Comparison of IncK-blaCMY-2 Plasmids in Extended-Spectrum Cephalosporin-Resistant Escherichia coli Isolated from Poultry and Humans in Denmark, Finland, and Germany
Abstract
1. Introduction
2. Results
2.1. Phenotypic Antimicrobial-Resistance Profiles
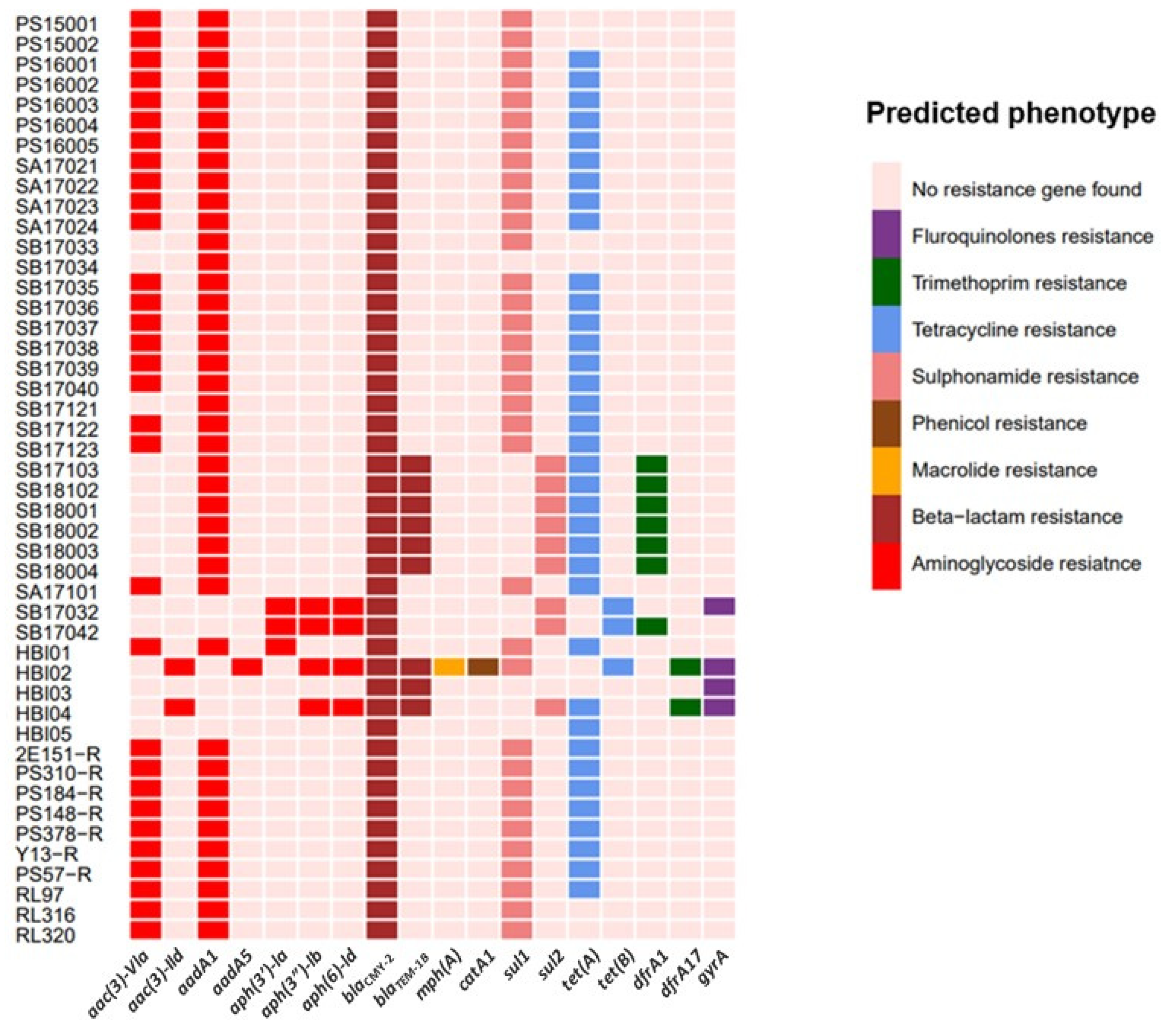
2.2. High Prevalence of Antimicrobial-Resistance Determinants
2.3. Results from Conjugation Assays
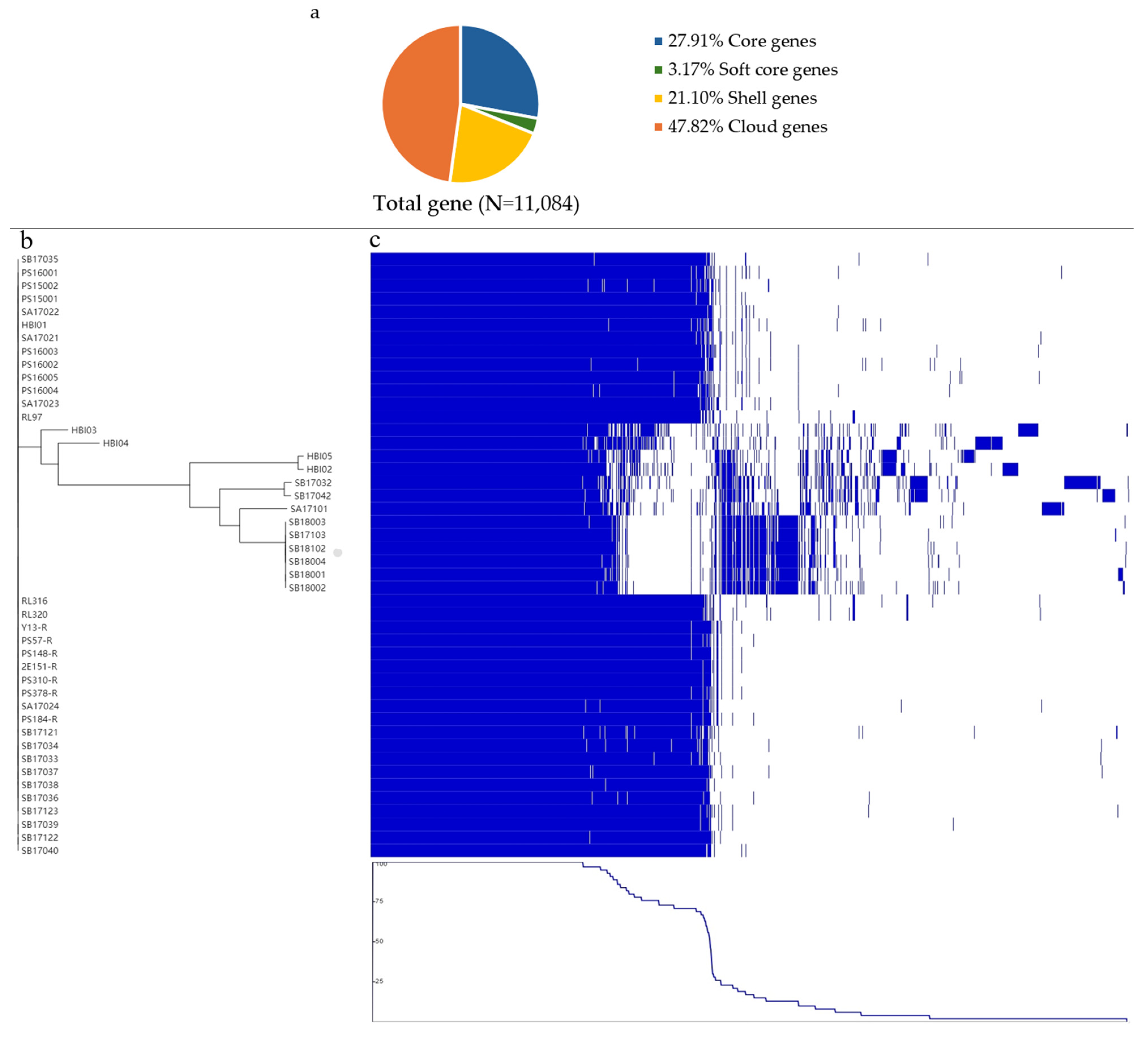
2.4. Pangenome Analysis
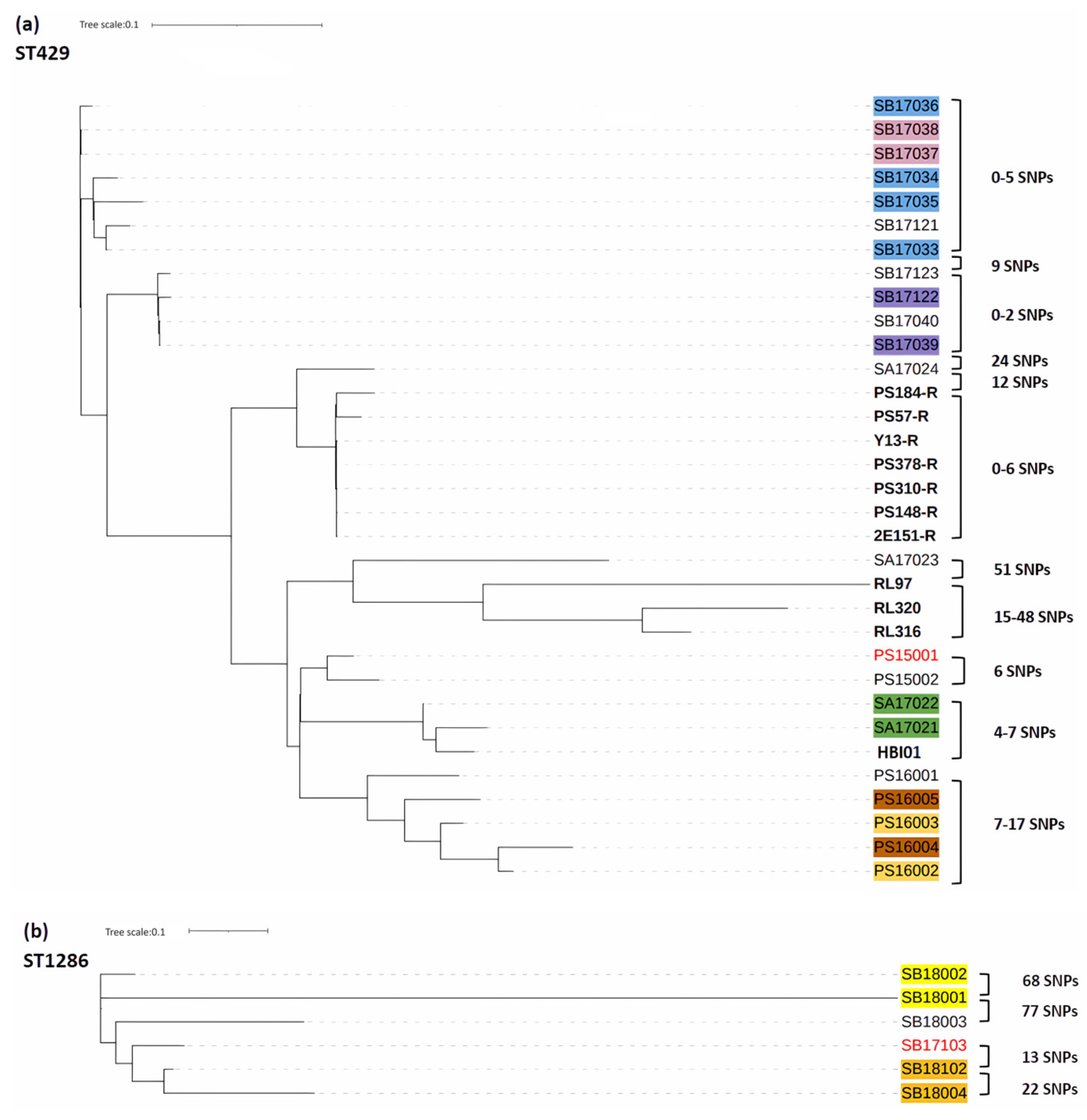
2.5. Phylogenetic Tree Analysis
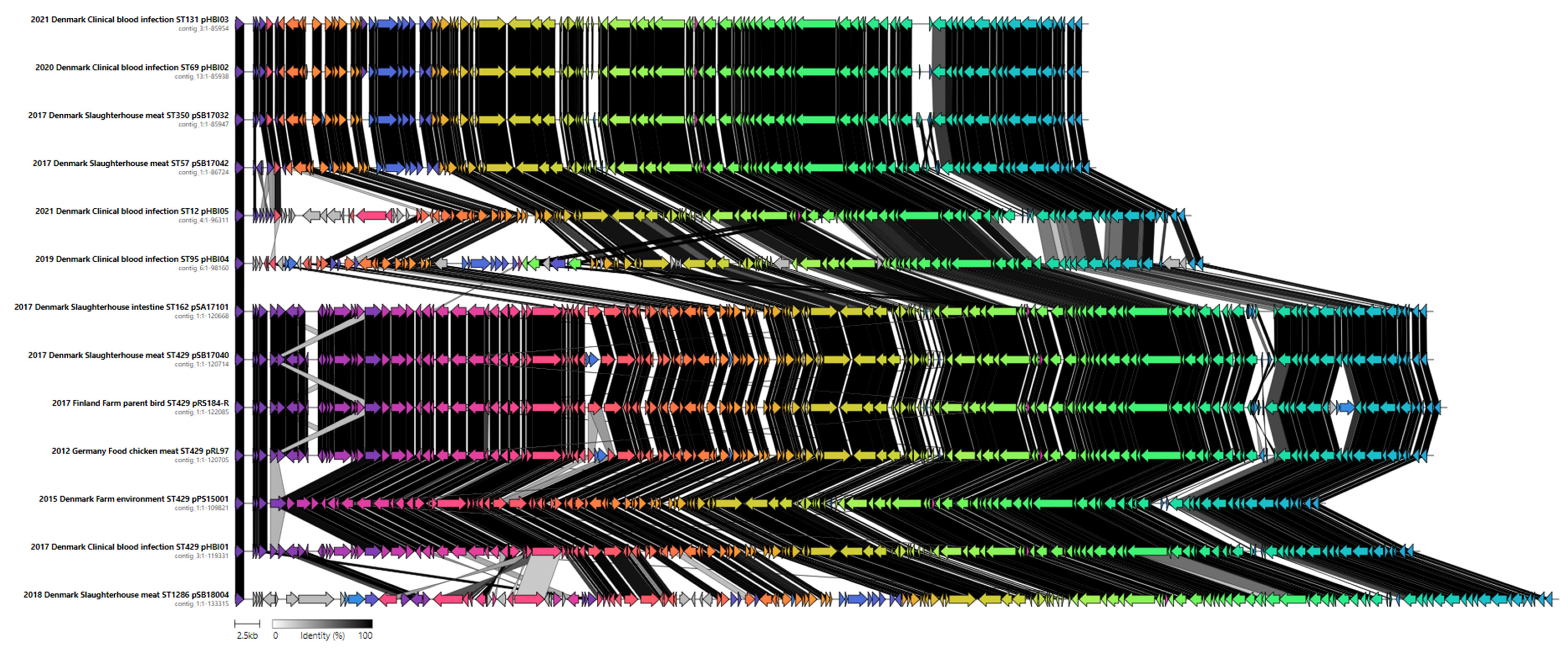
2.6. Plasmid Characterization
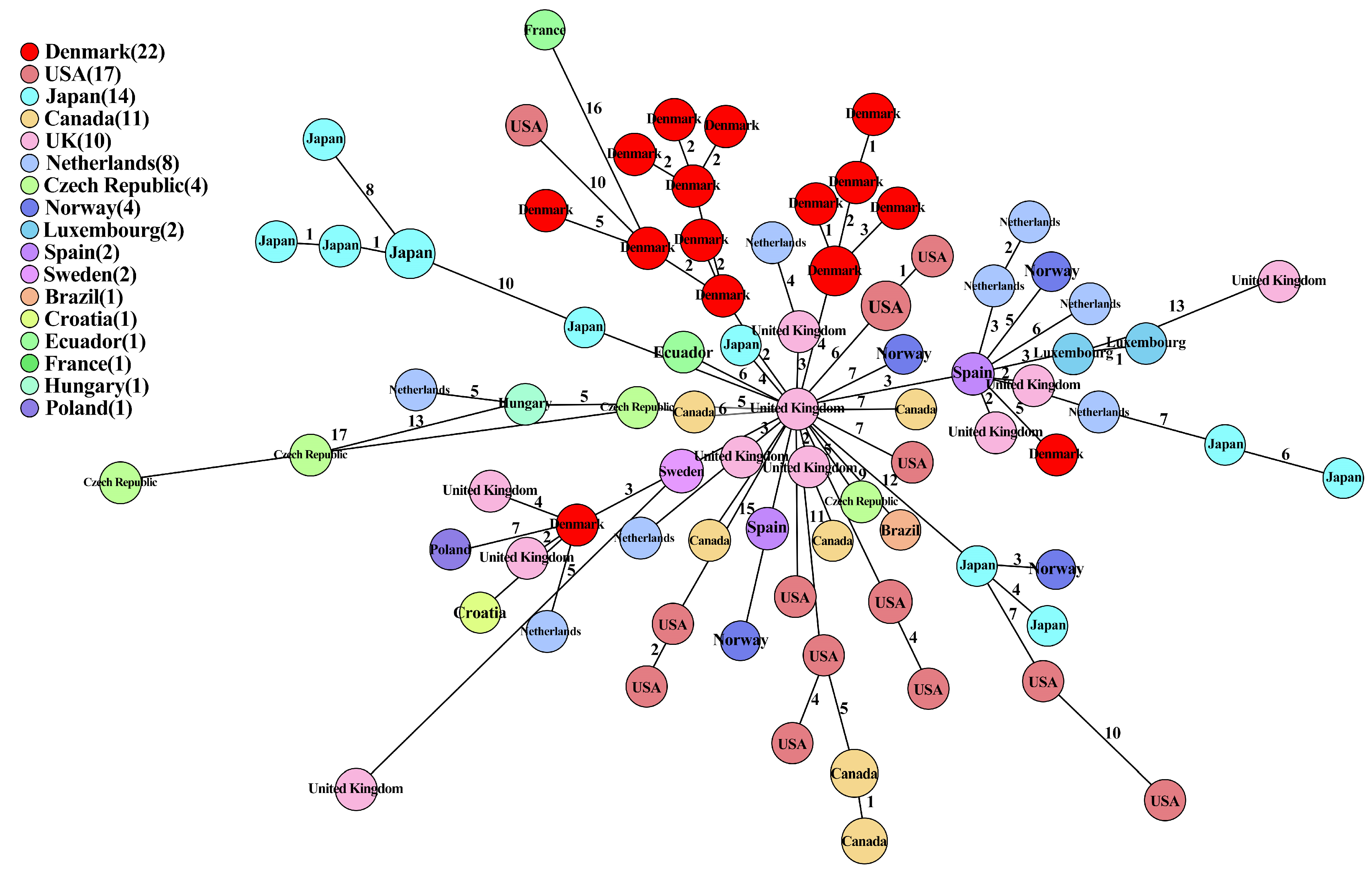
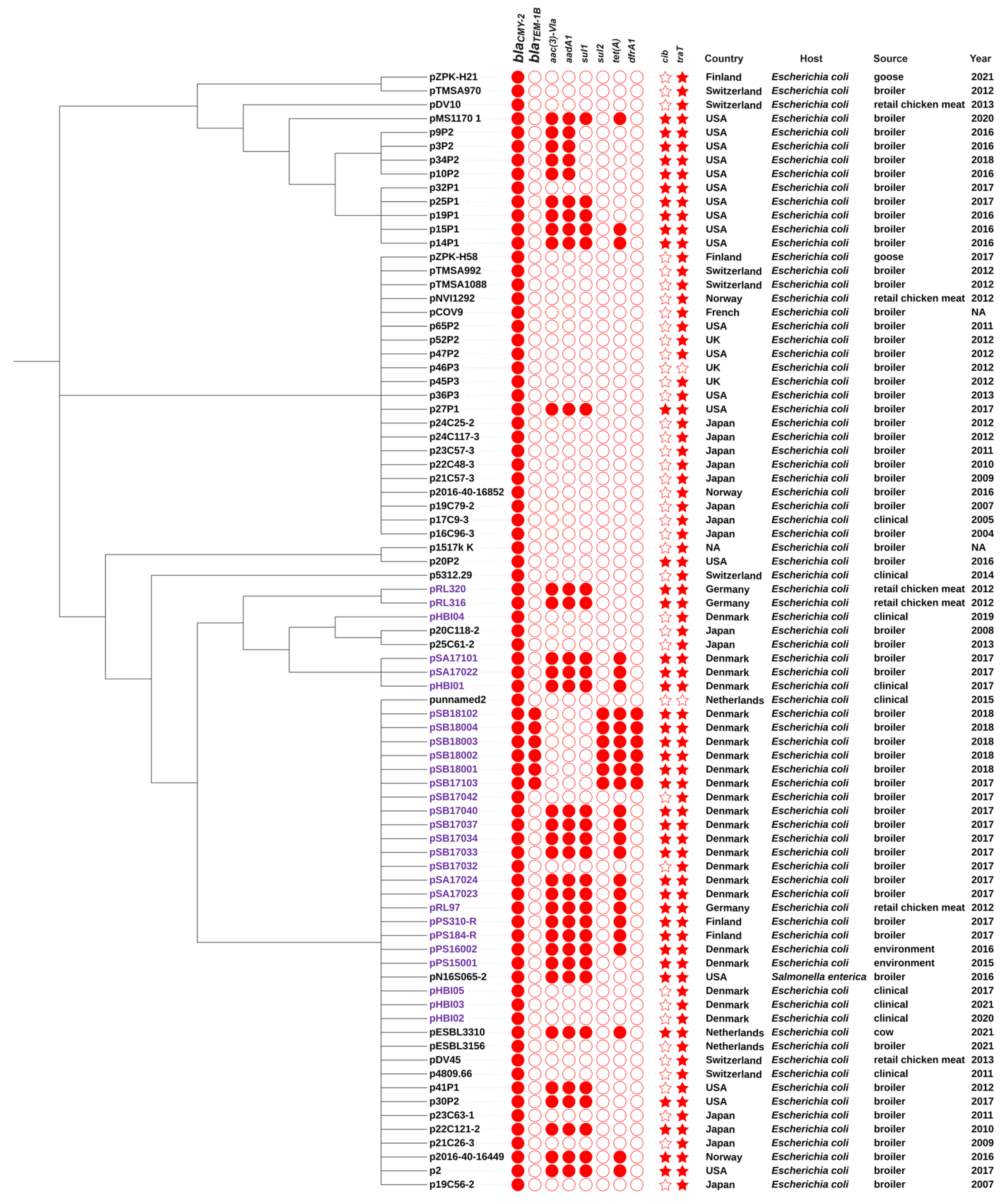
2.7. Comparison of IncK-blaCMY-2 Plasmids from This Study and Public Databases
3. Discussion
4. Materials and Methods
4.1. IncK-blaCMY-2-Positive ESC-Resistant E. coli Isolates Included in This Study
4.2. Antimicrobial Susceptibility Testing
4.3. Plasmid Conjugation
4.4. Genome Annotation and Pangenome Analysis
4.5. In Silico Analysis for Sequence Type and Resistance Genes
4.6. Isolate Phylogenetic Analysis
4.7. Plasmid Sequencing
4.8. Plasmid Comparison
4.9. Plasmid Phylogenetic Tree
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO List of Critically Important Antimicrobials for Human Medicine: 6th Revision. 2019. Available online: https://www.who.int/publications-detail-redirect/9789241515528 (accessed on 23 June 2023).
- European Medicines Agency (EMA). Categorization of Antibiotics for Use in Animals. 2019. Available online: https://fve.org/cms/wp-content/uploads/infographic-categorisation-antibiotics-use-animals-prudent-responsible-use_en2.pdf (accessed on 25 March 2024).
- Imkamp, F.; Kolesnik-Goldmann, N.; Bodendoerfer, E.; Zbinden, R.; Mancini, S. Detection of Extended-Spectrum β-Lactamases (ESBLs) and AmpC in Class A and Class B Carbapenemase-Producing Enterobacterales. Microbiol. Spectr. 2022, 10, e02137-22. [Google Scholar] [CrossRef] [PubMed]
- Meini, S.; Tascini, C.; Cei, M.; Sozio, E.; Rossolini, G.M. AmpC β-lactamase-producing Enterobacterales: What a clinician should know. Infection 2019, 47, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, V.D.C.; Kumar, M.; Das, N.G.N. AmpC and Extended Spectrum Beta Lactamase Producing Isolates of E. coli, Klebsiella spp. and P. mirabilis in a Tertiary Care Center and their Sensitivity to Other Antibiotics. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 868–877. [Google Scholar] [CrossRef]
- Nesse, L.L.; Mo, S.S.; Ramstad, S.N.; Witsø, I.L.; Sekse, C.; Bruvoll, A.E.E.; Urdahl, A.M.; Vestby, L.K. The Effect of Antimicrobial Resistance Plasmids Carrying blaCMY-2 on Biofilm Formation by Escherichia coli from the Broiler Production Chain. Microorganisms 2021, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-H.; Lee, J.-J.; Wang, M.-H.; Chu, C. Genetic analysis and plasmid-mediated blaCMY-2 in Salmonella and Shigella and the Ceftriaxone Susceptibility regulated by the ISEcp-1 tnpA-blaCMY-2-blc-sugE. J. Microbiol. Immunol. Infect. 2021, 54, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Hassen, B.; Abbassi, M.S.; Ruiz-Ripa, L.; Mama, O.M.; Hassen, A.; Torres, C.; Hammami, S. High prevalence of mcr-1 encoding colistin resistance and first identification of blaCTX-M-55 in ESBL/CMY-2-producing Escherichia coli isolated from chicken faeces and retail meat in Tunisia. Int. J. Food Microbiol. 2020, 318, 108478. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.-R.; Wei, B.; Cha, S.-Y.; Shang, K.; Zhang, J.-F.; Jang, H.-K.; Kang, M. Characterization of Extended-Spectrum Cephalosporin (ESC) Resistance in Salmonella Isolated from Chicken and Identification of High Frequency Transfer of blaCMY-2 Gene Harboring Plasmid In Vitro and In Vivo. Animals 2021, 11, 1778. [Google Scholar] [CrossRef]
- Awosile, B.; Reyes-Velez, J.; Cuesta-Astroz, Y.; Rodríguez-Lecompte, J.C.; Saab, M.E.; Heider, L.C.; Keefe, G.; Sánchez, J.; McClure, J.T. Whole-genome sequence analysis of 4 fecal blaCMY-2-producing Escherichia coli isolates from Holstein dairy calves. J. Dairy Sci. 2020, 103, 877–883. [Google Scholar] [CrossRef]
- Marchetti, V.M.; Bitar, I.; Mercato, A.; Nucleo, E.; Marchesini, F.; Mancinelli, M.; Prati, P.; Scarsi, G.S.; Hrabak, J.; Pagani, L.; et al. Deadly Puppy Infection Caused by an MDR Escherichia coli O39 blaCTX-M-15, blaCMY-2, blaDHA-1, and aac (6)-Ib-cr–Positive in a Breeding Kennel in Central Italy. Front. Microbiol. 2020, 11, 584. [Google Scholar] [CrossRef]
- Athanasakopoulou, Z.; Tsilipounidaki, K.; Sofia, M.; Chatzopoulos, D.C.; Giannakopoulos, A.; Karakousis, I.; Giannakis, V.; Spyrou, V.; Touloudi, A.; Satra, M.; et al. Poultry and wild birds as a reservoir of CMY-2 producing Escherichia coli: The first large-scale study in Greece. Antibiotics 2021, 10, 235. [Google Scholar] [CrossRef]
- Carvalho, I.; Cunha, R.; Martins, C.; Martínez-Álvarez, S.; Chenouf, N.S.; Pimenta, P.; Pereira, A.R.; Ramos, S.; Sadi, M.; Martins, A.; et al. Antimicrobial resistance genes and diversity of clones among faecal ESBL-producing Escherichia coli isolated from healthy and sick dogs living in Portugal. Antibiotics 2021, 10, 1013. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, M.; Irrgang, A.; Roschanski, N.; Brenner Michael, G.; Hamprecht, A.; Rieber, H.; Käsbohrer, A.; Schwarz, S.; Rösler, U.; Kreienbrock, L.; et al. Whole genome analyses of CMY-2-producing Escherichia coli isolates from humans, animals, and food in Germany. BMC Genom. 2018, 19, 601. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Saras, E.; Métayer, V.; Médaille, C.; Madec, J.-Y. High prevalence of blaCTX-M-1/IncI1/ST3 and blaCMY-2/IncI1/ST2 plasmids in healthy urban dogs in France. Antimicrob. Agents Chemother. 2014, 58, 5358–5362. [Google Scholar] [CrossRef] [PubMed]
- Kurittu, P.E.; Khakipoor, B.; Brouwer, M.; Heikinheimo, A. Plasmids conferring resistance to extended-spectrum beta-lactamases including a rare IncN+ IncR multireplicon carrying blaCTX-M-1 in Escherichia coli recovered from migrating barnacle geese (Branta leucopsis). Open Res. Eur. 2021, 1, 46. [Google Scholar] [CrossRef] [PubMed]
- Myrenås, M.; Slettemeås, J.S.; Thorsteinsdottir, T.R.; Bengtsson, B.; Börjesson, S.; Nilsson, O.; Landén, A.; Sunde, M. Clonal spread of Escherichia coli resistant to cephalosporins and quinolones in the Nordic broiler production. Vet. Microbiol. 2018, 213, 123–128. [Google Scholar] [CrossRef] [PubMed]
- DANMAP 2016—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. 2017. Available online: https://www.danmap.org/reports/2016 (accessed on 23 December 2023).
- Roer, L.; Overballe-Petersen, S.; Hansen, F.; Johannesen, T.B.; Stegger, M.; Bortolaia, V.; Leekitcharoenphon, P.; Korsgaard, H.B.; Seyfarth, A.M.; Mossong, J.; et al. ST131 fimH22 Escherichia coli isolate with a blaCMY-2/IncI1/ST12 plasmid obtained from a patient with bloodstream infection: Highly similar to E. coli isolates of broiler origin. J. Antimicrob. Chemother. 2019, 74, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Che, M.; Birk, T.; Hansen, L.T. Prevalence and Transmission of Extended-Spectrum Cephalosporin (ESC) Resistance Genes in Escherichia coli Isolated from Poultry Production Systems and Slaughterhouses in Denmark. Antibiotics 2023, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- Golden, A.R.; Karlowsky, J.A.; Walkty, A.; Baxter, M.R.; Denisuik, A.J.; McCracken, M.; Mulvey, M.R.; Adam, H.J.; Bay, D.; Zhanel, G.G. Comparison of phenotypic antimicrobial susceptibility testing results and WGS-derived genotypic resistance profiles for a cohort of ESBL-producing Escherichia coli collected from Canadian hospitals: CANWARD 2007–2018. J. Antimicrob. Chemother. 2021, 76, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Oikarainen, P.E.; Pohjola, L.K.; Pietola, E.S.; Heikinheimo, A. Direct vertical transmission of ESBL/pAmpC-producing Escherichia coli limited in poultry production pyramid. Vet. Microbiol. 2019, 231, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.H.; Bortolaia, V.; Nielsen, C.A.; Nielsen, J.B.; Schønning, K.; Agersø, Y.; Guardabassi, L. Host-specific patterns of genetic diversity among IncI1-Iγ and IncK plasmids encoding CMY-2 β-lactamase in Escherichia coli isolates from humans, poultry meat, poultry, and dogs in Denmark. Appl. Environ. Microbiol. 2016, 82, 4705–4714. [Google Scholar] [CrossRef]
- Hong, J.S.; Song, W.; Park, H.-M.; Oh, J.-Y.; Chae, J.-C.; Jeong, S.; Jeong, S.H. Molecular characterization of fecal extended-spectrum β-lactamase-and AmpC β-lactamase-producing Escherichia coli from healthy companion animals and cohabiting humans in South Korea. Front. Microbiol. 2020, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.M.; Zakaria, A.S.; Edward, E.A. Genomic Characterization of International High-Risk Clone ST410 Escherichia coli Co-Harboring ESBL-Encoding Genes and blaNDM-5 on IncFIA/IncFIB/IncFII/IncQ1 Multreplicon Plasmid and Carrying a Chromosome-Borne blaCMY-2 from Egypt. Antibiotics 2022, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Aldea, I.; Gibello, A.; Hernández, M.; Leekitcharoenphon, P.; Bortolaia, V.; Moreno, M.A. Clonal and plasmid-mediated flow of ESBL/AmpC genes in Escherichia coli in a commercial laying hen farm. Vet. Microbiol. 2022, 270, 109453. [Google Scholar] [CrossRef] [PubMed]
- DANMAP 2017—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. 2018. Available online: https://www.danmap.org/reports/2017 (accessed on 16 January 2023).
- Poulsen, L.L.; Bisgaard, M.; Christensen, H. Prevalence of Potential Pathogenic and Antimicrobial Resistant Escherichia coli in Danish Broilers. Antibiotics 2023, 12, 344. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.W.; Avery, B.P.; Parmley, E.J.; Irwin, R.J.; Reid-Smith, R.J.; Deckert, A.E.; Finley, R.L.; Daignault, D.; Zhanel, G.G.; Mulvey, M.R.; et al. A One Health Genomic Investigation of Gentamicin Resistance in Escherichia coli from Human and Chicken Sources in Canada, 2014 to 2017. Antimicrob. Agents Chemother. 2022, 66, e00677-22. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Van Kessel, J.A.S.; Haley, B.J. Genome sequences of antibiotic-resistant Escherichia coli isolated from veal calves in the USA. J. Glob. Antimicrob. 2021, 26, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.-W.; Do, K.-H.; Shin, M.-K.; Lee, W.-K.; Lee, W.-K. Comparative genetic characterization of CMY-2-type beta-lactamase producing pathogenic Escherichia coli isolated from humans and pigs suffering from diarrhea in Korea. Ann. Clin. Microbiol. 2023, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Heisig, P.; Schedletzky, H.; Falkenstein-Paul, H. Mutations in the gyrA gene of a highly fluoroquinolone-resistant clinical isolate of Escherichia coli. Antimicrob. Agents Chemother. 1993, 37, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Regeimbal, J.M.; Jacobs, A.C.; Corey, B.W.; Henry, M.S.; Thompson, M.G.; Pavlicek, R.L.; Quinones, J.; Hannah, R.M.; Ghebremedhin, M.; Crane, N.J.; et al. Fate of CMY-2-encoding plasmids introduced into the human fecal microbiota by exogenous Escherichia coli. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Oladeinde, A.; Cook, K.; Lakin, S.M.; Woyda, R.; Abdo, Z.; Looft, T.; Herrington, K.; Zock, G.; Lawrence, J.P.; Thomas, J.C.; et al. Horizontal gene transfer and acquired antibiotic resistance in Salmonella enterica serovar Heidelberg following in vitro incubation in broiler ceca. Appl. Environ. Microbiol. 2019, 85, e01903-19. [Google Scholar] [CrossRef]
- Li, C.; Tyson, G.H.; Hsu, C.-H.; Harrison, L.; Strain, E.; Tran, T.-T.; Tillman, G.E.; Dessai, U.; McDermott, P.F.; Zhao, S. Long-read sequencing reveals evolution and acquisition of antimicrobial resistance and virulence genes in Salmonella enterica. Front. Microbiol. 2021, 12, 777817. [Google Scholar] [CrossRef] [PubMed]
- Segerman, B. The genetic integrity of bacterial species: The core genome and the accessory genome, two different stories. Front. Cell. Infect. Microbiol. 2012, 2, 116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Das, T.; Islam, Z.; Herrero-Fresno, A.; Biswas, P.K.; Olsen, J.E. High prevalence of mcr-1-encoded colistin resistance in commensal Escherichia coli from broiler chicken in Bangladesh. Sci. Rep. 2020, 10, 18637. [Google Scholar] [CrossRef] [PubMed]
- Lukjancenko, O.; Wassenaar, T.M.; Ussery, D.W. Comparison of 61 sequenced Escherichia coli genomes. Microb. Ecol. 2010, 60, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Touchon, M.; Hoede, C.; Tenaillon, O.; Barbe, V.; Baeriswyl, S.; Bidet, P.; Bingen, E.; Bonacorsi, S.; Bouchier, C.; Bouvet, O.; et al. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 2009, 5, e1000344. [Google Scholar] [CrossRef] [PubMed]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Donati, C.; Medini, D.; Ward, N.L.; Angiuoli, S.V.; Crabtree, J.; Jones, A.L.; Durkin, A.S.; et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: Implications for the microbial “pan-genome”. Proc. Natl. Acad. Sci. USA 2005, 102, 13950–13955. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liu, X.; Qian, C.; Sun, L.; Pang, S.; Liu, J.; Li, W.; Huang, W.; Cui, S.; Zhang, C.; et al. Pan-genome analysis of Delftia tsuruhatensis reveals important traits concerning the genetic diversity, pathogenicity, and biotechnological properties of the species. Microbiol. Spectr. 2022, 10, e02072-21. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, N.; Kim, B.; Lee, C.-M.; Oh, T.-J. Comparative genome analysis among Variovorax species and genome guided aromatic compound degradation analysis emphasizing 4-hydroxybenzoate degradation in Variovorax sp. PAMC26660. BMC Genom. 2022, 23, 375. [Google Scholar] [CrossRef] [PubMed]
- Cummins, E.A.; Hall, R.J.; Connor, C.; McInerney, J.O.; McNally, A. Distinct evolutionary trajectories in the Escherichia coli pangenome occur within sequence types. Microb. Genom. 2022, 8, 000903. [Google Scholar] [CrossRef]
- Wei, H.; Kong, L.; Wang, Y.; Huang, Z.; Yang, X.; Zhou, C.; Li, C.; Ma, B.; Li, C.; Lei, C.; et al. Characterization and Public Health Insights of the New Delhi Metallo-β-Lactamase-Producing Enterobacterales from Laying Hens in China. Microorganisms 2022, 10, 800. [Google Scholar] [CrossRef]
- Moreno, M.A.; García-Soto, S.; Hernández, M.; Bárcena, C.; Rodríguez-Lázaro, D.; Ugarte-Ruíz, M.; Domínguez, L. Day-old chicks are a source of antimicrobial resistant bacteria for laying hen farms. Vet. Microbiol. 2019, 230, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Höfle, U.; Gonzalez-Lopez, J.J.; Camacho, M.C.; Solà-Ginés, M.; Moreno-Mingorance, A.; Hernández, J.M.; De La Puente, J.; Pineda-Pampliega, J.; Aguirre, J.I.; Torres-Medina, F.; et al. Foraging at solid urban waste disposal sites as risk factor for cephalosporin and colistin resistant Escherichia coli carriage in white storks (Ciconia ciconia). Front. Microbiol. 2020, 11, 1397. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lin, W.; Chen, Y.; He, F. Genomic and phylogenetic analysis of a community-acquired extended-spectrum β-lactamase-producing Escherichia coli ST429 strain recovered from a urinary tract infection. J. Glob. Antimicrob. Resist. 2020, 22, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; de Jong, A.; Prenger-Berninghoff, E.; El Garch, F.; Leidner, U.; Tiwari, S.K.; Semmler, T. Genomic diversity and virulence potential of ESBL-and AmpC-β-lactamase-producing Escherichia coli strains from healthy food animals across Europe. Front. Microbiol. 2021, 12, 626774. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Zomer, A.L.; Bossers, A.; Harders, F.; Mevius, D.J.; Wagenaar, J.A.; Hordijk, J. Plasmids of distinct IncK lineages show compatible phenotypes. Antimicrob. Agents Chemother. 2017, 61, e01954-16. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, S.N.; Carattoli, A.; Schwendener, S.; Collaud, A.; Endimiani, A.; Perreten, V. Plasmids carrying blaCMY-2/4 in Escherichia coli from poultry, poultry meat, and humans belong to a novel IncK subgroup designated IncK2. Front. Microbiol. 2017, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Casella, T.; Nogueira, M.C.L.; Saras, E.; Haenni, M.; Madec, J.-Y. High prevalence of ESBLs in retail chicken meat despite reduced use of antimicrobials in chicken production, France. Int. J. Food Microbiol. 2017, 257, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, R.; Boerlin, P.; Beyrouthy, R.; Madec, J.-Y.; Schwarz, S.; Mulvey, M.R.; Zhanel, G.G.; Cormier, A.; Chalmers, G.; Bonnet, R.; et al. Dynamics of extended-spectrum cephalosporin resistance genes in Escherichia coli from Europe and North America. Nat. Commun. 2022, 13, 7490. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.S.; Norström, M.; Slettemeås, J.S.; Urdahl, A.M.; Telke, A.A.; Sunde, M. Longitudinal Sampling Reveals Persistence of and Genetic Diversity in Extended-Spectrum Cephalosporin-Resistant Escherichia coli From Norwegian Broiler Production. Front. Microbiol. 2021, 12, 795127. [Google Scholar] [CrossRef]
- Touzain, F.; Le Devendec, L.; de Boisséson, C.; Baron, S.; Jouy, E.; Perrin-Guyomard, A.; Blanchard, Y.; Kempf, I. Characterization of plasmids harboring blaCTX-M and blaCMY genes in E. coli from French broilers. PLoS ONE 2018, 13, e0188768. [Google Scholar] [CrossRef]
- Zhuge, X.; Jiang, J.; Pan, Z.; Hu, L.; Wang, S.; Wang, H.; Leung, F.C.; Dai, J.; Fan, H. Comparative genomic analysis shows that avian pathogenic Escherichia coli isolate IMT5155 (O2: K1: H5; ST complex 95, ST140) shares close relationship with ST95 APEC O1: K1 and human ExPEC O18: K1 strains. PLoS ONE 2014, 9, e112048. [Google Scholar] [CrossRef]
- DANMAP 2022—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. 2023. Available online: https://www.danmap.org/Reports/2022 (accessed on 22 January 2024).
- Helldal, L.; Karami, N.; Welinder-Olsson, C.; Moore, E.R.B.; Åhren, C. Evaluation of MLVA for epidemiological typing and outbreak detection of ESBL-producing Escherichia coli in Sweden. BMC Microbiol. 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Manga, I.; Hasman, H.; Smidkova, J.; Medvecky, M.; Dolejska, M.; Cizek, A. Fecal carriage and whole-genome sequencing-assisted characterization of CMY-2 beta-lactamase-producing Escherichia coli in calves at Czech dairy cow farm. Foodborne Pathog. Dis. 2019, 16, 42–53. [Google Scholar] [CrossRef]
- DANMAP 2018—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. 2019. Available online: https://www.danmap.org/reports/2018 (accessed on 28 December 2023).
- Berg, E.; Wester, A.; Ahrenfeldt, J.; Mo, S.; Slettemeås, J.; Steinbakk, M.; Samuelsen, M.; Grude, N.; Simonsen, G.; Løhr, I.; et al. Norwegian patients, and retail chicken meat share cephalosporin-resistant Escherichia coli and IncK/blaCMY-2 resistance plasmids. Clin. Microbiol. Infect. 2017, 23, 407.e9–407.e15. [Google Scholar] [CrossRef] [PubMed]
- Dash, H.R.; Das, S. Bioremediation of mercury and the importance of bacterial mer genes. Int. Biodeterior. Biodegrade 2012, 75, 207–213. [Google Scholar] [CrossRef]
- Adamse, P.; Van der Fels-Klerx, H.J.; de Jong, J. Arsenic, Lead, Cadmium and Mercury in Animal Feed and Feed Materials: Trend Analysis of Monitoring Result Collected in the Netherlands; RIKILT Wageningen University & Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- Rozwandowicz, M.; Brouwer, M.S.M.; Mughini-Gras, L.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Mevius, D.J.; Hordijk, J. Successful host adaptation of IncK2 plasmids. Front. Microbiol. 2019, 10, 2384. [Google Scholar] [CrossRef]
- Vázquez, X.; García, V.; Fernández, J.; Bances, M.; de Toro, M.; Ladero, V.; Rodicio, R.; Rodicio, M.R. Colistin resistance in monophasic isolates of Salmonella enterica ST34 collected from meat-derived products in Spain, with or without CMY-2 co-production. Front. Microbiol. 2022, 12, 735364. [Google Scholar] [CrossRef]
- Ali, S.; Song, H.-J.; Moon, B.-Y.; Kim, S.-J.; Kang, H.Y.; Moon, D.C.; Lee, Y.-H.; Kwon, D.-H.; Yoon, S.-S.; Lim, S.-K. Antibiotic Resistance Profiles and Molecular Characteristics of blaCMY-2-Carrying Salmonella enterica Serovar Albany Isolated from Chickens During 2013–2020 in South Korea. Foodborne Pathog. Dis. 2023, 20, 492–501. [Google Scholar] [CrossRef]
- De Koster, S.; Ringenier, M.; Xavier, B.B.; Lammens, C.; De Coninck, D.; De Bruyne, K.; Mensaert, K.; Bergh, M.K.-V.D.; Kluytmans, J.; Dewulf, J.; et al. Genetic characterization of ESBL-producing and ciprofloxacin-resistant Escherichia coli from Belgian broilers and pigs. Front. Microbiol. 2023, 14, 1150470. [Google Scholar] [CrossRef]
- Cottell, J.L.; Saw, H.T.H.; Webber, M.A.; Piddock, L.J. Functional genomics to identify the factors contributing to successful persistence and global spread of an antibiotic resistance plasmid. BMC Microbiol. 2014, 14, 168. [Google Scholar] [CrossRef]
- Hasman, H.; (Statens Serum Institut, Copenhagen, Denmark). Personal communication, 2022.
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Giske, C.G.; Turnidge, J.; Cantón, R.; Kahlmeter, G. EUCAST Steering Committee. Update from the European Committee on Antimicrobial Susceptibility Testing (EUCAST). J. Clin. Microbiol. 2022, 60, e0027621. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Rey, I.; Bello Gonzalez, T.D.J.; van der Goot, J.; Ceccarelli, D.; Bouwhuis, G.; Schillemans, D.; Jurburg, S.D.; Veldman, K.T.; de Visser, M.; Brouwer, M.S.M. Succession in the caecal microbiota of developing broilers colonised by extended-spectrum β-lactamase-producing Escherichia coli. Anim. Microbiome 2022, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Pallares-Vega, R.; Macedo, G.; Brouwer, M.S.M.; Leal, L.H.; van der Maas, P.; van Loosdrecht, M.C.M.; Weissbrodt, D.G.; Heederik, D.; Mevius, D.; Schmitt, H. Temperature and nutrient limitations decrease transfer of conjugative IncP-1 plasmid pKJK5 to wild Escherichia coli strains. Front. Microbiol. 2021, 12, 656250. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.V.; Mellerup, A.; Christiansen, L.E.; Ståhl, M.; Olsen, J.E.; Angen, Ø. Sampling and Pooling Methods for Capturing Herd Level Antibiotic Resistance in Swine Feces using qPCR and CFU Approaches. PLoS ONE 2015, 10, e0131672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pangenome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Taylor, B.; Delaney, A.J.; Soares, J.; Seemann, T.; Keane, J.A.; Harris, S.R. SNP-sites: Rapid efficient extraction of SNPs from multi-FASTA-alignments. Microb. Genom. 2016, 2, e000056. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Hadfield, J.; Croucher, N.J.; Goater, R.J.; Abudahab, K.; Aanensen, D.M.; Harris, S.R. Phandango: An interactive viewer for bacterial population genomics. Bioinformatics 2018, 34, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Kaas, R.S.; Seyfarth, A.M.; Agersø, Y.; Lund, O.; Larsen, M.V.; Aarestrup, F.M. Genotyping using whole-genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. J. Antimicrob. Chemother. 2013, 68, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Alikhan, N.-F.; Mohamed, K.; Fan, Y.; Achtman, M.; the Agama Study Group; Brown, D.; Chattaway, M.; Dallman, T.; Delahay, R.; et al. The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genom. Res. 2020, 30, 138–152. Available online: http://www.genome.org/cgi/doi/10.1101/gr.251678.119 (accessed on 10 October 2023). [CrossRef]
- Jünemann, S.; Sedlazeck, F.J.; Prior, K.; Albersmeier, A.; John, U.; Kalinowski, J.; Mellmann, A.; Goesmann, A.; von Haeseler, A.; Stoye, J.; et al. Updating benchtop sequencing performance comparison. Nat. Biotechnol. 2013, 31, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb. Genom. 2017, 3, e000132. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Feng, A.; Akter, S.; Leigh, S.A.; Wang, H.; Pharr, G.T.; Evans, J.; Branton, S.L.; Landinez, M.P.; Pace, L.; Wan, X.-F. Genomic diversity, pathogenicity, and antimicrobial resistance of Escherichia coli isolated from poultry in the southern United States. BMC Microbiol. 2023, 23, 15. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Hasman, H. PlasmidFinder and In Silico pMLST: Identification and Typing of Plasmid Replicons in Whole-Genome Sequencing (WGS). Methods Mol. Biol. 2020, 2075, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.M.; Chooi, Y.H. Clinker & cluster map.js: Automatic generation of gene cluster comparison figures. Bioinformatics 2021, 37, 2473–2475. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
| Resistant Strains (%) | Distribution (%) of Strains Linked to a Specific MIC Value (mg/L) * | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | ≥512 | |
| Amikacin | 0 | 100 | |||||||||||||||
| Ampicillin | 100 | 2.2 | 97.8 | ||||||||||||||
| Azithromycin | 2.2 | 13.0 | 78.3 | 6.5 | 2.2 | ||||||||||||
| Cefotaxime | 100 | 2.2 | 8.7 | 89.1 | |||||||||||||
| Ceftazidime | 100 | 2.2 | 2.2 | 45.6 | 50.0 | ||||||||||||
| Chloramphenicol | 2.2 | 93.5 | 4.3 | 2.2 | |||||||||||||
| Ciprofloxacin | 8.6 | 69.6 | 21.8 | 4.3 | 4.3 | ||||||||||||
| Colistin | 0 | 100 | |||||||||||||||
| Gentamicin | 71.7 | 8.7 | 17.4 | 2.2 | 23.9 | 47.8 | |||||||||||
| Meropenem | 0 | 100 | |||||||||||||||
| Nalidixic acid | 8.7 | 91.3 | 8.7 | ||||||||||||||
| Sulfamethoxazole | 93.5 | 6.5 | 93.5 | ||||||||||||||
| Tetracycline | 84.8 | 15.2 | 84.8 | ||||||||||||||
| Tigecycline | 0 | 97.8 | 2.2 | ||||||||||||||
| Trimethoprim | 19.6 | 52.2 | 23.9 | 4.3 | 19.6 | ||||||||||||
| Isolate ID | Host Species | Sample Type | Year | Country * | ST | IncK Plasmid Characterization | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ONT Sequenced | Plasmid Name | Plasmid Size (bp) | ESC-Resistance Gene | Other AMR Genes | Virulence Genes & Mercury-Resistance Genes | Conjugative Transferability of blaCMY-2 | ||||||
| PS15001 | Farm a | Environment | 2015 | DK | 429 | + | pPS25001 | 109821bp | blaCMY-2 | sul1, aac (3)-Via, aadA1 | cib, traT | Positive |
| PS15002 | Farm | Environment | 2015 | DK | 429 | Positive | ||||||
| PS16001 | Farm | Environment | 2016 | DK | 429 | Positive | ||||||
| PS16002 | Farm | Environment | 2016 | DK | 429 | + | pPS16002 | 119378bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| PS16003 | Farm | Environment | 2016 | DK | 429 | Positive | ||||||
| PS16004 | Farm | Environment | 2016 | DK | 429 | Positive | ||||||
| PS16005 | Farm | Environment | 2016 | DK | 429 | Positive | ||||||
| SA17021 | Slaughterhouse b | Thighs | 2017 | DK | 429 | Positive | ||||||
| SA17022 | Slaughterhouse | Thighs | 2017 | DK | 429 | + | pSA17022 | 119378bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| SA17023 | Slaughterhouse | Thighs | 2017 | DK | 429 | + | pSA17023 | 119378bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| SA17024 | Slaughterhouse | Thighs | 2017 | DK | 429 | + | pSA17024 | 120714bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| SB17033 | Slaughterhouse | Thighs | 2017 | DK | 429 | + | pSB17033 | 120714bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| SB17034 | Slaughterhouse | Thighs | 2017 | DK | 429 | + | pSB17034 | 120714bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| SB17035 | Slaughterhouse | Thighs | 2017 | DK | 429 | Positive | ||||||
| SB17036 | Slaughterhouse | Thighs | 2017 | DK | 429 | Positive | ||||||
| SB17037 | Slaughterhouse | Thighs | 2017 | DK | 429 | + | pSB17037 | 120714bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| SB17038 | Slaughterhouse | Thighs | 2017 | DK | 429 | Positive | ||||||
| SB17039 | Slaughterhouse | Thighs | 2017 | DK | 429 | Positive | ||||||
| SB17040 | Slaughterhouse | Thighs | 2017 | DK | 429 | + | pSB17040 | 120714bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| SB17121 | Slaughterhouse | Intestine | 2017 | DK | 429 | Positive | ||||||
| SB17122 | Slaughterhouse | Intestine | 2017 | DK | 429 | Positive | ||||||
| SB17123 | Slaughterhouse | Intestine | 2017 | DK | 429 | Positive | ||||||
| SB17103 | Slaughterhouse | Intestine | 2017 | DK | 1286 | + | pSB17103 | 132580bp | blaCMY-2, blaTEM-1 | dfrA1, sul2, tet(A) | cib, traT | Positive |
| SB18102 | Slaughterhouse | Intestine | 2018 | DK | 1286 | + | pSB18102 | 133311bp | blaCMY-2, blaTEM-1 | dfrA1, sul2, tet(A) | cib, traT | Positive |
| SB18001 | Slaughterhouse | Thighs | 2018 | DK | 1286 | + | pSB18001 | 132529bp | blaCMY-2, blaTEM-1 | dfrA1, sul2, tet(A) | cib, traT | Positive |
| SB18002 | Slaughterhouse | Thighs | 2018 | DK | 1286 | + | pSB18002 | 133297bp | blaCMY-2, blaTEM-1 | dfrA1, sul2, tet(A) | cib, traT | Positive |
| SB18003 | Slaughterhouse | Thighs | 2018 | DK | 1286 | + | pSB18003 | 133303bp | blaCMY-2, blaTEM-1 | dfrA1, sul2, tet(A) | cib, traT | Positive |
| SB18004 | Slaughterhouse | Thighs | 2018 | DK | 1286 | + | pSB18004 | 133315bp | blaCMY-2, blaTEM-1 | dfrA1, sul2, tet(A) | cib, traT | Positive |
| SA17101 | Slaughterhouse | Intestine | 2017 | DK | 162 | + | pSA17101 | 120668bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| SB17032 | Slaughterhouse | Thighs | 2017 | DK | 350 | + | pSB17032 | 85947bp | blaCMY-2 | - | traT | Positive |
| SB17042 | Slaughterhouse | Thighs | 2017 | DK | 57 | + | pSB17042 | 86724bp | blaCMY-2 | - | traT | Positive |
| HBI01 | Clinical c | Blood | 2017 | DK | ST429 | + | pHBI01 | 119331bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT | Positive |
| HBI02 | Clinical | Blood | 2020 | DK | ST69 | + | pHBI02 | 85938bp | blaCMY-2 | - | traT | Positive |
| HBI03 | Clinical | Blood | 2021 | DK | ST131 | + | pHBI03 | 85954bp | blaCMY-2 | - | traT | Positive |
| HBI04 | Clinical | Blood | 2019 | DK | ST95 | + | pHBI04 | 98160bp | blaCMY-2 | - | traT | Positive |
| HBI05 | Clinical | Blood | 2017 | DK | ST12 | + | pHBI05 | 96311bp | blaCMY-2 | - | traT | Positive |
| 2E151-R | FI farm d | Egg | 2017 | FI b | ST429 | Positive | ||||||
| PS310-R | FI farm | Parent bird | 2017 | FI | ST429 | + | pPS310-R | 122067bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| PS184-R | FI farm | Parent bird | 2017 | FI | ST429 | + | pPS184-R | 122085bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| PS148-R | FI farm | Parent bird | 2017 | FI | ST429 | Positive | ||||||
| PS378-R | FI farm | Parent bird | 2017 | FI | ST429 | Positive | ||||||
| Y13-R | FI farm | Environment | 2017 | FI | ST429 | Positive | ||||||
| PS57-R | FI farm | Parent bird | 2017 | FI | ST429 | Positive | ||||||
| RL97 | Food e | Chicken Meat | 2012 | DE c | ST429 | + | pRL97 | 120705bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| RL316 | Food | Chicken Meat | 2012 | DE | ST429 | + | pRL316 | 117209bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
| RL320 | Food | Chicken Meat | 2012 | DE | ST429 | + | pRL320 | 117209bp | blaCMY-2 | sul1, aac (3)-Via, aadA1, tet(A) | cib, traT, mer | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, M.; Fresno, A.H.; Calvo-Fernandez, C.; Hasman, H.; Kurittu, P.E.; Heikinheimo, A.; Hansen, L.T. Comparison of IncK-blaCMY-2 Plasmids in Extended-Spectrum Cephalosporin-Resistant Escherichia coli Isolated from Poultry and Humans in Denmark, Finland, and Germany. Antibiotics 2024, 13, 349. https://doi.org/10.3390/antibiotics13040349
Che M, Fresno AH, Calvo-Fernandez C, Hasman H, Kurittu PE, Heikinheimo A, Hansen LT. Comparison of IncK-blaCMY-2 Plasmids in Extended-Spectrum Cephalosporin-Resistant Escherichia coli Isolated from Poultry and Humans in Denmark, Finland, and Germany. Antibiotics. 2024; 13(4):349. https://doi.org/10.3390/antibiotics13040349
Chicago/Turabian StyleChe, Meiyao, Ana Herrero Fresno, Cristina Calvo-Fernandez, Henrik Hasman, Paula E. Kurittu, Annamari Heikinheimo, and Lisbeth Truelstrup Hansen. 2024. "Comparison of IncK-blaCMY-2 Plasmids in Extended-Spectrum Cephalosporin-Resistant Escherichia coli Isolated from Poultry and Humans in Denmark, Finland, and Germany" Antibiotics 13, no. 4: 349. https://doi.org/10.3390/antibiotics13040349
APA StyleChe, M., Fresno, A. H., Calvo-Fernandez, C., Hasman, H., Kurittu, P. E., Heikinheimo, A., & Hansen, L. T. (2024). Comparison of IncK-blaCMY-2 Plasmids in Extended-Spectrum Cephalosporin-Resistant Escherichia coli Isolated from Poultry and Humans in Denmark, Finland, and Germany. Antibiotics, 13(4), 349. https://doi.org/10.3390/antibiotics13040349







