AMPs as Host-Directed Immunomodulatory Agents against Skin Infections Caused by Opportunistic Bacterial Pathogens
Abstract
1. Introduction
2. Opportunistic Skin Pathogen-Associated Infections
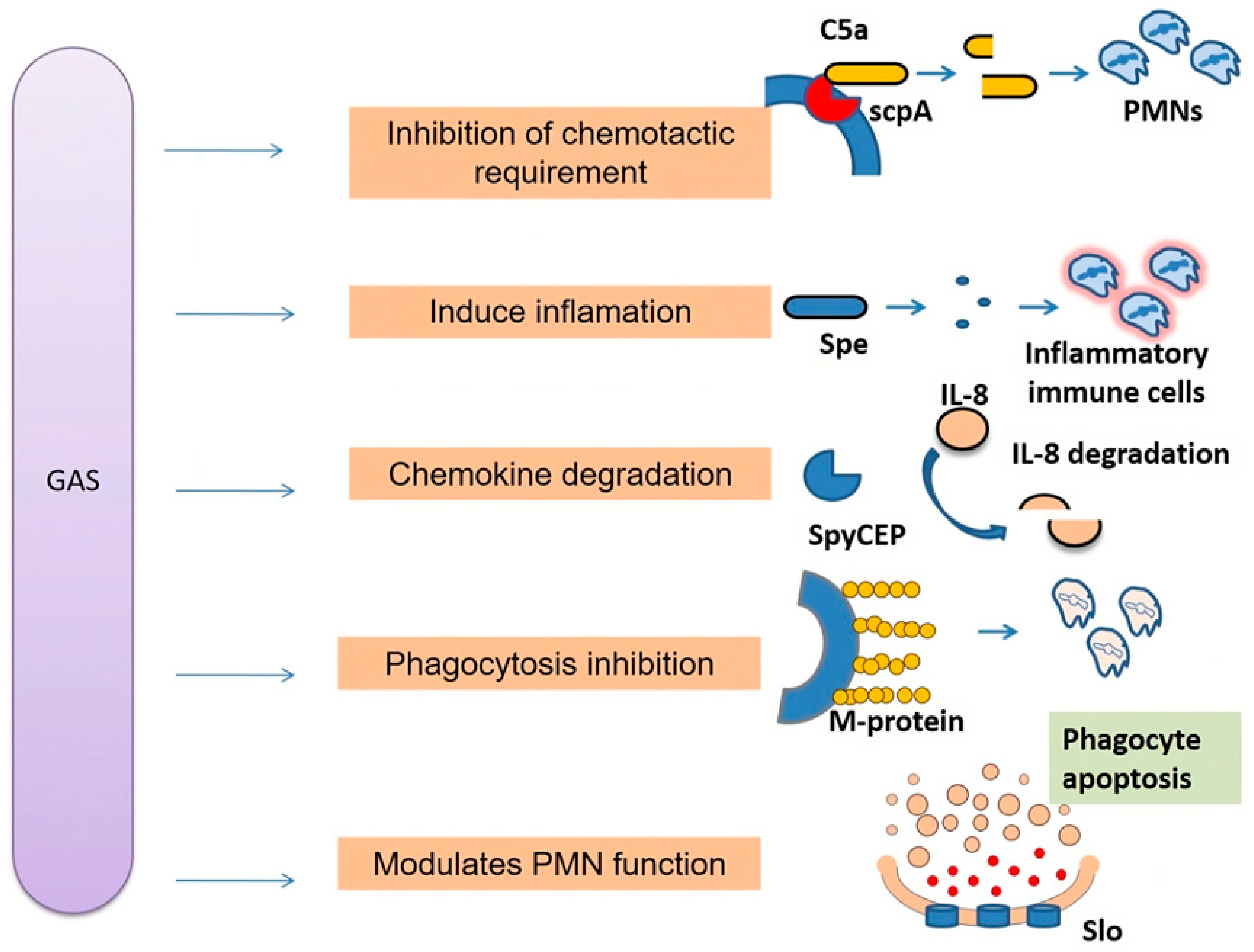
2.1. Group A Streptococcal Infections
2.2. Staphylococcal Infections
3. Skin Antimicrobial Peptides (AMPs)
Mode of Action
4. Immunomodulation of Host Immune Cells and Responses
4.1. Dendritic Cells
4.2. Mast Cells
4.3. Neutrophils
4.4. Macrophages
4.5. Gamma Delta (γδ) T Cells
4.6. NK Cells
4.7. Keratinocytes
4.8. Melanocytes
5. AMPs in Adjunct Therapy
Challenges of AMP Therapies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Kolarsick, P.A.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Woodley, D.T. Distinct fibroblasts in the papillary and reticular dermis: Implications for wound healing. Dermatol. Clin. 2017, 35, 95–100. [Google Scholar] [CrossRef]
- Ng, K.W.; Lau, W.M. Skin deep: The basics of human skin structure and drug penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar]
- Salmon, J.K.; Armstrong, C.A.; Ansel, J.C. The skin as an immune organ. Western J. Med. 1994, 160, 146. [Google Scholar]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef]
- Kim, M.; Truong, N.R.; James, V.; Bosnjak, L.; Sandgren, K.J.; Harman, A.N.; Nasr, N.; Bertram, K.M.; Olbourne, N.; Sawleshwarkar, S.; et al. Relay of herpes simplex virus between Langerhans cells and dermal dendritic cells in human skin. PLoS Pathog. 2015, 11, e1004812. [Google Scholar] [CrossRef]
- Heath, W.R.; Carbone, F.R. The skin-resident and migratory immune system in steady state and memory: Innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013, 14, 978–985. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Duckney, P.; Wong, H.K.; Serrano, J.; Yaradou, D.; Oddos, T.; Stamatas, G.N. The role of the skin barrier in modulating the effects of common skin microbial species on the inflammation, differentiation and proliferation status of epidermal keratinocytes. BMC Res. Notes. 2013, 6, 474. [Google Scholar] [CrossRef]
- Jusko, M.; Potempa, J.; Kantyka, T.; Bielecka, E.; Miller, H.K.; Kalinska, M.; Dubin, G.; Garred, P.; Shaw, L.N.; Blom, A.M. Staphylococcal proteases aid in evasion of the human complement system. J. Innate Immun. 2014, 6, 31–46. [Google Scholar] [CrossRef]
- Syed, S.; Viazmina, L.; Mager, R.; Meri, S.; Haapasalo, K. Streptococci and the complement system: Interplay during infection, inflammation and autoimmunity. FEBS Lett. 2020, 594, 2570–2585. [Google Scholar] [CrossRef]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Castro, S.A.; Dorfmueller, H.C. A brief review on Group A Streptococcus pathogenesis and vaccine development. R. Soc. Open Sci. 2021, 8, 201991. [Google Scholar] [CrossRef]
- Rohde, M.; Cleary, P.P. Adhesion and invasion of Streptococcus pyogenes into host cells and clinical relevance of intracellular streptococci. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2022. [Google Scholar]
- Reglinski, M.; Sriskandan, S. The contribution of group A streptococcal virulence determinants to the pathogenesis of sepsis. Virulence 2014, 5, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Travers, P.; Walport, M. Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2008; Volume 3. [Google Scholar]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, D.; Grywalska, E. Staphylococcus aureus and host immunity in recurrent furunculosis. Dermatology 2019, 235, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Grumann, D.; Nübel, U.; Bröker, B.M. Staphylococcus aureus toxins–their functions and genetics. Infect. Genet. Evol. 2014, 21, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Foster Foster, T.J. Immune evasion by staphylococci. Nat. Rev. Microbiol. 2005, 3, 948–958. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Antimicrobial peptides activity in the skin. Skin Res. Technol. 2019, 25, 111–117. [Google Scholar] [CrossRef]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef]
- Harder, J.; Schröder, J.M.; Gläser, R. The skin surface as antimicrobial barrier: Present concepts and future outlooks. Exp. Dermatol. 2013, 22, 1–5. [Google Scholar] [CrossRef]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.H.; Shah, P.; Chen, Y.W.; Chen, C.S. Systematic Analysis of Intracellular-targeting Antimicrobial Peptides, Bactenecin 7, Hybrid of Pleurocidin and Dermaseptin, Proline-Arginine-rich Peptide, and Lactoferricin B, by Using Escherichia coli Proteome Microarrays. Mol. Cell. Proteom. MCP 2016, 15, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Moreno-Morales, J.; Ballesté-Delpierre, C. Current landscape in the discovery of novel antibacterial agents. Clin. Microbiol. Infect. 2020, 26, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Puri, S.; McCall, A.; Norris, H.L.; Russo, T.; Edgerton, M. Human Salivary Protein Histatin 5 Has Potent Bactericidal Activity against ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2017, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Backbone Cyclization and Dimerization of LL-37-Derived Peptides Enhance Antimicrobial Activity and Proteolytic Stability. Front. Microbiol. 2020, 11, 168. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Lee, H.R.; You, D.G.; Kim, H.K.; Sohn, J.W.; Kim, M.J.; Park, J.K.; Lee, G.Y.; Yoo, Y.D. Romo1-derived antimicrobial peptide is a new antimicrobial agent against multidrug-resistant bacteria in a murine model of sepsis. Mbio 2020, 11, e03258-19. [Google Scholar] [CrossRef]
- Hakim, A.; Braun, H.; Thornton, D.; Strymish, J. Successful treatment of methicillin-sensitive Staphylococcus aureus tricuspid-valve endocarditis with dalbavancin as an outpatient in a person who injects drugs: A case report. Int. J. Infect. Dis. 2020, 91, 202–205. [Google Scholar] [CrossRef]
- Mirski, T.; Niemcewicz, M.; Bartoszcze, M.; Gryko, R.; Michalski, A. Utilisation of peptides against microbial infections—A review. Ann. Agric. Environ. Med. 2018, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Bartoletti, M.; Gatti, M.; Viale, P. Advances in the therapy of bacterial bloodstream infections. Clin. Microbiol. Infect. 2020, 26, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Pan, C.Y.; Wu, H.Y.; Chen, J.Y. Antimicrobial peptide Epinecidin-1 promotes complete skin regeneration of methicillin-resistant Staphylococcus aureus-infected burn wounds in a swine model. Oncotarget 2017, 8, 21067–21080. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919. [Google Scholar] [PubMed]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-membrane permeabilizing modes of action of antimicrobial peptides on bacteria. Curr. Top. Med. Chem. 2016, 16, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Hancock, R.E. Peptide design for antimicrobial and immunomodulatory applications. Pept. Sci. 2013, 100, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.R.; Bottazzi, B.; De Nardo, D.; Lawlor, K.E. Immunomodulation of innate immune cells. Front. Immunol. 2020, 11, 101. [Google Scholar] [CrossRef]
- Gestal, M.C.; Johnson, H.M.; Harvill, E.T. Immunomodulation as a novel strategy for prevention and treatment of Bordetella spp. infections. Front. Immunol. 2019, 10, 2869. [Google Scholar] [CrossRef] [PubMed]
- Cruvinel, W.D.M.; Mesquita Júnior, D.; Araújo, J.A.P.; Catelan, T.T.T.; Souza, A.W.S.D.; Silva, N.P.D.; Andrade, L.E.C. Immune system: Part I. Fundamentals of innate immunity with emphasis on molecular and cellular mechanisms of inflammatory response. Rev. Bras. Reumatol. 2010, 50, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; Sia, J.K.; Bizzell, E.; Madan-Lala, R.; Rengarajan, J. Mycobacterium tuberculosis GroEL2 modulates dendritic cell responses. Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Young, J.W.; Banchereau, J. Dendritic cells. Adv. Immunol. 1999, 72, 255–324. [Google Scholar] [PubMed]
- Austyn, J.M. Dendritic System—History, Tissues, and Immunity Tolerance. In Myeloid Cells in Health and Disease: A Synthesis; American Society for Microbiology: Washington, DC, USA, 2020; p. 155. [Google Scholar]
- Muñoz-Carrillo, J.L.; Contreras-Cordero, J.F.; Gutiérrez-Coronado, O.; Villalobos-Gutiérrez, P.T.; Ramos-Gracia, L.G.; Vargas-Barboza, J.M. Role of Dendritic Cells in Pathogen Infections: A Current Perspective. In Cell Interaction-Molecular and Immunological Basis for Disease Management; IntechOpen: London, UK, 2021. [Google Scholar]
- Jongbloed, S.L.; Kassianos, A.J.; McDonald, K.J.; Clark, G.J.; Ju, X.; Angel, C.E.; Chen, C.J.J.; Dunbar, P.R.; Wadley, R.B.; Jeet, V.; et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010, 207, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Darisipudi, M.N.; Nordengrün, M.; Bröker, B.M.; Péton, V. Messing with the sentinels—The interaction of Staphylococcus aureus with dendritic cells. Microorganisms 2018, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, N.S.; Richardson, J.R.; Schreiner, J.; Klenk, J.; Günter, M.; Autenrieth, S.E. Staphylococcus aureus PSM peptides induce tolerogenic dendritic cells upon treatment with ligands of extracellular and intracellular TLRs. Int. J. Med. Microbiol. 2016, 306, 666–674. [Google Scholar] [CrossRef]
- Van Der Does, A.M.; Joosten, S.A.; Vroomans, E.; Bogaards, S.J.; Van Meijgaarden, K.E.; Ottenhoff, T.H.; Van Dissel, J.T.; Nibbering, P.H. The antimicrobial peptide hLF1–11 drives monocyte-dendritic cell differentiation toward dendritic cells that promote antifungal responses and enhance Th17 polarization. J. Innate Immun. 2012, 4, 284–292. [Google Scholar] [CrossRef]
- Ferris, L.K.; Mburu, Y.K.; Mathers, A.R.; Fluharty, E.R.; Larregina, A.T.; Ferris, R.L.; Falo, L.D., Jr. Human beta-defensin 3 induces maturation of human Langerhans cell–like dendritic cells: An antimicrobial peptide that functions as an endogenous adjuvant. J. Investig. Dermatol. 2013, 133, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Johnzon, C.F.; Rönnberg, E.; Pejler, G. The role of mast cells in bacterial infection. Am. J. Pathol. 2016, 186, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Urb, M.; Sheppard, D.C. The role of mast cells in the defence against pathogens. PLoS Pathog. 2012, 8, e1002619. [Google Scholar] [CrossRef] [PubMed]
- von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, R.; Gao, Z.; Thankavel, K.; van der Merwe, P.A.; Abraham, S.N. The mast cell tumor necrosis factor α response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc. Natl. Acad. Sci. USA 1999, 96, 8110–8115. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Kotian, A.; Subramanian, H.; Daniell, H.; Ali, H. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget 2015, 6, 28573. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Neutrophils and bacterial immune evasion. J. Innate Immun. 2018, 10, 432–441. [Google Scholar] [CrossRef]
- Haas, P.J.; de Haas, C.J.; Kleibeuker, W.; Poppelier, M.J.; van Kessel, K.P.; Kruijtzer, J.A.; Liskamp, R.M.; van Strijp, J.A. N-terminal residues of the chemotaxis inhibitory protein of Staphylococcus aureus are essential for blocking formylated peptide receptor but not C5a receptor. J. Immunol. 2004, 173, 5704–5711. [Google Scholar] [CrossRef]
- Cress, B.F.; Englaender, J.A.; He, W.; Kasper, D.; Linhardt, R.J.; Koffas, M.A. Masquerading microbial pathogens: Capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol. Rev. 2014, 38, 660–697. [Google Scholar] [CrossRef]
- Lesiuk, M.; Paduszyńska, M.; Greber, K.E. Synthetic Antimicrobial Immunomodulatory Peptides: Ongoing Studies and Clinical Trials. Antibiotics 2022, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Pidwill, G.R.; Gibson, J.F.; Cole, J.; Renshaw, S.A.; Foster, S.J. The role of macrophages in Staphylococcus aureus infection. Front. Immunol. 2021, 11, 3506. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J. Immune evasion by Mycobacterium tuberculosis: Living with the enemy. Curr. Opin. Immunol. 2003, 15, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kruse, T.; Kristensen, H.H. Using antimicrobial host defense peptides as anti-infective and immunomodulatory agents. Expert Rev. Anti-Infect. Ther. 2008, 6, 887–895. [Google Scholar] [CrossRef]
- Castillo-González, R.; Cibrian, D.; Sánchez-Madrid, F. Dissecting the complexity of γδ T-cell subsets in skin homeostasis, inflammation, and malignancy. J. Allergy Clin. Immunol. 2021, 147, 2030–2042. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, L.; Xiao, Z.; Li, M.; Wu, X.; Li, W.; Li, X.; Zhao, Q.; Wu, Y.; Zhang, H.; et al. Protective role of γδ T cells in different pathogen infections and its potential clinical application. J. Immunol. Res. 2018, 2018, 5081634. [Google Scholar] [CrossRef]
- Kaufmann, S.H. Immunity to intracellular bacteria. Annu. Rev. Immunol. 1993, 11, 129–163. [Google Scholar] [CrossRef]
- Nijnik, A.; Madera, L.; Ma, S.; Waldbrook, M.; Elliott, M.R.; Easton, D.M.; Mayer, M.L.; Mullaly, S.C.; Kindrachuk, J.; Jenssen, H.; et al. Synthetic cationic peptide IDR-1002 provides protection against bacterial infections through chemokine induction and enhanced leukocyte recruitment. J. Immunol. 2010, 184, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Small, C.L.; McCormick, S.; Gill, N.; Kugathasan, K.; Santosuosso, M.; Donaldson, N.; Heinrichs, D.E.; Ashkar, A.; Xing, Z. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J. Immunol. 2008, 180, 5558–5568. [Google Scholar] [CrossRef] [PubMed]
- McSharry, B.P.; Gardiner, C.M. The role of NK cells in bacterial infections. In Natural Killer Cells; Springer: Berlin/Heidelberg, Germany, 2010; pp. 153–175. [Google Scholar]
- Clark, S.E.; Filak, H.C.; Guthrie, B.S.; Schmidt, R.L.; Jamieson, A.; Merkel, P.; Knight, V.; Cole, C.M.; Raulet, D.H.; Lenz, L.L. Bacterial manipulation of NK cell regulatory activity increases susceptibility to Listeria monocytogenes infection. PLoS Pathog. 2016, 12, e1005708. [Google Scholar] [CrossRef] [PubMed]
- Artamonov, A.Y.; Shanin, S.N.; Orlov, D.S.; Shamova, O.V.; Kolodkin, N.I.; Rybakina, E.G. Immunomodulatory activity of antimicrobial peptide indolicidin and its structural analogues. Immunol. Med. 2014, 11, 101–104. [Google Scholar] [CrossRef]
- Menon, G.K. Skin basics; structure and function. In Lipids and Skin Health; Springer: Cham, Switzerland, 2015; pp. 9–23. [Google Scholar]
- Wang, J.N.; Li, M. The immune function of keratinocytes in anti-pathogen infection in the skin. Int. J. Dermatol. Venereol. 2020, 3, 231–238. [Google Scholar] [CrossRef]
- Sayedyahossein, S.; Xu, S.X.; Rudkouskaya, A.; McGavin, M.J.; McCormick, J.K.; Dagnino, L. Staphylococcus aureus keratinocyte invasion is mediated by integrin-linked kinase and Rac1. FASEB J. 2015, 29, 711–723. [Google Scholar] [CrossRef]
- Kisich, K.O.; Howell, M.D.; Boguniewicz, M.; Heizer, H.R.; Watson, N.U.; Leung, D.Y. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on β-defensin 3. J. Investig. Dermatol. 2007, 127, 2368–2380. [Google Scholar] [CrossRef]
- Ulland, T.K.; Ferguson, P.J.; Sutterwala, F.S. Evasion of inflammasome activation by microbial pathogens. J. Clin. Investig. 2015, 125, 469–477. [Google Scholar] [CrossRef]
- Schauber, J.; Gallo, R.L. Antimicrobial peptides and the skin immune defense system. J. Allergy Clin. Immunol. 2008, 122, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mohanty, S.; Zhang, Y.; Lüchow, M.; Qin, L.; Fortuin, L.; Malkoch, M. Dendritic hydrogels induce immune modulation in human keratinocytes and effectively eradicate bacterial pathogens. J. Am. Chem. Soc. 2021, 143, 17180–17190. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Song, B. Melanocytes and skin immunity. In Journal of Investigative Dermatology Symposium Proceedings; Elsevier: Amsterdam, The Netherlands, 2015; Volume 17, pp. 37–39. [Google Scholar]
- Speeckaert, R.; Belpaire, A.; Speeckaert, M.; van Geel, N. The delicate relation between melanocytes and skin immunity: A game of hide and seek. Pigment. Cell Melanoma Res. 2022, 35, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Dell’Angelica, E.C.; Mullins, C.; Caplan, S.; Bonifacino, J.S. Lysosome-related organelles. FASEB J. 2000, 14, 1265–1278. [Google Scholar] [PubMed]
- Ahn, J.H.; Park, T.J.; Jin, S.H.; Kang, H.Y. Human melanocytes express functional Toll-like receptor 4. Exp. Dermatol. 2008, 17, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Brumell, J.H.; Perrin, A.J.; Goosney, D.L.; Finlay, B.B. Microbial pathogenesis: New niches for Salmonella. Curr. Biol. 2002, 12, R15–R17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, B.; Zhang, Z.; Huang, Y.; Xu, Z.; Chen, X.; Huang, Y.; Jian, J.; Yan, Q. α-MSH is partially involved in the immunomodulation of Nile tilapia (Oreochromis niloticus) antibacterial immunity. Fish Shellfish Immunol. 2022, 131, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Steinstraesser, L.; Hirsch, T.; Schulte, M.; Kueckelhaus, M.; Jacobsen, F.; Mersch, E.A.; Kindrachuk, J. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS ONE 2012, 7, e39373. [Google Scholar] [CrossRef]
- Hung, C.C.; Chiang, C.T.; Wu, R.C.; Lee, C. Positive Effect of Severe Nakagami-m Fading on the Performance of Multiuser TAS/MRC Systems with High Selection Gain. Int. J. Antennas Propag. 2012, 2012, 987631. [Google Scholar] [CrossRef]
- Satyam, R.; Bhardwaj, T.; Jha, N.K.; Jha, S.K.; Nand, P. Toward a chimeric vaccine against multiple isolates of Mycobacteroides-An integrative approach. Life Sci. 2020, 250, 117541. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.C.; Schöllmann, C.; Stauss-Grabo, M.; Schäfer-Korting, M. Antimicrobial peptides and skin: A paradigm of translational medicine. Skin Pharmacol. Physiol. 2012, 25, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; McClean, S. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem. Pharmacol. 2006, 71, 1289–1298. [Google Scholar] [CrossRef]
- Kang, J.; Dietz, M.J.; Li, B. Antimicrobial peptide LL-37 is bactericidal against Staphylococcus aureus biofilms. PLoS ONE 2019, 14, e0216676. [Google Scholar] [CrossRef]
- Kanchanapally, R.; Nellore, B.P.V.; Sinha, S.S.; Pedraza, F.; Jones, S.J.; Pramanik, A.; Ray, P.C. Antimicrobial peptide-conjugated graphene oxide membrane for efficient removal and effective killing of multiple drug resistant bacteria. RSC Adv. 2015, 5, 18881–18887. [Google Scholar] [CrossRef]
- Dong, P.; Zhou, Y.; He, W.; Hua, D. A strategy for enhanced antibacterial activity against Staphylococcus aureus by the assembly of alamethicin with a thermo-sensitive polymeric carrier. ChemComm 2016, 52, 896–899. [Google Scholar] [CrossRef]

| Sl. No. | Antimicrobial Peptide | Mechanism of Action | Reference |
|---|---|---|---|
| 1 | LL37 | Barrel-stave mechanism of membrane disruption and inhibit LPS binding in Bacteria, fungi and viral pathogens; P. aeruginosa | [30] |
| 2 | OP-145 (LL-37 derived; phase II) | Membrane disruption in gram-positive | [31] |
| 3 | PAC113 (P113; histatin 5 analog; phase IIb) | Membrane disruption and immunomodulation ESKAPE Pathogens | [32] |
| 4 | Cys-KR12 | Membrane disruption E. coli, S. aureus | [33] |
| 5 | LTX-109 | Membrane disruption and cell lysis in MRSA | [34] |
| 6 | AMPR-11 | Disrupts bacterial membranes by interacting with cardiolipin and lipid A in sepsis-causing bacteria, including multidrug-resistant strains | [35] |
| 7 | Dalbavancin | Inhibition of bacterial cell wall synthesis in S. aureus | [36] |
| 8 | Polymyxins | Membrane disruption in P. aeruginosa | [37] |
| 9 | Vancomycin | Inhibition of bacterial cell wall synthesis in MRSA, VISA, VRSA | [38] |
| 10 | WRL3 | Membrane lysis in MRSA | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, S.; Barik, D.; Biswas, D. AMPs as Host-Directed Immunomodulatory Agents against Skin Infections Caused by Opportunistic Bacterial Pathogens. Antibiotics 2024, 13, 439. https://doi.org/10.3390/antibiotics13050439
Saha S, Barik D, Biswas D. AMPs as Host-Directed Immunomodulatory Agents against Skin Infections Caused by Opportunistic Bacterial Pathogens. Antibiotics. 2024; 13(5):439. https://doi.org/10.3390/antibiotics13050439
Chicago/Turabian StyleSaha, Subhasree, Devashish Barik, and Debabrata Biswas. 2024. "AMPs as Host-Directed Immunomodulatory Agents against Skin Infections Caused by Opportunistic Bacterial Pathogens" Antibiotics 13, no. 5: 439. https://doi.org/10.3390/antibiotics13050439
APA StyleSaha, S., Barik, D., & Biswas, D. (2024). AMPs as Host-Directed Immunomodulatory Agents against Skin Infections Caused by Opportunistic Bacterial Pathogens. Antibiotics, 13(5), 439. https://doi.org/10.3390/antibiotics13050439





