevisiae, Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes growth | NA | [155] |
| Metallic NP | Monodisperse spherical CuNPs | 6–20 nm | NR | In vitro application after extracellular production from Shewanella loihica PV-4 | 100 µg/mL CuNPs inhibits 86% of E. coli | NA | [165] |
| Metallic NP | AgNPs (mostly spherical in shape) | 6 nm and 18 nm | NR | In vitro application | 200 mg/L had the highest inhibition effect on Salmonella | NA | [159] |
| Metallic NP | AuNPs (spherical) | 80–120 nm | −41.4 | In vitro application | Adequate antimicrobial activity against S. typhi, P. aeruginosa, E. aerogenes, S. aureus, B. subtilis, and M. luteus | NA | [167] |
| Metallic NP | AuNP (mostly spherical; also, triangular and hexagonal) | 40–85 nm | NR | In vitro application | Adequate antimicrobial activity against P. aeruginosa, K. oxytoca, E. faecalis, K. pneumoniae, V. cholerae, E. coli, S. typhi, S. paratyphii, V. parahaemolyticus, and P. vulgaris | NA | [168] |
| Metallic NP | ZnONPs (mostly spherical in shape) | 32–40 nm | NR | In vitro application | Maximum zone of inhibition at 100 μg/mL for Shigella dysenteriae, Bacillus cereus, Salmonella paratyphi, Candida albicans, A. niger, Staphylococcus aureus, Salmonella paratyphi, and Bacillus cereus | NA | [169] |
| Metallic NP | ZnONPs (spherical) | 60 nm | NR | In vitro application | Inhibitory and bactericidal activity was demonstrated against P. aeruginosa, E. coli, S. aureus, and B. subtilis | NA | [177] |
| Quantum dots | 2.4 eV CdTe photoexcited quantum dots | 3 nm | NR | In vitro application | Quantum dots can kill MRSA, CR-Escherichia coli, and ESBL Klebsiella pneumoniae and Salmonella typhimurium | NA | [179] |
| Quantum dots | Positively charged carbon quantum dots | 2.5 nm | −12.77 mV | In vitro application (in vitro); local application to the skin (in vivo) | Potent antibacterial effect on S. aureus, MRSA, L. monocytogenes, E. faecalis, E. coli, S. marcescens, P. aeruginosa, drug-resistant E. coli, and drug-resistant P. aeruginosa. Better antibacterial effect on Gram-positive bacteria | Faster healing and faster white blood cell recovery in a mixed infected wound rat animal model with S. aureus and E. coli, with minimal in vivo toxicity | [180] |
| Lipid-based | Encochleated amphotericin B (MAT2203, Matinas Biopharma) | NR | NR | Oral administration | NA | Potent anticryptococcal activity in mice and humans with favorable safety profile | [138,185,186] |
| Lipid based | Liposomal amphotericin B | 80 nm | NR | Intravenous administration | NA | Potent activity in invasive aspergillosis, cryptococcal meningitis, and visceral leishmaniasis in humans | [188,189,190,191] |
| Nanozyme | Hydrogel-based artificial enzyme | 50–70 nm | NR | Direct application (in vitro); local application to the skin (in vivo) | Activity against drug-resistant S. aureus and drug-resistant E. coli | Excellent wound healing properties in mice | [195] |
| Nanozyme | Oxygenated nanodiamonds | 2–10 nm | −19–−36 mV | Direct application (in vitro); local application with oral cavity flushing (in vivo) | Potent activity against Fusobacterium nucleatum, Porphyromonas gingivalis, and S. sanguis | Acceleration of wound healing after periodontal infection | [196] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannou, P.; Baliou, S.; Samonis, G. Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections. Antibiotics 2024, 13, 121. https://doi.org/10.3390/antibiotics13020121
Ioannou P, Baliou S, Samonis G. Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections. Antibiotics. 2024; 13(2):121. https://doi.org/10.3390/antibiotics13020121
Chicago/Turabian StyleIoannou, Petros, Stella Baliou, and George Samonis. 2024. "Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections" Antibiotics 13, no. 2: 121. https://doi.org/10.3390/antibiotics13020121
APA StyleIoannou, P., Baliou, S., & Samonis, G. (2024). Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections. Antibiotics, 13(2), 121. https://doi.org/10.3390/antibiotics13020121






