Abstract
Aim: Periprosthetic joint infections (PJIs) of unicompartmental knee arthroplasties (UKAs) can lead to secondary osteoarthritis of the other compartments. The objective of this study was to identify the frequency of PJIs in cases of UKA with progressed secondary osteoarthritis and the result of septic one-stage revision in these cases to verify the value of preoperative aspiration in cases of secondary osteoarthritis of UKA. Methods: We retrospectively reviewed 97 patients with a unicompartmental arthroplasty who underwent revision surgery to a total knee arthroplasty (TKA) between January 2013 and March 2021 because of subsequent osteoarthritis. Preoperative aspiration and sample collection during the revision surgery were employed to identify potential periprosthetic joint infections (PJIs). The post-revision period was monitored for septic complications over an average duration of 55.7 ± 25.2 months (24–113). Results: PJIs were identified in 5.2% of cases through preoperative aspiration. In all instances of PJIs, a one-stage septic revision was performed, and notably, none of these cases experienced septic complications during the follow-up period. Conclusions: Preoperative aspiration is essential in order to exclude the presence of a PJI before performing revision surgery of UKA due to secondary osteoarthritis.
1. Introduction
Unicompartmental knee arthroplasty (UKA) is a successful treatment option for unicompartmental osteoarthritis of the knee. Several reports have demonstrated survival rates greater than 90% at 10 years after modern UKA implantation in clinics with experienced surgeons [1,2,3,4,5]. Advantages in UKA compared to total knee arthroplasty (TKA) such as less invasive surgery, shorter operative time and hospital stay, lower intraoperative blood loss, and higher postoperative range of motion and level of activity, have led to a considerable increase in primary UKA numbers in recent years [6,7,8,9]. Accepted indications for UKA are unicompartmental osteoarthritis of the medial or lateral compartment with no osteoarthritis in the other compartments as well as intact anterior crucial ligament and medial collateral ligament [1,2,5,6,9]. On the other hand, contraindications for unicompartmental knee arthroplasties are flexion contracture of more than 15 degrees, low range of motion with flexion below 100 degrees, septic arthritis of the joint, inflammatory arthritis with activity in the knee, and ligamentous instability, especially rupture of the anterior crucial ligament [1,2,5,6,9]. However, osteoarthritis of another compartment may eventually occur and is one of the most frequent reasons for revision of UKAs [8,10].
Citak et al. identified arthritis within the other compartment as the main cause of UKA revision with a percentage of 39.5 [10]. Pandit et al. researched the frequency of complications after UKA and detected arthritis within the lateral compartment as the most frequent complication in 0.9% [11].
Even periprosthetic joint infection (PJI) is less frequent in UKA than in TKA; it is the reason for revision of UKA in 0.2% to 1% of cases [12,13]. Because chronic periprosthetic joint infection can lead to secondary osteoarthritis of the other compartments [14,15], some revisions due to progression of osteoarthritis may have been the result of unrecognized periprosthetic joint infections. Preoperative diagnostics with aspiration of the joint represent an established method for ruling out or verifying periprosthetic joint infection but are not performed as a routine before revision of UKAs when osteoarthritis has been diagnosed in the other compartments. If a periprosthetic infection would be diagnosed preoperatively, a single-stage septic revision procedure could be performed so that preoperative aspiration as a routine may be helpful to reduce the amount of unrecognized periprosthetic joint infections in failed unicompartmental knee arthroplasties. To the best of our knowledge, there are no publications dealing with this topic of preoperative aspiration for ruling out or detecting periprosthetic joint infection in failed unicompartmental knee arthroplasties because of secondary osteoarthritis of other compartments. Therefore, the aim of the current study was to answer the following questions:
- How often is PJI recognized in UKAs where progressed osteoarthritis is given as the reason for revision?
- What are the success rates of the aseptic and septic one-stage revisions of UKAs to total knee arthroplasties?
2. Results
Preoperative aspiration revealed PJIs according to the criteria for a periprosthetic infection based on the ICM score in five (5.2%) patients (Figure 1, Table 1). In all cases, a one-stage septic revision was performed. Intraoperatively collected specimens confirmed PJIs in each case (Figure 1, Table 1). The postoperative course after 90 septic revisions was without recurrence of septic complications in all cases.
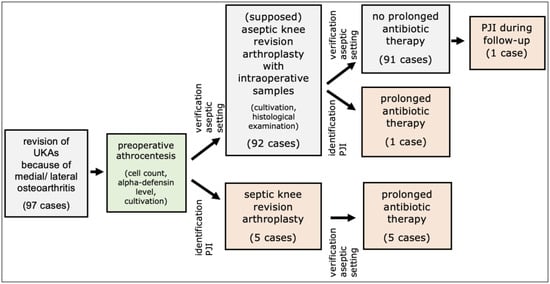
Figure 1.
Patient flowchart. PJI = periprosthetic joint infection.

Table 1.
Parameters of the 5 patients with preoperative detected periprosthetic joint infection (PJI) (1 to 5) and 1 patient (6) with preoperative unrecognized PJI. mo = months, HPF = high power field (×400).
Preoperative PJIs were identified in three females and two males with a mean age of 69.4 years; the average body mass index (BMI) was 28.4 kg/m2. Regarding the American Society of Anesthesiologists (ASA) score, three patients were categorized as ASA 2 and two patients as ASA 3. The Charlson Comorbidity Index (CCI) classifies one patient each within CCI-Score 1, CCI-Score 2, CCI-Score 4, CCI-Score 5, and CCI-Score 6. In three cases, Staphylococcus epidermidis, and in one case, Cutibacterium acnes was identified in the preoperative cultivation as well as in the intraoperative culture. Remarkably, in one case, the perioperative cultivation isolated Staphylococcus epidermidis and later on, the intraoperative culture showed Staphylococcus epidermidis and additionally Cutibacterium acnes. Following the ICM score, three patients reached an ICM score of 7 and two patients an ICM score of 10.
In 92 (94.8%) patient cases, the preoperative aspirate was negative for the presence of PJI. Therefore, aseptic revision of the unicompartmental prosthesis was performed (Figure 1).
Because the criteria of a PJI were met in the intraoperative specimens, prolonged postoperative antibiosis was carried out in one patient case (1.0%) (Figure 1, Table 1). In this case, Staphylococcus epidermidis was detected within the intraoperative culture. No reinfection occurred in the subsequent follow-up.
One case (1.0%), without evidence of PJI in the preoperative aspirate and intraoperative specimens, experienced a septic complication that was successfully treated with one-stage septic revision (Figure 1).
3. Discussion
Routine preoperative aspiration of joints with unicompartmental prostheses that are referred for prosthesis revision due to osteoarthritis revealed a periprosthetic infection in 5.2% of cases. To the best of our knowledge, this is the first study to systematically address this issue.
In previous studies of the literature concerning the outcome of revision surgery of UKAs, Leta et al. [16], analyzed 578 cases from the Norwegian Prosthesis Register and found that periprosthetic infection occurred postoperatively in 16% of cases. For the Australian Prosthesis Registry, Hang et al. [17] found an infection rate after revision surgery from UKAs to total knee arthroplasties (TKAs) of 14%. In a meta-analysis of 1373 revised UKAs, Shen et al. [18] found a rate of PJIs of 4.1%. Due to the lack of preoperative aspiration, it is possible that a PJI exists prior to revision surgery but was not diagnosed preoperatively. Consequently, the PJI could not adequately be addressed with local and systemic antibiotic therapy. Such local and systemic specific antibiotic therapy, if the microorganism had been known preoperatively, would in all likelihood have allowed successful one-stage septic revision and so avoided postoperative periprosthetic infection. This is supported by the favorable outcomes of septic one-stage revisions of UKAs in the studies by Singer et al. [19], with a 100% rate of infection control in 6 cases after a mean follow-up of 36 months (24–72 months) and a rate of infection control of 93.3% after 8 years for 15 patients in a report by Kocaoglu et al. [20]. Like the present study with 100% freedom from infection in five cases after 48.6 ± 29.5 months, they had preoperative knowledge of the pathogen and carried out specific local and systemic antibiotic therapy.
Moreover, if a periprosthetic joint infection as the reason of secondary osteoarthritis in the other components could be ruled out by routine preoperative aspiration, prolonged antibiotic usage can be prevented, which may otherwise be prescribed by some surgeons in unclear situations. By this, the risk of allergic reactions to antibiotics may be reduced also [21].
The study has some limitations. It is a retrospective analysis of data collected prospectively and entered into a database. This may result in a selection bias. The study quality could be further improved by future prospective multicenter studies with a larger number of cases. The number of periprosthetic infections of UKAs is low, which limits the evaluation of the therapeutic success of one-stage septic revisions. However, this is due to the low incidence of such infections in UKAs, and the case number is consistent with other studies [19,20].
Moreover, the accuracy of aspiration alone in diagnosing periprosthetic infection is not 100%. Diagnostic tests with high sensitivities mostly have lower specificities and result in a higher percentage of patients treated for periprosthetic joint infections. The opposite situation exists for tests with lower sensitivities and higher specificities. Fink et al. analyzed the serum C-reactive protein level, the synovial fluid obtained by joint aspiration and five synovial biopsies in 145 cases of knee replacements prior to revision to assess the value of these parameters in diagnosing PJI [22]. They showed a sensitivity of aspiration of 72.5%, a specificity of 95.2%, and an accuracy of 89%. The authors emphasize the diagnostic value of joint biopsy, which had a sensitivity of 100%, a specificity of 98.1%, and an accuracy of 98.6% [22]. Barrack et al. analyzed 78 cases of aspiration in order to detect PJI after TKA and showed a sensitivity of 65.4% and a specificity of 96.1% [23]. By performing several tests simultaneously (C-reactive serum level, leukocyte in serum culturing, leukocyte count in the aspirate, alpha-defensin determination), a very high accuracy can be achieved [24].
Moreover, the findings of the aspiration were confirmed in all cases by the tissue samples taken intraoperatively for culturing and histological study. We also observed one case of PJI which was not detected by the preoperative aspiration. However, in our opinion, this case does not argue against the benefit of preoperative aspiration before revision of a unicompartmental endoprosthesis with osteoarthritis.
4. Material and Methods
The study included 97 patients with a unicompartmental arthroplasty (UKA) who underwent revision surgery to a total knee arthroplasty (TKA) between January 2013 and March 2021 because of subsequent osteoarthritis. All patients underwent preoperative aspiration of the associated knee joint. Revisions of unicompartmental prosthesis because of other causes, such as loosening, lack of osteointegration, ligamentous instability, fracture, inlay dislocation, and arthrofibrosis, were excluded from the study because these indications for revision were not the topic of this study. However, preoperative aspirations before revision were also performed in these cases, because preoperative aspiration is performed in our clinic as a routine procedure before any revision surgery.
The cohort consisted of 65 females and 32 males, aged 70.6 ± 9.4 (40.0–98.0) years. There were 87 revisions (89.7%) of a medial unicompartmental prosthesis to bicondylar total knee arthroplasty (TKA), 7 revisions (7.2%) of a lateral unicompartmental prosthesis to TKA, and 3 revisions (3.1%) of a medial unicompartmental prosthesis to hinged TKA because of intraoperative collateral ligament instability. The time between primary surgery and revision surgery was 81.5 ± 47.9 (4.0–197.0) months. With respect to the ASA classification scores, 3 patients were classed as ASA 1, 58 patients were ASA 2, 36 patients ASA 3, and 0 patients ASA 4 [25,26]. Regarding the Charlson Comorbidity Index (CCI), there were 2 patients with CCI 0, 1 patient with CCI 1, 9 patients with CCI 2, 21 patients with CCI 3, 24 patients with CCI 4, 14 patients with CCI 5, 14 patient with CCI 6, 6 patients with CCI 7, 3 patients with CCI 8, 2 patients with CCI 9, and 1 patient with CCI 10 [26,27].
Preoperative aspiration was conducted, and the aspirate was subjected to microbial cultivation, cell count determination, and analysis of alpha-defensin levels (as of 2017). Cell quantification was performed by aspirating a minimum of 1 mL synovial fluid into an EDTA tube, followed by cell count determination using the ABX Pentra XL 80 laboratory diagnostic device (Horiba Medical, Montpellier, France). In addition, the collected fluid was promptly transferred into pediatric blood culture bottles containing BD BACTEC-PEDS-PLUS/F-Medium (Becton Dickinson, Heidelberg, Germany) and underwent a 14-day incubation period [28]. Alpha-defensin levels were assessed via ELISA test. Serum CRP-levels were determined across all cases.
During revision surgery, samples were extracted from five distinct areas close to the prosthesis (periprosthetic tissue and synovium). Additionally, five samples from the synovium and periprosthetic connective tissue membrane associated with the loosened prosthesis were procured for histological evaluation. Perioperative antibiotic administration occurred subsequent to the collection of all samples. Biopsy samples were placed in sterile tubes and promptly transported to the microbiological laboratory, similar to the aspirated fluid, within one hour of sampling. These samples were then streaked onto blood agar and inoculated into special nutrient broth for anaerobic organisms, with an incubation period of 14 days [28]. Tissue analyses and aspiration results were evaluated based on the ICM criteria [29,30,31], whereby a diagnosis of periprosthetic joint infection (PJI) was assigned if the cumulative diagnostic score reached at least 6. Morawietz and Krenn et al.’s classification system [32,33,34] was utilized for histological analysis of periprosthetic tissue to distinguish between wear particle type (I), infection type (II), combined type (III), and indeterminate type (IV). Furthermore, the count of polymorphonuclear leukocytes per high power microscope field was determined.
After the revision surgery, patients were followed up for at least 2 years. The data were collected prospectively in our database and were analyzed retrospectively. The mean follow-up was 55.7 ± 25.2 (24–113) months. In cases of PJI, a septic one-stage revision was performed with specific local antibiotics in the bone cement and systemic antibiotic therapy (2 weeks intravenous and 4 weeks oral) according to the susceptibility of the detected microorganism. Patients were categorized as reinfection-free according to Diaz-Ledezma et al. [35] if they fulfilled the subsequent conditions: absence of PJI-related mortality, absence of further PJI-related surgeries, and both microbiological and clinical absence of infection for a minimum duration of 24 months. An internal CRP detection threshold of ≥10 mg/L was established [35].
Statistical analysis utilized SPSS for Windows (version 22; IBM Corp.; Armonk, NY, USA). All participants provided informed consent prior to participation. Unless otherwise stated, descriptive results are expressed as mean ± standard deviation (and range) or absolute number (percentage), respectively. The level of significance was set at p < 0.05. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Landesärztekammer Badenwürttemberg (committee’s reference number F-2023-115).
5. Conclusions
In summary, we suggest that the incidence of periprosthetic infection of 5.2% associated with osteoarthritis subsequent to UKA and the successful treatment of the PJI with a single-stage septic revision represents a clear indication of the benefit to be gained by routinely aspirating the joint requiring revision of a UKA due to subsequent osteoarthritis. We therefore recommend that before every revision of a UKA, the affected joint should be aspirated in order to exclude the presence of a periprosthetic infection.
Author Contributions
Conceptualization, B.P.B., F.H.S. and B.F.; methodology, B.P.B., F.H.S. and B.F.; software, B.P.B.; formal analysis, B.P.B.; investigation, B.P.B. and F.H.S.; resources, B.P.B. and B.F.; writing—original draft preparation, B.P.B. and B.F.; writing—review and editing, B.P.B. and B.F.; visualization, B.P.B.; supervision, B.F.; project administration, B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Landesärztekammer Badenwürttemberg (committee’s reference number F-2023-115).
Informed Consent Statement
All patients gave consent to treatment according to institutional guidelines and to pseudonymized assessment of clinical data and treatment outcome.
Data Availability Statement
We do not wish to share our data because of some of patients’ data regarding individual privacy, and according to the policy of our hospital, the data could not be shared to others without permission.
Acknowledgments
Special thanks go to Irina Berger, Pathological Institute, Klinikum Kassel who performed the histopathological analyses of the synovial tissue, and to Rüdiger Braun, MVZ-Labor Ludwigsburg, who performed the microbiological analyses, as well as Udo Geipel, Bioscientia MVZ-Labor Saarbrücken for the recommendation of the systemic antibiotic therapy.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ASA = American Society of Anesthesiologists, BMI = body mass index, CCI = Charlson Comorbidity Index, CRP = c-reactive protein, EDTA = ethylene diamine tetraacetic acid, ELISA = enzyme-linked immunosorbent assay, ICM = International Consensus Meeting, PJI = periprosthetic joint infection, TKA = total knee arthroplasty, UKA = unicompartmental knee arthroplasty
References
- Foran, J.R.; Brown, N.M.; Della Valle, C.J.; Berger, R.A.; Galante, J.O. Long-term survivorship and failure modes of unicompartmental 170 knee arthroplasty. Clin. Orthop. Relat. Res. 2013, 471, 102–108. [Google Scholar] [CrossRef]
- Koshino, T.; Sato, K.; Umemoto, Y.; Akamatsu, Y.; Kumagai, K.; Saito, T. Clinical results of unicompartmental arthroplasty for knee 180 osteoarthritis using a tibial component with screw fixation. Int. Orthop. 2015, 39, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Schlueter-Brust, K.; Kugland, K.; Stein, G.; Henckel, J.; Chrisst, H.; Eysel, P.; Bontemps, G. Ten year survivorship after cemented and uncemented medial Uniglide® unicompartmental knee arthroplasties. Knee 2014, 21, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Vasso, M.; Del Regno, C.; Perisano, C.; D’Amelio, A.; Corona, K.; Schiavone Panni, A. Unicompartmental knee arthroplasty is effective: Ten year results. Int. Orthop. 2015, 39, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Tada, M.; Yoshida, H.; Takei, S.; Fukuoka, S.; Nakamura, H. Oxford phase 3 unicompartmental knee arthroplasty in Japan—Clinical results in greater than one thousand cases over ten years. J. Arthroplast. 2013, 28 (Suppl. 9), 168–171. [Google Scholar] [CrossRef]
- Beard, D.J.; Davies, L.J.; Cook, J.A.; MacLennan, G.; Price, A.; Kent, S.; Hudson, J.; Carr, A.; Leal, J.; Campbell, H.; et al. Total versus partial knee replacement in patients with medial compartment knee osteoarthritis: The TOPKAT RCT. Health Technol. Assess. 2020, 24, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Jiranek, W.A.; McGlynn, F.J. Yearly incidence of unicompartmental knee arthroplasty in the United States. J. Arthroplast. 2008, 23, 408–412. [Google Scholar] [CrossRef]
- Vasso, M.; Coroa, K.; DÀpolito, R.; Mazzitelli, G.; Panni, A.S. Unicompartmental knee arthroplasty: Modes of failure and conversion to total knee arthroplasty. Joints 2017, 5, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.A.; Middleton, R.; Abram, S.G.F.; Smith, S.; Alvand, A.; Jackson, W.F.; Bottomley, N.; Hepewll, S.; Price, A.J. Patient relevant outcomes of unicompartmental versus total knee replacement: Systematic review and meta-analysis. BMJ 2019, 364, I352. [Google Scholar] [CrossRef]
- Citak, M.; Dersch, K.; Kamath, A.F.; Haasper, C.; Gehrke, T.; Kendoff, D. Common causes of failed unicompartmental knee arthroplasty: A single-centre analysis of four hundred and seventy one cases. Int. Orthop. 2014, 38, 961–965. [Google Scholar] [CrossRef]
- Pandit, H.; Jenkins, C.; Gill, H.S.; Barker, K.; Dodd, C.A.; Murray, D.W. Minimally invasive Oxford phase 3 unicompartmental knee replacement: Results of 1000 cases. J. Bone Jt. Surg. Br. 2011, 93, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Labruyère, C.; Zeller, V.; Lhotellier, L.; Desplaces, N.; Léonard, P.; Mamoudy, P.; Marmor, S. Chronic infection of unicompartmental knee arthroplasty: One-stage conversion to total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2015, 101, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Vasso, M.; Schiavone Panni, A.; De Martino, I.; Gasparini, G. Prosthetic knee infection by resistant bacteria: The worst-case scenario. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Kaandorp, C.J.; Krijnen, P.; Moens, H.J.B.; Habbema, J.D.F.; van Schaardenburg, D. The outcome of bacterial arthritis. A prospective community-based study. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1997, 40, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.; Cockayne, A.; Humphreys, H. Clinical and molecular aspects of the pathogenesis of Staphylococcus aureus bone and joint infections. J. Med. Microbiol. 1996, 44, 157–164. [Google Scholar] [CrossRef]
- Leta, T.H.; Lygre, S.H.; Skredderstuen, A.; Hallan, G.; Gjertsen, J.E.; Rokne, B.; Furnes, O. Outcomes of Unicompartmental Knee Arthroplasty after Aseptic Revision to Total Knee Arthroplasty: A Comparative Study of 768 TKAs and 578 UKAs Revised to TKAs from the Norwegian Arthroplasty Register (1994 to 2011). J. Bone Jt. Surg. Am. 2016, 98, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.R.; Stanford, T.E.; Graves, S.E.; Davidson, D.C.; de Steiger, R.N.; Miller, L.N. Outcome of revision of unicompartmental knee replacement. 1948 cases from the Australian Orthopaedic Association National Joint Replacement Registry, 1999–2008. Acta Orthoaedica 2010, 81, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Shen, D.; Fang, Y.; Li, X.; Cui, L.; Wei, B.; Wu, L. Clinical outcomes of revision total knee arthroplasty after high tibial osteotomy and unicompartmental knee arthroplasty: A systemic review and meta-analysis. Orthop. Surg. 2022, 14, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.; Merz, A.; Frommelt, L.; Fink, B. High Rate of Infection Control with One-stage Revision of Septic Knee Prostheses excluding MRSA and MRSE. Clin. Orthop. Related Res. 2012, 470, 1461–1471. [Google Scholar] [CrossRef]
- Kocaoglu, H.; Hennes, F.; Abdelaziz, H.; Neufeld, M.E.; Gehrke, T.; Citak, M. Survival analysis of one-stage exchange of infected unicompartmental knee arthroplasty: A single-center study with minimum 3 years follow-up. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 327–333. [Google Scholar] [CrossRef]
- Ovidiu, B.; Dumitru, M.; Vrinceanu, D.; Cergan, R.; Jeican, I.I.; Giurcaneanu, C.; Miron, A. Current approach to medico-legal aspects of allergic reactions. Rom. J. Leg. Med. 2021, 29, 328–331. [Google Scholar]
- Fink, B.; Makowiak, C.; Fuerst, M.; Berger, I.; Schäfer, P.; Frommelt, L. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J. Bone Jt. Surg. Br. 2008, 90, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Barrack, R.L.; Jennings, R.W.; Wolfe, M.W.; Bertot, A.J. The Coventry Award. The value of preoperative aspiration before total knee revision. Clin. Orthop. Relat. Res. 1997, 345, 8–16. [Google Scholar] [CrossRef]
- Quilan, N.D.; Jennings, J.M. Joint aspiration for diagnosis of chronic periprosthetic joint infection: When, how, and what tests? Arthroplasty 2023, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Teves, J.; Holc, F.; Castro Lalín, A.; García-Mansilla, A.; Vildoza, S.; Brandariz, R.; Carbó, L.; Costantini, J. Are frailty scores superior to the ASA score in predicting complications, hospital stay, and readmissions in total knee replacement? A comparative study between octogenarian and septuagenarian patients. Rev. Esp. Cir. Ortop. Traumatol. 2024, 30, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Quach, L.H.; Jayamaha, S.; Whitehouse, S.L.; Crawford, R.; Pulle, C.R.; Bell, J.J. Comparison of the Charlson Comorbidity Index with the ASA score for predicting 12-month mortality in acute hip fracture. Injury 2020, 51, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar] [PubMed]
- Schäfer, P.; Fink, B.; Sandow, D.; Margull, A.; Berger, I.; Frommelt, L. Prolonged bacterial culture to identify late periprosthetic joint infection: A promising strategy. Clin. Inf. Dis. 2008, 47, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314. [Google Scholar] [CrossRef]
- Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J. Arthroplast. 2011, 26, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Otto, M.; Morawietz, L.; Hopf, T.; Jakobs, M.; Klauser, W.; Schwantes, B.; Gehrke, T. Histopathologic diagnostics in endoprosthetics: Periprosthetic neosynovialitis, hypersensitivity reaction, and arthrofibrosis. Orthopäde 2009, 38, 520–530. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Perino, G.; Kienapfel, H.; Ascherl, R.; Hassenpflug, G.J.; Thomsen, M.; Thomas, P.; Huber, M.; Kendoff, D.; et al. Revised histopathological consensus classification of joint implant related pathology. Pathol. Res. Pract. 2014, 210, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Morawietz, L.; Hasart, O.; Strube, P.; Perka, C.; Tohtz, S. Histopathological diagnosis of periprosthetic joint infection 195 following total hip arthroplasty: Use of a standardized classification system of the periprosthetic interface membrane. Orthopade 2009, 38, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ledezma, C.; Higuera, C.A.; Parvizi, J. Success after treatment of periprosthetic joint infection: A Delphi-based international multidisciplinary consensus. Clin. Orthop. Relat. Res. 2013, 471, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).