Great Plasticity in a Great Pathogen: Capsular Types, Virulence Factors and Biofilm Formation in ESBL-Producing Klebsiella pneumoniae from Pediatric Infections in Uruguay
Abstract
:1. Introduction
2. Results
2.1. Isolates
2.2. K-Types, Virulence Factors, and Hypermucoviscosity
2.3. Biofilm
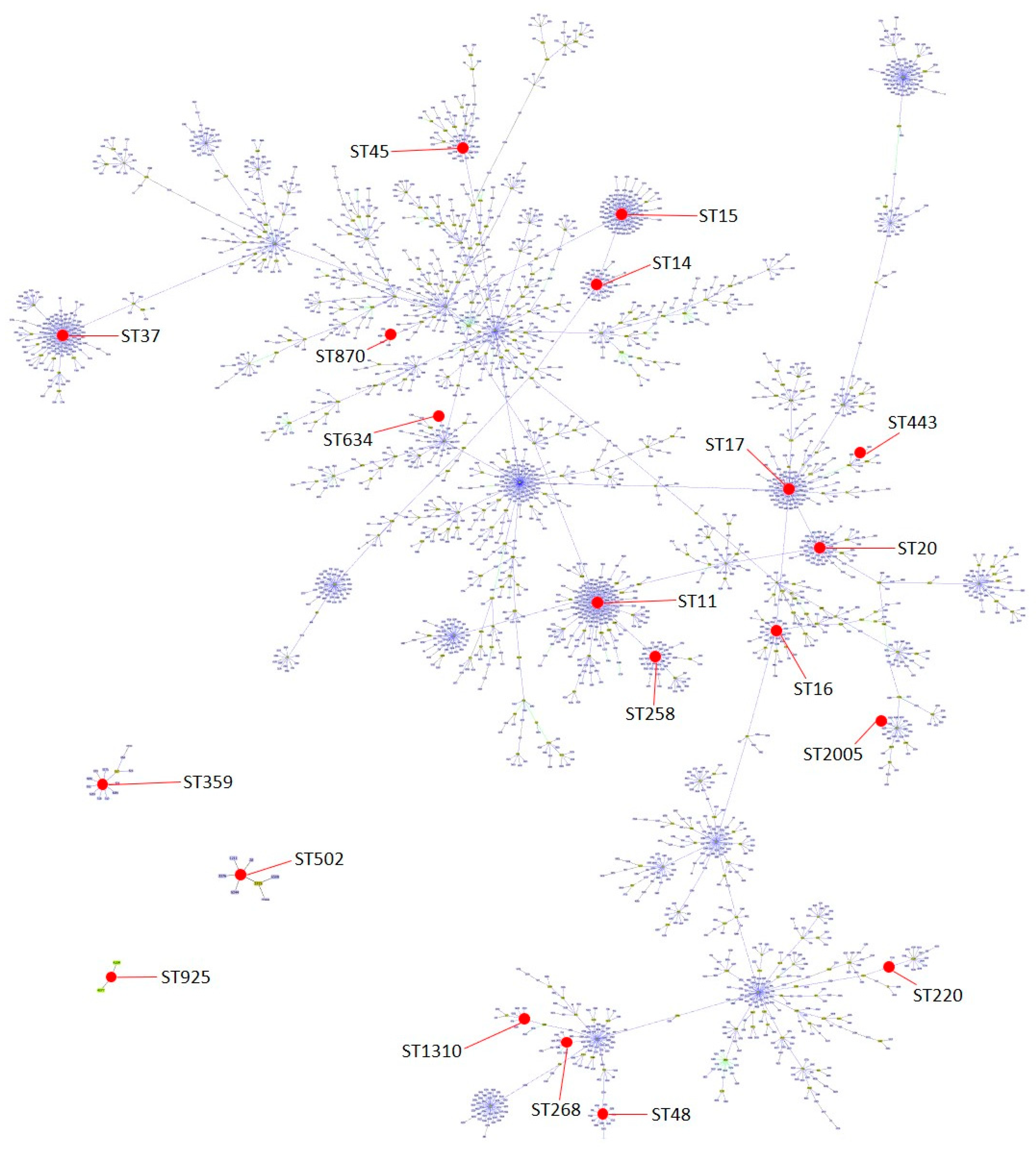
2.4. Sequence Types and Pulse Types
2.5. Antibiotic Susceptibility
2.6. Statistical Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. K-Types, Virulence Factors, and Hypermucoviscosity Characterization
4.3. Biofilm Quantification
4.4. Pulsed-Field Gel Electrophoresis (PFGE) and Multilocus Sequence Typing (MLST) Characterization
4.5. Antibiotic Susceptibility Testing
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet. Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A.; et al. GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Wyres, K.; Wick, R.; Judd, L.; Froumine, R.; Tokolyi, A.; Gorrie, C.; Lam, M.; Duchêne, S.; Jenney, A.; Holt, K. Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae. bioRxiv 2018. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Zong, Z. Worldwide transmission of ST11-KL64 carbapenem-resistant Klebsiella pneumoniae: An analysis of publicly available genomes. mSphere 2023, 8, e00173-23. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Lanza, V.F.; Peixe, L.; Coque, T.M.; Novais, Â. Phylogenomics of Globally Spread Clonal Groups 14 and 15 of Klebsiella pneumoniae. Microbiol. Spectr. 2023, 11, e03395-22. [Google Scholar] [CrossRef]
- Garcia-Fulgueiras, V.; Bado, I.; Mota, M.I.; Robino, L.; Cordeiro, N.F.; Varela, A.; Algorta, G.; Gutkind, G.; Ayala, J.A.; Vignoli, R. Extended-spectrum β-lactamases and plasmid-mediated quinolone resistance in enterobacterial clinical isolates in the paediatric hospital of Uruguay. J. Antimicrob. Chemother. 2011, 66, 1725–1729. [Google Scholar] [CrossRef]
- Garcia-Fulgueiras, V.; Araujo, L.; Bado, I.; Cordeiro, N.F.; Mota, M.I.; Laguna, G.; Algorta, G.; Vignoli, R. Allodemic distribution of plasmids co-harbouring blaCTX-M-15/aac(6′)-Ib-cr/qnrB in Klebsiella pneumoniae is the main source of extended-spectrum β-lactamases in Uruguay’s paediatric hospital. J. Glob. Antimicrob. Resist. 2017, 9, 68–73. [Google Scholar] [CrossRef]
- Bado, I.; Gutiérrez, C.; García-Fulgueiras, V.; Cordeiro, N.F.; Araújo Pirez, L.; Seija, V.; Bazet, C.; Rieppi, G.; Vignoli, R. CTX-M-15 in combination with aac(6′)-Ib-cr is the most prevalent mechanism of resistance both in Escherichia coli and Klebsiella pneumoniae, including K. pneumoniae ST258, in an ICU in Uruguay. J. Glob. Antimicrob. Resist. 2016, 6, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Papa-Ezdra, R.; Caiata, L.; Palacio, R.; Outeda, M.; Cabezas, L.; Bálsamo, A.; Vignoli, R.; Bado, I.; Seija, V. Prevalence and molecular characterisation of carbapenemase-producing Enterobacterales in an outbreak-free setting in a single hospital in Uruguay. J. Glob. Antimicrob. Resist. 2021, 24, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Brisse, S.; Fevre, C.; Passet, V.; Issenhuth-Jeanjean, S.; Tournebize, R.; Diancourt, L.; Grimont, P. Virulent Clones of Klebsiella pneumoniae: Identification and Evolutionary Scenario Based on Genomic and Phenotypic Characterization. PLoS ONE 2009, 4, e4982. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.X.; Lin, Z.W.; Chen, C.; Chen, Z.; Lin, F.J.; Wu, Y.; Yang, S.Y.; Sun, X.; Yao, W.M.; Li, D.Y.; et al. Biofilm Formation in Klebsiella pneumoniae Bacteremia Strains Was Found to be Associated with CC23 and the Presence of wcaG. Front. Cell. Infect. Microbiol. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Horváth, M.; Kovács, T.; Koderivalappil, S.; Ábrahám, H.; Rákhely, G.; Schneider, G. Identification of a newly isolated lytic bacteriophage against K24 capsular type, carbapenem resistant Klebsiella pneumoniae isolates. Sci. Rep. 2020, 10, 5891. [Google Scholar] [CrossRef]
- Horváth, M.; Kovács, T.; Kun, J.; Gyenesei, A.; Damjanova, I.; Tigyi, Z.; Schneider, G. Virulence Characteristics and Molecular Typing of Carbapenem-Resistant ST15 Klebsiella pneumoniae Clinical Isolates, Possessing the K24 Capsular Type. Antibiotics 2023, 12, 479. [Google Scholar] [CrossRef]
- Peirano, G.; Cheng, L.; Kreiswirth, B.N.; Pitout, J.D.D. Emerging Antimicrobial-Resistant High-Risk Klebsiella pneumoniae Clones ST307 and ST147. Antmicrobial Agents Chemother. 2020, 64, e01148-20. [Google Scholar] [CrossRef]
- Priante, E.; Minotti, C.; Contessa, C.; Boschetto, M.; Stano, P.; Dal Bello, F.; De Canale, E.; Lolli, E.; Baldo, V.; Baraldi, E.; et al. Successful Control of an Outbreak by Phenotypically Identified Extended-Spectrum Beta-Lactamase–Producing Klebsiella pneumoniae in a Neonatal Intensive Care Unit. Antibiotics 2022, 11, 1649. [Google Scholar] [CrossRef]
- Flores-Valdez, M.; Ares, M.A.; Rosales-Reyes, R.; Torres, J.; Girón, J.A.; Weimer, B.C.; Mendez-Tenorio, A.; De la Cruz, M.A. Whole Genome Sequencing of Pediatric Klebsiella pneumoniae Strains Reveals Important Insights Into Their Virulence-Associated Traits. Front. Microbiol. 2021, 12, 711577. [Google Scholar] [CrossRef]
- Fasciana, T.; Gentile, B.; Aquilina, M.; Ciammaruconi, A.; Mascarella, C.; Anselmo, A.; Fortunato, A.; Fillo, S.; Petralito, G.; Lista, F.; et al. Co-existence of virulence factors and antibiotic resistance in new Klebsiella pneumoniae clones emerging in south of Italy. BMC Infect. Dis. 2019, 19, 928. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.T.; Lai, S.Y.; Yi, W.C.; Hsueh, P.R.; Liu, K.L.; Chang, S.C. Klebsiella pneumoniae Genotype K1: An Emerging Pathogen That Causes Septic Ocular or Central Nervous System Complications from Pyogenic Liver Abscess. Clin. Infect. Dis. 2007, 45, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.C.; Fang, C.T.; Lee, C.Z.; Shun, C.T.; Wang, J.T. Genomic Heterogeneity in Klebsiella pneumoniae Strains Is Associated with Primary Pyogenic Liver Abscess and Metastatic Infection. J. Infect. Dis. 2005, 192, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Lev, A.I.; Astashkin, E.I.; Kislichkina, A.A.; Solovieva, E.V.; Kombarova, T.I.; Korobova, O.V.; Ershova, O.N.; Alexandrova, I.A.; Malikov, V.E.; Bogun, A.G.; et al. Comparative analysis of Klebsiella pneumoniae strains isolated in 2012–2016 that differ by antibiotic resistance genes and virulence genes profiles. Pathog. Glob. Health 2018, 112, 142–151. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhadury, P.; Mitra, S.; Naha, S.; Saha, B.; Dutta, S.; Basu, S. Hypervirulent Klebsiella pneumoniae Causing Neonatal Bloodstream Infections: Emergence of NDM-1-Producing Hypervirulent ST11-K2 and ST15-K54 Strains Possessing pLVPK-Associated Markers. Microbiol. Spectr. 2023, 11, e04121-22. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Mazumder, R.; Ahmed, A.; Saima, U.; Phelan, J.E.; Campino, S.; Ahmed, D.; Asadulghani, M.; Clark, T.G.; Mondal, D. Genome dynamics of high-risk resistant and hypervirulent Klebsiella pneumoniae clones in Dhaka, Bangladesh. Front. Microbiol. 2023, 14, 1184196. [Google Scholar] [CrossRef]
- Zemmour, A.; Dali-Yahia, R.; Maatallah, M.; Saidi-Ouahrani, N.; Rahmani, B.; Benhamouche, N.; Al-Farsi, H.M.; Giske, C.G. High-risk clones of extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from the University Hospital Establishment of Oran, Algeria (2011–2012). PLoS ONE 2021, 16, e0254805. [Google Scholar] [CrossRef]
- Awoke, T.; Teka, B.; Seman, A.; Sebre, S.; Yeshitela, B.; Aseffa, A.; Mihret, A.; Abebe, T. High Prevalence of Multidrug-Resistant Klebsiella pneumoniae in a Tertiary Care Hospital in Ethiopia. Antibiotics 2021, 10, 1007. [Google Scholar] [CrossRef]
- Poletajew, S.; Pawlik, K.; Bonder-Nowicka, A.; Pakuszewski, A.; Nyk, Ł.; Kryst, P. Multi-Drug Resistant Bacteria as Aetiological Factors of Infections in a Tertiary Multidisciplinary Hospital in Poland. Antibiotics 2021, 10, 1232. [Google Scholar] [CrossRef]
- Wyres, K.L.; Holt, K.E. Klebsiella pneumoniae Population Genomics and Antimicrobial-Resistant Clones. Trends Microbiol. 2016, 24, 944–956. [Google Scholar] [CrossRef]
- Sennati, S.; Santella, G.; Di Conza, J.; Pallecchi, L.; Pino, M.; Ghiglione, B.; Rossolini, G.M.; Radice, M.; Gutkind, G. Changing Epidemiology of Extended-Spectrum β-Lactamases in Argentina: Emergence of CTX-M-15. Antimicrob. Agents Chemother. 2012, 56, 6003–6005. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.R.; Pinto, V.P.T.; Barbosa, F.C.B. The Spread of CTX-M-Type Extended-Spectrum β-Lactamases in Brazil: A Systematic Review. Microb. Drug Resist. 2016, 22, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, B.Y.; Choi, M.H.; Yoon, E.J.; Lee, H.; Lee, K.J.; Park, Y.S.; Shin, J.H.; Uh, Y.; Shin, K.S.; et al. Antimicrobial resistance and virulence factors of Klebsiella pneumoniae affecting 30 day mortality in patients with bloodstream infection. J. Antimicrob. Chemother. 2019, 74, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Chuang, Y.; Shun, C.; Chang, S.; Wang, J. A Novel Virulence Gene in Klebsiella pneumoniae Strains Causing Primary Liver Abscess and Septic Metastatic Complications. J. Exp. Med. 2004, 199, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Villegas, A.; Baronetti, N.; José Albesa, I.; Polifroni, R.; Parma, A.; Etcheverría, A.; Becerra, M.; Padola, N.; Paraje, M. Relevance of Biofilms in the Pathogenesis of Shiga-Toxin-Producing Escherichia coli Infection. Sci. World J. 2013, 2013, 607258. [Google Scholar] [CrossRef]
- Francisco, A.P.; Bugalho, M.; Ramirez, M.; Carriço, J.A. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinform. 2009, 10, 152. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Robino, L.; Telechea, H.; Speranza, N.; García-Fulgueiras, V.; Cordeiro, N.; Bado, I.; Mota, M.I.; Giachetto, G.; Algorta, G.; Vignoli, R. Risk factors for the acquisition of extended-spectrum beta-lactamase-producing Enterobacteriaceae in hospitalized children. J. Infect. Dev. Ctries. 2013, 7, 361–364. [Google Scholar] [CrossRef]

| MIC (mg/L) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Sample | Resistance Mechanisms | K/wzi | kfuBC | fyuA | BF | ST | PT | CTX | CAZ | AK | GN | CIP | SXT | Antibiotype |
| 11 | csf | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K2 | + | − | M | 14 | XVII | ≥64 | 16 | 8 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT |
| 14 | blood | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K2 | + | − | S | 14 | XII | ≥64 | 16 | 4 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT |
| 29 | urine | blaCTX-M-8/aac(6′)Ib | K2 | − | − | M | 14 | XVII | 32 | ≤1 | 16 | 8 | 1 | ≤20 | 3GC/GN/CIP */AK * |
| 737 | blood | blaCTX-M-8/aac(6′)Ib/qnrB | K2 | + | − | S | 14 | XXV | 16 | ≤1 | 16 | 8 | 1.5 | ≤20 | 3GC/GN/CIP/AK * |
| 16 | sw | blaSHV-5 | K17 | + | − | W | 870 | VIII | 4 | ≥64 | ≤2 | ≤1 | ≤0.25 | ≤20 | 3GC |
| 17 | urine | blaSHV-5 | K17 | + | − | M | 870 | VIII | 32 | ≥64 | ≤2 | ≤1 | ≤0.25 | ≤20 | 3GC |
| 18 | urine | blaSHV-5 | K17 | + | − | NO | 870 | VIII | 4 | ≥64 | ≤2 | ≤1 | ≤0.25 | ≤20 | 3GC |
| 04 | blood | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K20 | − | − | W | 268 | XVIII | ≥64 | ≥64 | 8 | ≥16 | 2 | ≤20 | 3GC/GN/CIP |
| 025 | urine | blaCTX-M-2/aac(6′)Ib | K24 | − | − | NO | 45 | XXIII | ≥256 | 4 | 16 | ≥16 | 0.032 | ≤20 | 3GC/GN/AK * |
| 05 | urine | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K24 | − | + | NO | 45 | XV | ≥64 | 16 | 16 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT/AK * |
| 06 | blood | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K24 | − | + | NO | 45 | XIII | ≥64 | 16 | 16 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT/AK * |
| 07 | blood | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K24 | + | + | M | 14 | XI | ≥64 | 16 | 16 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT/AK * |
| 08 | urine | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K24 | − | + | NO | 45 | XV | ≥64 | 8 | 4 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT |
| 09 | ct | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K24 | − | + | W | 14 | XII | ≥64 | 16 | 4 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT |
| 12 | urine | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K24 | − | + | NO | 45 | XV | ≥64 | 8 | 4 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT |
| 13 | csf | blaCTX-M-15/aac(6′)Ib-cr/qnrB | K24 | + | + | W | 45 | X | ≥64 | 16 | 16 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT/AK * |
| 327 | urine | blaSHV-5/aac(6′)Ib | K24 | − | − | NO | 45 | XXIV | ≥256 | ≥64 | 12 | ≤1 | 0.023 | ≥320 | 3GC/SXT/AK * |
| 33 | sw | blaSHV-2 | K24 | + | + | S | 14 | XVII | ≤1 | 16 | ≤2 | ≤1 | ≤0.25 | ≤20 | 3GC |
| 954b | urine | blaCTX-M-8 | K24 | + | − | S | 15 | XXVIII | 64 | ≤1 | 1.5 | ≤1 | ≥32 | ≥320 | 3GC/CIP/SXT |
| 23 | urine | blaCTX-M-2/aac(6′)Ib | K27 | − | − | M | 11 | XIX | ≥64 | 16 | 16 | ≥16 | ≥4 | 80 | 3GC/GN/CIP/SXT/AK * |
| 836 | urine | blaSHV-2/aac(6′)Ib | K28 | − | + | W | 20 | XXVII | 32 | ≤1 | 24 | 8 | 0.032 | ≤20 | 3GC/AK/GN |
| 742 | blood | blaSHV-2 | K62 | − | − | S | 17 | XXVI | 32 | 4 | 16 | ≤1 | 0.023 | ≤20 | 3GC/AK * |
| 30 | urine | blaCTX-M-9/aac(6′)Ib/qnrA | wzi050 | − | − | M | 502 | I | ≥64 | ≤1 | 16 | 8 | 0.25 | ≥320 | 3GC/GN/SXT/AK * |
| 31 | urine | blaCTX-M-15/aac(6′)Ib-cr | wzi050 | − | − | NO | 16 | II | ≥64 | ≥64 | ≤2 | ≤1 | ≥4 | ≥320 | 3GC/CIP/SXT |
| 15 | blood | blaSHV-5 | wzi086 | − | − | NO | 1310 | VI | ≥256 | ≥64 | ≤2 | ≤1 | ≤0.25 | ≥320 | 3GC/SXT |
| 10 | blood | blaCTX-M-15/aac(6′)Ib-cr/qnrB | wzi118 | − | + | W | 443 | XIV | ≥64 | ≥64 | 16 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT/AK * |
| 19 | urine | blaSHV-5/aac(6′)Ib | wzi154 | − | − | W | 258 | IX | 8 | ≥64 | ≥64 | ≥16 | ≥4 | 40 | 3GC/AK/GN/CIP |
| 20 | blood | blaSHV-5/aac(6′)Ib | wzi154 | − | − | NO | 258 | IX | ≥256 | ≥64 | ≥64 | ≥16 | ≥4 | 40 | 3GC/AK/GN/CIP |
| 28 | blood | blaCTX-M-2/aac(6′)Ib | wzi154 | − | − | NO | 37 | VII | ≥64 | ≤1 | 16 | ≥16 | ≤0.25 | ≤20 | 3GC/GN/AK * |
| 32 | urine | blaCTX-M-15/aac(6′)Ib-cr | wzi154 | − | − | NO | 258 | IX | ≥64 | ≥64 | ≥64 | 4 | ≥4 | ≥320 | 3GC/AK/CIP/SXT/GN * |
| 01 | blood | blaCTX-M-15/aac(6′)Ib-cr/qnrB | wzi167 | − | + | NO | 48 | XVI | ≥64 | 8 | ≤2 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT |
| 02 | urine | blaCTX-M-15/aac(6′)Ib-cr/qnrB | wzi167 | − | + | NO | 48 | XVI | ≥64 | 8 | ≤2 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT |
| 03 | sw | blaCTX-M-15/aac(6′)Ib-cr/qnrB | wzi167 | − | + | NO | 48 | XVI | ≥64 | 8 | ≤2 | ≥16 | 2 | ≥320 | 3GC/GN/CIP/SXT |
| 22 | urine | blaSHV-5/aac(6′)Ib | wzi177 | − | − | M | 220 | V | 16 | ≥64 | ≤2 | ≥16 | 0.5 | ≥320 | 3GC/GN/SXT |
| 21 | urine | blaSHV-5/aac(6′)Ib | wzi209 | − | + | NO | 2005 | IV | 32 | ≥64 | 8 | 8 | 0.5 | ≤20 | 3GC/GN |
| 004 | sf | blaCTX-M-9/aac(6′)Ib | wzi231 | + | − | W | 925 | XXII | 8 | ≥64 | 8 | ≥16 | 0.023 | ≥320 | 3GC/GN/SXT |
| 24 | urine | blaCTX-M-2/aac(6′)Ib | wzi236 | − | − | W | 359 | XX | ≥64 | 4 | 16 | ≥16 | ≤0.25 | ≤20 | 3GC/GN/AK * |
| 26 | urine | blaCTX-M-2/aac(6′)Ib | wzi236 | − | − | NO | 359 | XXI | ≥64 | 16 | 16 | ≥16 | ≤0.25 | ≤20 | 3GC/GN/AK * |
| 25 | urine | blaCTX-M-2/aac(6′)Ib | wzi281 | − | − | M | 634 | III | ≥64 | 16 | 16 | ≥16 | ≤0.25 | 40 | 3GC/GN/AK * |
| 27 | urine | blaCTX-M-2/aac(6′)Ib | wzi281 | − | − | M | 634 | III | ≥64 | 4 | 16 | ≥16 | ≤0.25 | 40 | 3GC/GN/AK * |
| Breakpoint a S (mg/L) | Breakpoint I b (mg/L) | Breakpoint R (mg/L) | MIC Range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) | % S | % I | % R | |
|---|---|---|---|---|---|---|---|---|---|
| CTX | ≤1 | 2 | ≥4 | ≤1–≥256 | ≥64 | ≥64 | 2 | 0 | 98 |
| CAZ | ≤1 | 2–4 | ≥8 | ≤1–≥64 | 16 | ≥64 | 15 | 10 | 75 |
| AK | ≤8 | 16 | ≥32 | ≤2–≥64 | 16 | 16 | 47 | 43 | 10 |
| GN | ≤2 | 4 | ≥8 | ≤1–≥16 | ≥16 | ≥16 | 23 | 2 | 75 |
| CIP | ≤0.5 | 1 | ≥2 | ≤0.25–≥32 | 2 | ≥4 | 45 | 2 | 53 |
| SXT | ≤20 | 40 | ≥80 | ≤20–≥320 | ≥320 | ≥320 | 35 | 10 | 55 |
| Variable | K24 (11) | No-K24 (29) | p-Value | OR (CI95%) |
|---|---|---|---|---|
| kfuBC | 4 | 7 | - § | |
| fyuA | 8 | 6 | 0.01 | 10.22 (2.06–50.76) |
| Strong/Moderate * | 3 | 11 | - | |
| Weak/NO * | 8 | 18 | - | |
| ST14 | 3 | 4 | - | |
| ST45 | 7 | 0 | 0.00 | 2.75 (1.26–6.01) |
| blaCTX-M-15 | 7 | 9 | - | |
| blaCTX-M-2 | 1 | 6 | - | |
| blaSHV-5 | 1 | 8 | - | |
| Invasive sample ‡ | 3 | 11 | - | |
| MDR | 9 | 16 | - | |
| kfuBC (11) | No-kfuBC (29) | p-value | OR (CI95%) | |
| Strong/Moderate | 7 | 7 | 0.03 | 5.50 (1.23–24.50) |
| Weak/NO | 4 | 22 | 0.03 | 0.18 (0.04–0.81) |
| ST14 | 5 | 2 | 0.01 | 11.25 (1.75–72.50) |
| ST45 | 1 | 6 | - | |
| blaCTX-M-15 | 4 | 12 | - | |
| blaCTX-M-2 | 0 | 7 | - | |
| blaSHV-5 | 3 | 6 | - | |
| Invasive sample | 6 | 8 | - | |
| MDR | 7 | 18 | - | |
| fyuA (14) | No-fyuA (26) | p-value | OR (CI95%) | |
| Strong/Moderate | 2 | 12 | - | |
| Weak/NO | 12 | 14 | - | |
| ST14 | 3 | 4 | - | |
| ST45 | 5 | 2 | 0.04 | 6.67 (1.09–40.73) |
| blaCTX-M-15 | 11 | 5 | 0.00 | 15.40 (3.09–76.78) |
| blaCTX-M-2 | 0 | 7 | - | |
| blaSHV-5 | 1 | 8 | - | |
| Invasive sample | 5 | 9 | - | |
| MDR | 11 | 14 | - | |
| Biofilm producer S/M # (14) | No-Biofilm producer S/M # (26) | p-value | OR (CI95%) | |
| ST14 | 6 | 1 | 0.00 | 18.75 (1.95–180.00) |
| ST45 | 0 | 7 | - | |
| blaCTX-M-15 | 3 | 13 | - | |
| blaCTX-M-2 | 3 | 4 | - | |
| blaSHV-5 | 2 | 7 | - | |
| Invasive sample | 5 | 9 | - | |
| MDR | 8 | 17 | - | |
| ST14 (7) | No-ST14 (33) | p-value | OR (CI95%) | |
| blaCTX-M-15 | 4 | 12 | - | |
| blaCTX-M-2 | 0 | 7 | - | |
| blaSHV-5 | 0 | 9 | - | |
| Invasive sample | 4 | 10 | - | |
| MDR | 5 | 20 | - | |
| ST45 (7) | No-ST45 (33) | p-value | OR (CI95%) | |
| blaCTX-M-15 | 5 | 11 | - | |
| blaCTX-M-2 | 1 | 6 | - | |
| blaSHV-5 | 1 | 8 | - | |
| Invasive sample | 2 | 12 | - | |
| MDR | 6 | 19 | - | |
| Invasive sample (14) | No-Invasive sample (26) | p-value | OR (CI95%) | |
| blaCTX-M-15 | 8 | 8 | - | |
| blaCTX-M-2 | 1 | 6 | - | |
| blaSHV-5 | 2 | 7 | - | |
| MDR | 11 | 14 | - | |
| MDR (25) | No-MDR (15) | p-value | OR (CI95%) | |
| blaCTX-M-15 | 16 | 0 | 0.00 | 2.78 (1.65–4.68) |
| blaCTX-M-2 | 1 | 6 | 0.01 | 0.06 (0.01–0.59) |
| blaSHV-5 | 4 | 5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, L.; Papa-Ezdra, R.; Ávila, P.; Iribarnegaray, V.; Bado, I.; Telechea, H.; Garcia-Fulgueiras, V.; Vignoli, R. Great Plasticity in a Great Pathogen: Capsular Types, Virulence Factors and Biofilm Formation in ESBL-Producing Klebsiella pneumoniae from Pediatric Infections in Uruguay. Antibiotics 2024, 13, 170. https://doi.org/10.3390/antibiotics13020170
Araújo L, Papa-Ezdra R, Ávila P, Iribarnegaray V, Bado I, Telechea H, Garcia-Fulgueiras V, Vignoli R. Great Plasticity in a Great Pathogen: Capsular Types, Virulence Factors and Biofilm Formation in ESBL-Producing Klebsiella pneumoniae from Pediatric Infections in Uruguay. Antibiotics. 2024; 13(2):170. https://doi.org/10.3390/antibiotics13020170
Chicago/Turabian StyleAraújo, Lucía, Romina Papa-Ezdra, Pablo Ávila, Victoria Iribarnegaray, Inés Bado, Hector Telechea, Virginia Garcia-Fulgueiras, and Rafael Vignoli. 2024. "Great Plasticity in a Great Pathogen: Capsular Types, Virulence Factors and Biofilm Formation in ESBL-Producing Klebsiella pneumoniae from Pediatric Infections in Uruguay" Antibiotics 13, no. 2: 170. https://doi.org/10.3390/antibiotics13020170
APA StyleAraújo, L., Papa-Ezdra, R., Ávila, P., Iribarnegaray, V., Bado, I., Telechea, H., Garcia-Fulgueiras, V., & Vignoli, R. (2024). Great Plasticity in a Great Pathogen: Capsular Types, Virulence Factors and Biofilm Formation in ESBL-Producing Klebsiella pneumoniae from Pediatric Infections in Uruguay. Antibiotics, 13(2), 170. https://doi.org/10.3390/antibiotics13020170






