Antibiotic Consumption 2017–2022 in 30 Private Hospitals in France: Impact of Antimicrobial Stewardship Tools and COVID-19 Pandemic
Abstract
1. Introduction
2. Results
2.1. Main Characteristics of Institutions
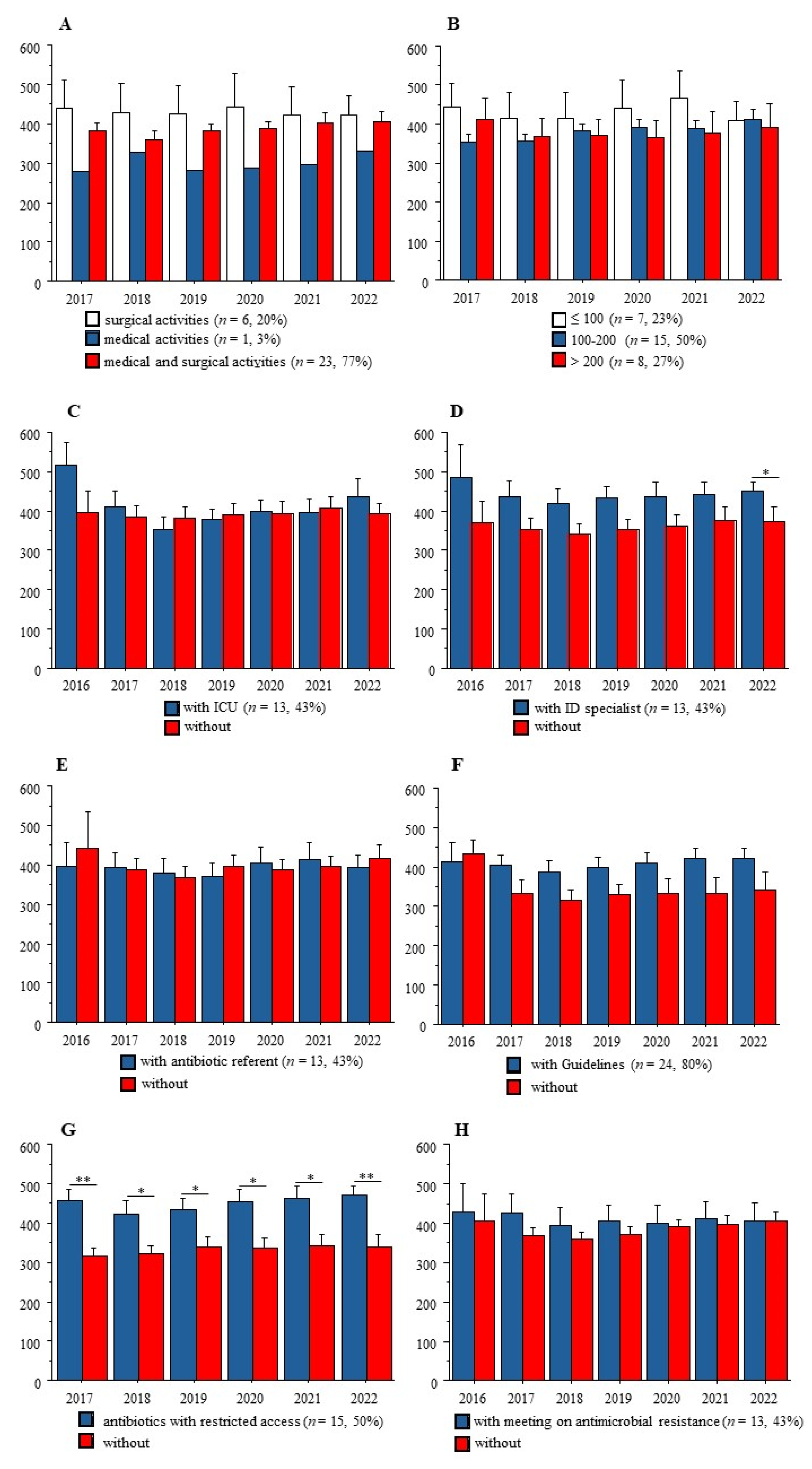
2.2. Antimicrobial Stewardship Tools
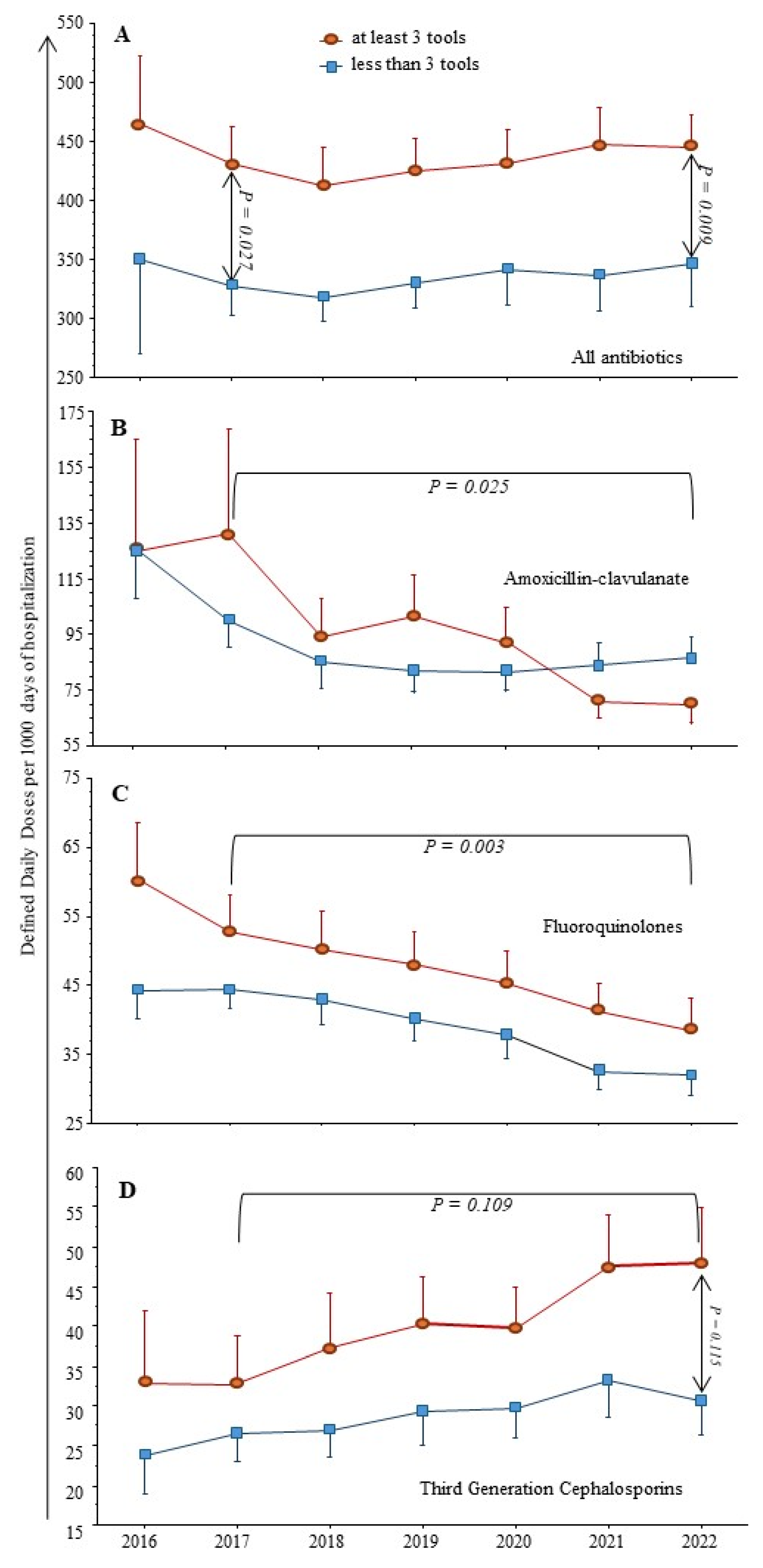
2.3. Antimicrobial Consumption
2.4. COVID-19 Pandemic and Antimicrobial Consumption
3. Discussion
4. Materials and Methods
4.1. Antibiotic Consumption
4.2. Antimicrobial Stewardship
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Core Elements of Hospital Antibiotic Stewardship Programs: Antibiotic Use|CDC. Available online: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed on 1 January 2020).
- Binda, F.; Tebano, G.; Kallen, M.C.; Ten Oever, J.; Hulscher, M.E.; Schouten, J.A.; Pulcini, C. Nationwide survey of hospital antibiotic stewardship programs in France. Med. Mal. Infect. 2020, 50, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Etienne, P.; Roger, P.-M.; Brofferio, P.; Labate, C.; Blanc, V.; Tiger, F.; Négrin, N.; Léotard, S. Antimicrobial stewardship program and quality of antibiotic prescriptions. Med. Mal. Infect. 2011, 41, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Roger, P.-M.; Espinet, A.; Ravily, D.; Meyer, M.-J.; Moll, F.; Montera, E.; Rancezot, A.; Dautezac, V.; Pantaloni, O. Simplified therapeutic guidelines: The main tool of antimicrobial stewardship programs associated with optimal antibiotic therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Avdic, E.; Keenan, J.F.; Zhao, Y.; Anand, G.; Cooper, J.; Dezube, R.; Hsu, S.; Cosgrove, S.E. What is the more effective antibiotic stewardship intervention: Preprescription authorization or postprescription review with feedback? Clin. Infect. Dis. 2017, 64, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Schuts, E.C.; Hulscher, M.E.J.L.; Mouton, J.W.; Verduin, C.M.; Stuart, J.W.T.C.; Overdiek, H.W.P.M.; van der Linden, P.D.; Natsch, S.; Hertogh, C.M.P.M.; Wolfs, T.F.W.; et al. Current evidence on hospital antimicrobial stewardship objectives: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, X. A survey on antimicrobial stewardship in 116 tertiary hospitals in China. Clin. Microbiol. Infect. 2019, 25, 759.e9–759.e14. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2021 (accessed on 24 September 2023).
- Mendelson, M.; Morris, A.M.; Thursky, K.; Pulcini, C. How to start an antimicrobial stewardship programme in a hospital. Clin. Microbiol. Infect. 2020, 26, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Friedli, O.; Gasser, M.; Cusini, A.; Fulchini, R.; Vuichard-Gysin, D.; Tobler, R.H.; Wassilew, N.; Plüss-Suard, C.; Kronenberg, A. Impact of the COVID-19 pandemic on inpatient antibiotic consumption in Switzerland. Antibiotics 2022, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hasan, S.S.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Antimicrobial consumption in patients with COVID-19: A systematic review and meta-analysis. Expert Rev. Anti-infective Ther. 2022, 20, 749–772. [Google Scholar] [CrossRef] [PubMed]
- Viel, S.; Markowicz, S.; Ait-Medjber, L.; Ouissa, R.; Delta, D.; Portecop, P.; Foucan, T.; Roger, P.-M. Dedicated team to ambulatory care for patients with COVID-19 requiring oxygen: Low rate of hospital readmission. Int. J. Infect. Dis. 2022, 123, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Amadeo, B.; Dumartin, C.; Parneix, P.; Fourrier-Réglat, A.; Rogues, A.-M. Relationship between antibiotic consumption and antibiotic policy: An adjusted analysis in the French healthcare system. J. Antimicrob. Chemother. 2011, 66, 434–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pakyz, A.L.; Moczygemba, L.R.; Wang, H.; Stevens, M.P.; Edmond, M.B. An evaluation of the association between an antimicrobial stewardship score and antimicrobial usage. J. Antimicrob. Chemother. 2015, 70, 1588–1591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamel, A.M.; Monem, M.S.A.; Sharaf, N.A.; Magdy, N.; Farid, S.F. Efficacy and safety of azithromycin in Covid-19 patients: A systematic review and meta-analysis of randomized clinical trials. Rev. Med. Virol. 2022, 32, e2258. [Google Scholar] [CrossRef] [PubMed]
- Aldeyab, M.A.; Crowe, W.; Karasneh, R.A.; Patterson, L.; Sartaj, M.; Ewing, J.; Lattyak, W.J.; Al-Azzam, S.; Araydah, M.; Elhajji, F.D.; et al. The impact of the COVID-19 pandemic on antibiotic consumption and prevalence of pathogens in primary and secondary healthcare settings in Northern Ireland. Br. J. Clin. Pharmacol. 2023, 89, 2851–2866. [Google Scholar] [CrossRef] [PubMed]
- Enquete Prevalence IAS 2022.pdf. Available online: https://www.cpias-ile-de-france.fr/surveillance/enp/2022/SpF-ENP-2022-Guide-Enqueteur.pdf (accessed on 4 August 2023).
- Abubakar, U.; Awaisu, A.; Khan, A.H.; Alam, K. Impact of COVID-19 pandemic on healthcare-associated infections: A systematic review and meta-analysis. Antibiotics 2023, 12, 1600. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; Soucy, J.-P.R.; Leung, V.; So, M.; Kwan, A.T.; Portnoff, J.S.; Bertagnolio, S.; Raybardhan, S.; MacFadden, D.R.; Daneman, N. Antibiotic resistance associated with the COVID-19 pandemic: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Boussat, S.; Demoré, B.; Lozniewski, A.; Aissa, N.; Rabaud, C. How to improve the collection and analysis of hospital antibiotic consumption: Preliminary results of the ConsoRes software experimental implementation. Med. Mal. Infect. 2012, 42, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Santé Publique France Enquêtes Flash: Évaluation de la Circulation des Variants du SARS-CoV-2 en France. Saint-Maurice: Santé publique France. Available online: https://www.santepubliquefrance.fr/etudes-et-enquetes/enquetes-flash-evaluation-de-la-circulation-des-variants-du-sars-cov-2-en-france#block-33727 (accessed on 25 September 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roger, P.-M.; Lesselingue, D.; Gérard, A.; Roghi, J.; Quint, P.; Un, S.; Chincholle, A.; Assi, A.; Bouchard, O.; Javaudin, V.; et al. Antibiotic Consumption 2017–2022 in 30 Private Hospitals in France: Impact of Antimicrobial Stewardship Tools and COVID-19 Pandemic. Antibiotics 2024, 13, 180. https://doi.org/10.3390/antibiotics13020180
Roger P-M, Lesselingue D, Gérard A, Roghi J, Quint P, Un S, Chincholle A, Assi A, Bouchard O, Javaudin V, et al. Antibiotic Consumption 2017–2022 in 30 Private Hospitals in France: Impact of Antimicrobial Stewardship Tools and COVID-19 Pandemic. Antibiotics. 2024; 13(2):180. https://doi.org/10.3390/antibiotics13020180
Chicago/Turabian StyleRoger, Pierre-Marie, Diane Lesselingue, Anouk Gérard, Jacques Roghi, Pauline Quint, Sophie Un, Agnès Chincholle, Assi Assi, Odile Bouchard, Véronique Javaudin, and et al. 2024. "Antibiotic Consumption 2017–2022 in 30 Private Hospitals in France: Impact of Antimicrobial Stewardship Tools and COVID-19 Pandemic" Antibiotics 13, no. 2: 180. https://doi.org/10.3390/antibiotics13020180
APA StyleRoger, P.-M., Lesselingue, D., Gérard, A., Roghi, J., Quint, P., Un, S., Chincholle, A., Assi, A., Bouchard, O., Javaudin, V., & Denes, E. (2024). Antibiotic Consumption 2017–2022 in 30 Private Hospitals in France: Impact of Antimicrobial Stewardship Tools and COVID-19 Pandemic. Antibiotics, 13(2), 180. https://doi.org/10.3390/antibiotics13020180




