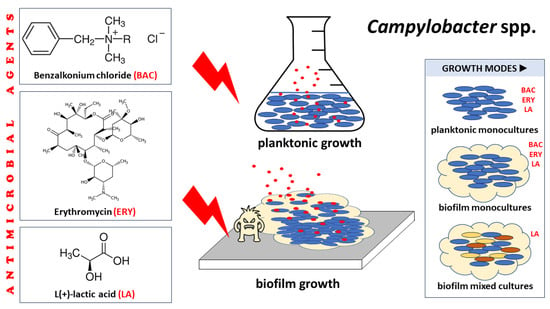
Comparative Assessment of the Antibacterial and Antibiofilm Actions of Benzalkonium Chloride, Erythromycin, and L(+)-Lactic Acid against Raw Chicken Meat Campylobacter spp. Isolates
Abstract
1. Introduction
2. Results
2.1. The Determination of the MICs, MBCs, and MBICs of Antimicrobial Agents against Campylobacter Cultures
2.2. The Inhibitory Effect of LA against the Biofilm and Planktonic Growth of Each Member Isolate of the Mixed-Culture Campylobacter Consortia
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Agents (Chemicals) and Preparation of Their Stock Solutions
4.2. The Preparation of Sterile Chicken Juice (CJ)
4.3. Campylobacter Isolates and the Preparation of Their Working Cultures
4.4. The Determination of the MICs and MBCs of Antimicrobial Agents against Campylobacter Planktonic Monocultures
4.5. The Determination of the MBICs of Antimicrobial Agents against Campylobacter Monocultures
4.6. The Determination of the MBICs of LA against Campylobacter Mixed Cultures
4.7. The Selective Quantification of the Planktonic and Biofilm Populations of Each Campylobacter Isolate of Mixed Cultures (Consortia), with or without LA
4.8. Statistics
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nastasijevic, I.; Proscia, F.; Boskovic, M.; Glisic, M.; Blagojevic, B.; Sorgentone, S.; Kirbis, A.; Ferri, M. The European Union control strategy for Campylobacter spp. in the broiler meat chain. J. Food Saf. 2020, 40, e12819. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 zoonoses report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED). Campylobacter (Campylobacteriosis). 2021. Available online: https://www.cdc.gov/campylobacter/ (accessed on 3 February 2024).
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Tobolowsky, F.; Laughlin, M.; Aubert, R.; Payne, D. Campylobacteriosis|CDC Yellow Book 2024. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/campylobacteriosis (accessed on 3 February 2024).
- Berndtson, E.; Emanuelson, U.; Engvall, A.; Danielsson-Tham, M.-L.A. 1-year epidemiological study of campylobacters in 18 Swedish chicken farms. Prev. Vet. Med. 1996, 26, 167–185. [Google Scholar] [CrossRef]
- Newell, D.G.; Fearnley, C. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003, 69, 4343–4351. [Google Scholar] [CrossRef]
- Berndtson, E.; Tivemo, M.; Engvall, A. Distribution and numbers of Campylobacter in newly slaughtered broiler chickens and hens. Int. J. Food Microbiol. 1992, 15, 45–50. [Google Scholar] [CrossRef]
- Berrang, M.E.; Buhr, R.J.; Cason, J.A. Campylobacter recovery from external and internal organs of commercial broiler carcass prior to scalding. Poult. Sci. 2000, 79, 286–290. [Google Scholar] [CrossRef]
- Wallace, J.S.; Stanley, K.N.; Currie, J.E.; Diggle, P.J.; Jones, K.S. Seasonality of thermophilic Campylobacter populations in chickens. J. Appl. Microbiol. 1997, 82, 219–224. [Google Scholar] [CrossRef]
- Hodges, L.M.; Carrillo, C.D.; Upham, J.; Borza, A.; Eisebraun, M.; Kenwell, R.; Mutschall, S.K.; Haldane, D.; Schleihauf, E.; Taboada, E.N. A strain comparison of Campylobacter isolated from retail poultry and human clinical cases in Atlantic Canada. PLoS ONE 2019, 14, e0215928. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Ferreira, V.; Truninger, M.; Maia, R.; Teixeira, P. Cross-contamination events of Campylobacter spp. in domestic kitchens associated with consumer handling practices of raw poultry. Int. J. Food Microbiol. 2020, 338, 108984. [Google Scholar] [CrossRef]
- Alter, T.; Scherer, K. Stress Response of Campylobacter spp. and its role in food processing. J. Vet. Med. 2006, 53, 351–357. [Google Scholar] [CrossRef]
- Pokhrel, D.; Thames, H.T.; Zhang, L.; Dinh, T.T.N.; Schilling, W.; White, S.B.; Ramachandran, R.; Theradiyil Sukumaran, A. Roles of aerotolerance, biofilm formation, and viable but non-culturable state in the survival of Campylobacter jejuni in poultry processing environments. Microorganisms 2022, 10, 2165. [Google Scholar] [CrossRef]
- Kalmokoff, M.; Lanthier, P.; Tremblay, T.-L.; Foss, M.; Lau, P.C.; Sanders, G.; Austin, J.; Kelly, J.; Szymanski, C.M. Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J. Bacteriol. 2006, 188, 4312–4320. [Google Scholar] [CrossRef]
- Skirrow, M.B. Campylobacter enteritis: A “new” disease. BMJ 1977, 2, 9–11. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- Washington, J.A., 2nd; Wilson, W.R. Erythromycin: A microbial and clinical perspective after 30 years of clinical use (1). Mayo Clin. Proc. 1985, 60, 189–203. [Google Scholar] [CrossRef]
- Qin, X.; Wang, X.; Shen, Z. The rise of antibiotic resistance in Campylobacter. Curr. Opin. Gastroenterol. 2023, 39, 9–15. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 3 February 2024).
- Karatzas, K.-A.G.; Randall, L.P.; Webber, M.A.; Piddock, L.; Humphrey, T.J.; Woodward, M.J.; Coldham, N.G. Phenotypic and proteomic characterization of multiply antibiotic-resistant variants of Salmonella enterica serovar Typhimurium selected following exposure to disinfectants. Appl. Environ. Microbiol. 2008, 74, 1508–1516. [Google Scholar] [CrossRef]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef]
- Mavri, A.; Ribič, U.; Smole Možina, S. The biocide and antibiotic resistance in Campylobacter jejuni and Campylobacter coli. Food Eng. Ser. 2015, 271, 269–283. [Google Scholar] [CrossRef]
- White, D.G.; McDermott, P.F. Biocides, drug resistance and microbial evolution. Curr. Opin. Microbiol. 2001, 4, 313–317. [Google Scholar] [CrossRef]
- Cadena, M.; Kelman, T.; Marco, M.L.; Pitesky, M. Understanding antimicrobial resistance (AMR) profiles of Salmonella biofilm and planktonic bacteria challenged with disinfectants commonly used during poultry processing. Foods 2019, 8, 275. [Google Scholar] [CrossRef]
- Capita, R.; Riesco-Peláez, F.; Alonso-Hernando, A.; Alonso-Calleja, C. Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl. Environ. Microbiol. 2014, 80, 1268–1280. [Google Scholar] [CrossRef]
- Ziech, R.E.; Perin, A.P.; Lampugnani, C.; Sereno, M.J.; Viana, C.; Soares, V.M.; Pereira, J.G.; Paes, J.; dos Santos Bersot, L. Biofilm-producing ability and tolerance to industrial sanitizers in Salmonella spp. isolated from Brazilian poultry processing plants. LWT 2016, 68, 85–90. [Google Scholar] [CrossRef]
- Beier, R.C.; Byrd, J.A.; Andrews, K.; Caldwell, D.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Disinfectant and antimicrobial susceptibility studies of the foodborne pathogen Campylobacter jejuni isolated from the litter of broiler chicken houses. Poult. Sci. 2021, 100, 1024–1033. [Google Scholar] [CrossRef]
- Pereira, B.M.P.; Tagkopoulos, I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef]
- Avrain, L.; Allain, L.; Vernozy-Rozand, C.; Kempf, I. Disinfectant susceptibility testing of avian and swine Campylobacter isolates by a filtration method. Vet. Microbiol. 2003, 96, 35–40. [Google Scholar] [CrossRef]
- Carvalho, D.; Menezes, R.; Chitolina, G.Z.; Kunert, C.; Wilsmann, D.E.; Borges, K.A.; Furian, T.Q.; Tadeu, C.; de Souza Moraes, H.L.; Pinheiro, V. Antibiofilm activity of the biosurfactant and organic acids against foodborne pathogens at different temperatures, times of contact, and concentrations. Braz. J. Microbiol. 2022, 53, 1051–1064. [Google Scholar] [CrossRef]
- Šimunović, K.; Zajkoska, S.; Bezek, K.; Klančnik, A.; Barlič Maganja, D.; Smole Možina, S. Comparison of Campylobacter jejuni slaughterhouse and surface-water isolates indicates better adaptation of slaughterhouse isolates to the chicken host environment. Microorganisms 2020, 8, 1693. [Google Scholar] [CrossRef]
- Trachoo, N.; Frank, J.F. Effectiveness of chemical sanitizers against Campylobacter jejuni–containing biofilms. J. Food Prot. 2002, 65, 1117–1121. [Google Scholar] [CrossRef]
- Beier, R.C.; Harvey, R.B.; Hernandez, C.; Andrews, K.; Droleskey, R.E.; Hume, M.E.; Davidson, M.K.; Bodeis-Jones, S.; Young, S.; Anderson, R.C.; et al. Disinfectant and antimicrobial susceptibility profiles of Campylobacter coli isolated in 1998 to 1999 and 2015 from swine and commercial pork chops. J. Food Sci. 2019, 84, 1501–1512. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention strategies to control Campylobacter at different stages of the food chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef]
- Iplikcioglu Cil, G.; Ozdemir, H.; Onaran, B.; Cengiz, G.; Sen, E. Effect of lactic acid and steam treatments on Campylobacter jejuni on chicken skin. Emir. J. Food Agric. 2019, 31, 143. [Google Scholar] [CrossRef]
- Liu, A.; Peng, Z.; Zou, L.; Zhou, K.; Ao, X.; He, L.; Chen, S.; Liu, S. The effects of lactic acid-based spray washing on bacterial profile and quality of chicken carcasses. Food Control 2016, 60, 615–620. [Google Scholar] [CrossRef]
- Ford, E.; Davis, M.; Kim, B.; Katen, T.; Zuelly, S.Μ.S. Impact of antimicrobial carcass washes and processing techniques on quality attributes of beef frankfurters. Foods 2022, 11, 1891. [Google Scholar] [CrossRef]
- Keener, K.M.; Bashor, M.P.; Curtis, P.A.; Sheldon, B.W.; Kathariou, S. Comprehensive review of Campylobacter and poultry processing. Compr. Rev. Food Sci. Food Saf. 2004, 3, 105–116. [Google Scholar] [CrossRef]
- Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. The use of organic acids (lactic and acetic) as a microbial decontaminant during the slaughter of meat animal species: A review. Foods 2021, 10, 2293. [Google Scholar] [CrossRef]
- Byrd, J.A.; Hargis, B.M.; Caldwell, D.J.; Bailey, R.H.; Herron, K.L.; McReynolds, J.L.; Brewer, R.L.; Anderson, R.C.; Bischoff, K.M.; Callaway, T.R.; et al. Effect of lactic acid administration in the drinking water during preslaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poult. Sci. 2001, 80, 278–283. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Cudjoe, K.S.; Kapperud, G. The effect of lactic acid sprays on Campylobacter jejuni inoculated onto poultry carcasses. Acta Vet. Scand. 1991, 32, 491–498. [Google Scholar] [CrossRef]
- Theron, Μ.Μ.; Lues, J.F.R. Organic acids and meat preservation: A review. Food Rev. Int. 2007, 23, 141–158. [Google Scholar] [CrossRef]
- Meredith, H.; McDowell, D.; Bolton, D.J. An evaluation of trisodium phosphate, citric acid and lactic acid cloacal wash treatments to reduce Campylobacter, total viable counts (TVC) and total Enterobacteriaceae counts (TEC) on broiler carcasses during processing. Food Control 2013, 32, 149–152. [Google Scholar] [CrossRef]
- Giaouris, E.; Heir, E.; Desvaux, M.; Hébraud, M.; Møretrø, T.; Langsrud, S.; Doulgeraki, A.; Nychas, G.J.; Kačániová, M.; Czaczyk, K.; et al. Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens. Front. Microbiol. 2015, 6, 841. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic colorimetric proliferation assays: MTT, WST, and resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar] [CrossRef]
- Park, S.F. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int. J. Food Microbiol. 2002, 74, 177–188. [Google Scholar] [CrossRef]
- Pickett, C.L.; Auffenberg, T.; Pesci, E.C.; Sheen, V.L.; Jusuf, S.S. Iron acquisition and hemolysin production by Campylobacter jejuni. Infect. Immun. 1992, 60, 3872–3877. [Google Scholar] [CrossRef]
- Barclay, R. The role of iron in infection. Med. Lab. Sci. 1985, 42, 166–177. [Google Scholar]
- Mavri, A.; Smole Mozina, S. Involvement of efflux mechanisms in biocide resistance of Campylobacter jejuni and Campylobacter coli. J. Med. Microbiol. 2012, 61 Pt 6, 800–808. [Google Scholar] [CrossRef]
- Smole-Možina, S.; Kurinčič, M.; Kramar, A.; Uršič, S.; Katalinić, V. Prevalence and Resistance against Different Antimicrobial Compounds of Campylobacter spp. in/from Retail Poultry Meat. International 55th Meat Industry Conference, Tara Mauntain. 2009. Available online: https://journalmeattechnology.com/index.php/meat_technology/article/view/329/269 (accessed on 3 February 2024).
- Gutiérrez-Martín, C.B.; Yubero, S.; Martínez, S.; Frandoloso, R.; Rodríguez-Ferri, E.F. Evaluation of efficacy of several disinfectants against Campylobacter jejuni strains by a suspension test. Res. Vet. Sci. 2011, 91, e44–e47. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Clinical Breakpoints—Breakpoints and Guidance. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 3 February 2024).
- Beier, R.C.; Harvey, R.B.; Hernandez, C.A.; Hume, M.E.; Andrews, K.; Droleskey, R.E.; Davidson, M.K.; Bodeis-Jones, S.; Young, S.; Duke, S.E.; et al. Interactions of organic acids with Campylobacter coli from swine. PLoS ONE 2018, 13, e0202100. [Google Scholar] [CrossRef]
- Shin, E.; Lee, Y. Characterization of erythromycin-resistant porcine isolates of Campylobacter coli. Microb. Drug Resist. 2010, 16, 231–239. [Google Scholar] [CrossRef]
- Corcoran, D.; Quinn, T.; Cotter, L.; Fanning, S. An investigation of the molecular mechanisms contributing to high-level erythromycin resistance in Campylobacter. Int. J. Antimicrob. Agents 2006, 27, 40–45. [Google Scholar] [CrossRef]
- Kurinčič, M.; Botteldoorn, N.; Herman, L.; Smole Možina, S. Mechanisms of erythromycin resistance of Campylobacter spp. isolated from food, animals and humans. Int. J. Food Microbiol. 2007, 120, 186–190. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Ma, L.; de la Fuente Núñez, C.; Gölz, G.; Lu, X. Effects of meat juice on biofilm formation of Campylobacter and Salmonella. Int. J. Food Microbiol. 2017, 253, 20–28. [Google Scholar] [CrossRef]
- Birk, T.; Ingmer, H.; Andersen, M.T.; Jørgensen, K.; Brøndsted, L. Chicken juice, a food-based model system suitable to study survival of Campylobacter jejuni. Lett. Appl. Microbiol. 2004, 38, 66–71. [Google Scholar] [CrossRef]
- Brown, H.L.; Reuter, M.; Salt, L.J.; Cross, K.L.; Betts, R.P.; van Vliet, A.H.M. Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl. Environ. Microbiol. 2014, 80, 7053–7060. [Google Scholar] [CrossRef]
- Rossi, D.A.; Dumont, C.F.; de Souza Santos, A.C.; de Lourdes Vaz, M.E.; Prado, R.R.; Monteiro, G.P.; da Silva Melo, C.B.; Stamoulis, V.J.; Dos Santos, J.P.; de Melo, R.T. Antibiotic resistance in the alternative lifestyles of Campylobacter jejuni. Front. Cell Infect. 2021, 11, 535757. [Google Scholar] [CrossRef] [PubMed]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Belmehdi, O.; Kounnoun, A.; Cheyadmi, S.; Ouzakar, S.; Senhaji, N.S.; Abrini, J. Evaluation of the combined effect of antibiotics and essential oils against Campylobacter multidrug resistant strains and their biofilm formation. S. Afr. J. Bot. 2022, 150, 451–465. [Google Scholar] [CrossRef]
- Brizzolara, D.; Cantow, H.-J.; Diederichs, K.; Keller, E.; Domb, A.J. Mechanism of the stereocomplex formation between enantiomeric poly(lactide)s. Macromol. 1996, 2, 191–197. [Google Scholar] [CrossRef]
- Bouchoux, A.; Roux-de Balmann, H.; Lutin, F. Investigation of nanofiltration as a purification step for lactic acid production processes based on conventional and bipolar electrodialysis operations. Sep. Purif. Technol. 2006, 52, 266–273. [Google Scholar] [CrossRef]
- Hábová, V.; Melzoch, K.; Rychtera, M.; Sekavová, B. Electrodialysis as a useful technique for lactic acid separation from a model solution and a fermentation broth. Desalination 2004, 162, 361–372. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Q.; Zhao, W.; Ma, H.; Sakata, K. Extraction and purification of lactic acid from fermentation broth by esterification and hydrolysis method. Sep. Purif. Technol. 2006, 49, 43–48. [Google Scholar] [CrossRef]
- Yuan, L.; Hansen, M.F.; Røder, H.L.; Wang, N.; Burmølle, M.; He, G. Mixed-species biofilms in the food industry: Current knowledge and novel control strategies. Crit. Rev. Food Sci. Nutr. 2019, 60, 2277–2293. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, Q.; Zhang, J.; Ma, Z.; Wang, J.; Nie, X.; Ding, Y.; Xue, L.; Chen, M.; Wu, S.; et al. Campylobacter jejuni biofilm formation under aerobic conditions and inhibition by ZnO nanoparticles. Front. Microbiol. 2020, 11, 207. [Google Scholar] [CrossRef]
- Teh, A.H.T.; Lee, S.M.; Dykes, G.A. Association of some Campylobacter jejuni with Pseudomonas aeruginosa biofilms increases attachment under conditions mimicking those in the environment. PLoS ONE 2019, 14, e0215275. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou, D.; Simoni, M.; Vafeiadis, G.; Kaftantzis, N.-M.; Giaouris, E. Prevalence of Campylobacter spp., Salmonella spp., and Listeria monocytogenes, and population levels of food safety indicator microorganisms in retail raw chicken meat and ready-to-eat fresh leafy greens salads sold in Greece. Foods 2023, 12, 4502. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Vetas, D.; Dimitropoulou, E.; Mitropoulou, G.; Kourkoutas, Y.; Giaouris, E. Disinfection efficiencies of sage and spearmint essential oils against planktonic and biofilm Staphylococcus aureus cells in comparison with sodium hypochlorite. Int. J. Food Microbiol. 2017, 257, 19–25. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). EUCAST Reading Guide for Broth Microdilution. 2022, p. 9. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Reading_guide_BMD_v_4.0_2022.pdf (accessed on 3 February 2024).
- Coban, A.Y. Rapid determination of methicillin resistance among Staphylococcus aureus clinical isolates by colorimetric methods. J. Clin. Microbiol. 2012, 50, 2191–2193. [Google Scholar] [CrossRef]
- Dimou, I.; Dritsas, S.; Aggelopoulou, P.; Vassilatou, K.; Damianaki, S.; Giaouris, E. Development of a herbal mouthwash containing a mixture of essential oils and plant extracts and in vitro testing of its antimicrobial efficiency against the planktonic and biofilm-enclosed cariogenic bacterium Streptococcus mutans. Biofouling 2021, 37, 397–409. [Google Scholar] [CrossRef]




| Antimicrobial Agent | Campylobacter/Consortium Code | MIC 1 | MBC 2 | MIC | MBC | MBIC 3 | |
|---|---|---|---|---|---|---|---|
| Species | μg/mL | ||||||
| in MH 4 | in MH-HB 5 | in MH-CJ 6 | |||||
| BAC | ATCC 33291 | C. jejuni | 2 | 4 | 4 | 8 | nbf 7 |
| CAMP_005 | C. coli | 2 | 4 | 8 | 16 | 2 | |
| CAMP_022 | C. jejuni | 2 | 2 | 4 | 8 | 2 | |
| CAMP_025 | C. coli | 2 | 4 | 8 | 16 | 4 | |
| CAMP_048 | C. jejuni | 2 | 2 | 4 | 8 | 1 | |
| CAMP_071 | C. coli | 0.5 | 1 | 2 | 4 | nbf | |
| CAMP_074 | C. jejuni | 4 | 4 | 16 | 32 | nbf | |
| CAMP_083 | C. coli | 8 | 8 | 16 | 32 | 16 | |
| CAMP_091 | C. jejuni | 8 | 8 | 16 | 32 | 8 | |
| CAMP_097 | C. coli | 1 | 1 | 8 | 8 | nbf | |
| CAMP_114 | C. jejuni | 2 | 4 | 1 | 1 | 2 | |
| CAMP_130 | C. jejuni | 4 | 4 | 8 | 16 | 8 | |
| CAMP_132 | C. jejuni | 1 | 1 | 4 | 4 | nbf | |
| ERY | ATCC_33291 | C. jejuni | 2 | 2 | 1 | 2 | nbf |
| CAMP_005 | C. coli | 2 | 4 | 2 | 4 | 2 | |
| CAMP_022 | C. jejuni | 4 | 4 | 1 | 2 | 1 | |
| CAMP_025 | C. coli | 4 | 16 | 4 | 8 | 2 | |
| CAMP_048 | C. jejuni | 1 | 2 | 0.5 | 1 | 0.5 | |
| CAMP_071 | C. coli | 0.5 | 0.5 | 0.25 | 0.25 | nbf | |
| CAMP_074 | C. jejuni | 256 | 256 | 4 | 64 | nbf | |
| CAMP_083 | C. coli | 1024 | 1024 | 32 | 128 | 32 | |
| CAMP_091 | C. jejuni | 1024 | 1024 | 16 | 64 | 32 | |
| CAMP_097 | C. coli | 2 | 4 | 1 | 2 | nbf | |
| CAMP_114 | C. jejuni | 4 | 4 | 0.5 | 1 | 0.25 | |
| CAMP_130 | C. jejuni | 0.5 | 0.5 | 0.25 | 0.5 | 0.25 | |
| CAMP_132 | C. jejuni | 2 | 2 | 1 | 1 | nbf | |
| LA | ATCC_33291 | C. jejuni | 1024 | 1024 | 2048 | 2048 | nbf |
| CAMP_005 | C. coli | 1024 | 1024 | 2048 | 2048 | 1024 | |
| CAMP_022 | C. jejuni | 1024 | 1024 | 2048 | 2048 | 1024 | |
| CAMP_025 | C. coli | 1024 | 1024 | 1024 | 1024 | 1024 | |
| CAMP_048 | C. jejuni | 2048 | 2048 | 2048 | 2048 | 1024 | |
| CAMP_071 | C. coli | 1024 | 1024 | 2048 | 2048 | nbf | |
| CAMP_074 | C. jejuni | 1024 | 1024 | 2048 | 2048 | nbf | |
| CAMP_083 | C. coli | 1024 | 2048 | 2048 | 2048 | 2048 | |
| CAMP_091 | C. jejuni | 2048 | 2048 | 2048 | 2048 | 1024 | |
| CAMP_097 | C. coli | 1024 | 1024 | 2048 | 2048 | nbf | |
| CAMP_114 | C. jejuni | 1024 | 1024 | 2048 | 2048 | 1024 | |
| CAMP_130 | C. jejuni | 1024 | 1024 | 2048 | 2048 | 2048 | |
| CAMP_132 | C. jejuni | 1024 | 1024 | 2048 | 2048 | nbf | |
| CONS1 (CAMP_048/083/130) | C. jejuni/C. coli/C. jejuni | nd 8 | nd | nd | nd | 4096 | |
| CONS2 (CAMP_022/091/130) | C. jejuni/C. jejuni/C. jejuni | nd | nd | nd | nd | 4096 | |
| CONS3 (CAMP_005/083/130) | C. jejuni/C. coli/C. jejuni | nd | nd | nd | nd | 4096 | |
| CONS4 (CAMP_048/074/130) | C. jejuni/C. jejuni/C. jejuni | nd | nd | nd | nd | 4096 | |
| Consortium Code | Group A 1 | Group B 2 | Group C 3 |
|---|---|---|---|
| CONS1 | C. jejuni (CAMP_130) | C. coli (CAMP_083) | C. jejuni (CAMP_048) |
| CONS2 | C. jejuni (CAMP_130) | C. jejuni (CAMP_091) | C. jejuni (CAMP_022) |
| CONS3 | C. jejuni (CAMP_130) | C. coli (CAMP_083) | C. jejuni (CAMP_005) |
| CONS4 | C. jejuni (CAMP_130) | C. jejuni (CAMP_074) | C. jejuni (CAMP_048) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostoglou, D.; Vass, A.; Giaouris, E. Comparative Assessment of the Antibacterial and Antibiofilm Actions of Benzalkonium Chloride, Erythromycin, and L(+)-Lactic Acid against Raw Chicken Meat Campylobacter spp. Isolates. Antibiotics 2024, 13, 201. https://doi.org/10.3390/antibiotics13030201
Kostoglou D, Vass A, Giaouris E. Comparative Assessment of the Antibacterial and Antibiofilm Actions of Benzalkonium Chloride, Erythromycin, and L(+)-Lactic Acid against Raw Chicken Meat Campylobacter spp. Isolates. Antibiotics. 2024; 13(3):201. https://doi.org/10.3390/antibiotics13030201
Chicago/Turabian StyleKostoglou, Dimitra, Athina Vass, and Efstathios Giaouris. 2024. "Comparative Assessment of the Antibacterial and Antibiofilm Actions of Benzalkonium Chloride, Erythromycin, and L(+)-Lactic Acid against Raw Chicken Meat Campylobacter spp. Isolates" Antibiotics 13, no. 3: 201. https://doi.org/10.3390/antibiotics13030201
APA StyleKostoglou, D., Vass, A., & Giaouris, E. (2024). Comparative Assessment of the Antibacterial and Antibiofilm Actions of Benzalkonium Chloride, Erythromycin, and L(+)-Lactic Acid against Raw Chicken Meat Campylobacter spp. Isolates. Antibiotics, 13(3), 201. https://doi.org/10.3390/antibiotics13030201