The MraY Inhibitor Muraymycin D2 and Its Derivatives Induce Enlarged Cells in Obligate Intracellular Chlamydia and Wolbachia and Break the Persistence Phenotype in Chlamydia
Abstract
:1. Introduction
2. Results
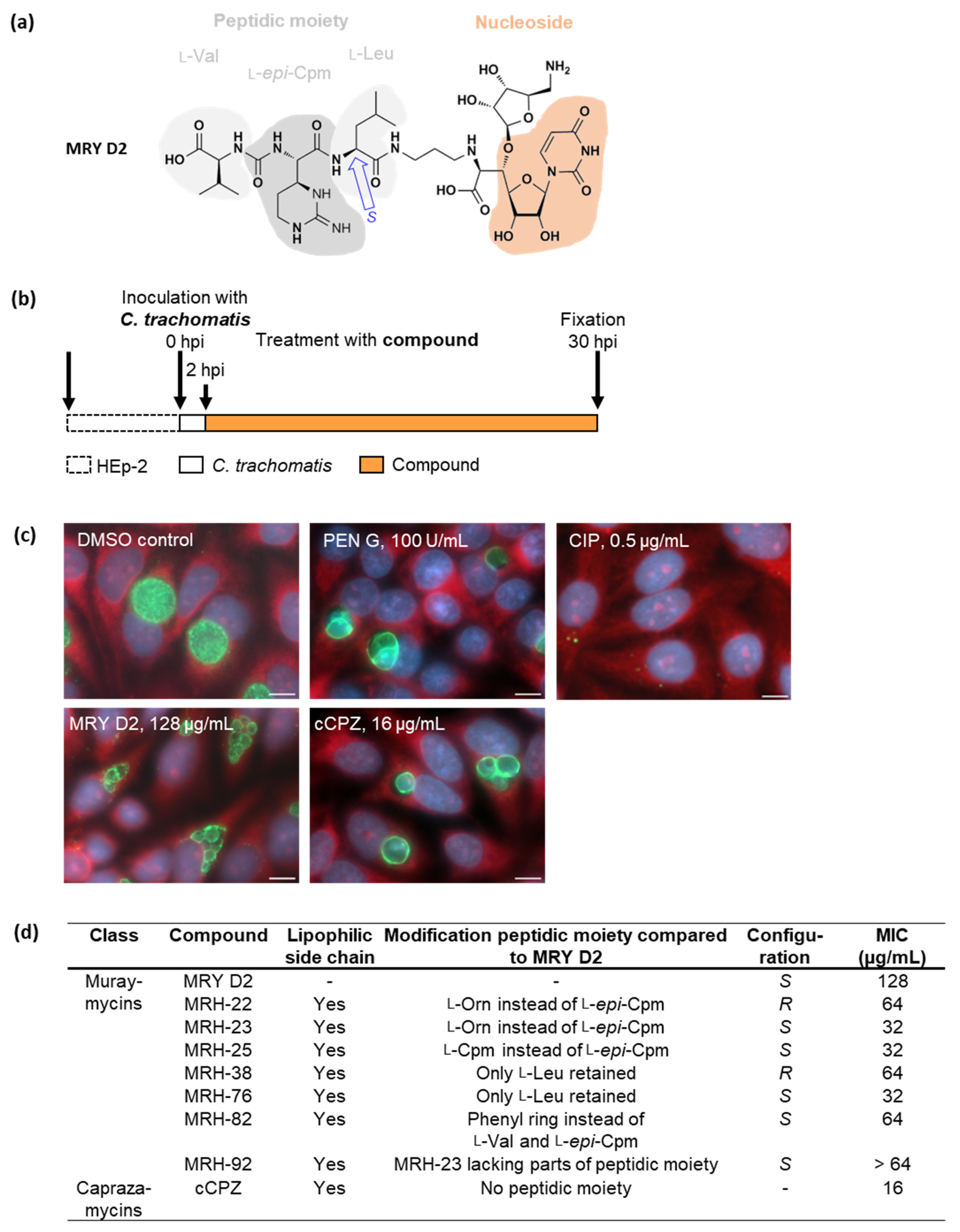
2.1. Muraymycin D2 and its Derivatives Induce the Formation of Enlarged Cells in C. trachomatis upon Treatment at an Early Stage of the Chlamydial Developmental Cycle
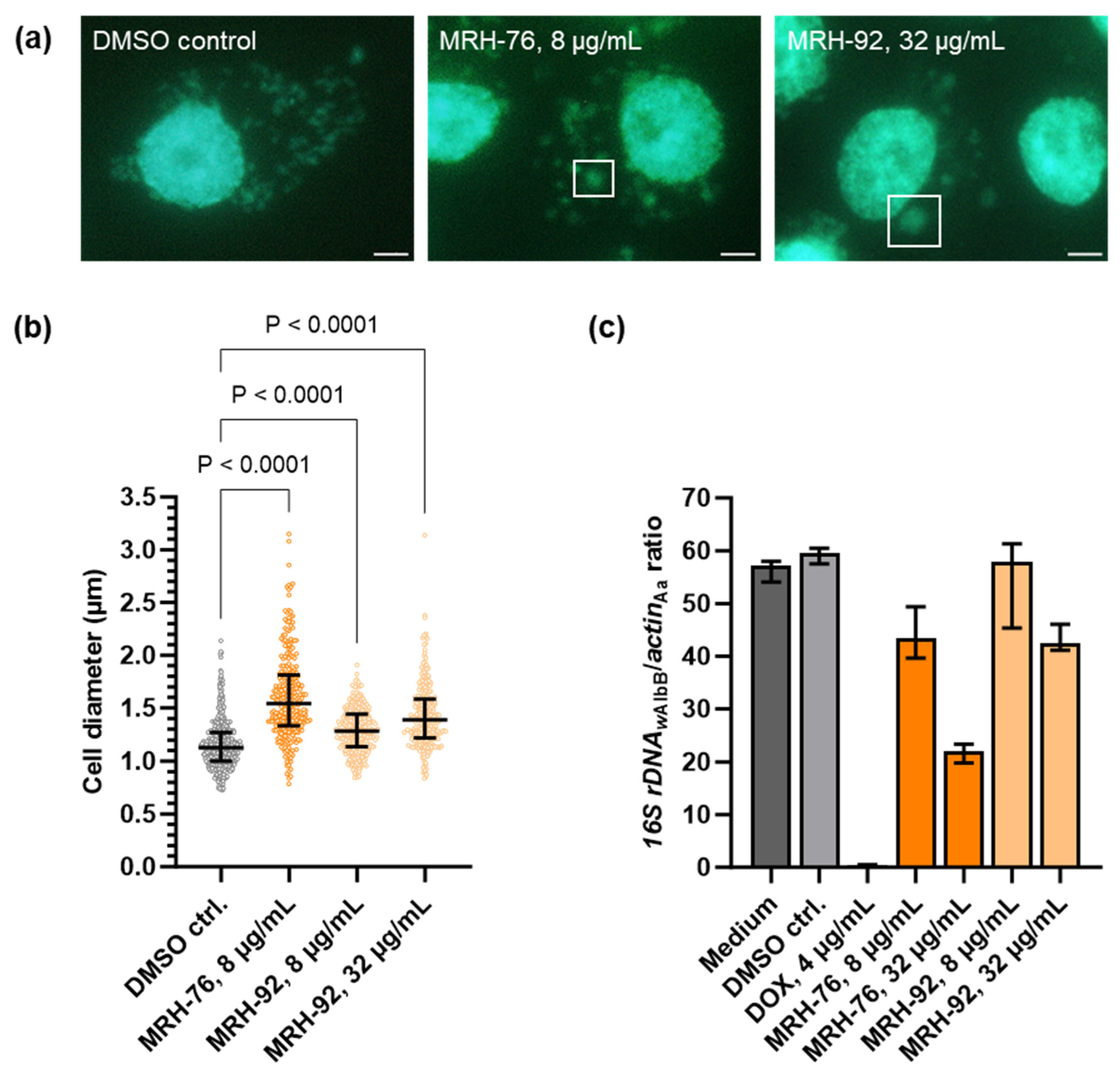
2.2. Muraymycins Induce the Formation of Enlarged Cells in Wolbachia
2.3. Muraymycin D2 and the Derivative MRH-92 Inhibit the Chlamydial Transferase MraY In Vitro
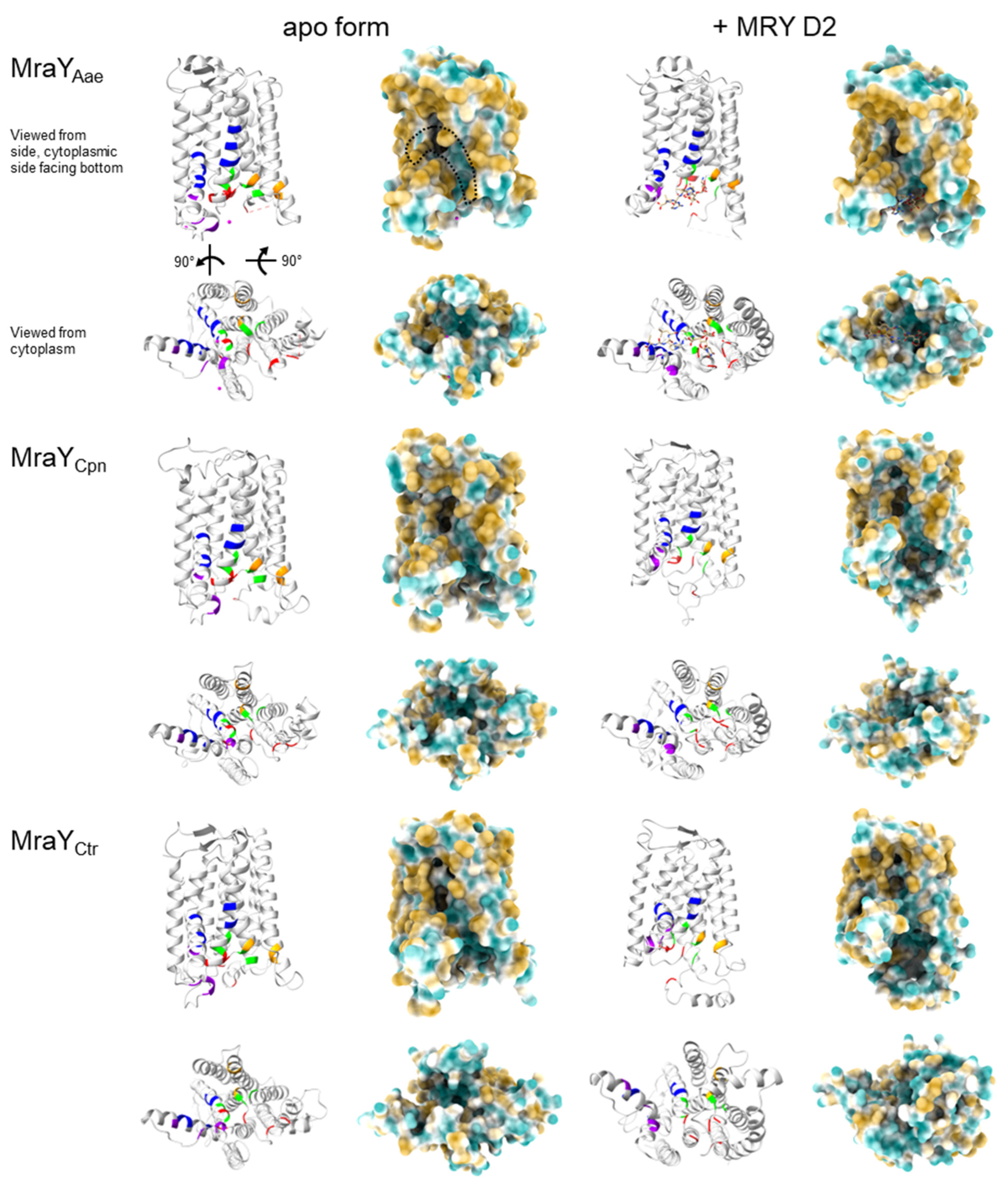
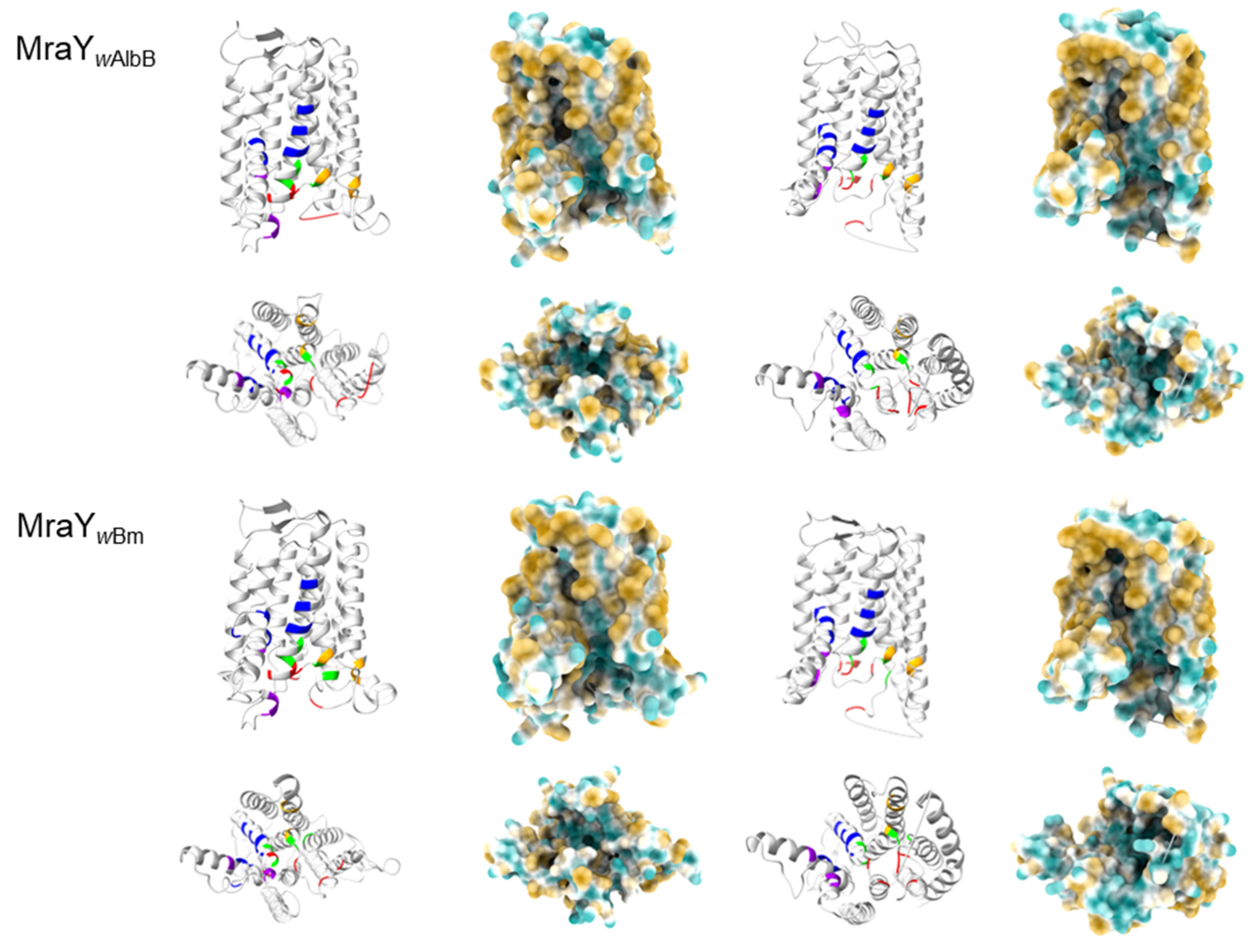
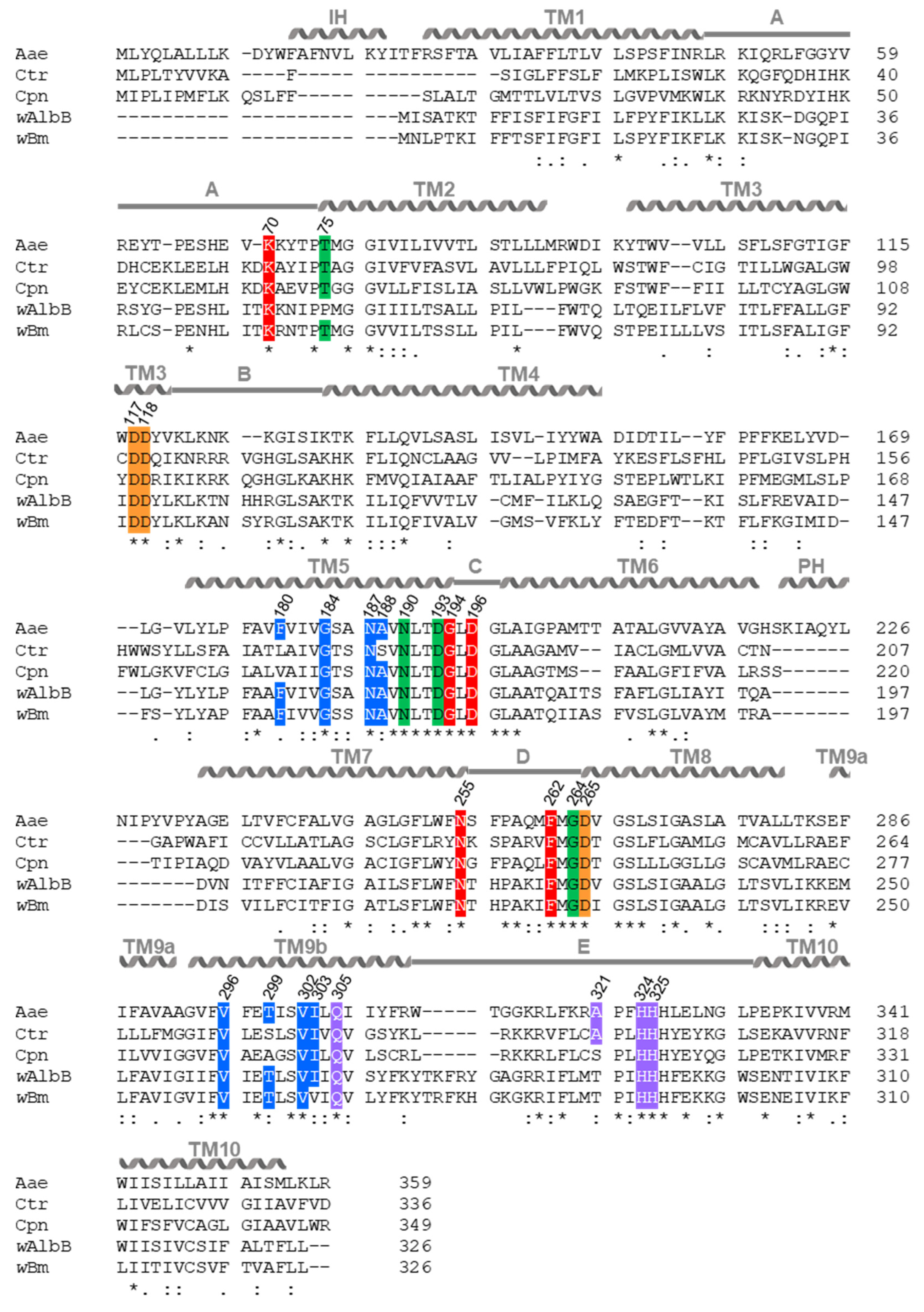
2.4. In Silico Analysis Reveals That Chlamydia and Wolbachia MraY Retain All Amino Acid Residues Important for Binding of UDP-MurNAc-Pentapeptide and Muraymycins
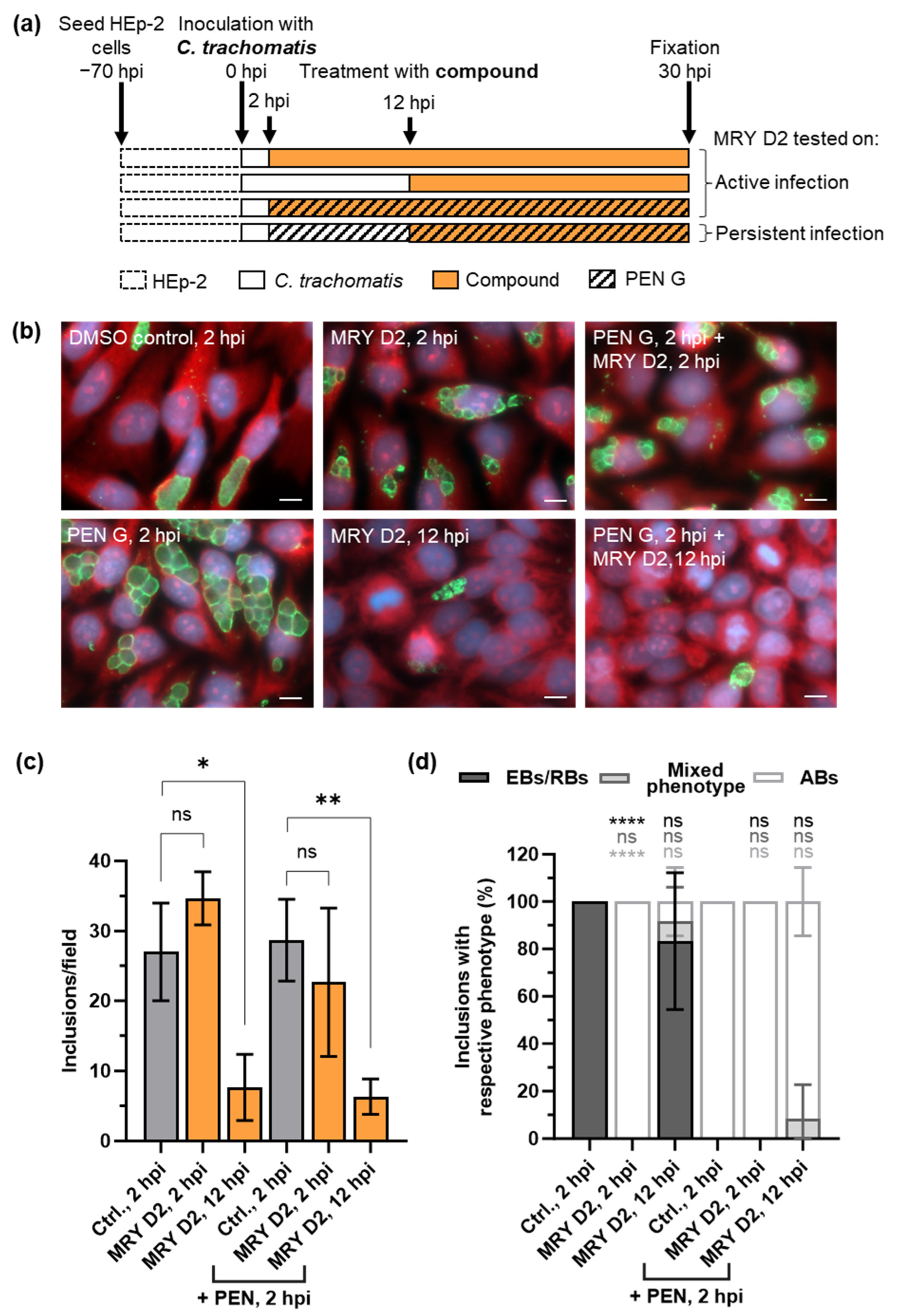
2.5. Muraymycin D2 Eliminates Persistent C. trachomatis Cells from an Established PEN-Induced Persistent Infection upon Addition at the Mid-Stage of the Chlamydial Developmental Cycle
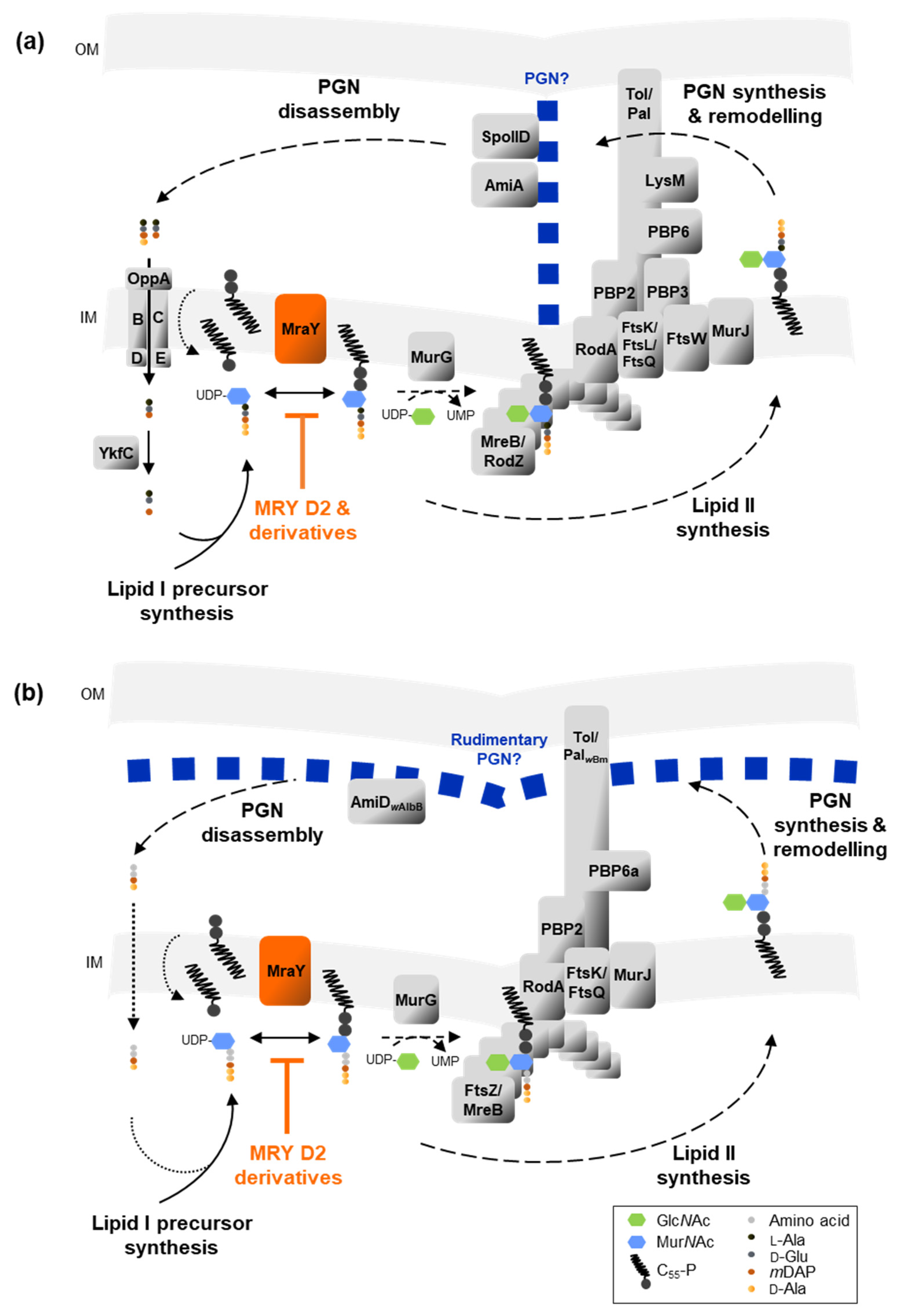
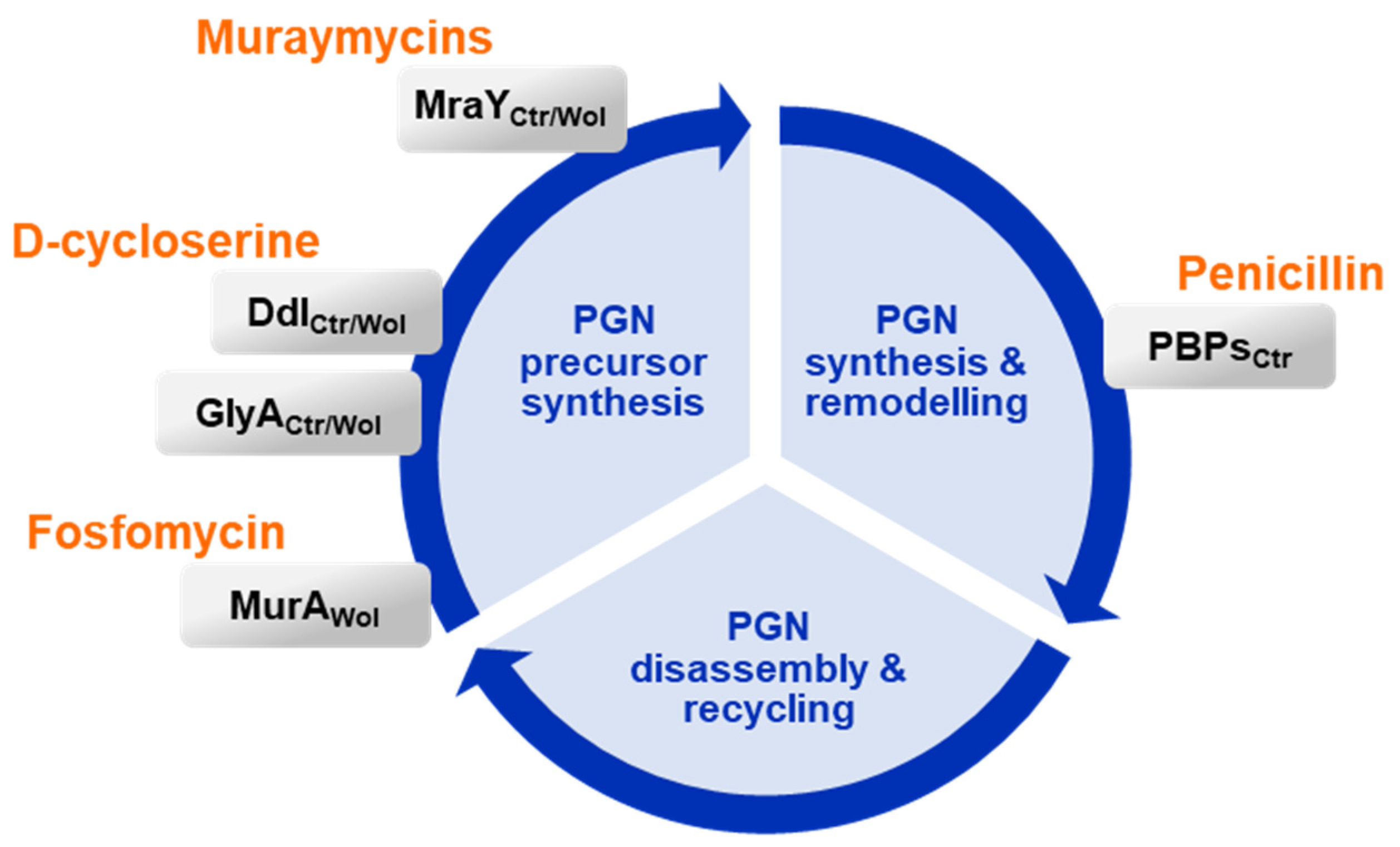
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Cell Culture and Bacterial Strains
4.3. Cytotoxicity of Compounds toward HEp-2 Cells
4.4. Effect of Compounds on Active and Penicillin G-Induced Persistent C. trachomatis Infections
4.5. Fluorescence Microscopy of Muraymycin-Treated Wolbachia
4.6. Quantification of Wolbachia
4.7. Purification of C. pneumoniae MraY
4.8. Inhibition of C. pneumoniae MraY In Vitro
4.9. In Silico Analysis of MraY
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, H.R.; Turner, A.; Taylor, H.R. Trachoma. Lancet 2008, 371, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Chlamydia Infection. In ECDC. Annual Epidemiological Report for 2019; ECDC: Stockholm, Switzerland, 2022. [Google Scholar]
- Weström, L.; Joesoef, R.; Reynolds, G.; Hagdu, A.; Thompson, S.E. Pelvic Inflammatory Disease and Fertility: A Cohort Study of 1844 Women with Laparoscopically Verified Disease and 657 Control Women with Normal Laparoscopic Results. Sex. Transm. Dis. 1992, 19, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Cates, W.; Wasserheit, J.N. Genital Chlamydial Infections: Epidemiology and Reproductive Sequelae. Am. J. Obstet. Gynecol. 1991, 164, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines for the Treatment of Chlamydia Trachomatis; World Health Organization: Geneva, Switzerland, 2016.
- Bragina, E.; Gomberg, M.; Dmitriev, G. Electron Microscopic Evidence of Persistent Chlamydial Infection Following Treatment. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Hosenfeld, C.B.; Workowski, K.A.; Berman, S.; Zaidi, A.; Dyson, J.; Mosure, D.; Bolan, G.; Bauer, H.M. Repeat Infection with Chlamydia and Gonorrhea among Females: A Systematic Review of the Literature. Sex. Transm. Dis. 2009, 36, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Batteiger, B.E.; Tu, W.; Ofner, S.; Van Der Pol, B.; Stothard, D.R.; Orr, D.P.; Katz, B.P.; Fortenberry, J.D. Repeated Chlamydia Trachomatis Genital Infections in Adolescent Women. J. Infect. Dis. 2010, 201, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.R.; Whittington, W.L.H.; Handsfield, H.H.; Hughes, J.P.; Stamm, W.E.; Hogben, M.; Clark, A.; Malinski, C.; Helmers, J.R.L.; Thomas, K.K.; et al. Effect of Expedited Treatment of Sex Partners on Recurrent or Persistent Gonorrhea or Chlamydial Infection. N. Engl. J. Med. 2005, 352, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Spencer, N.; Bowlin, A.K.; Wang, Y.; Rank, R.G. Chlamydial Infection of the Gastrointestinal Tract: A Reservoir for Persistent Infection. Pathog. Dis. 2013, 68, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Phillips Campbell, R.; Kintner, J.; Whittimore, J.; Schoborg, R.V. Chlamydia Muridarum Enters a Viable but Non-Infectious State in Amoxicillin-Treated BALB/c Mice. Microbes Infect. 2012, 14, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.E.; Belland, R.J.; AbdelRahman, Y.M.; Beatty, W.L.; Aiyar, A.A.; Zea, A.H.; Greene, S.J.; Marrero, L.; Buckner, L.R.; Tate, D.J.; et al. Morphologic and Molecular Evaluation of Chlamydia Trachomatis Growth in Human Endocervix Reveals Distinct Growth Patterns. Front. Cell Infect. Microbiol. 2014, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Shemer, Y.; Sarov, I. Inhibition of Growth of Chlamydia Trachomatis by Human Gamma Interferon. Infect. Immun. 1985, 48, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Skilton, R.J.; Cutcliffe, L.T.; Barlow, D.; Wang, Y.; Salim, O.; Lambden, P.R.; Clarke, I.N. Penicillin Induced Persistence in Chlamydia Trachomatis: High Quality Time Lapse Video Analysis of the Developmental Cycle. PLoS ONE 2009, 4, e7723. [Google Scholar] [CrossRef] [PubMed]
- Beatty, W.L.; Morrison, R.P.; Byrne, G.I. Reactivation of Persistent Chlamydia Trachomatis Infection in Cell Culture. Infect. Immun. 1995, 63, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Wyrick, P.B.; Knight, S.T. Pre-Exposure of Infected Human Endometrial Epithelial Cells to Penicillin in Vitro Renders Chlamydia Trachomatis Refractory to Azithromycin. J. Antimicrob. Chemother. 2004, 54, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Reveneau, N.; Crane, D.D.; Fischer, E.; Caldwell, H.D. Bactericidal Activity of First-Choice Antibiotics against Gamma Interferon-Induced Persistent Infection of Human Epithelial Cells by Chlamydia Trachomatis. Antimicrob. Agents Chemother. 2005, 49, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Phillips-Campbell, R.; Kintner, J.; Schoborg, R.V. Induction of the Chlamydia Muridarum Stress/Persistence Response Increases Azithromycin Treatment Failure in a Murine Model of Infection. Antimicrob. Agents Chemother. 2014, 58, 1782–1784. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Bilo, K.; Cross, H.F.; Archer, J.P.; Underwood, A.P. 16S RDNA Phylogeny and Ultrastructural Characterization of Wolbachia Intracellular Bacteria of the Filarial Nematodes Brugia malayi, B. pahangi, and Wuchereria bancrofti. Exp. parasitol. 1999, 91, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Henkle-Dührsen, K.; Eckelt, V.H.O.; Wildenburg, G.; Blaxter, M.; Walter, R.D. Gene Structure, Activity and Localization of a Catalase from Intracellular Bacteria in Onchocerca volvulus. Mol. Biochem. Parasitol. 1998, 96, 69–81. [Google Scholar] [CrossRef]
- Brown, A.M.V.; Wasala, S.K.; Howe, D.K.; Peetz, A.B.; Zasada, I.A.; Denver, D.R. Genomic Evidence for Plant-Parasitic Nematodes as the Earliest Wolbachia hosts. Sci. Rep. 2016, 6, 34955. [Google Scholar] [CrossRef] [PubMed]
- Dudzic, J.P.; Curtis, C.I.; Gowen, B.E.; Perlman, S.J. A Highly Divergent Wolbachia with a Tiny Genome in an Insect-Parasitic Tylenchid Nematode. Proc. Royal Soc. B 2022, 289, 20221518. [Google Scholar] [CrossRef] [PubMed]
- Pfarr, K.; Hoerauf, A. Antibiotics Which Target the Wolbachia Endosymbionts of Filarial Parasites: A New Strategy for Control of Filariasis and Amelioration of Pathology. Mini Rev. Med. Chem. 2006, 6, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Pfarr, K.M.; Debrah, A.Y.; Specht, S.; Hoerauf, A. Filariasis and Lymphoedema. Parasite Immunol. 2009, 31, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, E.A.; Schmidt, C.A.; Kwong, K.T.; Pigott, D.M.; Mupfasoni, D.; Biswas, G.; Shirude, S.; Hill, E.; Donkers, K.M.; Abdoli, A.; et al. The Global Distribution of Lymphatic Filariasis, 2000–2018: A Geospatial Analysis. Lancet Glob. Health 2020, 8, e1186–e1194. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; WHO: Switzerland, Geneva, 2020.
- Karunakaran, I.; Ritter, M.; Pfarr, K.; Klarmann-Schulz, U.; Debrah, A.Y.; Debrah, L.B.; Katawa, G.; Wanji, S.; Specht, S.; Adjobimey, T.; et al. Filariasis Research—From Basic Research to Drug Development and Novel Diagnostics, over a Decade of Research at the Institute for Medical Microbiology, Immunology and Parasitology, Bonn, Germany. Front. Trop. Dis. 2023, 4, 1126173. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Hoerauf, A.; Bockarie, M. Lymphatic Filariasis and Onchocerciasis. Lancet 2010, 376, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Liechti, G.W.; Kuru, E.; Hall, E.; Kalinda, A.; Brun, Y.V.; VanNieuwenhze, M.; Maurelli, A.T. A New Metabolic Cell-Wall Labelling Method Reveals Peptidoglycan in Chlamydia trachomatis. Nature 2014, 506, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Liechti, G.; Kuru, E.; Packiam, M.; Hsu, Y.-P.; Tekkam, S.; Hall, E.; Rittichier, J.T.; VanNieuwenhze, M.; Brun, Y.V.; Maurelli, A.T. Pathogenic Chlamydia Lack a Classical Sacculus but Synthesize a Narrow, Mid-Cell Peptidoglycan Ring, Regulated by MreB, for Cell Division. PLoS Pathog. 2016, 12, e1005590. [Google Scholar] [CrossRef] [PubMed]
- Atwal, S.; Chuenklin, S.; Bonder, E.M.; Flores, J.; Gillespie, J.J.; Driscoll, T.P.; Salje, J. Discovery of a Diverse Set of Bacteria That Build Their Cell Walls without the Canonical Peptidoglycan Polymerase APBP. mBio 2021, 12, e01342-21. [Google Scholar] [CrossRef] [PubMed]
- Henrichfreise, B.; Schiefer, A.; Schneider, T.; Nzukou, E.; Poellinger, C.; Hoffmann, T.-J.; Johnston, K.L.; Moelleken, K.; Wiedemann, I.; Pfarr, K.; et al. Functional Conservation of the Lipid II Biosynthesis Pathway in the Cell Wall-Less Bacteria Chlamydia and Wolbachia: Why Is Lipid II Needed? Mol. Microbiol. 2009, 73, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J.; Schiefer, A.; Schneider, T.; Jülicher, K.; Johnston, K.L.; Taylor, M.J.; Sahl, H.-G.; Hoerauf, A.; Pfarr, K. Requirement of Lipid II Biosynthesis for Cell Division in Cell Wall-Less Wolbachia, Endobacteria of Arthropods and Filarial Nematodes. Int. J. Med. Microbiol. 2013, 303, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Henrichfreise, B.; Brunke, M.; Viollier, P.H. Bacterial Surfaces: The Wall That SEDS Built. Cur Biol. 2016, 26, R1158–R1160. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Y.; Ouellette, S.P.; Belland, R.J.; Cox, J.V. Polarized Cell Division of Chlamydia trachomatis. PLoS Pathog. 2016, 12, e1005822. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.V.; Abdelrahman, Y.M.; Ouellette, S.P. Penicillin-Binding Proteins Regulate Multiple Steps in the Polarized Cell Division Process of Chlamydia. Sci. Rep. 2020, 10, 12588. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, S.P.; Karimova, G.; Subtil, A.; Ladant, D. Chlamydia Co-Opts the Rod Shape-Determining Proteins MreB and Pbp2 for Cell Division. Mol. Microbiol. 2012, 85, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Liechti, G.W. Localized Peptidoglycan Biosynthesis in Chlamydia Trachomatis Conforms to the Polarized Division and Cell Size Reduction Developmental Models. Front. Microbiol. 2021, 12, 733850. [Google Scholar] [CrossRef] [PubMed]
- Storey, C.; Chopra, I. Affinities of β-Lactams for Penicillin Binding Proteins of Chlamydia Trachomatis and Their Antichlamydial Activities. Antimicrob. Agents Chemother. 2001, 45, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Hermans, P.G.; Hart, C.A.; Trees, A.J. In Vitro Activity of Antimicrobial Agents against the Endosymbiont Wolbachia pipientis. J. Antimicrob. Chemother. 2001, 47, 659–663. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Sandlin, R.C.; Maurelli, A.T. In Vitro and In Vivo Functional Activity of Chlamydia MurA, a UDP-N.-Acetylglucosamine Enolpyruvyl Transferase Involved in Peptidoglycan Synthesis and Fosfomycin Resistance. J. Bacteriol. 2003, 185, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Kahan, F.M.; Kahan, J.S.; Cassidy, P.J.; Kropp, H. The Mechanism of Action of Fosfomycin (Phosphonomycin). Ann. N. Y Acad. Sci. 1974, 235, 364–386. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.A.; Barbieri, L.R.; Carter, G.T.; Lenoy, E.; Lotvin, J.; Petersen, P.J.; Siegel, M.M.; Singh, G.; Williamson, R.T. Structures of the Muraymycins, Novel Peptidoglycan Biosynthesis Inhibitors. J. Am. Chem. Soc. 2002, 124, 10260–10261. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Nakagawa, N.; Doi, N.; Hattori, S.; Naganawa, H.; Hamada, M. Caprazamycin B, A Novel Anti-Tuberculosis Antibiotic, from Streptomyces sp. J. Antibiot. 2003, 56, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Tanino, T.; Ichikawa, S.; Al-Dabbagh, B.; Bouhss, A.; Oyama, H.; Matsuda, A. Synthesis and Biological Evaluation of Muraymycin Analogues Active against Anti-Drug-Resistant Bacteria. ACS Med. Chem. Lett. 2010, 1, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Takahashi, Y.; Shitara, T.; Nakamura, H.; Naganawa, H.; Miyake, T.; Akamatsu, Y. Caprazamycins, Novel Lipo-Nucleoside Antibiotics, from Streptomyces sp. J. Antibiot. 2005, 58, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Tanino, T.; Al-Dabbagh, B.; Mengin-Lecreulx, D.; Bouhss, A.; Oyama, H.; Ichikawa, S.; Matsuda, A. Mechanistic Analysis of Muraymycin Analogues: A Guide to the Design of MraY Inhibitors. J. Med. Chem. 2011, 54, 8421–8439. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y.; Tanino, T.; Sekiguchi, M.; Yonezawa, S.; Sakagami, M.; Takahashi, F.; Togame, H.; Tanaka, Y.; Takemoto, H.; Ichikawa, S.; et al. Expansion of Antibacterial Spectrum of Muraymycins toward Pseudomonas aeruginosa. ACS Med. Chem. Lett. 2014, 5, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Yamaguchi, M.; Hsuan, L.S.; Kato, Y.; Matsuda, A. Carbacaprazamycins: Chemically Stable Analogues of the Caprazamycin Nucleoside Antibiotics. ACS Infect. Dis. 2015, 1, 151–156. [Google Scholar] [CrossRef]
- Chung, B.C.; Mashalidis, E.H.; Tanino, T.; Kim, M.; Matsuda, A.; Hong, J.; Ichikawa, S.; Lee, S.-Y. Structural Insights into Inhibition of Lipid I Production in Bacterial Cell Wall Synthesis. Nature 2016, 533, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Miyairi, I.; Mahdi, O.S.; Ouellette, S.P.; Belland, R.J.; Byrne, G.I. Different Growth Rates of Chlamydia trachomatis Biovars Reflect Pathotype. J. Infect. Dis. 2006, 194, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Di Francesco, A.; D’Antuono, A.; Delucca, F.; Shurdhi, A.; Moroni, A.; Baldelli, R.; Cevenini, R. In Vitro Activities of Several Antimicrobial Agents against Recently Isolated and Genotyped Chlamydia trachomatis Urogenital Serovars D through K. Antimicrob. Agents Chemother. 2010, 54, 5379–5380. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, A.; Schmitz, A.; Schäberle, T.F.; Specht, S.; Lämmer, C.; Johnston, K.L.; Vassylyev, D.G.; König, G.M.; Hoerauf, A.; Pfarr, K. Corallopyronin A Specifically Targets and Depletes Essential Obligate Wolbachia Endobacteria from Filarial Nematodes In Vivo. J. Infect. Dis. 2012, 206, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mashalidis, E.H.; Kaeser, B.; Terasawa, Y.; Katsuyama, A.; Kwon, D.-Y.; Lee, K.; Hong, J.; Ichikawa, S.; Lee, S.-Y. Chemical Logic of MraY Inhibition by Antibacterial Nucleoside Natural Products. Nat. Commun. 2019, 10, 2917. [Google Scholar] [CrossRef] [PubMed]
- Bouhss, A.; Mengin-Lecreulx, D.; Le Beller, D.; van Heijenoort, J. Topological Analysis of the MraY Protein Catalysing the First Membrane Step of Peptidoglycan Synthesis. Mol. Microbiol. 1999, 34, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.J.; Brandish, P.E.; Gilbey, A.M.; Bugg, T.D.H. Phospho-N-Acetyl-Muramyl-Pentapeptide Translocase from Escherichia coli: Catalytic Role of Conserved Aspartic Acid Residues. J. Bacteriol. 2004, 186, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbagh, B.; Henry, X.; El Ghachi, M.; Auger, G.; Blanot, D.; Parquet, C.; Mengin-Lecreulx, D.; Bouhss, A. Active Site Mapping of MraY, a Member of the Polyprenyl-Phosphate N-Acetylhexosamine 1-Phosphate Transferase Superfamily, Catalyzing the First Membrane Step of Peptidoglycan Biosynthesis. Biochemistry 2008, 47, 8919–8928. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.C.; Zhao, J.; Gillespie, R.A.; Kwon, D.-Y.; Guan, Z.; Hong, J.; Zhou, P.; Lee, S.-Y. Crystal Structure of MraY, an Essential Membrane Enzyme for Bacterial Cell Wall Synthesis. Science 2013, 341, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Ichikawa, S.; Matsuda, A. Structure–Activity Relationship of Truncated Analogs of Caprazamycins as Potential Anti-Tuberculosis Agents. Bioorg Med. Chem. 2008, 16, 5123–5133. [Google Scholar] [CrossRef] [PubMed]
- Dini, C.; Drochon, N.; Guillot, J.C.; Mauvais, P.; Walter, P.; Aszodi, J. Synthesis of Analogues of the O-β-d-Ribofuranosyl Nucleoside Moiety of Liposidomycins. Part 2: Role of the Hydroxyl Groups upon the Inhibition of MraY. Bioorg Med. Chem. Lett. 2001, 11, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Dini, C.; Drochon, N.; Feteanu, S.; Guillot, J.C.; Peixoto, C.; Aszodi, J. Synthesis of Analogues of the O-β-d-Ribofuranosyl Nucleoside Moiety of Liposidomycins. Part 1: Contribution of the Amino Group and the Uracil Moiety upon the Inhibition of MraY. Bioorg Med. Chem. Lett. 2001, 11, 529–531. [Google Scholar] [CrossRef]
- Foschi, C.; Salvo, M.; Cevenini, R.; Marangoni, A. Chlamydia Trachomatis Antimicrobial Susceptibility in Colorectal and Endocervical Cells. J. Antimicrob. Chemother. 2018, 73, 409–413. [Google Scholar] [CrossRef]
- Peuchant, O.; Duvert, J.P.; Clerc, M.; Raherison, S.; Bébéar, C.; Bébéar, C.M.; de Barbeyrac, B. Effects of Antibiotics on Chlamydia Trachomatis Viability as Determined by Real-Time Quantitative PCR. J. Med. Microbiol. 2011, 60, 508–514. [Google Scholar] [CrossRef]
- Otten, C.; De Benedetti, S.; Gaballah, A.; Bühl, H.; Klöckner, A.; Brauner, J.; Sahl, H.-G.; Henrichfreise, B. Co-Solvents as Stabilizing Agents during Heterologous Overexpression in Escherichia coli—Application to Chlamydial Penicillin-Binding Protein 6. PLoS ONE 2015, 10, e0122110. [Google Scholar] [CrossRef] [PubMed]
- Shima, K.; Kaufhold, I.; Eder, T.; Käding, N.; Schmidt, N.; Ogunsulire, I.M.; Deenen, R.; Köhrer, K.; Friedrich, D.; Isay, S.E.; et al. Regulation of the Mitochondrion-Fatty Acid Axis for the Metabolic Reprogramming of Chlamydia Trachomatis during Treatment with β-Lactam Antimicrobials. mBio 2021, 12, e00023-21. [Google Scholar] [CrossRef] [PubMed]
- Belland, R.J.; Zhong, G.; Crane, D.D.; Hogan, D.; Sturdevant, D.; Sharma, J.; Beatty, W.L.; Caldwell, H.D. Genomic Transcriptional Profiling of the Developmental Cycle of Chlamydia trachomatis. Proc. Nat. Acad. Sci. USA 2003, 100, 8478–8483. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, S.; Bühl, H.; Gaballah, A.; Klöckner, A.; Otten, C.; Schneider, T.; Sahl, H.-G.; Henrichfreise, B. Characterization of Serine Hydroxymethyltransferase GlyA as a Potential Source of D-Alanine in Chlamydia pneumoniae. Front. Cell Infect. Microbiol. 2014, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Patin, D.; Bostock, J.; Chopra, I.; Mengin-Lecreulx, D.; Blanot, D. Biochemical Characterisation of the Chlamydial MurF Ligase, and Possible Sequence of the Chlamydial Peptidoglycan Pentapeptide Stem. Arch. Microbiol. 2012, 194, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Mengin-Lecreulx, D.; van Heijenoort, J. Effect of Growth Conditions on Peptidoglycan Content and Cytoplasmic Steps of Its Biosynthesis in Escherichia coli. J. Bacteriol. 1985, 163, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Lambden, P.R.; Pickett, M.A.; Clarke, I.N. The Effect of Penicillin on Chlamydia trachomatis DNA Replication. Microbiology 2006, 152, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Meeske, A.J.; Riley, E.P.; Robins, W.P.; Uehara, T.; Mekalanos, J.J.; Kahne, D.; Walker, S.; Kruse, A.C.; Bernhardt, T.G.; Rudner, D.Z. SEDS Proteins Are a Widespread Family of Bacterial Cell Wall Polymerases. Nature 2016, 537, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Welsh, M.A.; Marmont, L.S.; Lee, W.; Sjodt, M.; Kruse, A.C.; Kahne, D.; Bernhardt, T.G.; Walker, S. FtsW Is a Peptidoglycan Polymerase That Is Functional Only in Complex with Its Cognate Penicillin-Binding Protein. Nat. Microbiol. 2019, 4, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.; Verma, M.; Pathak, M.; Mitra, K.; Misra-Bhattacharya, S. Cloning, Expression and Characterization of UDP-N-Acetylglucosamine Enolpyruvyl Transferase (MurA) from Wolbachia endosymbiont of Human Lymphatic Filarial Parasite Brugia malayi. PLoS ONE 2014, 9, e99884. [Google Scholar] [CrossRef]
- McCoy, A.J.; Maurelli, A.T. Characterization of Chlamydia MurC-Ddl, a Fusion Protein Exhibiting D-Alanyl-D-Alanine Ligase Activity Involved in Peptidoglycan Synthesis and D-Cycloserine Sensitivity. Mol. Microbiol. 2005, 57, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Kuru, E.; Radkov, A.; Meng, X.; Egan, A.; Alvarez, L.; Dowson, A.; Booher, G.; Breukink, E.; Roper, D.I.; Cava, F.; et al. Mechanisms of Incorporation for D-Amino Acid Probes That Target Peptidoglycan Biosynthesis. ACS Chem. Biol. 2019, 14, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.; Deisinger, J.P.; Ulm, H.; Müller, A.; Li, W.; Hardt, P.; Wang, X.; Li, X.; Sylvester, M.; Engeser, M.; et al. Coordination of Capsule Assembly and Cell Wall Biosynthesis in Staphylococcus aureus. Nat. Commun. 2019, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Mann, P.A.; Müller, A.; Wolff, K.A.; Fischmann, T.; Wang, H.; Reed, P.; Hou, Y.; Li, W.; Müller, C.E.; Xiao, J.; et al. Chemical Genetic Analysis and Functional Characterization of Staphylococcal Wall Teichoic Acid 2-Epimerases Reveals Unconventional Antibiotic Drug Targets. PLoS Pathog. 2016, 12, e1005585. [Google Scholar] [CrossRef] [PubMed]
- Goodell, E.W.; Schwarz, U. Release of Cell Wall Peptides into Culture Medium by Exponentially Growing Escherichia coli. J. Bacteriol. 1985, 162, 391–397. [Google Scholar] [CrossRef]
- Jacquier, N.; Yadav, A.K.; Pillonel, T.; Viollier, P.H.; Cava, F.; Greub, G. A SpoIID Homolog Cleaves Glycan Strands at the Chlamydial Division Septum. mBio 2019, 10, e01128-19. [Google Scholar] [CrossRef] [PubMed]
- Klöckner, A.; Otten, C.; Derouaux, A.; Vollmer, W.; Bühl, H.; De Benedetti, S.; Münch, D.; Josten, M.; Mölleken, K.; Sahl, H.-G.; et al. AmiA Is a Penicillin Target Enzyme with Dual Activity in the Intracellular Pathogen Chlamydia pneumoniae. Nat. Commun. 2014, 5, 4201. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.; Otten, C.; Jacquier, N.; Lee, J.; Mengin-Lecreulx, D.; Löckener, I.; Kluj, R.; Mayer, C.; Corona, F.; Dannenberg, J.; et al. An NlpC/P60 Protein Catalyzes a Key Step in Peptidoglycan Recycling at the Intersection of Energy Recovery, Cell Division and Immune Evasion in the Intracellular Pathogen Chlamydia trachomatis. PLoS Pathog. 2023, 19, e1011047. [Google Scholar] [CrossRef] [PubMed]
- Chamaillard, M.; Hashimoto, M.; Horie, Y.; Masumoto, J.; Qiu, S.; Saab, L.; Ogura, Y.; Kawasaki, A.; Fukase, K.; Kusumoto, S.; et al. An Essential Role for NOD1 in Host Recognition of Bacterial Peptidoglycan Containing Diaminopimelic Acid. Nat. Immunol. 2003, 4, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N.; Ogura, Y.; Fontalba, A.; Gutierrez, O.; Pons, F.; Crespo, J.; Fukase, K.; Inamura, S.; Kusumoto, S.; Hashimoto, M.; et al. Host Recognition of Bacterial Muramyl Dipeptide Mediated through NOD2. J. Biol. Chem. 2003, 278, 5509–5512. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.M.; Murphy, A.J.; Barrett, K.T.; Wira, C.R.; Guyre, P.M.; Pioli, P.A. Functional Expression of Pattern Recognition Receptors in Tissues of the Human Female Reproductive Tract. J. Reprod. Immunol. 2009, 80, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Travassos, L.H.; Carneiro, L.A.M.; Girardin, S.; Philpott, D.J. Nod Proteins Link Bacterial Sensing and Autophagy. Autophagy 2010, 6, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Irving, A.T.; Mimuro, H.; Kufer, T.A.; Lo, C.; Wheeler, R.; Turner, L.J.; Thomas, B.J.; Malosse, C.; Gantier, M.P.; Casillas, L.N.; et al. The Immune Receptor NOD1 and Kinase RIP2 Interact with Bacterial Peptidoglycan on Early Endosomes to Promote Autophagy and Inflammatory Signaling. Cell Host Microbe 2014, 15, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Brockett, M.R.; Liechti, G.W. Persistence Alters the Interaction between Chlamydia trachomatis and Its Host Cell. Infect. Immun. 2021, 89, e685-20. [Google Scholar] [CrossRef] [PubMed]
- Packiam, M.; Weinrick, B.; Jacobs, W.R.; Maurelli, A.T. Structural Characterization of Muropeptides from Chlamydia trachomatis Peptidoglycan by Mass Spectrometry Resolves “Chlamydial Anomaly”. Proc. Nat. Acad. Sci. USA 2015, 112, 11660–11665. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.D.; Langley, R.S.; Johnston, K.L.; Egerton, G.; Wanji, S.; Taylor, M.J. Wolbachia Endosymbiotic Bacteria of Brugia malayi Mediate Macrophage Tolerance to TLR- and CD40-Specific Stimuli in a MyD88/TLR2-Dependent Manner. J. Immunol. 2006, 177, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in Speed, Utility and Usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Makepeace, B.L.; Rodgers, L.; Trees, A.J. Rate of Elimination of Wolbachia pipientis by Doxycycline in Vitro Increases Following Drug Withdrawal. Antimicrob. Agents Chemother. 2006, 50, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Klepsch, M.M.; Schlegel, S.; Appel, A.; Draheim, R.; Tarry, M.; Högbom, M.; van Wijk, K.J.; Slotboom, D.J.; Persson, J.O.; et al. Tuning Escherichia coli for Membrane Protein Overexpression. Proc. Nat. Acad. Sci. USA 2008, 105, 14371–14376. [Google Scholar] [CrossRef] [PubMed]
- Kohlrausch, U.; Höltje, J.-V. One-Step Purification Procedure for UDP-N-Acetylmuramyl-Peptide Murein Precursors from Bacillus cereus. FEMS Microbiol. Lett. 1991, 78, 253–258. [Google Scholar] [CrossRef]
- Rick, P.D.; Hubbard, G.L.; Kitaoka, M.; Nagaki, H.; Kinoshita, T.; Dowd, S.; Simplaceanu, V.; Ho, C. Characterization of the Lipid-Carrier Involved in the Synthesis of Enterobacterial Common Antigen (ECA) and Identification of a Novel Phosphoglyceride in a Mutant of Salmonella typhimurium Defective in ECA Synthesis. Glycobiol. 1998, 8, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löckener, I.; Behrmann, L.V.; Reuter, J.; Schiefer, A.; Klöckner, A.; Krannich, S.; Otten, C.; Mölleken, K.; Ichikawa, S.; Hoerauf, A.; et al. The MraY Inhibitor Muraymycin D2 and Its Derivatives Induce Enlarged Cells in Obligate Intracellular Chlamydia and Wolbachia and Break the Persistence Phenotype in Chlamydia. Antibiotics 2024, 13, 421. https://doi.org/10.3390/antibiotics13050421
Löckener I, Behrmann LV, Reuter J, Schiefer A, Klöckner A, Krannich S, Otten C, Mölleken K, Ichikawa S, Hoerauf A, et al. The MraY Inhibitor Muraymycin D2 and Its Derivatives Induce Enlarged Cells in Obligate Intracellular Chlamydia and Wolbachia and Break the Persistence Phenotype in Chlamydia. Antibiotics. 2024; 13(5):421. https://doi.org/10.3390/antibiotics13050421
Chicago/Turabian StyleLöckener, Iris, Lara Vanessa Behrmann, Jula Reuter, Andrea Schiefer, Anna Klöckner, Sebastian Krannich, Christian Otten, Katja Mölleken, Satoshi Ichikawa, Achim Hoerauf, and et al. 2024. "The MraY Inhibitor Muraymycin D2 and Its Derivatives Induce Enlarged Cells in Obligate Intracellular Chlamydia and Wolbachia and Break the Persistence Phenotype in Chlamydia" Antibiotics 13, no. 5: 421. https://doi.org/10.3390/antibiotics13050421
APA StyleLöckener, I., Behrmann, L. V., Reuter, J., Schiefer, A., Klöckner, A., Krannich, S., Otten, C., Mölleken, K., Ichikawa, S., Hoerauf, A., Schneider, T., Pfarr, K. M., & Henrichfreise, B. (2024). The MraY Inhibitor Muraymycin D2 and Its Derivatives Induce Enlarged Cells in Obligate Intracellular Chlamydia and Wolbachia and Break the Persistence Phenotype in Chlamydia. Antibiotics, 13(5), 421. https://doi.org/10.3390/antibiotics13050421






