Synergistic Effects of Oligochitosan and Pyraclostrobin in Controlling Leaf Spot Disease in Pseudostellaria heterophylla
Abstract
1. Introduction
2. Results
2.1. Effects of Pyraclostrobin and Oligochitosan on LSD
2.2. Effects of Pyraclostrobin and Oligochitosan on Disease Resistance
2.3. Effects of Pyraclostrobin and Oligochitosan on Photosynthetic Capability
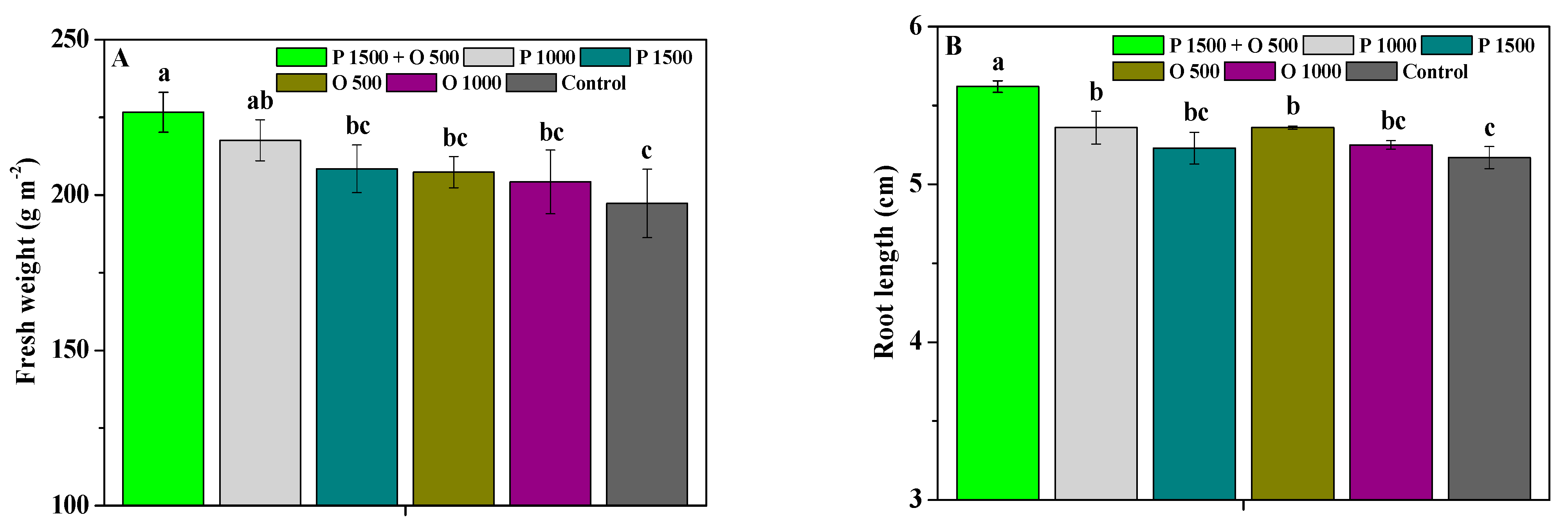
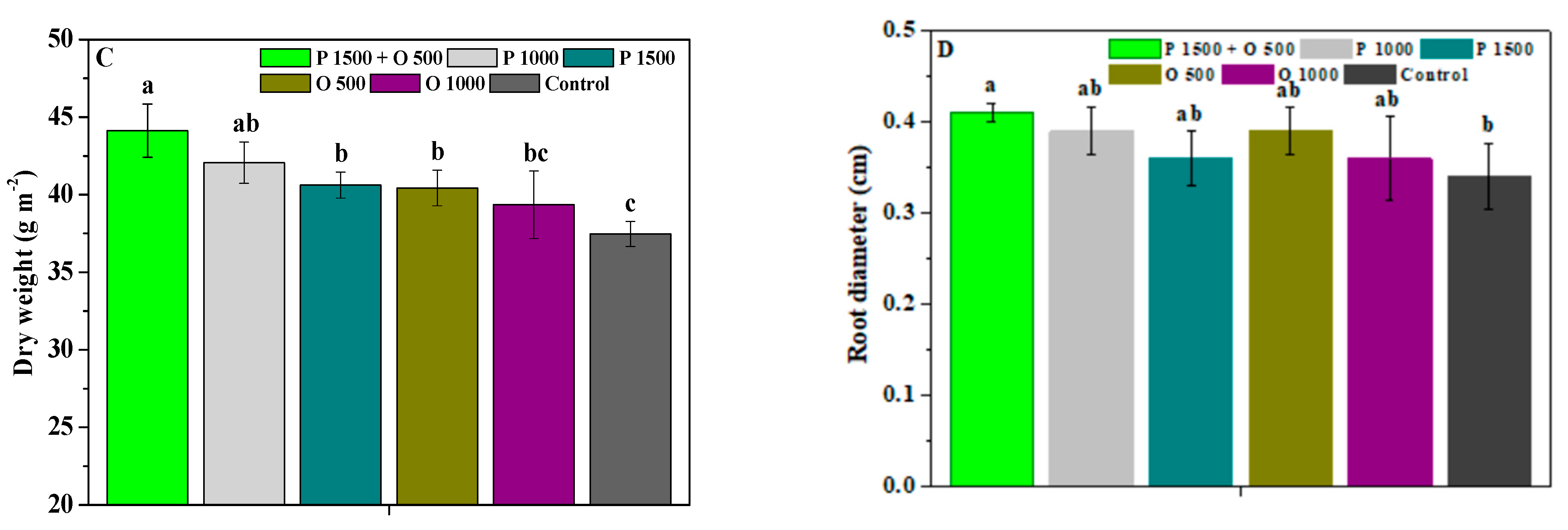
2.4. Effects of Pyraclostrobin and Oligochitosan on Agronomic Trait
2.5. Effects of Pyraclostrobin and Oligochitosan on Yield and Quality
3. Discussion
4. Materials and Methods
4.1. Oligochitosan, Pyraclostrobin, and Instruments
4.2. Field Herbal Orchard
4.3. Control Test
4.4. Methods
4.4.1. Control Effects
4.4.2. Resistance-Related Substance Content and Enzyme Activity
4.4.3. Photosynthetic Capability and Agronomic Trait Parameters
4.4.4. Tuberous Root Growth and Medicinal Quality
4.5. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Li, P.; Su, J.; Wang, D.; Kuang, Y.; Ye, Z.; Chen, M. EST-SSR Development and Genetic Diversity in the Medicinal Plant Pseudostellaria heterophylla (Miq.) Pax. J. Appl. Res. Med. Aromat. Plants 2023, 33, 100450. [Google Scholar] [CrossRef]
- Ma, J.; Sun, M.; Qiu, L.; Xie, Y.; Ma, Y.; Liang, W. The 5-Aminolevulinic Acid (5-ALA) Supplement Enhances PSII Photochemical Activity and Antioxidant Activity in the Late Growth Promotion of Pseudostellaria heterophylla. Plants 2022, 11, 3035. [Google Scholar] [CrossRef]
- Liao, X.; He, X.; Xu, G.; Wang, Z.; Li, J.; Guan, H.; Zhou, M.; Li, Y.; Wang, Y.; Gan, L.; et al. Two Nitrogen-containing compounds from Pseudostellaria heterophylla. Nat. Prod. Commun. 2018, 13, 343–346. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, S.; He, Y.; Li, J.; Zhong, R.; He, X.; Liu, Y.; Xiao, T.; Lan, Y.; Long, Q.; et al. Protective Effects of Fractions from Pseudostellaria heterophylla against Cobalt Chloride Induced Hypoxic Injury in H9c2 cell. J. Ethnopharmacol. 2013, 147, 540–545. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Z.; Gao, H.; Pen, X.; Li, Y.; Sun, S.; Lee, J.; Lin, W. Interaction of Pseudostellaria heterophylla with Quorum Sensing and Quorum Quenching Bacteria Mediated by Root Exudates in a Consecutive Monoculture System. J. Microbiol. Biotechnol. 2016, 26, 2159. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, M.; Ahn, K.; Um, J.; Lee, S.; Yang, W. Immunomodulatory Effects of Pseudostellaria heterophylla (Miquel) Pax on Regulation of Th1/Th2 Levels in Mice with Atopic Dermatitis. Mol. Med. Rep. 2017, 15, 649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, T.; Zhang, C.; Zheng, W.; Li, J.; Jiang, W.; Xiao, C.; Wei, D.; Yang, C.; Xu, R.; et al. Gibberellin Disturbs the Balance of Endogenesis Hormones and Inhibits Adventitious Root Development of Pseudostellaria Heterophylla Through Regulating Gene Expression Related to Hormone Synthesis. Saudi J. Biol. Sci. 2021, 28, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, J.; Wu, H.; Qin, X.; Wang, J.; Wu, Y.; Khan, M.U.; Lin, S.; Xiao, Z.; Luo, X. Insights into the Regulation of Rhizosphere Bacterial Communities by Application of Bio-organic Fertilizer in Pseudostellaria heterophylla Monoculture Regime. Front. Microbiol. 2016, 7, 1788. [Google Scholar] [CrossRef]
- He, J.; Liang, S.; Zhang, G.; Zhao, Z.; Li, Z. Pathogen Identification and Screening of Fungicides against Pseudostellaria heterophylla (Miq.) Pax Leaf Spot. J. South. Agric. 2021, 52, 2124–2132. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Y.; Guo, L.; Zhang, X.; Pei, G.; Zhang, H.; Zhu, G.; Wu, X.; Zhou, T. Common Diseases and Drug Use of Pseudostellaria heterophylla. China J. Chin. Mater. Medica 2023, 48, 3281–3286. [Google Scholar] [CrossRef]
- Long, G.; Ma, D.; Xia, Z.; Shao, C. Pseudostellaria heterophylla Main Disease Occurrence Regulation and Control Counter Measure in Shibing County. Cult. Plant. 2013, 2, 46. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Yang, Y. Pathogen Identification of Leaf Spot on Pseudostellaria heterophylla and Screening of Fungicides for Its Control. Plant Prot. 2018, 44, 182–185. [Google Scholar] [CrossRef]
- Shao, C.; Li, D.; Ma, D. Preliminary Study on Screening Pesticide of Prevention and Control of Pseudostellaria heterophylla Leaf Spot. Tillage Cultiv. 2016, 4, 29–30. [Google Scholar] [CrossRef]
- Balba, H. Review of Strobilurin Fungicide Chemicals. J. Environ. Sci. Health Part B 2007, 42, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Musso, L.; Fabbrini, A.; Dallavalle, S. Natural Compound-derived Cytochrome bc1 Complex Inhibitors as Antifungal Agents. Molecules 2020, 25, 4582. [Google Scholar] [CrossRef]
- Hunte, C.; Palsdottir, H.; Trumpower, B.L. Protonmotive Pathways and Mechanisms in the Cytochrome bc1 Complex. FEBS Lett. 2003, 545, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Bahia, K.H.; Pascal, L.; Dong-Woo, L.; Elisabeth, D.; Fevzi, D. Recent Advances in Cytochrome bc 1: Inter Monomer Electronic Communication? FEBS Lett. 2011, 586, 617–621. [Google Scholar]
- Pedersen, M.; Wegner, C.; Phansak, P.; Sarath, G.; Gaussoin, R.; Schlegel, V. Monitoring Wheat Mitochondrial Compositional and Respiratory Changes using Fourier transform Mid-infrared Spectroscopy in Response to Agrochemical Treatments. Spectrochim. Acta A 2017, 173, 727–732. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, X.; Fan, Y.; Luan, S.; Huang, Q. Effect of Membrane Integrity on Survival Competition of Botrytis cinerea upon QoI Fungicide Pyraclostrobin. J. Phytopathol. 2020, 168, 601–608. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.A.; Mercader, J.V.; Agulló, C.; Abad-Somovilla, A.; Abad-Fuentes, A. Development of Immunoaffinity Columns for Pyraclostrobin Extraction from Fruit Juices and Analysis by Liquid Chromatography with UV Detection. J. Chromatogr. A 2011, 1218, 4902–4909. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Li, J.; Su, Y.; Wu, X. Chitosan as an Adjuvant to Enhance the Control Efficacy of Low-Dosage Pyraclostrobin against Powdery Mildew of Rosa roxburghii and Improve Its Photosynthesis, Yield, and Quality. Biomolecules 2022, 12, 1304. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, M.; Yang, Y.; Shi, H.; Zhou, J. Strong Lethality and Teratogenicity of Strobilurins on Xenopus Tropicalis Embryos: Basing on Ten Agricultural Fungicides. Environ. Pollut. 2016, 208, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Zhang, S.; Zhu, L.; Wang, J. Acute and Subchronic Toxicity of Pyraclostrobin in Zebrafish (Danio rerio). Chemosphere 2017, 188, 510–516. [Google Scholar] [CrossRef]
- Domingues, C.E.D.C.; Inoue, L.V.B.; Silva-Zacarin, E.C.M.; Malaspina, O. Fungicide Pyraclostrobin Affects Midgut Morphophysiology and Reduces Survival of Brazilian Native Stingless bee Melipona scutellaris. Ecotox. Environ. Safe 2020, 206, 111395. [Google Scholar] [CrossRef]
- Huang, X.; Yang, S.; Li, B.; Wang, A.; Mu, W. Comparative Toxicity of Multiple Exposure Routes of Pyraclostrobin in Adult Zebrafish (Danio rerio). Sci. Total Environ. 2021, 777, 145957. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Sarojini, V. Pseudomonas syringae pv. actinidiae: Chemical Control, Resistance Mechanisms and Possible Alternatives. Plant. Pathol. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Ochoa-Meza, L.C.; Quintana-Obregón, E.A.; VargasArispuro, I.; Falcón-Rodríguez, A.B.; Aispuro-Hernández, E.; Virgen-Ortiz, J.J.; Martínez-Téllez, M.Á. Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers 2021, 13, 3105. [Google Scholar] [CrossRef]
- Hernández, V.; Hellín, P.; Botella, M.Á.; Vicente, E.; Fenoll, J.; Flores, P. Oligosaccharins Alleviate Heat Stress in Greenhouse-Grown Tomatoes during the Spring-Summer Season in a Semi-Arid Climate. Agronomy 2022, 12, 802. [Google Scholar] [CrossRef]
- Díaz, V.B. Oligosacarinas (Oligosacáridos): Producción Químicay Aplicación a Los Cultivos Vegetales, 1st ed.; Autores de Argentina: Buenos Aires, Argentina, 2018. [Google Scholar]
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef]
- Boyzo-Marín, J.; Silva-Rojas, H.V.; Rebollar-Alviter, A. Biorational Treatments to Manage Dryberry of Blackberry Caused by Peronospora sparsa. Crop Prot. 2015, 76, 121–126. [Google Scholar] [CrossRef]
- Cui, K.; Shu, C.; Zhao, H.; Fan, X.; Cao, J.; Jiang, W. Preharvest Chitosan Oligochitosan and Salicylic Acid Treatments Enhance Phenol Metabolism and Maintain the Postharvest Quality of Apricots (Prunus armeniaca L.). Sci. Hortic. 2020, 267, 109334. [Google Scholar] [CrossRef]
- Costales, D.; Martínez, L.; Núñez, M. Efecto del Tratamiento de Semillas con Una Mezcla de Oligogalacturónidos Sobre Elcrecimiento de Plántulas de Tomate (Lycopersicum esculentum Mill). Cultiv. Trop. 2007, 28, 85–91. [Google Scholar]
- Cabrera, J.C.; Wegria, G.; Onderwater, R.C.A.; González, G.; Napoles, M.C.; Falcon-Rodriguez, A.B.; Costales, D.; Rogers, H.J.; Diosdado, E.; González, S. Practical Use of Oligosaccharins in Agriculture. Acta Hortic. 2013, 1009, 195–212. [Google Scholar] [CrossRef]
- Wang, Q.; Long, Y.; Ai, Q.; Su, Y.; Lei, Y. Oligosaccharins Used Together with Tebuconazole Enhances Resistance of Kiwifruit against Soft Rot Disease and Improves Its Yield and Quality. Horticulturae 2022, 8, 624. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Liu, J.; Su, Y.; Zhang, Q. Chitosan Enhances Low-Dosage Difenoconazole to Efficiently Control Leaf Spot Disease in Pseudostellaria heterophylla (Miq.) Pax. Molecules 2023, 28, 6170. [Google Scholar] [CrossRef]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic Propagation of Immunity in Plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Villarreal, M.; Vargas-Arispuro, I.; Islas-Osuna, M.A.; Gonzalez-Aguilar, G.; Martínez-Tellez, A.M. Pectin-derived oligosaccharides increase color and anthocyanin content in flame seedless grapes. J. Sci. Food Agric. 2011, 91, 1928–1930. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-harvest treatment of chitosan oligosaccharides improved strawberry fruit quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef]
- Chen, F.; Li, Q.; Su, Y.; Lei, Y.; Zhang, C. Chitosan Spraying Enhances the Growth, Photosynthesis, and Resistance of Continuous Pinellia ternata and Promotes Its Yield and Quality. Molecules 2023, 28, 2053. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs Over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Wu, X.; Su, Y.; Long, Y. Co-Application of Tetramycin and Chitosan in Controlling Leaf Spot Disease of Kiwifruit and Enhancing Its Resistance, Photosynthesis, Quality and Amino Acids. Biomolecules 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Su, Y.; Wu, X. Association of Physcion and Chitosan Can Efficiently Control Powdery Mildew in Rosa roxburghii. Antibiotics 2022, 11, 1661. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. General Principles of Four Parts of Chinese Pharmacopoeia 2020; China Medical Science and Technology Press: Beijing, China, 2019. [Google Scholar]
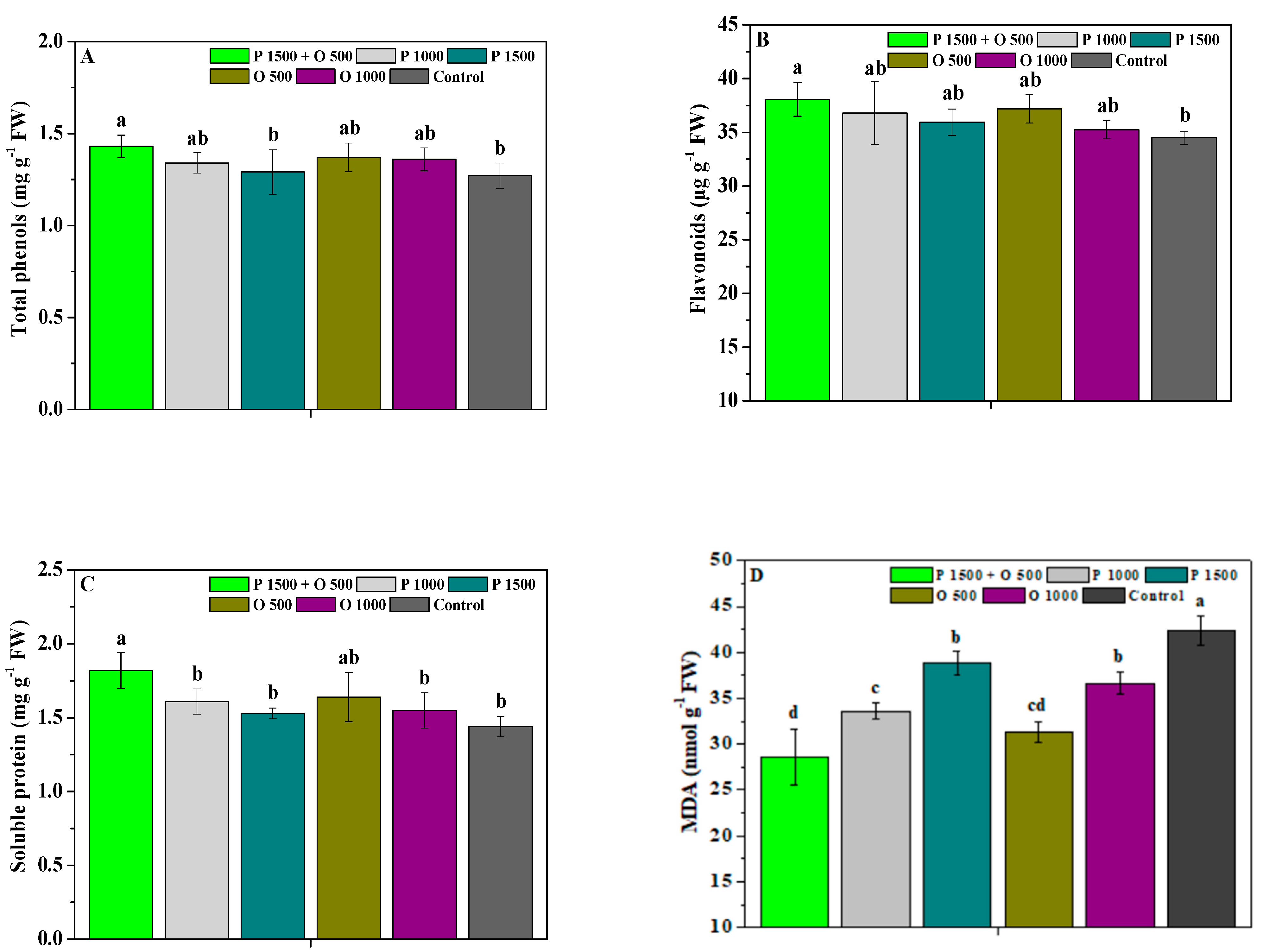

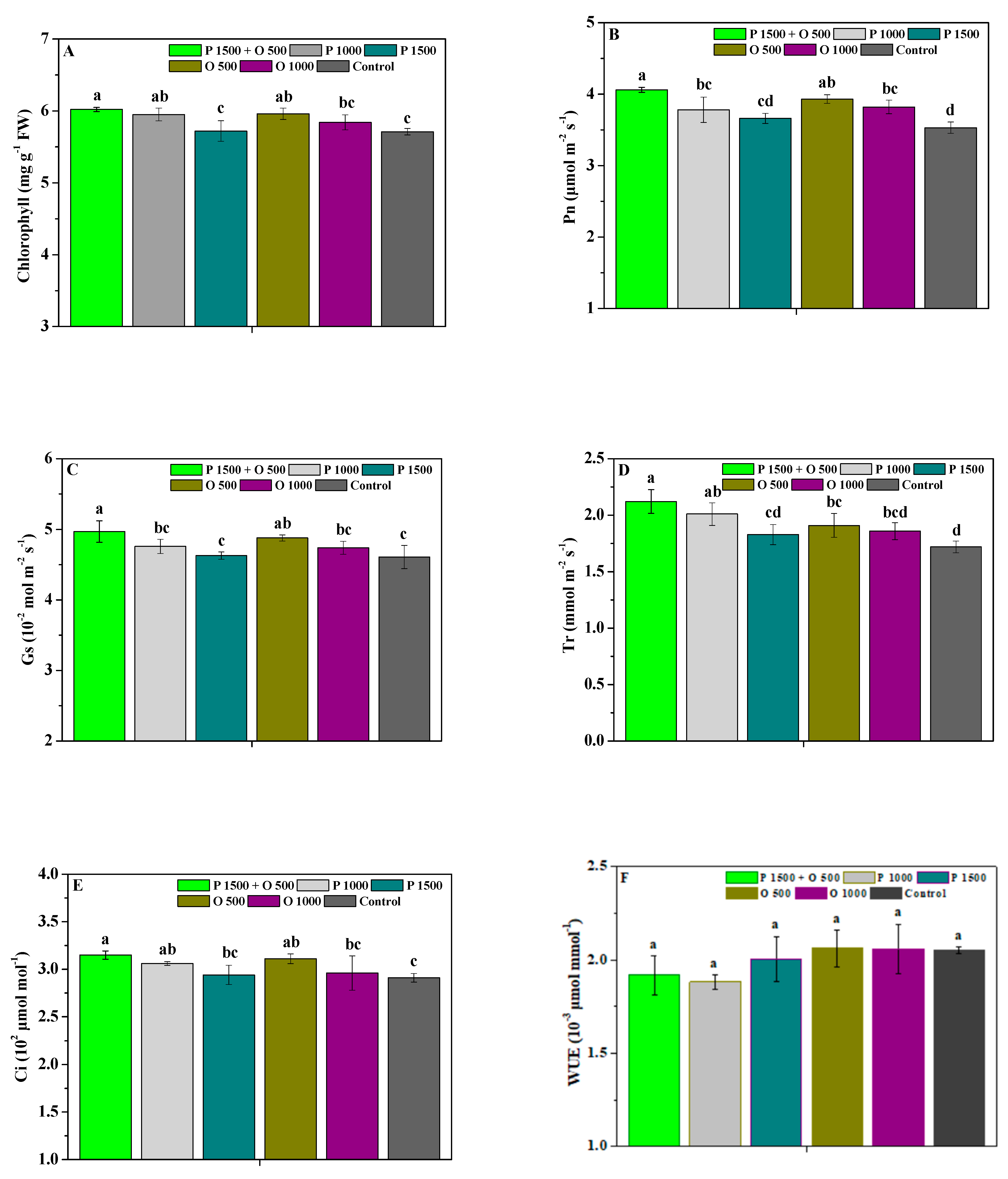
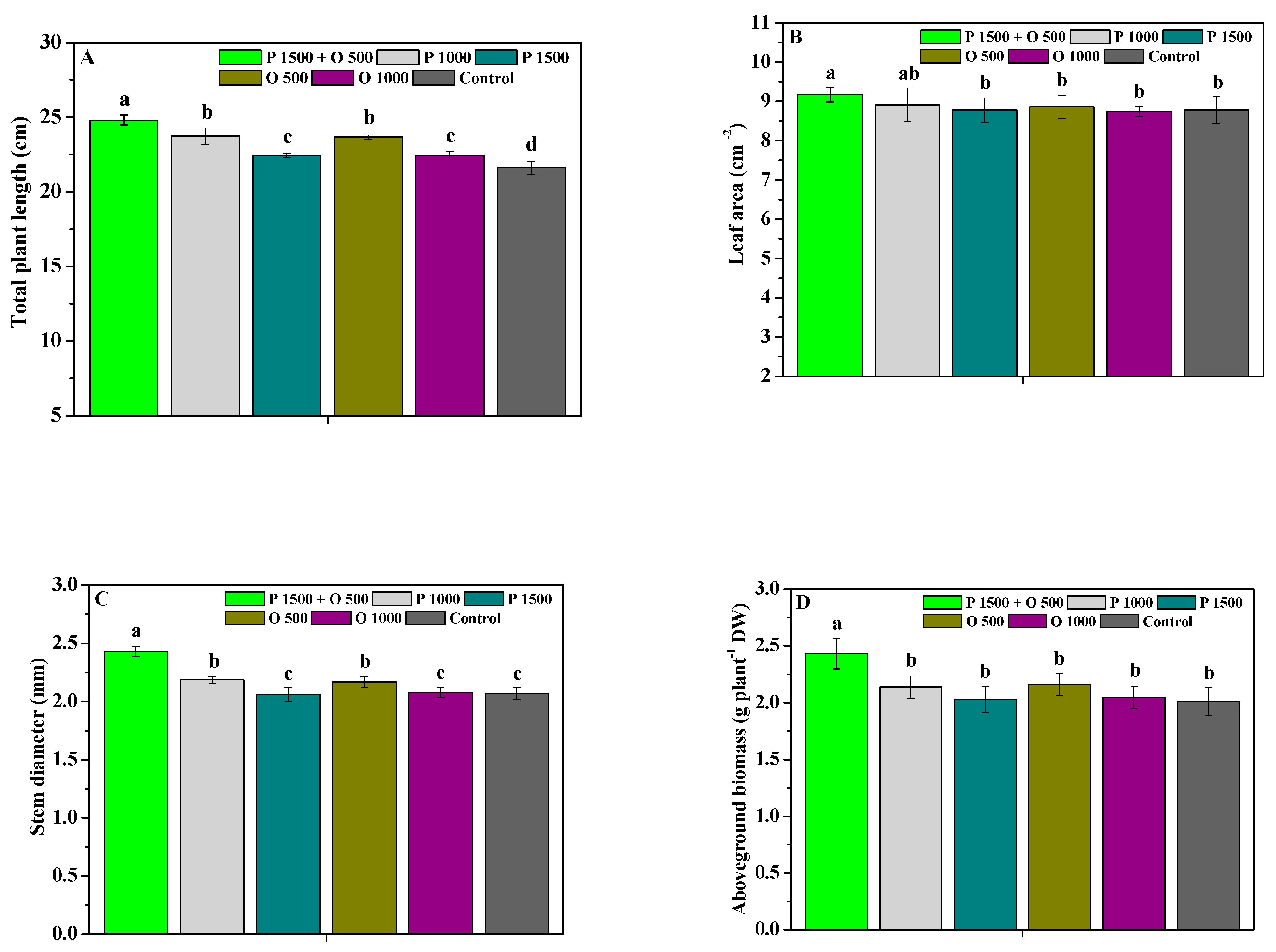
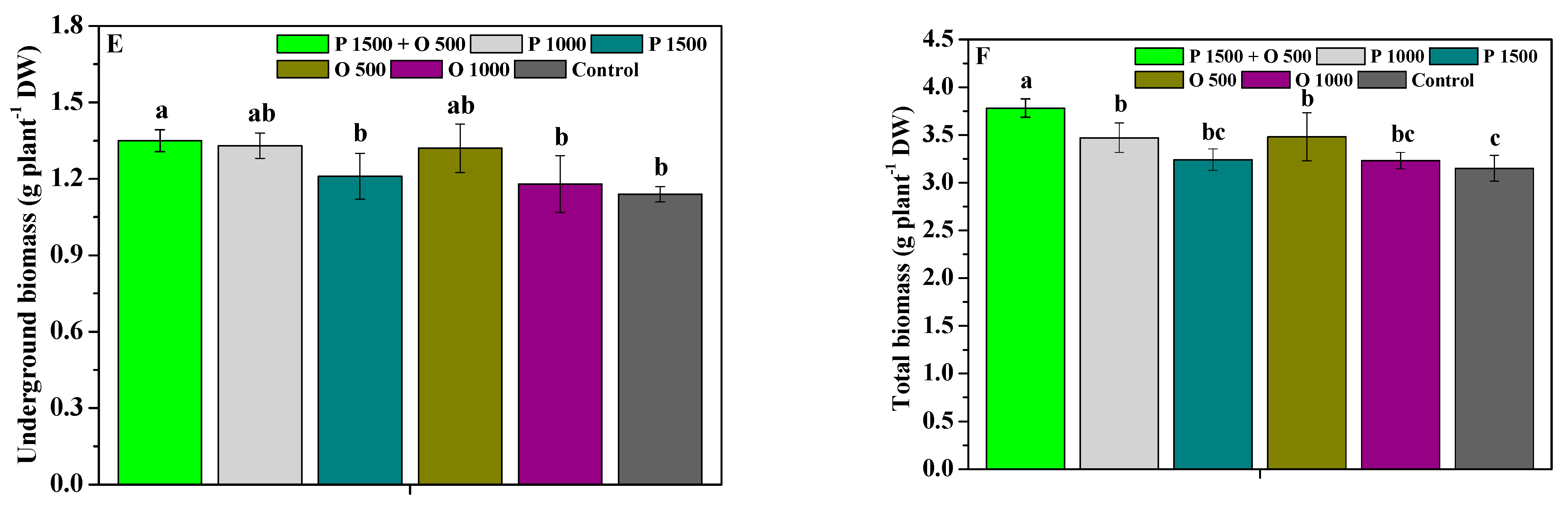


| Treatments | Applied Concentrations (g per 666.7 m2 Each Time) | 15 d after Spraying | 30 d after Spraying | ||
|---|---|---|---|---|---|
| Disease Index | Control Role (%) | Disease Index | Control Role (%) | ||
| P 1500 + O 500 | 12 g pyraclostrobin + 6 g oligochitosan | 0.68 ± 0.14 fD | 87.49 ± 2.27 aA | 0.98 ± 0.19 eE | 85.75 ± 2.21 aA |
| P 1000 | 18 g pyraclostrobin | 1.17 ± 0.24 eD | 78.45 ± 4.01 bA | 1.63 ± 0.21 dD | 76.17 ± 3.14 bB |
| P 1500 | 12 g pyraclostrobin | 1.75 ± 0.25 dC | 67.74 ± 4.03 cB | 2.31 ± 0.16 cC | 66.15 ± 3.91 cC |
| O 500 | 6 g oligochitosan | 2.34 ± 0.17 cB | 56.84 ± 0.67 dC | 2.85 ± 0.17 bBC | 58.31 ± 3.55 dCD |
| O 1000 | 3 g oligochitosan | 2.75 ± 0.15 bB | 49.03 ± 5.62 eC | 3.23 ± 0.23 bB | 52.86 ± 1.98 dD |
| Control | - | 5.42 ± 0.33 aA | 6.85 ± 0.24 aA | ||
| Soil Properties | Content | Soil Properties | Content (mg kg−1) |
|---|---|---|---|
| Temperature | 15.7 °C | Available zinc | 2.04 |
| Sunshine | 1188.7 h | Available phosphorus | 56.65 |
| Rainfall | 1004.4 mm | Available potassium | 129.72 |
| Frostless season | 325 days | Available iron | 8.42 |
| Average altitude | 1119 m | Available manganese | 18.25 |
| pH | 4.95 | Exchangeable magnesium | 288.68 |
| Organic matter | 36.04 g kg−1 | Available boron | 0.21 |
| Exchangeable calcium | 20.83 cmol kg−1 | Alkali hydrolyzed nitrogen | 114.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Tang, C.; Wang, Q.; Su, Y.; Zhang, Q. Synergistic Effects of Oligochitosan and Pyraclostrobin in Controlling Leaf Spot Disease in Pseudostellaria heterophylla. Antibiotics 2024, 13, 128. https://doi.org/10.3390/antibiotics13020128
Zhang C, Tang C, Wang Q, Su Y, Zhang Q. Synergistic Effects of Oligochitosan and Pyraclostrobin in Controlling Leaf Spot Disease in Pseudostellaria heterophylla. Antibiotics. 2024; 13(2):128. https://doi.org/10.3390/antibiotics13020128
Chicago/Turabian StyleZhang, Cheng, Chenglin Tang, Qiuping Wang, Yue Su, and Qinghai Zhang. 2024. "Synergistic Effects of Oligochitosan and Pyraclostrobin in Controlling Leaf Spot Disease in Pseudostellaria heterophylla" Antibiotics 13, no. 2: 128. https://doi.org/10.3390/antibiotics13020128
APA StyleZhang, C., Tang, C., Wang, Q., Su, Y., & Zhang, Q. (2024). Synergistic Effects of Oligochitosan and Pyraclostrobin in Controlling Leaf Spot Disease in Pseudostellaria heterophylla. Antibiotics, 13(2), 128. https://doi.org/10.3390/antibiotics13020128





