The Effect of Antimicrobial Peptide (PA-13) on Escherichia coli Carrying Antibiotic-Resistant Genes Isolated from Boar Semen
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Peptide Physicochemical Determination
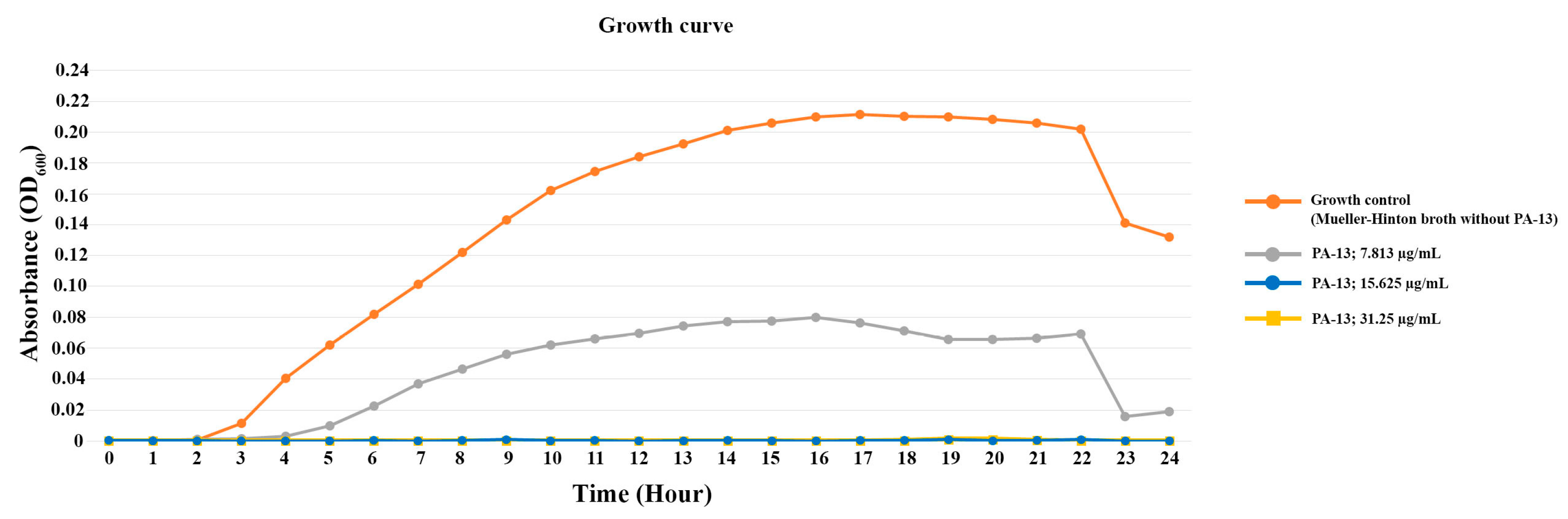
2.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.3. Leakage Assay
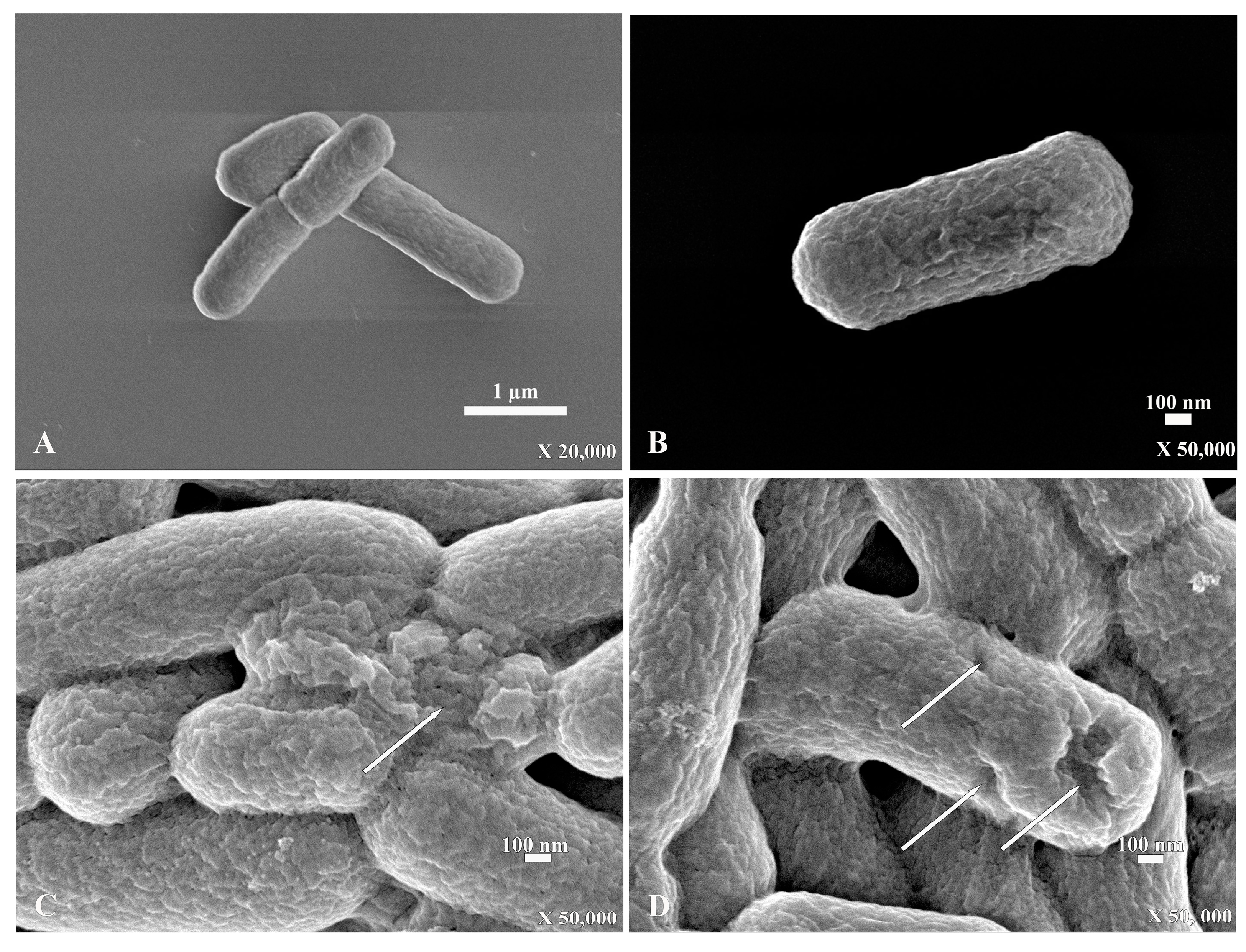
2.4. Scanning Electron Microscopy (SEM)
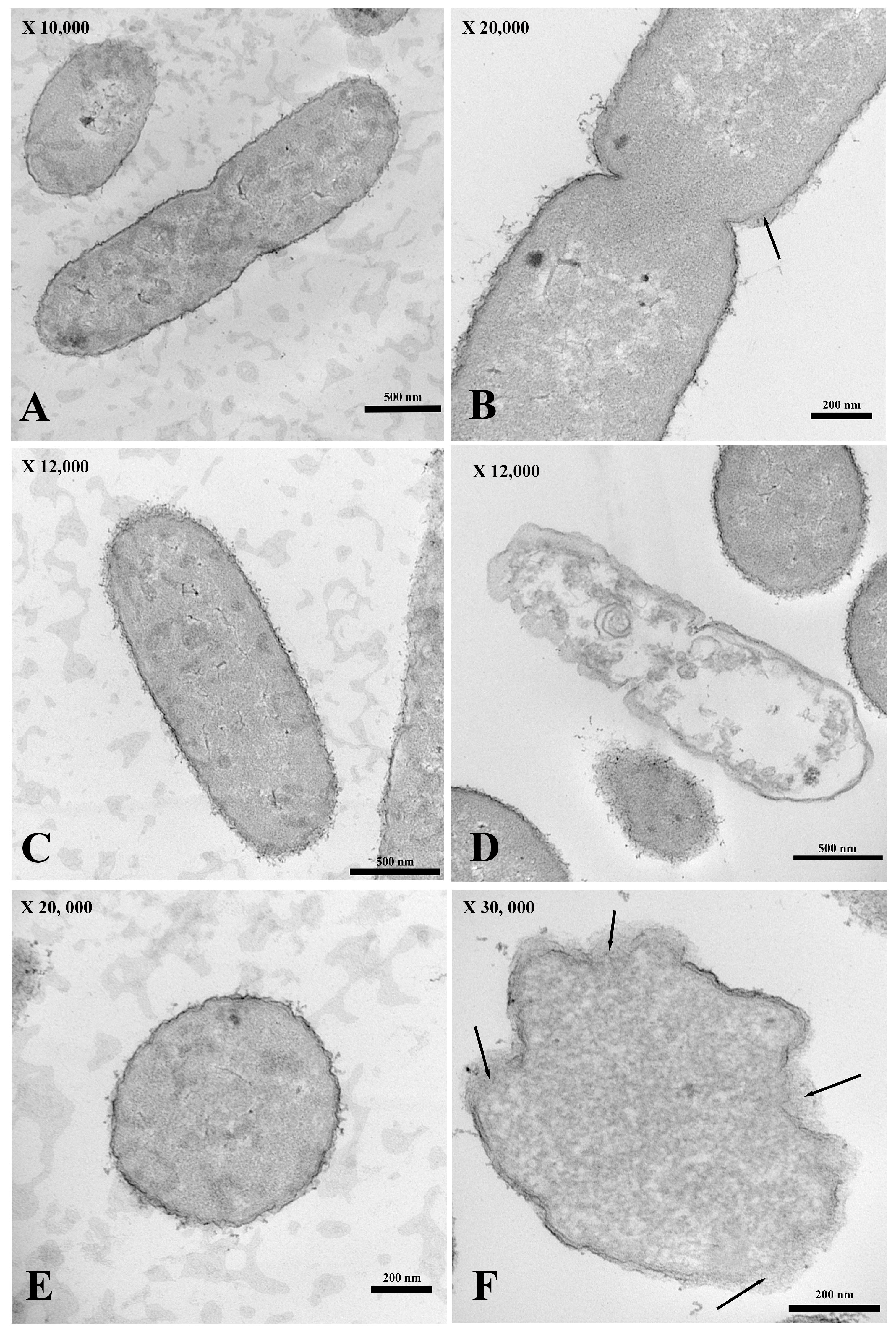
2.5. Transmission Electron Microscopy (TEM)
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Peptide Synthesis and Physicochemical Determination
4.3. Antimicrobial Activity
4.4. Bacterial Survival Assay
4.5. Leakage Assay
4.6. Scanning Electron Microscopy (SEM)
4.7. Transmission Electron Microscopy (TEM)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 17 November 2021).
- CDC. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services; CDC: Atlanta, GA, USA, 2019.
- Nguyet, L.T.Y.; Keeratikunakorn, K.; Kaeoket, K.; Ngamwongsatit, N. Antibiotic resistant Escherichia coli from diarrheic piglets from pig farms in Thailand that harbor colistin-resistant mcr genes. Sci. Rep. 2022, 12, 9083. [Google Scholar] [CrossRef] [PubMed]
- Tummaruk, P.; Kesdangsakonwut, S.; Prapasarakul, N.; Kaeoket, K. Endometritis in gilts: Reproductive data, bacterial culture, histopathology, and infiltration of immune cells in the endometrium. Comp. Clin. Path. 2010, 19, 575–584. [Google Scholar] [CrossRef]
- Kaewchomphunuch, T.; Charoenpichitnunt, T.; Thongbaiyai, V.; Ngamwongsatit, N.; Kaeoket, K. Cell-free culture supernatants of Lactobacillus spp. and Pediococcus spp. inhibit growth of pathogenic Escherichia coli isolated from pigs in Thailand. BMC Vet. Res. 2022, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Keeratikunakorn, K.; Kaewchomphunuch, T.; Kaeoket, K.; Ngamwongsatit, N. Antimicrobial activity of cell free supernatants from probiotics inhibits against pathogenic bacteria isolated from fresh boar semen. Sci. Rep. 2023, 13, 5995. [Google Scholar] [CrossRef] [PubMed]
- Farnum, D.; Riese, R.L. Urogenital infections in sows and gilts; differential diagnosis, diagnostic techniques and control. Iowa State Univ. Vet. 1989, 51, 98–102. [Google Scholar]
- de Winter, P.J.J.; Verdonck, M.; de Kruif, A.; Devriese, L.A.; Haesebrouck, F. Bacterial endometritis and vaginal discharge in the sow: Prevalence of different bacterial species and experimental reproduction of the syndrome. Anim. Reprod. Sci. 1995, 37, 325–335. [Google Scholar] [CrossRef]
- de Winter, P.J.J.; Verdonck, M.; de Kruif, A.; Devriese, L.A.; Haesebrouck, F. Endometritis and vaginal discharge in the sow. Anim. Reprod. Sci. 1992, 28, 51–58. [Google Scholar] [CrossRef]
- Dee, S.A. Porcine urogenital disease. Vet. Clin. N. Am. Food Anim. 1992, 8, 641–660. [Google Scholar] [CrossRef]
- Hussein, N.H.; Al-Kadmy, I.M.S.; Taha, B.M.; Hussein, J.D. Mobilized colistin resistance (mcr) genes from 1 to 10: A comprehensive review. Mol. Biol. Rep. 2020, 48, 2897–2907. [Google Scholar] [CrossRef]
- Morrell, J.M.; Nunez-Gonzalez, A.; Crespo-Felez, I.; Martinez-Martinez, S.; Alborcia, M.J.M.; Fernandez-Alegre, E.; Dominguez, J.; Gutiérrez-Martín, C.; Martínez-Pastor, F. Removal of bacteria from boar semen using a low-density colloid. Theriogenology 2019, 126, 272–278. [Google Scholar] [CrossRef]
- Jäkel, H.; Scheinpflug, K.; Mühldorfer, K.; Gianluppi, R.; Lucca, M.S.; Mellagi, A.G.; Bortolozzo, F.P.; Waberski, D. In vitro performance and in vivo fertility of antibiotic-free preserved boar semen stored at 5 °C. J. Anim. Sci. Biotechnol. 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Hensel, B.; Jakop, U.; Scheinpflug, K.; Mühldorfer, K.; Schröter, F.; Schäfer, J.; Greber, K.; Jung, M.; Schulze, M. Low temperature preservation of porcine semen: Influence of short antimicrobial lipopeptides on sperm quality and bacterial load. Sci. Rep. 2020, 10, 13225. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Czirjak, G.A.; Muller, K.; Bortfeldt, R.; Jung, M.; Jakop, U. Antibacterial defense and sperm quality in boar ejaculates. J. Reprod. Immunol. 2019, 131, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Shaoyong, W.K.; Li, Q.; Ren, Z.Q.; Xiao, J.Y.; Diao, Z.X.; Yang, G.S.; Pang, W. Effects of kojic acid on boar sperm quality and anti-bacterial activity during liquid preservation at 17 C. Theriogenology 2019, 140, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zietz, C.M.; Mudgapalli, A.; Wang, S.; Wang, Z. The evolution of the antimicrobial peptide database over 18 years: Milestones and new features. Protein Sci. 2022, 31, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, Y.X.; Shao, G.; Ma, J.J.; Cheng, X.R.; Lui, J.; Kang, J.; FU, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Seyedi, S.; Hoseini Goki, N.; Khameneh, B. Human antimicrobial peptides: Spectrum, mode of action and resistance mechanisms. Int. J. Pept. Res. 2021, 27, 801–816. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ali, S.A.; Singh, S.K.; Bhushan, V.; Mathur, M.; Jamwal, S.; Mohanty, A.K.; Kaushik, K.J.; Kumar, S. Antimicrobial peptides in farm animals: An updated review on its diversity, function, modes of action and therapeutic prospects. Vet. Sci. 2020, 7, 206. [Google Scholar] [CrossRef]
- Islam, M.M.; Asif, F.; Zaman, S.U.; Arnab, M.K.H.; Rahman, M.M.; Hasan, M. Effect of charge on the antimicrobial activity of alpha-helical amphibian antimicrobial peptide. Curr. Res. Microb. Sci. 2023, 4, 100182. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides-Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mechesso, A.F. Realistic and critical review of the state of systemic antimicrobial peptides. ADMET DMPK 2022, 10, 91–105. [Google Scholar] [CrossRef]
- Klubthawee, N.; Adisakwattana, P.; Hanpithakpong, W.; Somsri, S.; Aunpad, R. A novel, rationally designed, hybrid antimicrobial peptide, inspired by cathelicidin and aurein, exhibits membrane-active mechanisms against Pseudomonas aeruginosa. Sci. Rep. 2020, 10, 9117. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Q.; Cheng, Y.; Liu, R.; Zhao, R.; Wang, J.; Wang, Y.; Yang, S.; Chen, A. Effect of bacterial resistance of Escherichia coli from swine in large-scale pig farms in Beijing. Front. Microbiol. 2022, 13, 820833. [Google Scholar] [CrossRef] [PubMed]
- Pabón-Rodríguez, O.V.; López-López, K.; Casas-Bedoya, G.A.; Mogollón-Galvis, J.D.; Serna-Cock, L. Adhesion factors and antimicrobial resistance of Escherichia coli strains associated with colibacillosis in piglets in Colombia. Vet. World 2023, 16, 1231–1237. [Google Scholar] [CrossRef]
- Österberg, J.; Wingstrand, A.; Nygaard Jensen, A.; Kerouanton, A.; Cibin, V.; Barco, L.; Denis, M.; Aabo, S.; Bengtsson, B. Antibiotic resistance in Escherichia coli from pigs in organic and conventional farming in four European countries. PLoS ONE 2016, 11, e0157049. [Google Scholar] [CrossRef]
- Shi, J.; Chen, C.; Wang, D.; Wang, Z.; Liu, Y. The antimicrobial peptide LI14 combats multidrug-resistant bacterial infections. Commun. Biol. 2022, 5, 926. [Google Scholar] [CrossRef]
- Arthithanyaroj, S.; Chankhamhaengdecha, S.; Chaisri, U.; Aunpad, R.; Aroonnual, A. Effective inhibition of Clostridioides difficile by the novel peptide CM-A. PLoS ONE 2021, 16, e0257431. [Google Scholar] [CrossRef]
- Melo, M.N.; Ferre, R.; Castanho, M.A. Antimicrobial peptides: Linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009, 7, 245–250. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial peptides: A potent alternative to antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, S.; Gottlieb, C.T.; Vestergaard, M.; Hansen, P.R.; Gram, L.; Ingmer, H.; Thomsen, L.E. Amphibian antimicrobial peptide fallaxin analogue FL9 affects virulence gene expression and DNA replication in Staphylococcus aureus. J. Clin. Microbiol. 2015, 64, 1504–1513. [Google Scholar] [CrossRef]
- Polikanov, Y.S.; Aleksashin, N.A.; Beckert, B.; Wilson, D.N. The mechanisms of action of ribosome-targeting peptide antibiotics. Front. Mol. Biosci. 2018, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Rogozhin, E.A. Sub-inhibitory effects of antimicrobial peptides. Front. Microbiol. 2019, 10, 1160. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Baeder, D.Y.; Johnston, P.; Regoes, R.R.; Rolff, J. Bacteria primed by antimicrobial peptides develop tolerance and persist. PLoS Pathog. 2021, 17, e1009443. [Google Scholar] [CrossRef] [PubMed]
- López Cascales, J.J.; Zenak, S.; García de la Torre, J.; Lezama, O.G.; Garro, A.; Enriz, R.D. Small Cationic Peptides: Influence of Charge on Their Antimicrobial Activity. ACS Omega 2018, 3, 5390–5398. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Koh, J.J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef]
- Li, S.; She, P.; Zhou, L.; Zeng, X.; Xu, L.; Liu, Y.; Wu, Y. High-throughput identification of antibacterials against Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 591426. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Luther, A.M.; Nguyen, T.Q.; Verspohl, J.; Waberski, D. Antimicrobially active semen extenders allow the reduction of antibiotic use in pig insemination. Antibiotics 2021, 10, 1319. [Google Scholar] [CrossRef]
- Naik, L.S.; Ramana Devi, C.V. Phyto-fabricated silver nanoparticles inducing microbial cell death via reactive oxygen species-mediated membrane damage. IET Nanobiotechnol. 2021, 15, 492–504. [Google Scholar] [CrossRef]
- Phanchana, M.; Phetruen, T.; Harnvoravongchai, P.; Raksat, P.; Ounjai, P.; Chankhamhaengdecha, S.; Janvilisri, T. Repurposing a platelet aggregation inhibitor ticagrelor as an antimicrobial against Clostridioides difficile. Sci. Rep. 2020, 10, 6497. [Google Scholar] [CrossRef]

| Peptide | Amino Acid Sequence | Number of Amino Acids | Molecular Weight (g/mol) | Net Charge | Hydrophobicity | Percentage of Hydrophobic Residues |
|---|---|---|---|---|---|---|
| PA-13 | KIAKRIWKILRRR | 13 | 1736.25 | +7 | 0.678 | 46% |
| Sample | Antibiotic-Resistant Genes | Toxin Gene | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|---|
| E. coli ATCC 25922 | - | - | 7.813 | 7.813 |
| E. coli from boar semen | int1, mcr-3 | - | 15.625 | 15.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keeratikunakorn, K.; Aunpad, R.; Ngamwongsatit, N.; Kaeoket, K. The Effect of Antimicrobial Peptide (PA-13) on Escherichia coli Carrying Antibiotic-Resistant Genes Isolated from Boar Semen. Antibiotics 2024, 13, 138. https://doi.org/10.3390/antibiotics13020138
Keeratikunakorn K, Aunpad R, Ngamwongsatit N, Kaeoket K. The Effect of Antimicrobial Peptide (PA-13) on Escherichia coli Carrying Antibiotic-Resistant Genes Isolated from Boar Semen. Antibiotics. 2024; 13(2):138. https://doi.org/10.3390/antibiotics13020138
Chicago/Turabian StyleKeeratikunakorn, Krittika, Ratchaneewan Aunpad, Natharin Ngamwongsatit, and Kampon Kaeoket. 2024. "The Effect of Antimicrobial Peptide (PA-13) on Escherichia coli Carrying Antibiotic-Resistant Genes Isolated from Boar Semen" Antibiotics 13, no. 2: 138. https://doi.org/10.3390/antibiotics13020138






