Synthesis of Novel Artemisinin, Ciprofloxacin, and Norfloxacin Hybrids with Potent Antiplasmodial Activity
Abstract
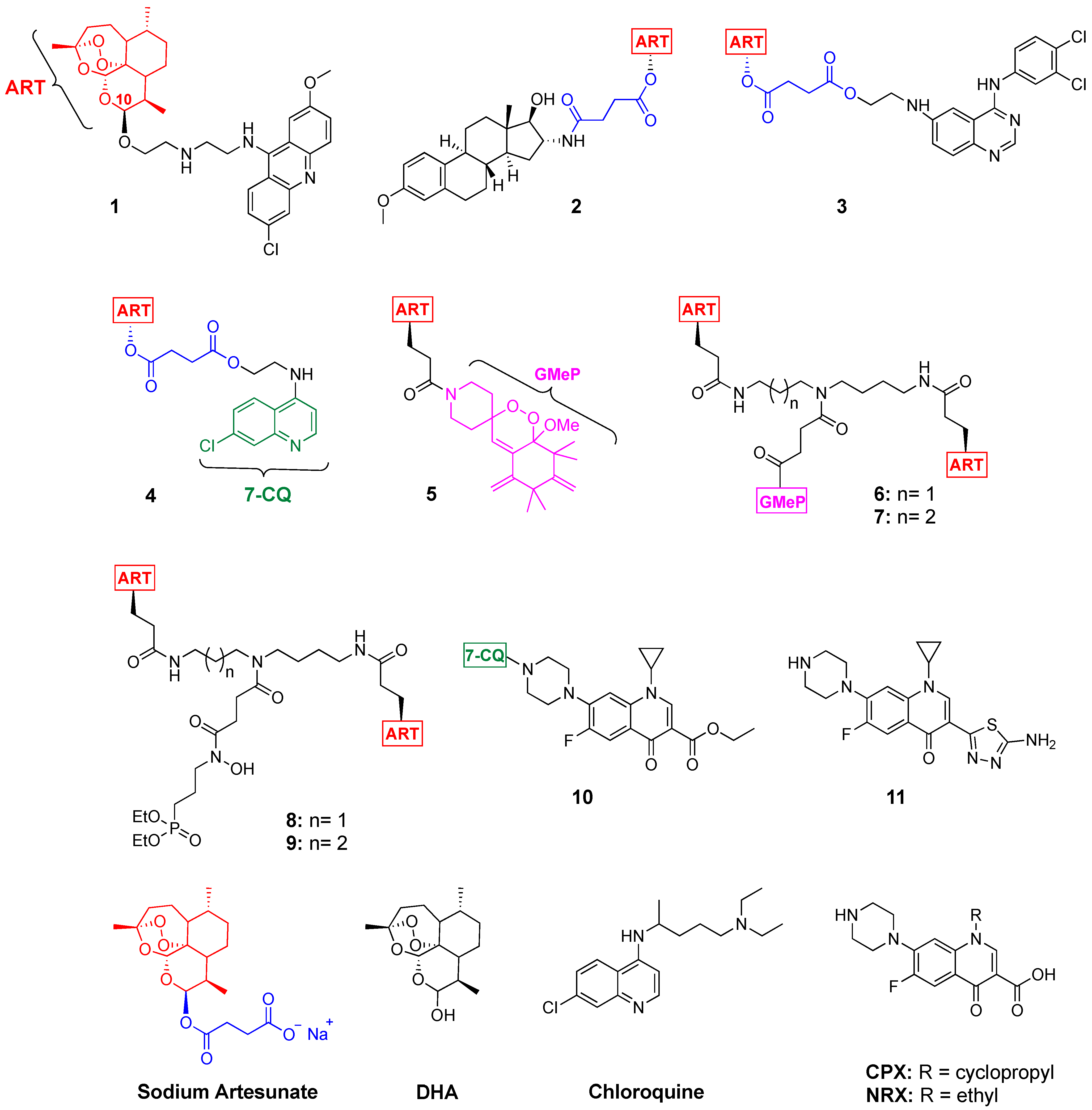
1. Introduction
2. Results and Discussion
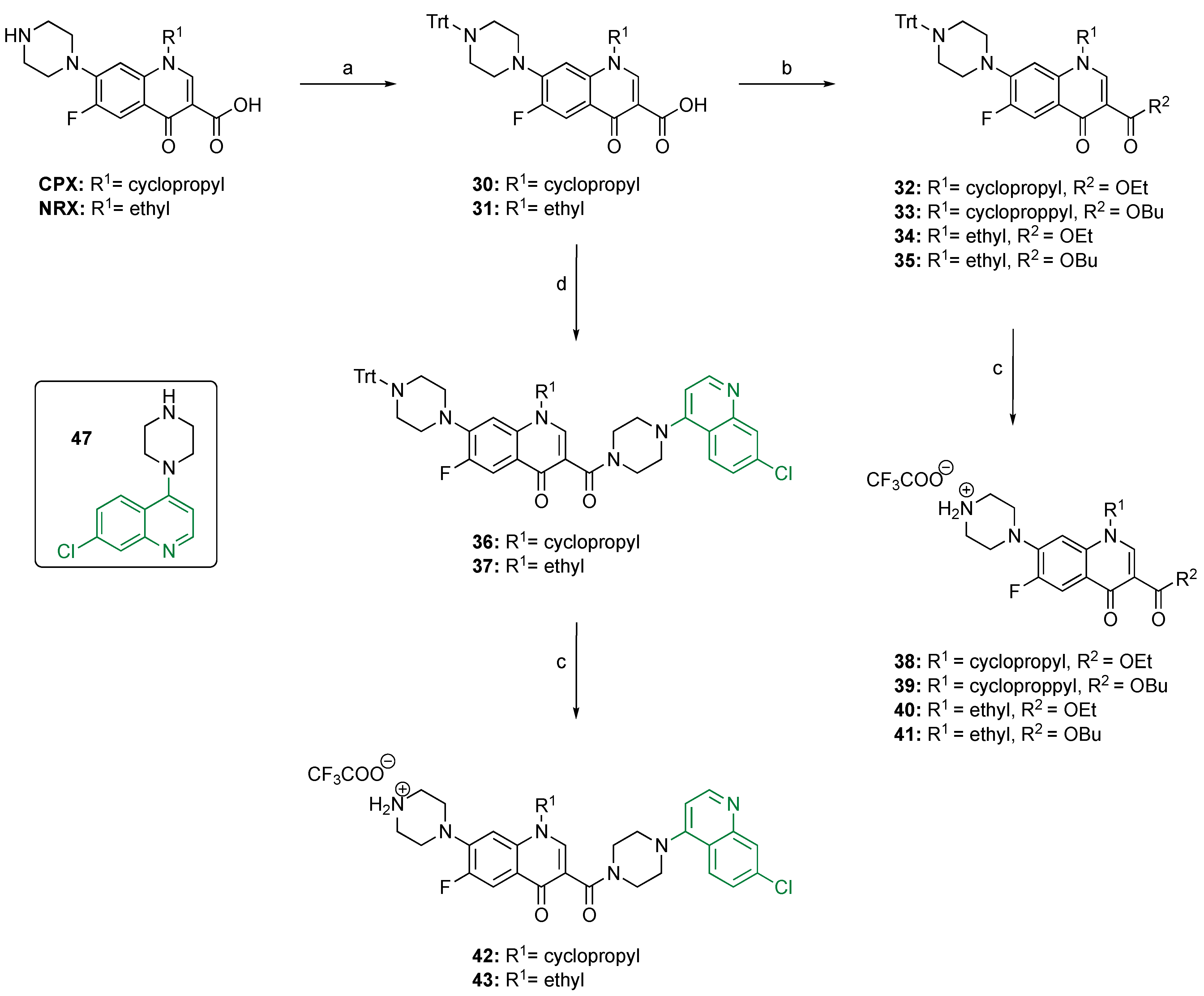
2.1. Synthesis of the Key Intermediates 38–43
2.2. Synthesis of the ART– and AS– CPX or NRX Hybrids 14–19 and 21–24
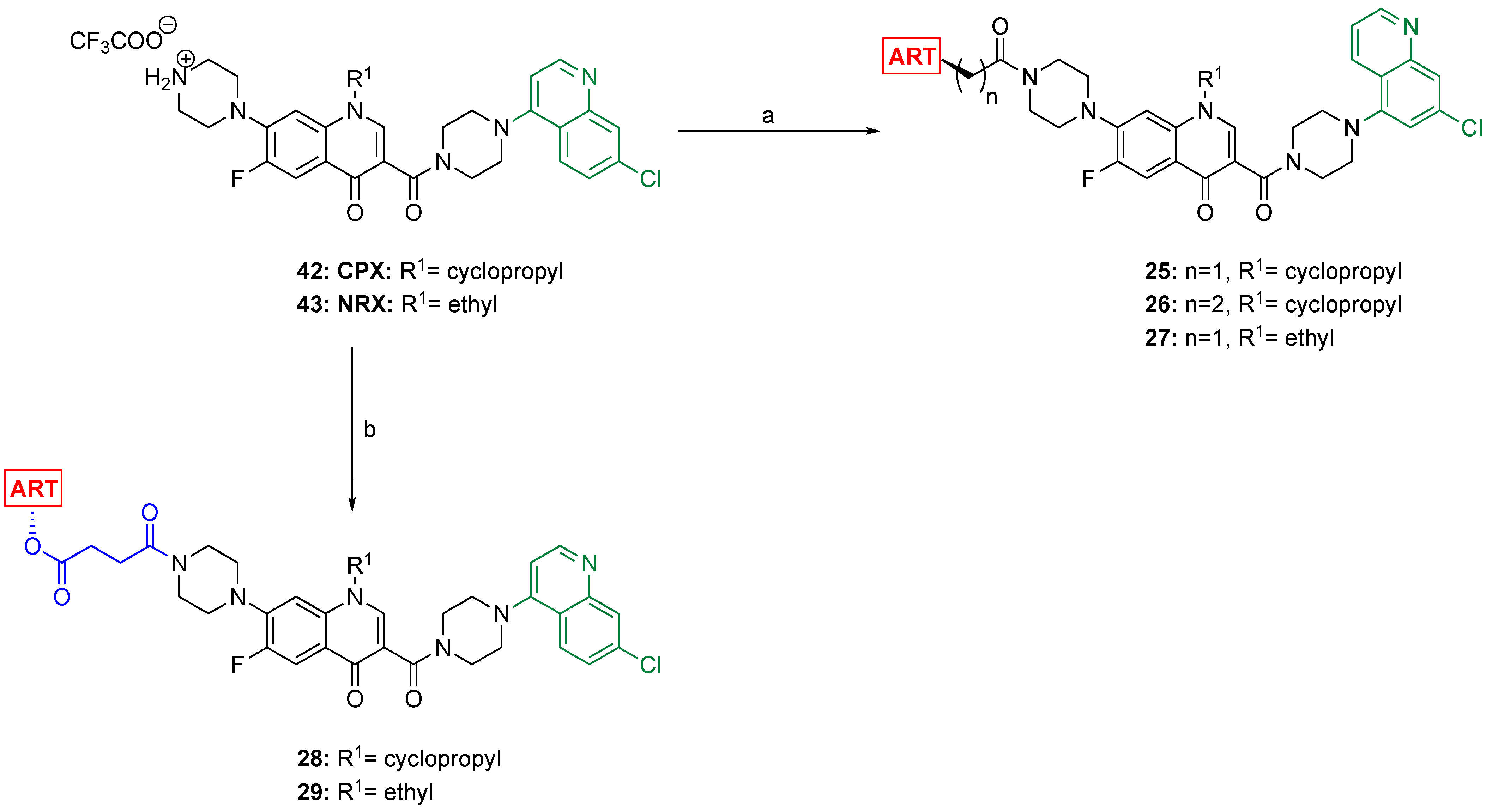
2.3. Synthesis of the ART–Drug–(7-CQ) Hybrids 25–29
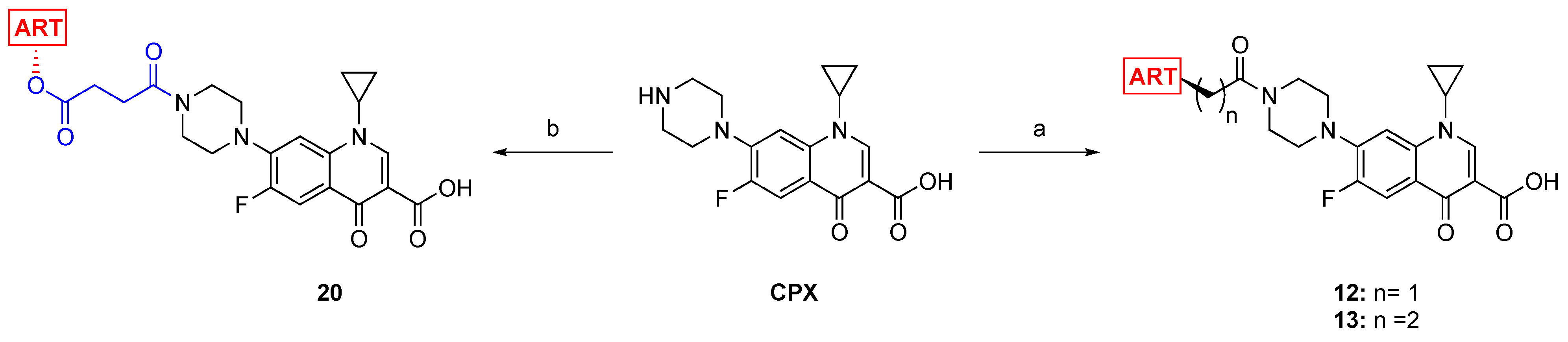
2.4. Synthesis of the ART–CPX and AS–CPX Hybrids 12, 13, and 20
2.5. Biological Investigation
| Entry | Compound | IC50 FcB1 (nM) | IC50 upon Fibroblasts AB943 (µM) a | Selectivity Index (IC50 AB943/IC50 FcB1) |
|---|---|---|---|---|
| 1 | CPX | 54,100 b | n.d. | |
| 2 | 12 | 14.8 +/− 1.9 c | 66.1 +/− 5.0 | 4453 |
| 3 | 13 | 2906 b | n.d. | |
| 4 | 14 | 121.3 b | n.d. | |
| 5 | 15 | 1466 b | n.d. | |
| 6 | 16 | 7.5 +/− 0.6 c | 14.9 +/− 3.8 | 2000 |
| 7 | 17 | 362.0 b | n.d. | |
| 8 | 20 | 4.0 +/− 0.4 c | 41.3 +/− 11.6 | 10,318 |
| 9 | 21 | 5.4 +/− 0.4 c | 52.8 +/− 1.0 | 9826 |
| 10 | 22 | 3.5 +/− 0.4 c | 38.9 +/− 10. | 11,303 |
| 11 | 25 | 7.5 +/− 2.5 c | 3.9 +/− 2.2 | 521 |
| 12 | 26 | 13.7 +/− 2.2 c | 36.5 +/− 9.4 | 2668 |
| 13 | 28 | 4.9 +/− 0.4 c | 19.6 +/− 7.5 | 4046 |
| 14 | 32 | 41,900 b | n.d. | |
| 15 | 33 | 3900 b | n.d. | |
| 16 | 36 | 26,500 b | n.d. | |
| 17 | 38 | 53,600 b | n.d. | |
| 18 | 39 | 13,600 b | n.d. | |
| 19 | 42 | 359.9 b | n.d. | |
| 20 | 44 | 401.4 b | n.d. | |
| 21 | 45 | 264.4 b | n.d. | |
| 22 | AS (46) | 18.5 +/− 3.1 c | >130.0 | >7042 |
| Entry | Compound | IC50 FcB1 (nM) | IC50 upon Fibroblast AB943 (µM) a | Selectivity Index (IC50 AB943/IC50 FcB1 |
|---|---|---|---|---|
| 1 | NRX | 81,410 b | n.d. | |
| 2 | 18 | 25.5 +/− 7.5 c | 54.3 +/− 12.4 | 2129 |
| 3 | 19 | 13.9 +/− 2.5 c | 18.0 +/− 2.3 | 1294 |
| 4 | 23 | 1.5 +/− 0.1 c | 43.7 +/− 14.7 | 28,382 |
| 5 | 24 | 1.9 +/− 0.3 c | 30.3 +/− 9.6 | 16,047 |
| 6 | 27 | 6.6 +/− 1.5 c | n.d. | |
| 7 | 29 | 5.0 +/− 0.8 c | 30.3 +/− 9.1 | 6027 |
| 8 | 34 | 84,787 b | n.d. | |
| 9 | 35 | 26,220 b | n.d. | |
| 10 | 37 | 184.1 +/− 22.6 c | n.d. | |
| 11 | 40 | 19,670 b | n.d. | |
| 12 | 41 | 13,890 b | n.d. | |
| 13 | 43 | 476.4 +/− 45.5 c | 12.7 +/− 4.2 | 27 |
| 14 | 44 | 401.4 b | n.d. | |
| 15 | 45 | 264.4 b | n.d. | |
| 16 | AS (46) | 18.5 +/− 3.1 c | >130.0 | >7042 |
| Entry | CPX-Comp. | IC50 FcB1 (nM) a | IC50 Dd2 (nM) a | IC50 upon Fibroblast AB943 (µM) a | Selectivity Index (IC50 AB943/IC50 FcB1) | Selectivity Index (IC50 AB943/IC50 Dd2) |
|---|---|---|---|---|---|---|
| 1 | 12 | 14.8 +/− 1.9 | 32.5 +/− 1.9 | 66.1 +/− 5.0 | 4453 | 2034 |
| 2 | 16 | 7.5 +/− 0.6 | 24.0 +/− 4.2 | 14.9 +/− 3.8 | 2000 | 622 |
| 3 | 20 | 4.0 +/− 0.4 | 8.0 +/− 0.4 | 41.3 +/− 11.6 | 10,318 | 5146 |
| 4 | 21 | 5.4 +/− 0.4 | 16.0 +/− 1.4 | 52.8 +/− 1.0 | 9826 | 3302 |
| 5 | 22 | 3.5 +/− 0.4 | 13.0 +/− 0.8 | 38.9 +/− 10.0 | 11,303 | 3002 |
| 6 | 25 | 7.5 +/− 2.5 | 17.7 +/− 2.0 | 3.9 +/− 2.2 | 521 | 220 |
| 7 | 26 | 13.7 +/− 2.2 | 41.5 +/− 2.5 | 36.5 +/− 9.4 | 2668 | 880 |
| 8 | 28 | 4.9 +/− 0.4 | 12.4 +/− 0.8 | 19.6 +/− 7.5 | 4046 | 1582 |
| 9 | AS (46) | 18.5 +/− 3.1 | 41.9 +/− 6.7 | >130.0 | >7042 | >3104 |
| Entry | NRX-Comp. | IC50 FcB1 (nM) a | IC50 Dd2 (nM) a | IC50 upon Fibroblast AB943 (µM) a | Selectivity Index (IC50 AB943/IC50 FcB1) | Selectivity Index (IC50 AB943/IC50 Dd2) |
|---|---|---|---|---|---|---|
| 1 | 18 | 25.5 +/− 7.5 | 58.7 +/− 10.8 | 54.3 +/− 12,4 | 2129 | 925 |
| 2 | 19 | 13.9 +/− 2.5 | 38.4 +/− 7.0 | 18.0 +/− 2.3 | 1294 | 469 |
| 3 | 23 | 1.5 +/− 0.1 | 3.5 +/− 0.7 | 43.7 +/− 14.7 | 28,382 | 12,488 |
| 4 | 24 | 1.9 +/− 0.3 | 9.2 +/− 3.5 | 30.3 +/− 9.6 | 16,047 | 3311 |
| 5 | 27 | 6.6 +/− 1.5 | 28.5 +/− 13.1 | n.d. | ||
| 6 | 29 | 5.0 +/− 0.8 | 18.2 +/− 9.1 | 30.3 +/− 9.1 | 6027 | 1658 |
| 7 | 37 | 184.1 +/− 22.6 | 307.3 +/− 171.4 | n.d. | ||
| 8 | 43 | 476.4 +/− 45.5 | 482.6 +/− 8.4 | 12.7 +/− 4.2 | 27 | 26 |
| 9 | AS (46) | 18.5 +/− 3.1 | 41.9 +/− 6.7 | >130.0 | >7042 | >3104 |
3. Materials and Methods
3.1. General Methods
3.2. Experimental Procedures
3.2.1. Synthesis of the N-Trt-Protected CPX 30 and NRX 31
3.2.2. Synthesis of the Ethyl and Butyl Ester of CPX (32 and 33) and NRX (34 and 35)
3.2.3. Synthesis of the Piperazine–Quinoline–CPX and Piperazine–Quinoline–NRX Conjugates 36 and 37, Respectively
3.2.4. Deprotection of Compounds 32–37
3.2.5. General Procedure for the Synthesis of the Hybrids 12–14, 20, and 21
3.2.6. General Procedure for the Synthesis of the Hybrids 15–19 and 22–29
3.3. Biological Evaluation
3.3.1. Cytototoxicity upon Human Primary fibroblast Cell Line AB934
3.3.2. In Vitro Growth Inhibition of P. falciparum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, P.G.; Ward, S.A.; O’Neill, P.M. Quinolines and Artemisinin: Chemistry, Biology and History BT-Malaria: Drugs, Disease and Post-Genomic Biology; Compans, R.W., Cooper, M.D., Honjo, T., Koprowski, H., Melchers, F., Oldstone, M.B.A., Olsnes, S., Potter, M., Vogt, P.K., Wagner, H., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 3–38. ISBN 978-3-540-29088-9. [Google Scholar]
- Shalini; Kumar, V. Have molecular hybrids delivered effective anti-cancer treatments and what should future drug discovery focus on? Expert Opin. Drug Discov. 2021, 16, 335–363. [Google Scholar] [CrossRef]
- Prasad Raiguru, B.; Panda, J.; Mohapatra, S.; Nayak, S. Recent developments in the synthesis of hybrid antimalarial drug discovery. Bioorg. Chem. 2023, 139, 106706. [Google Scholar] [CrossRef]
- Uddin, A.; Chawla, M.; Irfan, I.; Mahajan, S.; Singh, S.; Abid, M. Medicinal chemistry updates on quinoline- and endoperoxide-based hybrids with potent antimalarial activity. RSC Med. Chem. 2021, 12, 24–42. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Gao, C.; Zhang, S.; Xu, L.; Xu, Z.; Feng, L.-S.; Wu, X.; Zhao, F. Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2017, 139, 22–47. [Google Scholar] [CrossRef]
- Walsh, J.J.; Coughlan, D.; Heneghan, N.; Gaynor, C.; Bell, A. A novel artemisinin-quinine hybrid with potent antimalarial activity. Bioorg. Med. Chem. Lett. 2007, 17, 3599–3602. [Google Scholar] [CrossRef]
- Joubert, J.P.; Smit, F.J.; du Plessis, L.; Smith, P.J.; N’Da, D.D. Synthesis and in vitro biological evaluation of aminoacridines and artemisinin-acridine hybrids. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2014, 56, 16–27. [Google Scholar] [CrossRef]
- Fröhlich, T.; Kiss, A.; Wölfling, J.; Mernyák, E.; Kulmány, Á.E.; Minorics, R.; Zupkó, I.; Leidenberger, M.; Friedrich, O.; Kappes, B.; et al. Synthesis of Artemisinin–Estrogen Hybrids Highly Active against HCMV, P. falciparum, and Cervical and Breast Cancer. ACS Med. Chem. Lett. 2018, 9, 1128–1133. [Google Scholar] [CrossRef]
- Fröhlich, T.; Reiter, C.; Ibrahim, M.M.; Beutel, J.; Hutterer, C.; Zeitträger, I.; Bahsi, H.; Leidenberger, M.; Friedrich, O.; Kappes, B.; et al. Synthesis of Novel Hybrids of Quinazoline and Artemisinin with High Activities against Plasmodium falciparum, Human Cytomegalovirus, and Leukemia Cells. ACS Omega 2017, 2, 2422–2431. [Google Scholar] [CrossRef]
- Çapcı, A.; Lorion, M.M.; Wang, H.; Simon, N.; Leidenberger, M.; Borges Silva, M.C.; Moreira, D.R.M.; Zhu, Y.; Meng, Y.; Chen, J.Y.; et al. Artemisinin-(Iso)quinoline Hybrids by C-H Activation and Click Chemistry: Combating Multidrug-Resistant Malaria. Angew. Chem. Int. Ed. Engl. 2019, 58, 13066–13079. [Google Scholar] [CrossRef]
- Pepe, D.A.; Toumpa, D.; André-Barrès, C.; Menendez, C.; Mouray, E.; Baltas, M.; Grellier, P.; Papaioannou, D.; Athanassopoulos, C.M. Synthesis of Novel G Factor or Chloroquine-Artemisinin Hybrids and Conjugates with Potent Antiplasmodial Activity. ACS Med. Chem. Lett. 2020, 11, 921–927. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M. The MEP pathway: A new target for the development of herbicides, antibiotics and antimalarial drugs. Curr. Pharm. Des. 2004, 10, 2391–2400. [Google Scholar] [CrossRef]
- Lienau, C.; Konzuch, S.; Gräwert, T.; Illarionov, B.; Bacher, A.; Fischer, M.; Tanaka, N.; Kurz, T. Inhibition of the Non-Mevalonate Isoprenoid Pathway by Reverse Hydroxamate Analogues of Fosmidomycin. Procedia Chem. 2015, 14, 108–116. [Google Scholar] [CrossRef][Green Version]
- Fan, Y.-L.; Cheng, X.-W.; Wu, J.-B.; Liu, M.; Zhang, F.-Z.; Xu, Z.; Feng, L.-S. Antiplasmodial and antimalarial activities of quinolone derivatives: An overview. Eur. J. Med. Chem. 2018, 146, 1–14. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.-L. Ciprofloxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef]
- Patel, K.B.; Kumari, P. A review: Structure-activity relationship and antibacterial activities of Quinoline based hybrids. J. Mol. Struct. 2022, 1268, 133634. [Google Scholar] [CrossRef]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef]
- Cilliers, P.; Seldon, R.; Smit, F.J.; Aucamp, J.; Jordaan, A.; Warner, D.F.; N’Da, D.D. Design, synthesis, and antimycobacterial activity of novel ciprofloxacin derivatives. Chem. Biol. Drug Des. 2019, 94, 1518–1536. [Google Scholar] [CrossRef]
- Asif, M.; Husain, A.; Ahmad, A.; Khan, S.; Rashid, M.; Arora, K.; Bahl, D.; Iram, F. An overview on antitubercular activities of fluoroquinolones and other related analogues. Eur. J. Exp. Biol. 2015, 5, 96–109. [Google Scholar]
- Pasternak, J.; Rajtar, B.; Stec, A.; Polz-Dacewicz, M. Antiviral activity of ciprofloxacin on BK virus in an in vitro culture–Pilot study. J. Pre-Clin. Clin. Res. 2017, 11, 116–119. [Google Scholar] [CrossRef][Green Version]
- Scroggs, S.L.P.; Offerdahl, D.K.; Flather, D.P.; Morris, C.N.; Kendall, B.L.; Broeckel, R.M.; Beare, P.A.; Bloom, M.E. Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV. Viruses 2021, 13, 8. [Google Scholar] [CrossRef]
- Gao, F.; Sun, Z.; Kong, F.; Xiao, J. Artemisinin-derived hybrids and their anticancer activity. Eur. J. Med. Chem. 2020, 188, 112044. [Google Scholar] [CrossRef]
- Kloskowski, T.; Szeliski, K.; Fekner, Z.; Rasmus, M.; Dąbrowski, P.; Wolska, A.; Siedlecka, N.; Adamowicz, J.; Drewa, T.; Pokrywczyńska, M. Ciprofloxacin and Levofloxacin as Potential Drugs in Genitourinary Cancer Treatment—The Effect of Dose–Response on 2D and 3D Cell Cultures. Int. J. Mol. Sci. 2021, 22, 11970. [Google Scholar] [CrossRef]
- Gupta, P.; Gao, H.-L.; Ashar, Y.V.; Karadkhelkar, N.M.; Yoganathan, S.; Chen, Z.-S. Ciprofloxacin Enhances the Chemosensitivity of Cancer Cells to ABCB1 Substrates. Int. J. Mol. Sci. 2019, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Sączewski, J. Modifications of quinolones and fluoroquinolones: Hybrid compounds and dual-action molecules. Monatshefte Chem.-Chem. Mon. 2018, 149, 1199–1245. [Google Scholar] [CrossRef] [PubMed]
- Suaifan, G.A.R.Y.; Mohammed, A.A.M. Fluoroquinolones structural and medicinal developments (2013–2018): Where are we now? Bioorg. Med. Chem. 2019, 27, 3005–3060. [Google Scholar] [CrossRef]
- Mokaber-Esfahani, M.; Eshghi, H.; Akbarzadeh, M.; Gholizadeh, M.; Mirzaie, Y.; Hakimi, M.; Lari, J. Synthesis and Antibacterial Evaluation of New Pyrimidyl N-Ciprofloxacin Derivatives. ChemistrySelect 2019, 4, 8930–8933. [Google Scholar] [CrossRef]
- Dubar, F.; Anquetin, G.; Pradines, B.; Dive, D.; Khalife, J.; Biot, C. Enhancement of the Antimalarial Activity of Ciprofloxacin Using a Double Prodrug/Bioorganometallic Approach. J. Med. Chem. 2009, 52, 7954–7957. [Google Scholar] [CrossRef]
- Sadeghi, M.; Sarvi, S.; Emami, S.; Khalilian, A.; Hosseini, S.A.; Montazeri, M.; Shahdin, S.; Nayeri, T.; Daryani, A. Evaluation of anti-parasitic activities of new quinolones containing nitrofuran moiety against Toxoplasma gondii. Exp. Parasitol. 2022, 240, 108344. [Google Scholar] [CrossRef]
- Azéma, J.; Guidetti, B.; Korolyov, A.; Kiss, R.; Roques, C.; Constant, P.; Daffé, M.; Malet-Martino, M. Synthesis of lipophilic dimeric C-7/C-7-linked ciprofloxacin and C-6/C-6-linked levofloxacin derivatives. Versatile in vitro biological evaluations of monomeric and dimeric fluoroquinolone derivatives as potential antitumor, antibacterial or antimycobacte. Eur. J. Med. Chem. 2011, 46, 6025–6038. [Google Scholar] [CrossRef]
- Dana, S.; Valissery, P.; Kumar, S.; Gurung, S.K.; Mondal, N.; Dhar, S.K.; Mukhopadhyay, P. Synthesis of Novel Ciprofloxacin-Based Hybrid Molecules toward Potent Antimalarial Activity. ACS Med. Chem. Lett. 2020, 11, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Osman, E.O.; Attia, H.; Samir, R.; Mahmoud, Z. Design, synthesis, and antibacterial activity of a new series of ciprofloxacin-thiadiazole hybrid. J. Mol. Struct. 2023, 1282, 135135. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; dos Reis, D.B.; Reis, I.F.; de Carvalho, A.N.; Lourenço, M.C.S.; de Souza, M.V.N.; Pinheiro, A.C.; Saraiva, M.F. Synthesis and antimycobacterial evaluation of fluoroquinolones derivatives coupled with isoprenyl moiety at the C-7 position. Med. Chem. Res. 2022, 31, 949–959. [Google Scholar] [CrossRef]
- Ruiz, J.; Azema, J.; Payrastre, C.; Baltas, M.; Tuccio, B.; Vial, H.; Andre-Barres, C. Antimalarial bicyclic peroxides belonging to the G-factor family: Mechanistic aspects of their formation and iron (II) induced reduction. Curr. Top. Med. Chem. 2014, 14, 1668–1683. [Google Scholar] [CrossRef]
- Presser, A.; Feichtinger, A.; Buzzi, S. A simplified and scalable synthesis of artesunate. Monatshefte Chem.-Chem. Mon. 2017, 148, 63–68. [Google Scholar] [CrossRef]
- Palla, D.; Antoniou, A.I.; Baltas, M.; Menendez, C.; Grellier, P.; Mouray, E.; Athanassopoulos, C.M. Synthesis and Antiplasmodial Activity of Novel Fosmidomycin Derivatives and Conjugates with Artemisinin and Aminochloroquinoline. Molecules 2020, 25, 4858. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Grellier, P.; Labaied, M.; Sonnet, P.; Léger, J.-M.; Déprez-Poulain, R.; Forfar-Bares, I.; Dallemagne, P.; Lemaître, N.; Péhourcq, F.; et al. Synthesis, antimalarial activity, and molecular modeling of new pyrrolo[1,2-a]quinoxalines, bispyrrolo[1,2-a]quinoxalines, bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines, and bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J. Med. Chem. 2004, 47, 1997–2009. [Google Scholar] [CrossRef]
- Farjallah, A.; Chiarelli, L.R.; Forbak, M.; Degiacomi, G.; Danel, M.; Goncalves, F.; Carayon, C.; Seguin, C.; Fumagalli, M.; Záhorszká, M.; et al. A Coumarin-Based Analogue of Thiacetazone as Dual Covalent Inhibitor and Potential Fluorescent Label of HadA in Mycobacterium tuberculosis. ACS Infect. Dis. 2021, 7, 552–565. [Google Scholar] [CrossRef]
- Herrmann, L.; Leidenberger, M.; Sacramento de Morais, A.; Mai, C.; Çapci, A.; da Cruz Borges Silva, M.; Plass, F.; Kahnt, A.; Moreira, D.R.M.; Kappes, B.; et al. Autofluorescent antimalarials by hybridization of artemisinin and coumarin: In vitro/in vivo studies and live-cell imaging. Chem. Sci. 2023, 14, 12941–12952. [Google Scholar] [CrossRef]


| Compound | IC50 FcB1 |
|---|---|
| 39 | 7.0 +/− 0.4 μM |
| 40 | 45.7 +/− 7.6 μM |
| 41 | 8.6 +/− 1.0 μM |
| AS | 4.5 +/− 0.4 nM |
| 39 + AS | 4.4 +/− 0.4 nM a |
| 22 | 3.5 +/− 0.4 nM |
| 40 + AS | 5.1 +/− 0.6 nM a |
| 23 | 1.5 +/− 0.1 nM |
| 41 + AS | 5.1 +/−0.6 nM a |
| 24 | 1.9 +/− 0.3 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamvoukaki, G.; Antoniou, A.I.; Baltas, M.; Mouray, E.; Charneau, S.; Grellier, P.; Athanassopoulos, C.M. Synthesis of Novel Artemisinin, Ciprofloxacin, and Norfloxacin Hybrids with Potent Antiplasmodial Activity. Antibiotics 2024, 13, 142. https://doi.org/10.3390/antibiotics13020142
Vamvoukaki G, Antoniou AI, Baltas M, Mouray E, Charneau S, Grellier P, Athanassopoulos CM. Synthesis of Novel Artemisinin, Ciprofloxacin, and Norfloxacin Hybrids with Potent Antiplasmodial Activity. Antibiotics. 2024; 13(2):142. https://doi.org/10.3390/antibiotics13020142
Chicago/Turabian StyleVamvoukaki, Georgia, Antonia I. Antoniou, Michel Baltas, Elisabeth Mouray, Sebastien Charneau, Philippe Grellier, and Constantinos M. Athanassopoulos. 2024. "Synthesis of Novel Artemisinin, Ciprofloxacin, and Norfloxacin Hybrids with Potent Antiplasmodial Activity" Antibiotics 13, no. 2: 142. https://doi.org/10.3390/antibiotics13020142
APA StyleVamvoukaki, G., Antoniou, A. I., Baltas, M., Mouray, E., Charneau, S., Grellier, P., & Athanassopoulos, C. M. (2024). Synthesis of Novel Artemisinin, Ciprofloxacin, and Norfloxacin Hybrids with Potent Antiplasmodial Activity. Antibiotics, 13(2), 142. https://doi.org/10.3390/antibiotics13020142






