Bacterial Community- and Hospital-Acquired Pneumonia in Patients with Critical COVID-19—A Prospective Monocentric Cohort Study
Abstract
:1. Introduction
2. Results
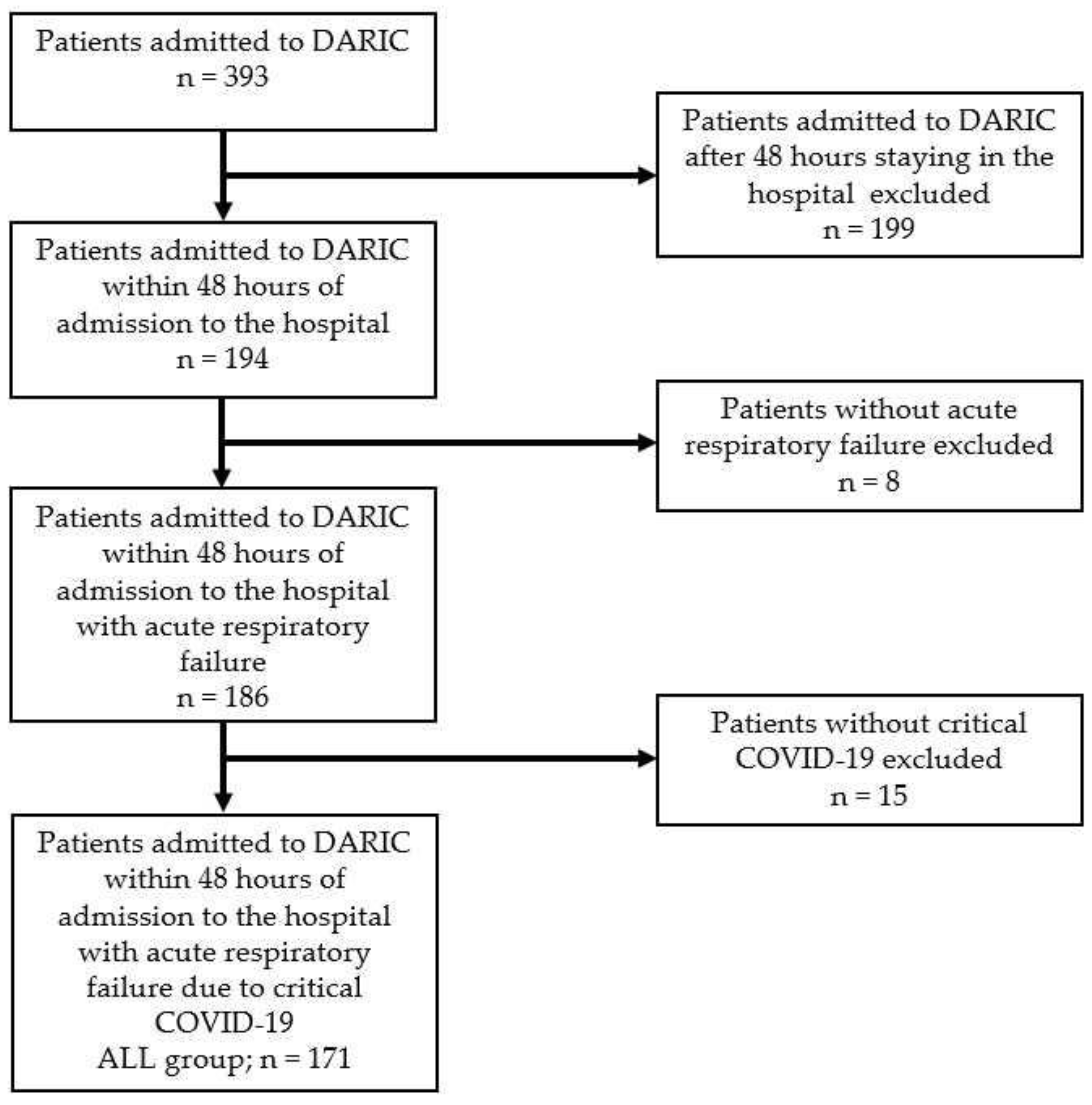
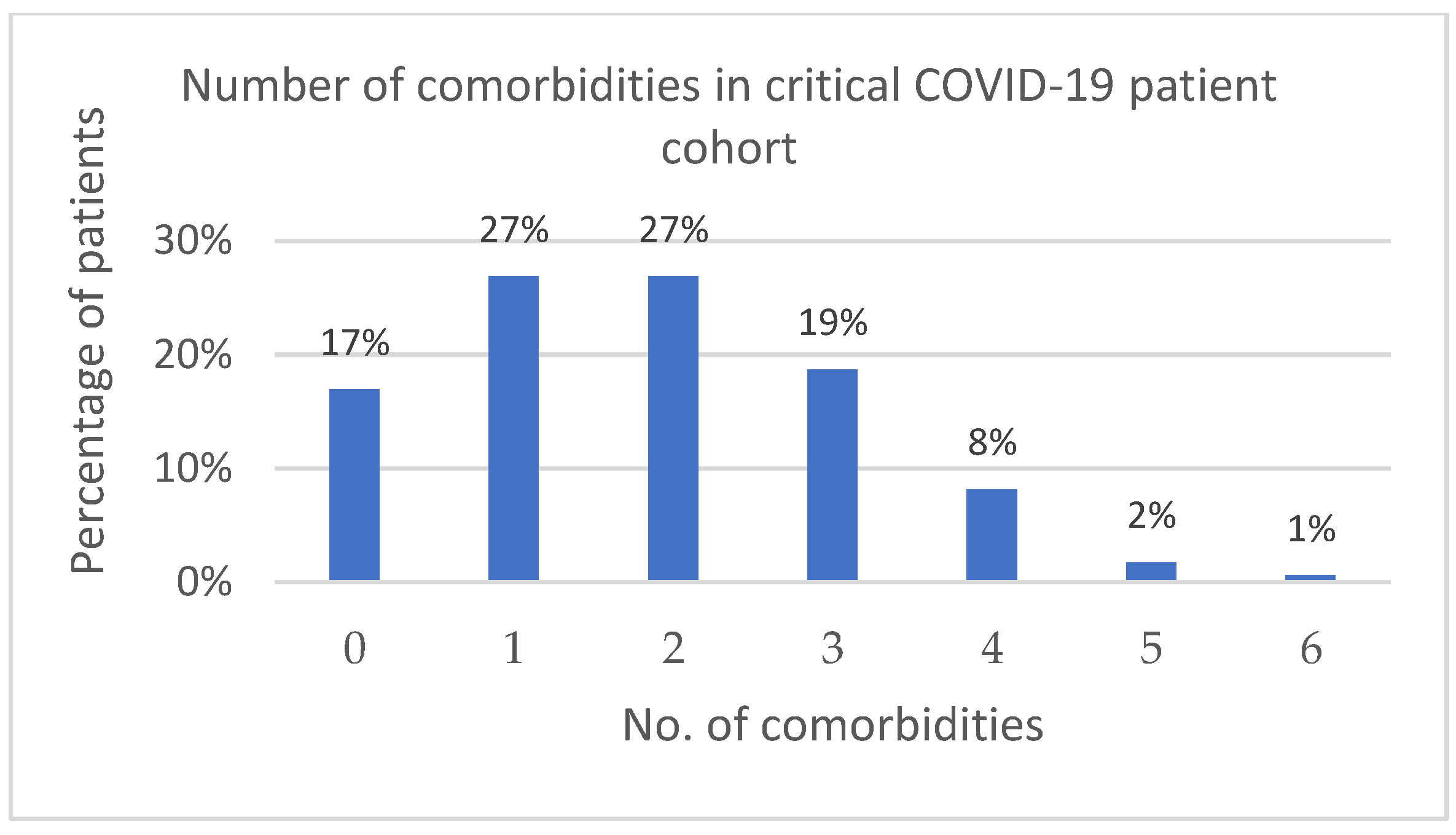
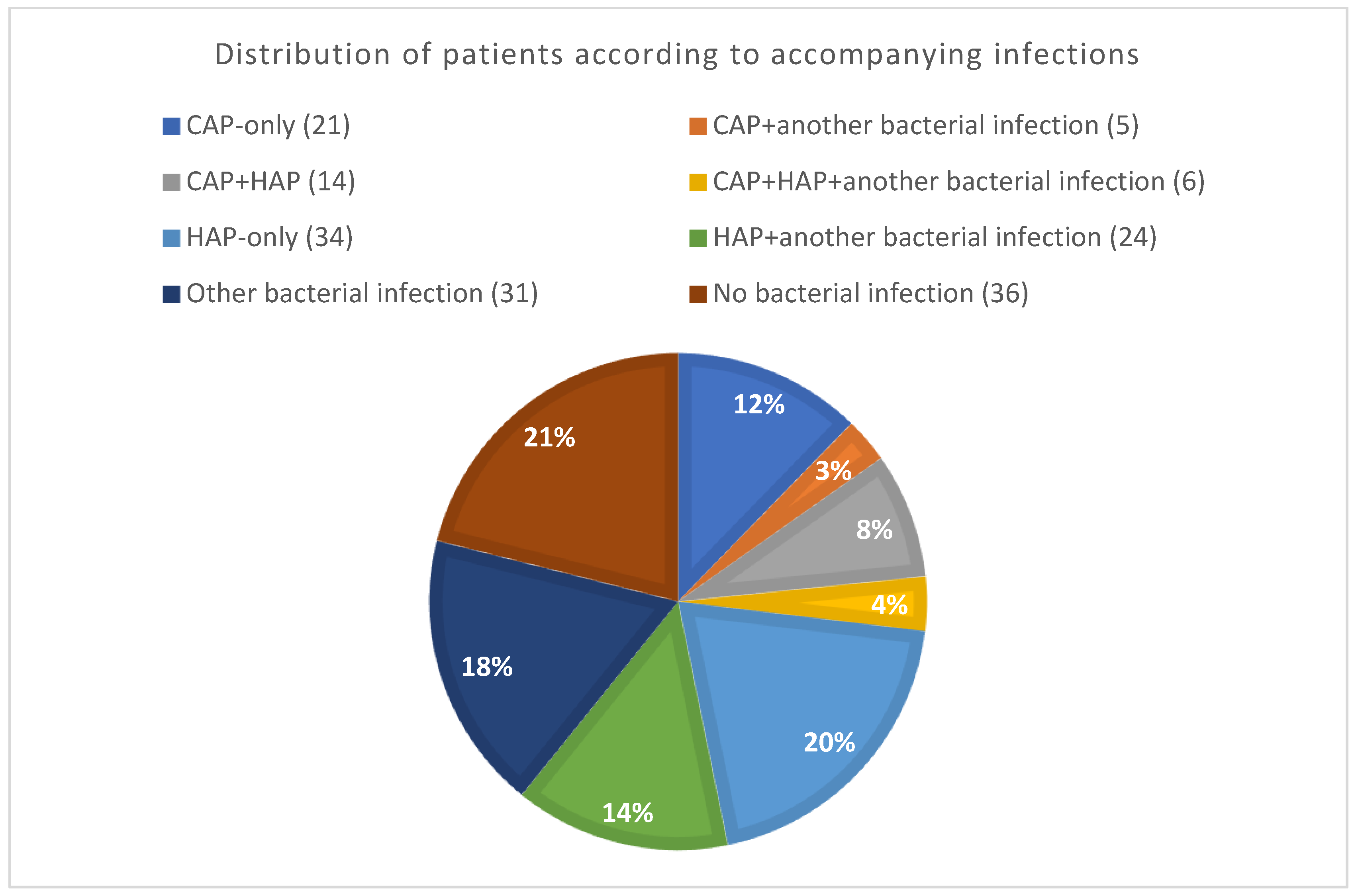
2.1. Classification of ALL Group Patients according to Presence of Infections
2.2. Bacterial CAP, HAP, and Other Infection Groups
2.3. Critical COVID-19 Patients with Bacterial CAP
2.4. Initial Inflammatory Markers in Bacterial CAP-Only Patients
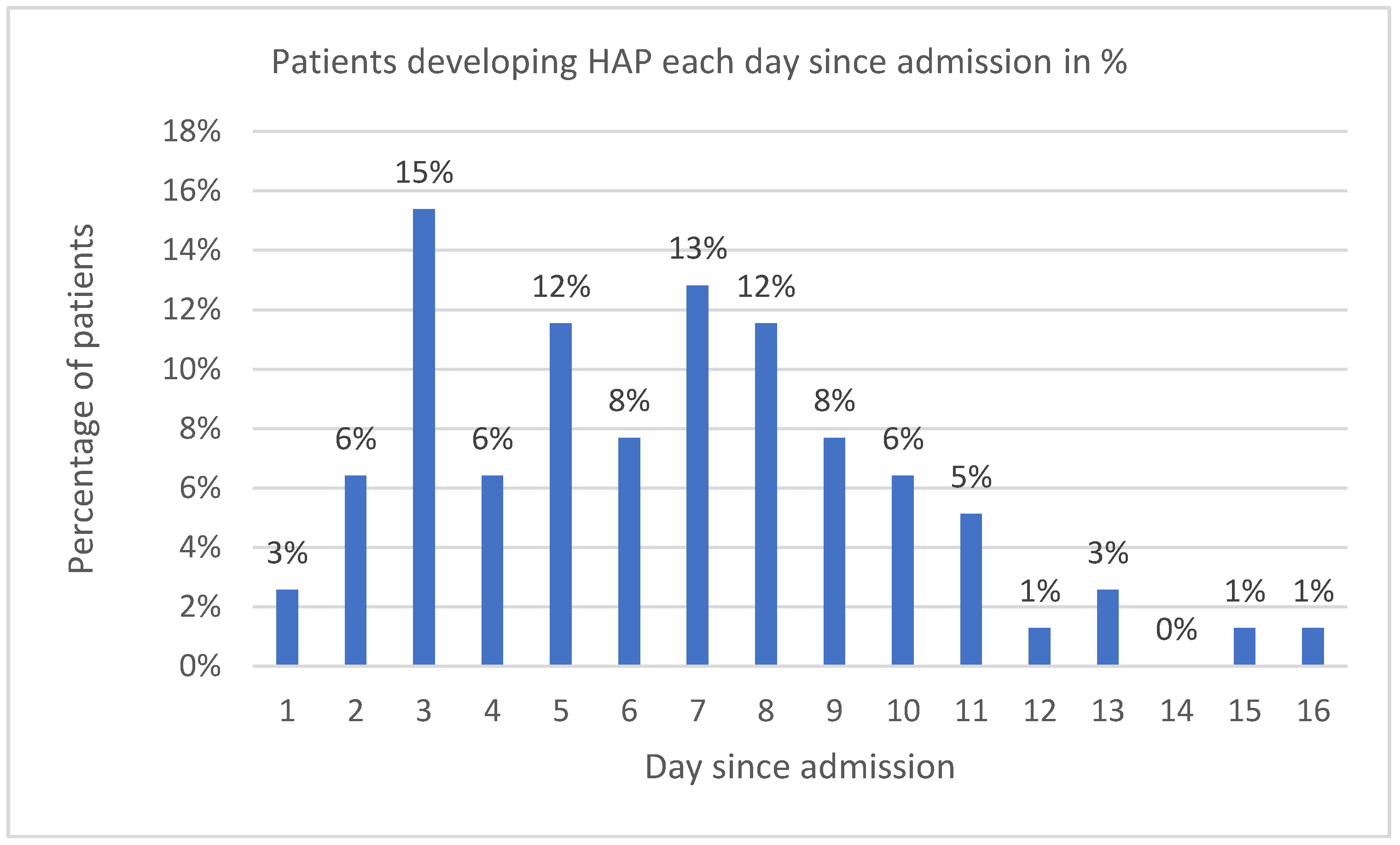
2.5. Critical COVID-19 Patients with Bacterial HAP
2.6. Inflammatory Markers in Bacterial HAP-Only Patients
2.7. Signs and Symptoms of HAP
3. Material and Methods
3.1. Study Design
3.2. Setting
3.3. Study Group of Patients
3.4. Definition of Bacterial CAP and HAP
3.5. Diagnostic Criteria for Bacterial CAP and HAP
- Positive detection of bacterial pathogens;
- PCT ≥ 1.0 µg/L, or CRP ≥ 100 mg/L;
- Clinical signs of bacterial CAP (sputum production, auscultation);
- Consolidations in lung tissue consistent with bacterial pneumonia shown by X-ray or CT scan;
- Dynamics in inflammatory biomarkers: if a new peak occurred after the initial decline (at least three of the following: PCT, CRP, IL-6, and white blood cell count (WBC)). Thresholds have been set CRP ≥ 50 mg/L, PCT ≥ 0.5 µg/L, IL-6 ≥ 300 ng/L or WBC ≥ 11 × 109;
- Positive detection of bacterial pathogens.
- And at least three of the six following criteria:
- Deterioration of clinical condition
- Respiratory insufficiency progression (increase in FiO2, PEEP, pressure support, need for intubation)
- New sputum production (change in amount/colour)
- Fever (new onset)
- Circulation instability (decrease in mean arterial pressure ≤ 65 torr, onset or increase in vasopressor support)
- New infiltrate on lung X-ray or CT scan
3.6. Data Collected during the Data Collection Phase
- Age;
- Gender;
- BMI;
- APACHE II;
- LOS;
- Mortality on day 28 (D28);
- Palliative care;
- Comorbidities;
- Biomarkers (CRP, PCT, IL-6, WBC);
- Body temperature;
- Clinical signs of sepsis;
- Microbiological findings.
3.7. Microbiological Examination
- Cultivation and microscopy of the clinical sample from the lower respiratory tract;
- Blood cultivation;
- Direct serological detection of pneumococcal and legionella antigens from urine;
- PCR detection of bacterial nucleic acid;
- Serological method for detection of antibodies against mycoplasma and chlamydophila.
3.8. Statistical Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://covid19treatmentguidelines.nih.gov (accessed on 28 August 2023).
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Clinical Management of COVID-19: Living Guideline. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.2 (accessed on 21 May 2023).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, L.A.; Costa, I.B.S.D.S.; Rizk, S.I.; Biselli, B.; Gomes, B.R.; Bittar, C.S.; de Oliveira, G.Q.; de Almeida, J.P.; de Oliveira Bello, M.V.; Garzillo, C.; et al. Intensive care management of patients with COVID-19: A practical approach. Ann. Intensive Care 2021, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef]
- Abate, S.M.; Ahmed, A.S.; Mantfardo, B.; Basu, B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: A systematic review and Meta-analysis. PLoS ONE 2020, 15, e0235653. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wunsch, H. Mechanical Ventilation in COVID-19: Interpreting the Current Epidemiology. Am. J. Respir. Crit. Care Med. 2020, 202, 1–4. [Google Scholar] [CrossRef]
- Johnson, J.A.; Mallari, K.F.; Pepe, V.M.; Treacy, T.; McDonough, G.; Khaing, P.; McGrath, C.; George, B.J.; Yoo, E.J. Mechanically ventilated COVID-19 patients admitted to the intensive care unit in the United States with or without respiratory failure secondary to COVID-19 pneumonia: A retrospective comparison of characteristics and outcomes. Acute Crit. Care 2023, 38, 298–307. [Google Scholar] [CrossRef]
- Ouyang, L.; Yu, M.; Zhu, Y.; Gong, J. Respiratory Supports Of COVID-19 Patients in Intensive Care Unit: A Systematic Review. Heliyon 2021, 7, e06813. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Bindo, F.; Motos, A.; Fernández-Barat, L.; Barbeta, E.; Gabarrús, A.; Ceccato, A.; Bermejo-Martin, J.F.; Ferrer, R.; Riera, J.; et al. Procalcitonin And C-Reactive Protein to Rule Out Early Bacterial Coinfection In COVID-19 Critically Ill Patients. Intensive Care Med. 2023, 49, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Anderson, R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 2021, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.; Rejman, D.; Bardoň, J. Zásady Antibiotické Léčby [Principles of Antibiotic Treatment]; Palacký University Olomouc: Olomouc, Czech Republic, 2020; pp. 102–107. ISBN 978-80-244-5740-6. [Google Scholar]
- De Santis, V.; Corona, A.; Vitale, D.; Nencini, C.; Potalivo, A.; Prete, A.; Zani, G.; Malfatto, A.; Tritapepe, L.; Taddei, S.; et al. Bacterial infections in critically ill patients with SARS-2-COVID-19 infection: Results of a prospective observational multicenter study. Infection 2022, 50, 139–148. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, C.; Luo, L.; Fang, F.; Chen, Y.; Li, J.; Peng, Z.; Pan, H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020, 127, 104364. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Creti, R.; Palma, C.; Pantosti, A.; Unit of Antibiotic Resistance and Special Pathogens; The Unit of Antibiotic Resistance and Special Pathogens. Bacterial coinfections in COVID-19: An underestimated adversary. Ann. Dell’Istituto Super. Sanita 2020, 56, 359–364. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W. Treatment of Community-Acquired Pneumonia During the Coronavirus Disease 2019 (COVID-19) Pandemic. Ann. Intern. Med. 2020, 173, 304–305. [Google Scholar] [CrossRef]
- Elabbadi, A.; Turpin, M.; Gerotziafas, G.T.; Teulier, M.; Voiriot, G.; Fartoukh, M. Bacterial coinfection in critically ill COVID-19 patients with severe pneumonia. Infection 2021, 49, 559–562. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Verroken, A.; Scohy, A.; Gérard, L.; Wittebole, X.; Collienne, C.; Laterre, P.F. Co-infections in COVID-19 critically ill and antibiotic management: A prospective cohort analysis. Crit. Care 2020, 24, 410. [Google Scholar] [CrossRef] [PubMed]
- Youngs, J.; Wyncoll, D.; Hopkins, P.; Arnold, A.; Ball, J.; Bicanic, T. Improving antibiotic stewardship in COVID-19: Bacterial co-infection is less common than with influenza. J. Infect. 2020, 81, e55–e57. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Coronavirus Disease 2019, Superinfections, and Antimicrobial Development: What Can We Expect? Clin. Infect. Dis. 2020, 71, 2736–2743. [Google Scholar] [CrossRef]
- Yoon, S.M.; Lee, J.; Lee, S.M.; Lee, H.Y. Incidence and clinical outcomes of bacterial superinfections in critically ill patients with COVID-19. Front. Med. 2023, 10, 1079721. [Google Scholar] [CrossRef]
- De Francesco, M.A.; Signorini, L.; Piva, S.; Pellizzeri, S.; Fumarola, B.; Corbellini, S.; Piccinelli, G.; Simonetti, F.; Carta, V.; Mangeri, L.; et al. Bacterial and fungal superinfections are detected at higher frequency in critically ill patients affected by SARS CoV-2 infection than negative patients and are associated to a worse outcome. J. Med. Virol. 2023, 95, e28892. [Google Scholar] [CrossRef]
- Schouten, J.; De Waele, J.; Lanckohr, C.; Koulenti, D.; Haddad, N.; Rizk, N.; Sjövall, F.; Kanj, S.S.; Alliance for the Prudent Use of Antibiotics (APUA). Antimicrobial stewardship in the ICU in COVID-19 times: The known unknowns. Int. J. Antimicrob. Agents 2021, 58, 106409. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.A.; Markov, N.S.; Stoeger, T.; Pawlowski, A.; Kang, M.; Nannapaneni, P.; Grant, R.A.; Pickens, C.; Walter, J.M.; Kruser, J.M.; et al. Machine Learning Links Unresolving Secondary Pneumonia to Mortality in Patients with Severe Pneumonia, Including COVID-19. J. Clin. Investig. 2023, 133, e170682. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Wu, H.Y.; Chang, P.H.; Chen, K.Y.; Lin, I.F.; Hsih, W.H.; Tsai, W.L.; Chen, J.A.; Lee, S.S.; GREAT working group. Coronavirus disease 2019 (COVID-19) associated bacterial coinfection: Incidence, diagnosis and treatment. J. Microbiol. Immunol. Infect. 2022, 55, 985–992. [Google Scholar] [CrossRef]
- Mandell, L.A.; Zhanel, G.G.; Rotstein, C.; Muscedere, J.; Loeb, M.; Johnstone, J. Community-Acquired Pneumonia in Canada During Coronavirus Disease 2019. Open Forum Infect. Dis. 2022, 9, ofac043. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, C.; Pludermann, A.; Mahtani, K. Differentiating Viral from Bacterial Pneumonia. Available online: https://www.cebm.net/covid-19/differentiating-viral-from-bacterial-pneumonia (accessed on 14 October 2023).
- COVID-19 Rapid Guideline: Managing: Managing COVID-19. Available online: https://www.nice.org.uk/guidance/ng191 (accessed on 22 June 2023).
- Naranje, P.; Bhalla, A.S.; Jana, M.; Garg, M.; Nair, A.D.; Singh, S.K.; Banday, I. Imaging of Pulmonary Superinfections and Co-Infections in COVID-19. Curr. Probl. Diagn. Radiol. 2022, 51, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Altmayer, S.; Zanon, M.; Pacini, G.S.; Watte, G.; Barros, M.C.; Mohammed, T.L.; Verma, N.; Marchiori, E.; Hochhegger, B. Comparison of the computed tomography findings in COVID-19 and other viral pneumonia in immunocompetent adults: A systematic review and meta-analysis. Eur. Radiol. 2020, 30, 6485–6496. [Google Scholar] [CrossRef]
- Parekh, M.; Donuru, A.; Balasubramanya, R.; Kapur, S. Review of the Chest CT Differential Diagnosis of Ground-Glass Opacities in the COVID Era. Radiology 2020, 297, E289–E302. [Google Scholar] [CrossRef]
- Duzgun, S.A.; Durhan, G.; Demirkazik, F.B.; Akpinar, M.G.; Ariyurek, O.M. COVID-19 pneumonia: The great radiological mimicker. Insights Imaging 2020, 11, 118. [Google Scholar] [CrossRef]
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L.; Deng, G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int. J. Infect. Dis. 2020, 96, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.Y.; Yin, C.H.; Yao, Y.M. Update Advances on C-Reactive Protein in COVID-19 and Other Viral Infections. Front. Immunol. 2021, 12, 720363. [Google Scholar] [CrossRef] [PubMed]
- Sidhwani, S.K.; Mirza, T.; Khatoon, A.; Shaikh, F.; Khan, R.; Shaikh, O.A.; Nashwan, A.J. Inflammatory markers and COVID-19 disease progression. J. Infect. Public Health 2023, 16, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Waterer, G. Advances in the causes and management of community acquired pneumonia in adults. BMJ 2017, 358, j2471. [Google Scholar] [CrossRef] [PubMed]
- Kamat, I.S.; Ramachandran, V.; Eswaran, H.; Guffey, D.; Musher, D.M. Procalcitonin to Distinguish Viral from Bacterial Pneumonia: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2020, 70, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Sabahat, U.; Thomas, L.M.; Shaikh, N.A.; Ali, N.M. An Unexpected Cause of Raised Procalcitonin. Dubai Med. J. 2023, 6, 310–314. [Google Scholar] [CrossRef]
- Cuquemelle, E.; Soulis, F.; Villers, D.; Roche-Campo, F.; Ara Somohano, C.; Fartoukh, M.; Kouatchet, A.; Mourvillier, B.; Dellamonica, J.; Picard, W.; et al. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med. 2011, 37, 796–800. [Google Scholar] [CrossRef]
- Pfister, R.; Kochanek, M.; Leygeber, T.; Brun-Buisson, C.; Cuquemelle, E.; Machado, M.B.; Piacentini, E.; Hammond, N.E.; Ingram, P.R.; Michels, G. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: A prospective cohort study, systematic review and individual patient data meta-analysis. Crit. Care 2014, 18, R44. [Google Scholar] [CrossRef]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef]
- May, M.; Chang, M.; Dietz, D.; Shoucri, S.; Laracy, J.; Sobieszczyk, M.E.; Uhlemann, A.C.; Zucker, J.; Kubin, C.J. Limited Utility of Procalcitonin in Identifying Community-Associated Bacterial Infections in Patients Presenting with Coronavirus Disease 2019. Antimicrob. Agents Chemother. 2021, 65, e02167-20. [Google Scholar] [CrossRef]
- Harte, E.; Kumarasamysarma, S.; Phillips, B.; Mackay, O.; Rashid, Z.; Malikova, N.; Mukit, A.; Ramachandran, S.; Biju, A.; Brown, K.; et al. Procalcitonin Values Fail to Track the Presence of Secondary Bacterial Infections in COVID-19 Icu Patients. Antibiotics 2023, 12, 709. [Google Scholar] [CrossRef]
- van Berkel, M.; Kox, M.; Frenzel, T.; Pickkers, P.; Schouten, J.; RCI-COVID-19 study group. Biomarkers for antimicrobial stewardship: A reappraisal in COVID-19 times? Crit. Care 2020, 24, 600. [Google Scholar] [CrossRef]
- Lidman, C.; Burman, L.G.; Lagergren, A.; Ortqvist, A. Limited value of routine microbiological diagnostics in patients hospitalized for community-acquired pneumonia. Scand. J. Infect. Dis. 2002, 34, 873–879. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.S.; Yang, X.L.; Hu, B.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; Shi, Z.L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Available online: http://www.eucast.org/clinical_breakpoints (accessed on 12 July 2023).
- Htoutou Sedlakova, M.; Hanulik, V.; Chroma, M.; Hricova, K.; Kolar, M.; Latal, T.; Schaumann, R.; Rodloff, A.C. Phenotypic detection of broad-spectrum beta-lactamases in microbiological practice. Med. Sci. Monit. 2011, 17, BR147–BR152. [Google Scholar] [CrossRef]
- Tamma, P.D.; Simner, P.J. Phenotypic Detection of Carbapenemase-Producing Organisms from Clinical Isolates. J. Clin. Microbiol. 2018, 56, e01140-18. [Google Scholar] [CrossRef]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Mlynarcik, P.; Dolejska, M.; Vagnerova, I.; Kutilová, I.; Kolar, M. Detection of clinically important β-lactamases by using PCR. FEMS Microbiol. Lett. 2021, 368, fnab068. [Google Scholar] [CrossRef]
- Pérez-Pérez, F.J.; Hanson, N.D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 2002, 40, 2153–2162. [Google Scholar] [CrossRef]
- Oliveira, D.C.; de Lencastre, H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995, 33, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Schoettler, J.J.; Sandrio, S.; Boesing, C.; Bauer, L.; Miethke, T.; Thiel, M.; Krebs, J. Bacterial Co- or Superinfection in Patients Treated in Intensive Care Unit with COVID-19- and Influenza-Associated Pneumonia. Pathogens 2023, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Gandhi, T.N.; Petty, L.A.; Patel, P.K.; Prescott, H.C.; Malani, A.N.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S.A. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin. Infect. Dis. 2021, 72, e533–e541. [Google Scholar] [CrossRef]
- Ramanan, M.; Burrell, A.; Paul, E.; Trapani, T.; Broadley, T.; McGloughlin, S.; French, C.; Udy, A. Nosocomial infections amongst critically ill COVID-19 patients in Australia. J. Clin. Virol. Plus 2021, 4, 100054. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, E.; Cultrera, R.; Quaranta, A.; Cricca, V.; Marangoni, E.; La Rosa, R.; Bertacchini, S.; Bellonzi, A.; Ragazzi, R.; Volta, C.A.; et al. Effect of the Pandemic Outbreak on ICU-Associated Infections and Antibiotic Prescription Trends in Non-COVID19 Acute Respiratory Failure Patients. J. Clin. Med. 2022, 11, 7080. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Terrier, O. Viral and Bacterial Co-Infections in The Lungs: Dangerous Liaisons. Viruses 2021, 13, 1725. [Google Scholar] [CrossRef]
- Bogdanová, K.; Doubravská, L.; Vágnerová, I.; Hricová, K.; Pudová, V.; Röderová, M.; Papajk, J.; Uvízl, R.; Langová, K.; Kolář, M. Clostridioides difficile and Vancomycin-Resistant Enterococci in COVID-19 Patients with Severe Pneumonia. Life 2021, 11, 1127. [Google Scholar] [CrossRef]
- Doubravská, L.; Htoutou Sedláková, M.; Fišerová, K.; Pudová, V.; Urbánek, K.; Petrželová, J.; Röderová, M.; Langová, K.; Mezerová, K.; Kučová, P.; et al. Bacterial Resistance to Antibiotics and Clonal Spread in COVID-19-Positive Patients on a Tertiary Hospital Intensive Care Unit, Czech Republic. Antibiotics 2022, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.; Tang, J.W.; Hui, D.S.; Wong, A.L.N.; Chan, M.T.V.; Joynt, G.M.; So, A.T.P.; Hall, S.D.; Chan, P.K.S.; Sung, J.J.Y. Airflow and droplet spreading around oxygen masks: A simulation model for infection control research. Am. J. Infect. Control 2007, 35, 684–689. [Google Scholar] [CrossRef]
- Leung, C.C.H.; Joynt, G.M.; Gomersall, C.D.; Wong, W.T.; Lee, A.; Ling, L.; Chan, P.K.S.; Lui, P.C.W.; Tsoi, P.C.Y.; Ling, C.M.; et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: A randomized controlled crossover trial. J. Hosp. Infect. 2019, 101, 84–87. [Google Scholar] [CrossRef]
- Kotoda, M.; Hishiyama, S.; Mitsui, K.; Tanikawa, T.; Morikawa, S.; Takamino, A.; Matsukawa, T. Assessment of the potential for pathogen dispersal during high-flow nasal therapy. J. Hosp. Infect. 2020, 104, 534–537. [Google Scholar] [CrossRef]
- Li, J.; Fink, J.B.; Ehrmann, S. High-flow nasal cannula for COVID-19 patients: Low risk of bio-aerosol dispersion. Eur. Respir. J. 2020, 55, 2000892. [Google Scholar] [CrossRef]
- Elshof, J.; Hebbink, R.; Duiverman, M.L.; Hagmeijer, R. High-flow nasal cannula for COVID-19 patients: Risk of bio-aerosol dispersion. Eur. Respir. J. 2020, 56, 2003004. [Google Scholar] [CrossRef]
- Johns, M.; George, S.; Taburyanskaya, M.; Poon, Y.K. A Review of the Evidence for Corticosteroids in COVID-19. J. Pharm. Pract. 2022, 35, 626–637. [Google Scholar] [CrossRef]
- Moreno-Torres, V.; de Mendoza, C.; de la Fuente, S.; Sánchez, E.; Martínez-Urbistondo, M.; Herráiz, J.; Gutiérrez, A.; Gutiérrez, Á.; Hernández, C.; Callejas, A.; et al. Bacterial Infections in Patients Hospitalized with COVID-19. Intern. Emerg. Med. 2022, 17, 431–438. [Google Scholar] [CrossRef]
- Sinha, P.; Furfaro, D.; Cummings, M.J.; Abrams, D.; Delucchi, K.; Maddali, M.V.; He, J.; Thompson, A.; Murn, M.; Fountain, J.; et al. Latent Class Analysis Reveals COVID-19–Related Acute Respiratory Distress Syndrome Subgroups with Differential Responses to Corticosteroids. Am. J. Respir. Crit. Care Med. 2021, 204, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Kane, A.D.; Kursumovic, E.; Oglesby, F.C.; Cook, T.M. Mortality in patients admitted to intensive care with COVID-19: An updated systematic review and meta-analysis of observational studies. Anaesthesia 2021, 76, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, A.; Oliva, A.; Siccardi, G.; Tramontano, A.; Pellegrino, D.; Mastroianni, C.M.; Venditti, M.; Palange, P. Risk Factors and Effect on Mortality of Superinfections in A Newly Established COVID-19 Respiratory Sub-Intensive Care Unit at University Hospital in Rome. BMC Pulm. Med. 2023, 23, 30. [Google Scholar] [CrossRef]
- Nassar, Y.; Mokhtar, A.; Elhadidy, A.; Elsayed, M.; Mostafa, F.; Rady, A.; Eladawy, A.; Elshazly, M.; Saeed, M.; Mokhtar, S.; et al. Outcomes and Risk Factors for Death in Patients with Coronavirus Disease-2019 (COVID-19) Pneumonia Admitted to The Intensive Care Units of An Egyptian University Hospital. A Retrospective Cohort Study. J. Infect. Public Health 2021, 14, 1381–1388. [Google Scholar] [CrossRef]
- Pickens, C.O.; Gao, C.A.; Cuttica, M.J.; Smith, S.B.; Pesce, L.L.; Grant, R.A.; Kang, M.; Morales-Nebreda, L.; Bavishi, A.A.; Arnold, J.M.; et al. Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated For COVID-19 Pneumonia. Am. J. Respir. Crit. Care Med. 2021, 204, 921–932. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yu, X.; Hu, D.; Bao, M.; Liu, H.; Zhou, J.; Jiang, H. Coronavirus Disease 2019 in Elderly Patients: Characteristics and Prognostic Factors Based On 4-Week Follow-Up. J. Infect. 2020, 80, 639–645. [Google Scholar] [CrossRef]
- Ferrando, C.; Mellado-Artigas, R.; Gea, A.; Arruti, E.; Aldecoa, C.; Bordell, A.; Adalia, R.; Zattera, L.; Ramasco, F.; Monedero, P.; et al. Características, Evolución Clínica Y Patient characteristics, clinical course and factors associat-ed to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: A prospective, cohort, multicentre study. Rev Esp Anestesiol Reanim (Engl Ed). Rev. Española Anestesiol. Reanim. 2020, 67, 425–437. [Google Scholar] [CrossRef]
- Michailides, C.; Paraskevas, T.; Karalis, I.; Koniari, I.; Pierrakos, C.; Karamouzos, V.; Marangos, M.; Velissaris, D. Impact of Bacterial Infections On COVID-19 Patients: Is Timing Important? Antibiotics 2023, 12, 379. [Google Scholar] [CrossRef]
- Heer, R.S.; Mandal, A.K.J.; Kho, J.; Szawarski, P.; Csabi, P.; Grenshaw, D.; Walker, I.A.L.; Missouris, C.G. Elevated Procalcitonin Concentrations in Severe COVID-19 May Not Reflect Bacterial Co-Infection. Ann. Clin. Bio-Chem. Int. J. Lab. Med. 2021, 58, 520–527. [Google Scholar] [CrossRef]
- Vazzana, N.; Dipaola, F.; Ognibene, S. Procalcitonin and Secondary Bacterial Infections in Covid-19: Association with Disease Severity and Outcomes. Acta Clin. Belg. 2022, 77, 268–272. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 Puzzle: Deciphering Pathophysiology and Phenotypes of a New Disease Entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Adre, L.A.B.; Catangui, J.S.; Bondoc, M.K.V.; Abdurahman, K.M. Prevalence of Hospital-Acquired Pneumonia Among Patients with Severe to Critical COVID-19 Pneumonia Given Tocilizumab. Cureus 2023, 15, e39604. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, L.; Xu, M.D.; Wu, J.; Luo, D.; Zhu, Y.S.; Li, B.X.; Song, X.Y.; Zhou, X. Prognostic Value of Interleukin-6, C-Reactive Protein, And Procalcitonin in Patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Barrasa, H.; Martín, A.; Maynar, J.; Rello, J.; Fernández-Torres, M.; Aguirre-Quiñonero, A.; Canut-Blasco, A.; Alava COVID-19 Study Investigators. High rate of infections during ICU admission of patients with severe SARS-CoV-2 pneumonia: A matter of time? J. Infect. 2021, 82, 186–230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-19 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Longuet Flandre, P.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensive Care 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Herkel, T.; Uvizl, R.; Doubravska, L.; Adamus, M.; Gabrhelik, T.; Htoutou Sedlakova, M.; Kolar, M.; Hanulik, V.; Pudova, V.; Langova, K.; et al. Epidemiology of hospital-acquired pneumonia: Results of a Central European multicenter, prospective, observational study compared with data from the European region. Biomed. Pap. 2016, 160, 448–455. [Google Scholar] [CrossRef] [PubMed]



| CAP | HAP |
|---|---|
| Streptococcus pneumoniae | Klebsiella pneumoniae |
| Haemophilus influenzae | Pseudomonas aeruginosa |
| Mycoplasma pneumoniae | Escherichia coli and other Enterobacterales |
| Chlamydophila pneumoniae | Staphylococcus aureus |
| Chlamydophila psittaci | Burkholderia cepacia complex |
| Moraxella catarrhalis | Acinetobacter baumannii |
| Staphylococcus aureus | Stenotrophomonas maltophilia |
| Legionella pneumophila | |
| Bordetella pertussis and parapertussis |
| Variables | ALL Group (n = 171) | HAP Group (n = 78) | CAP Group (n = 46) | No Bacterial Infection Group (n = 36) |
|---|---|---|---|---|
| Age, mean (SD) | 62.9 (±12.3) | 62.6 (±11.5) | 63.3 (±13.6) | 64 (±13.23) |
| Male, n (%) | 110 (64.3) | 55 (70.5) | 33 (71.7) | 23 (63.9) |
| BMI, kg/m2, mean (SD) | 33.3 (±7.2) | 32.8 (±7.7) | 33 (±7.3) | 32.5 (±5.7) |
| LOS (ICU), mean (SD) | 13 (±5.2) | 15.6 (±4.9) | 13.1 (±6) | 9.7 (±3.4) |
| Mortality (D28), n (%) | 76 (44.4) | 51 (65.4) | 27 (58.7) | 4 (11.1) |
| Palliative care, n (%) | 42 (24.6) | 28 (35.9) | 20 (43.5) | 1 (2.8) |
| Mech. ventilation, n (%) | 118 (69.0) | 66 (84.6) | 35 (76.1) | 17 (47.2) |
| HFOT, n (%) | 148 (86.5) | 66 (84.6) | 36 (78.3) | 32 (88.9) |
| APACHE II, mean (SD) | 13.9 (±8.5) | 13.9 (±7.8) | 17.4 (±10.4) | 12.7 (±7.55) |
| Corticosteroids, n (%) | 148 (86.5) | 68 (87.2) | 42 (91.3) | 32 (88.9) |
| Variables | CAP-Only (n = 21) | No Bacterial Infection (n = 36) | p-Value |
|---|---|---|---|
| Age, mean (SD) | 63.3 (±13.44) | 64 (±13.23) | 0.709 |
| Male, n (%) | 15 (71.4) | 23 (63.9) | 0.772 |
| BMI, kg/m2, mean (SD) | 33.6 (±7.53) | 32.5 (±5.7) | 0.785 |
| LOS (ICU), mean (SD) | 11.6 (±6.1) | 9.7 (±3.4) | 0.231 |
| Mortality (D28), n (%) | 8 (38.1) | 4 (11.1) | 0.022 |
| Palliative care, n (%) | 5 (23.8) | 1 (2.8) | 0.022 |
| Mech. ventilation, n (%) | 13 (61.9) | 17 (47.2) | 0.41 |
| HFOT, n (%) | 19 (90.5) | 32 (88.9) | 1.0 |
| APACHE II, mean (SD) | 17.1 (±11.82) | 12.7 (±7.55) | 0.177 |
| Corticosteroids | 19 (90.5) | 32 (88.9) | 1.0 |
| Variables | CAP-Only (n = 21) | No Bacterial Infection (n = 36) | p-Value |
|---|---|---|---|
| CRP, mean (SD) | 209 (±91.1) | 148 (±78.2) | 0.014 |
| PCT, mean (SD) | 7.1 (±21.8) | 0.3 (±0.3) | <0.0001 |
| IL-6, mean (SD) | 1282.3 (±5122.7) | 57.7 (±129.2) | 0.007 |
| WBC × 109, mean (SD) | 12.4 (±6) | 9.3 (±3.7) | 0.024 |
| Temperature, mean (SD) | 37.1 (±1.1) | 36.9 (±0.9) | 0.216 |
| Variables | HAP-Only (n = 34) | No Bacterial Infections (n = 36) | p-Value |
|---|---|---|---|
| Age, mean (SD) | 64.6 (±10.4) | 64 (±13.23) | 0.558 |
| Male, n (%) | 28 (82.4) | 23 (63.9) | 0.109 |
| BMI, kg/m2, mean (SD) | 32.5 (±5.7) | 32.5 (±5.7) | 0.698 |
| LOS (ICU), mean (SD) | 15.6 (±5.0) | 9.7 (±3.4) | 0.227 |
| Mortality (D28), n (%) | 19 (55.9) | 4 (11.1) | <0.0001 |
| Palliative care, n (%) | 9 (26.5) | 1 (2.8) | 0.006 |
| Mech. ventilation, n (%) | 25 (73.5) | 17 (47.2) | 0.03 |
| HFOT, n (%) | 31 (91.2) | 32 (88.9) | 1.0 |
| APACHE II, mean (SD) | 14.2 (±7.7) | 12.7 (±7.55) | 0.393 |
| Corticosteroids | 27 (79.4) | 32 (88.9) | 0.336 |
| Variables Initial Values | HAP-Only (n = 34) | No Bacterial Infections (n = 36) | p-Value |
|---|---|---|---|
| CRP, mean (SD) | 140.5 (±65.3) | 148 (±78.2) | 0.716 |
| PCT, mean (SD) | 1.3 (±2.9) | 0.3 (±0.3) | 0.032 |
| IL-6, mean (SD) | 192.7 (±639.4) | 57.7 (±129.2) | 0.005 |
| WBC × 109, mean (SD) | 8.19 (±9.9) | 9.3 (±3.7) | 0.145 |
| Temperature, mean (SD) | 37.2 (±1.2) | 36.9 (±0.9) | 0.285 |
| OR | 95% CI for OR | Sig. | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Sputum (1) | 3.629 | 1.122 | 11.737 | 0.31 |
| Vasopressor (1) | 1.924 | 0.689 | 5.371 | 0.212 |
| Respiratory deterioration (1) | 20.453 | 6.866 | 60.929 | <0.0001 |
| X-ray (1) | 6.435 | 1.959 | 21.133 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doubravská, L.; Htoutou Sedláková, M.; Fišerová, K.; Klementová, O.; Turek, R.; Langová, K.; Kolář, M. Bacterial Community- and Hospital-Acquired Pneumonia in Patients with Critical COVID-19—A Prospective Monocentric Cohort Study. Antibiotics 2024, 13, 192. https://doi.org/10.3390/antibiotics13020192
Doubravská L, Htoutou Sedláková M, Fišerová K, Klementová O, Turek R, Langová K, Kolář M. Bacterial Community- and Hospital-Acquired Pneumonia in Patients with Critical COVID-19—A Prospective Monocentric Cohort Study. Antibiotics. 2024; 13(2):192. https://doi.org/10.3390/antibiotics13020192
Chicago/Turabian StyleDoubravská, Lenka, Miroslava Htoutou Sedláková, Kateřina Fišerová, Olga Klementová, Radovan Turek, Kateřina Langová, and Milan Kolář. 2024. "Bacterial Community- and Hospital-Acquired Pneumonia in Patients with Critical COVID-19—A Prospective Monocentric Cohort Study" Antibiotics 13, no. 2: 192. https://doi.org/10.3390/antibiotics13020192







