Linalool Reduces Virulence and Tolerance to Adverse Conditions of Listeria monocytogenes
Abstract
1. Introduction
2. Results
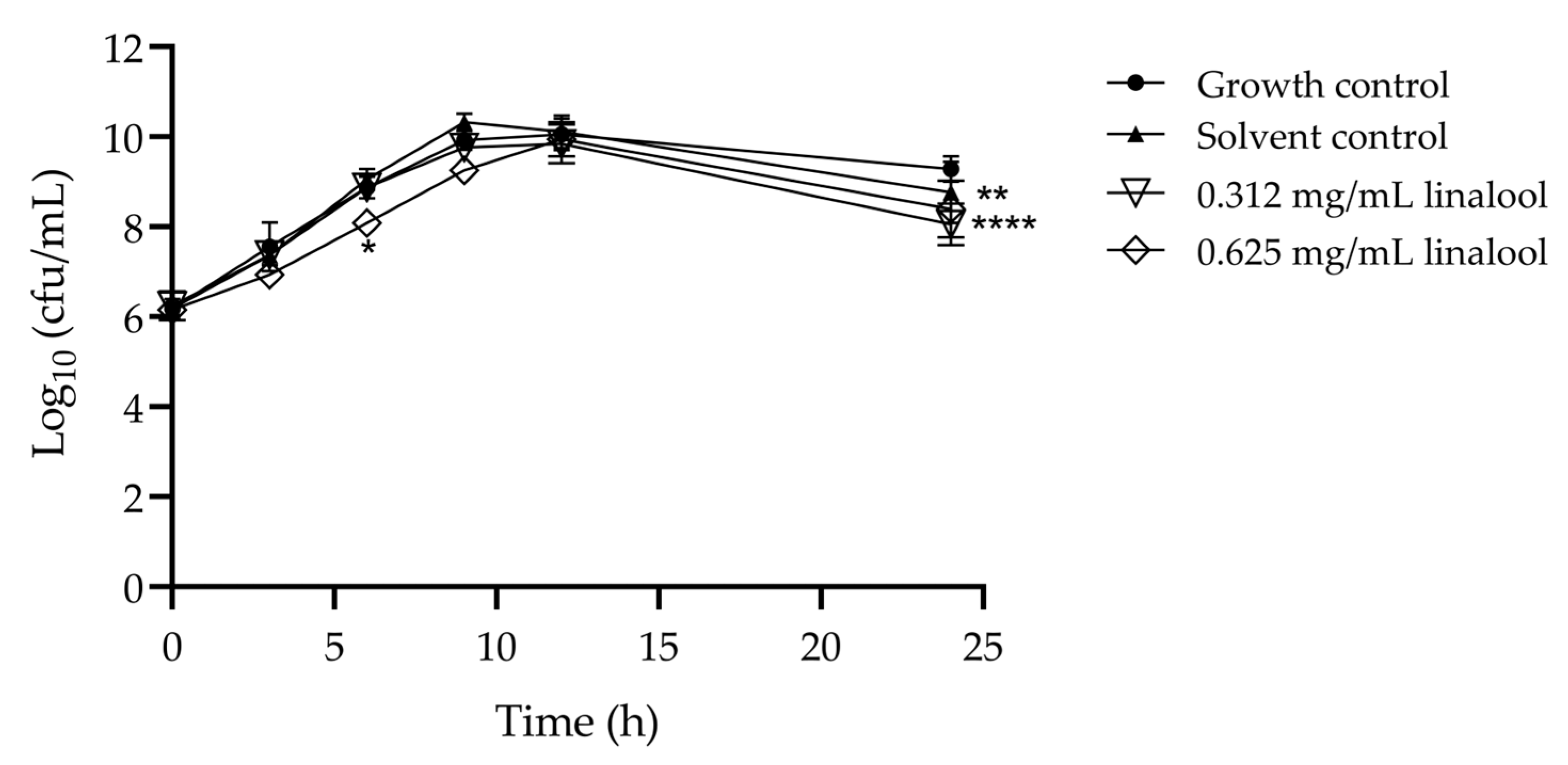
2.1. Effect of Linalool on Listeria monocytogenes Growth
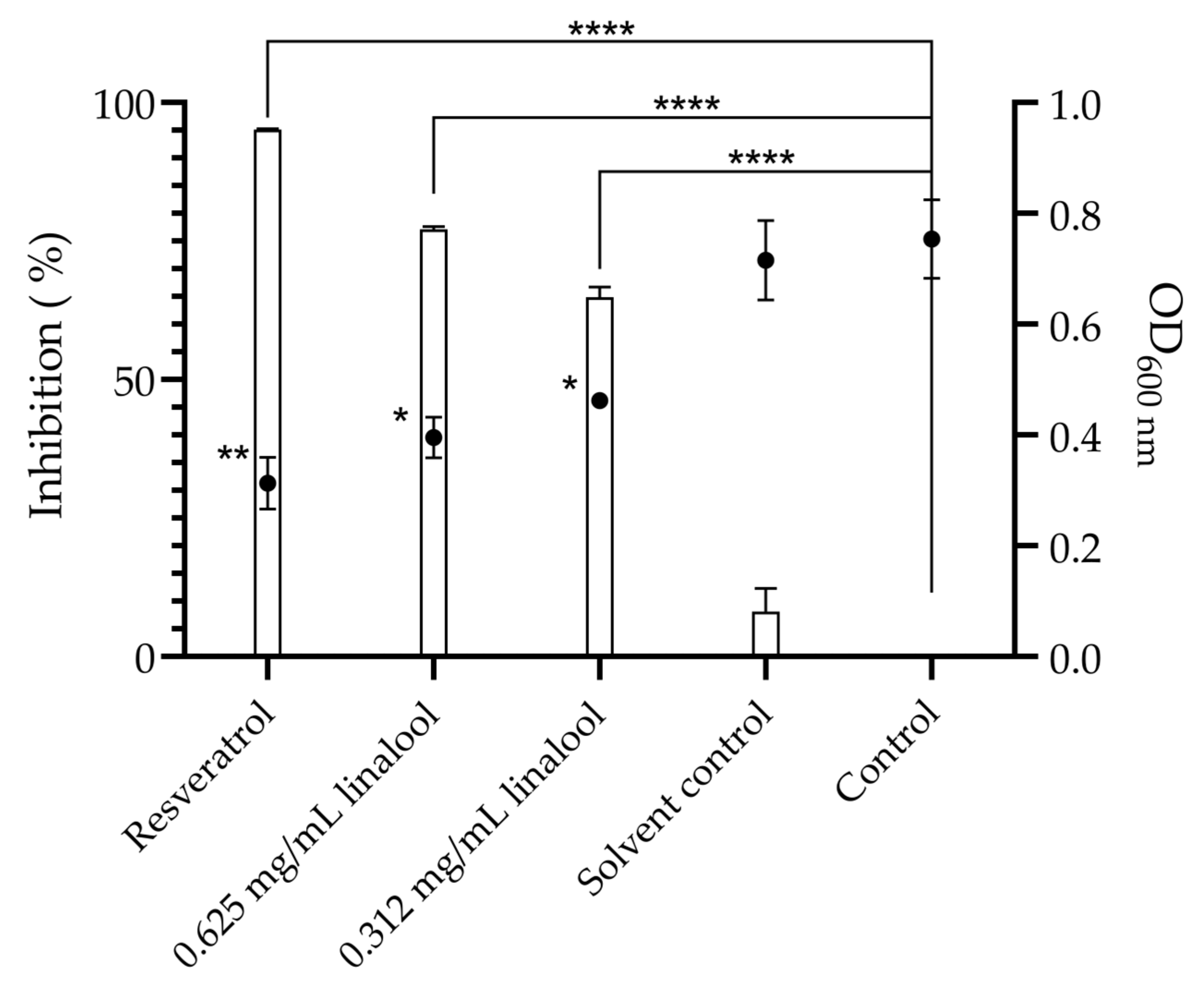
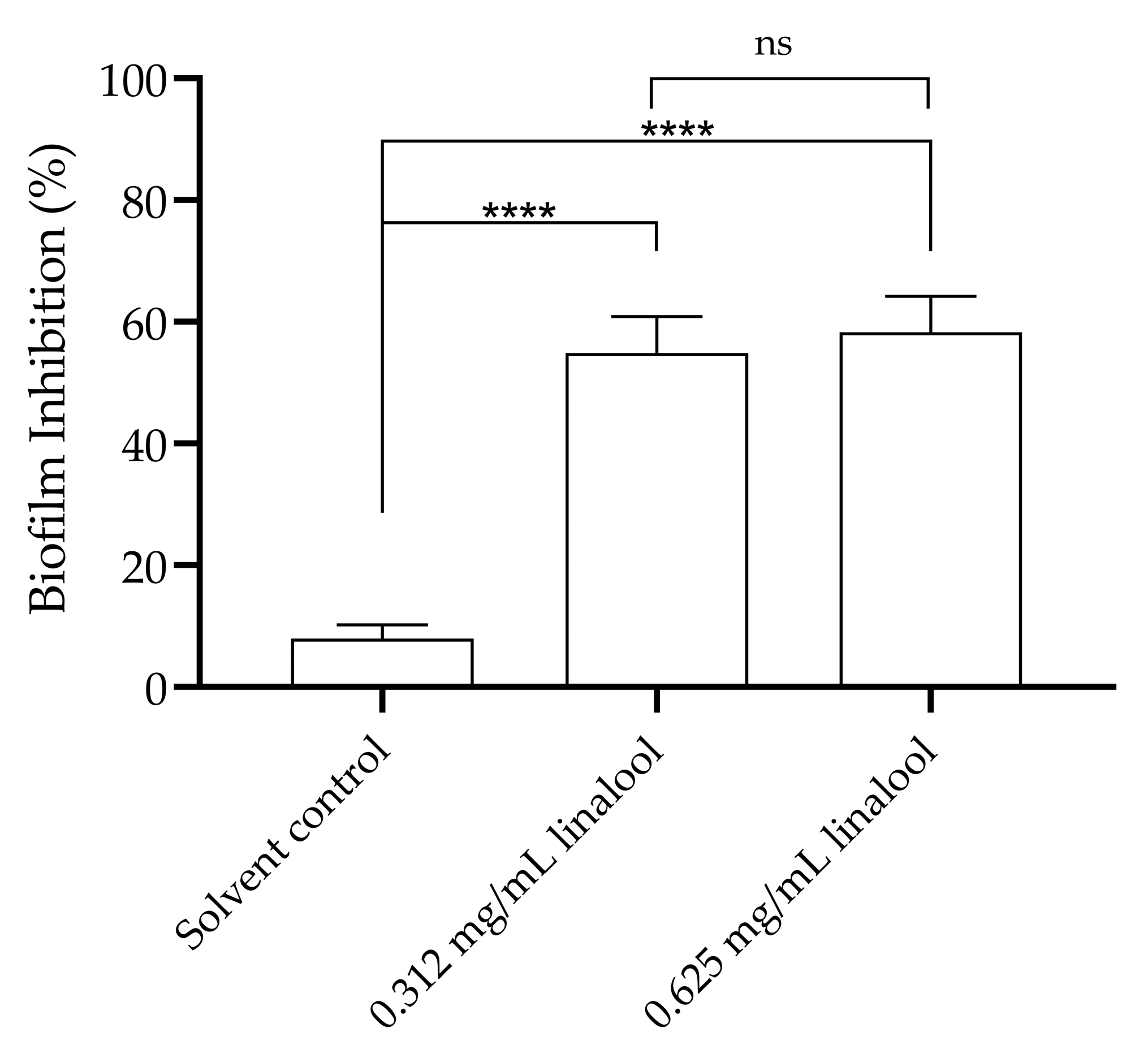
2.2. Inhibition of Quorum Sensing in the Biosensor Bacterium Chromobacterium violaceum, and Motility and Biofilm Formation of Listeria monocytogenes, in the Presence of Linalool
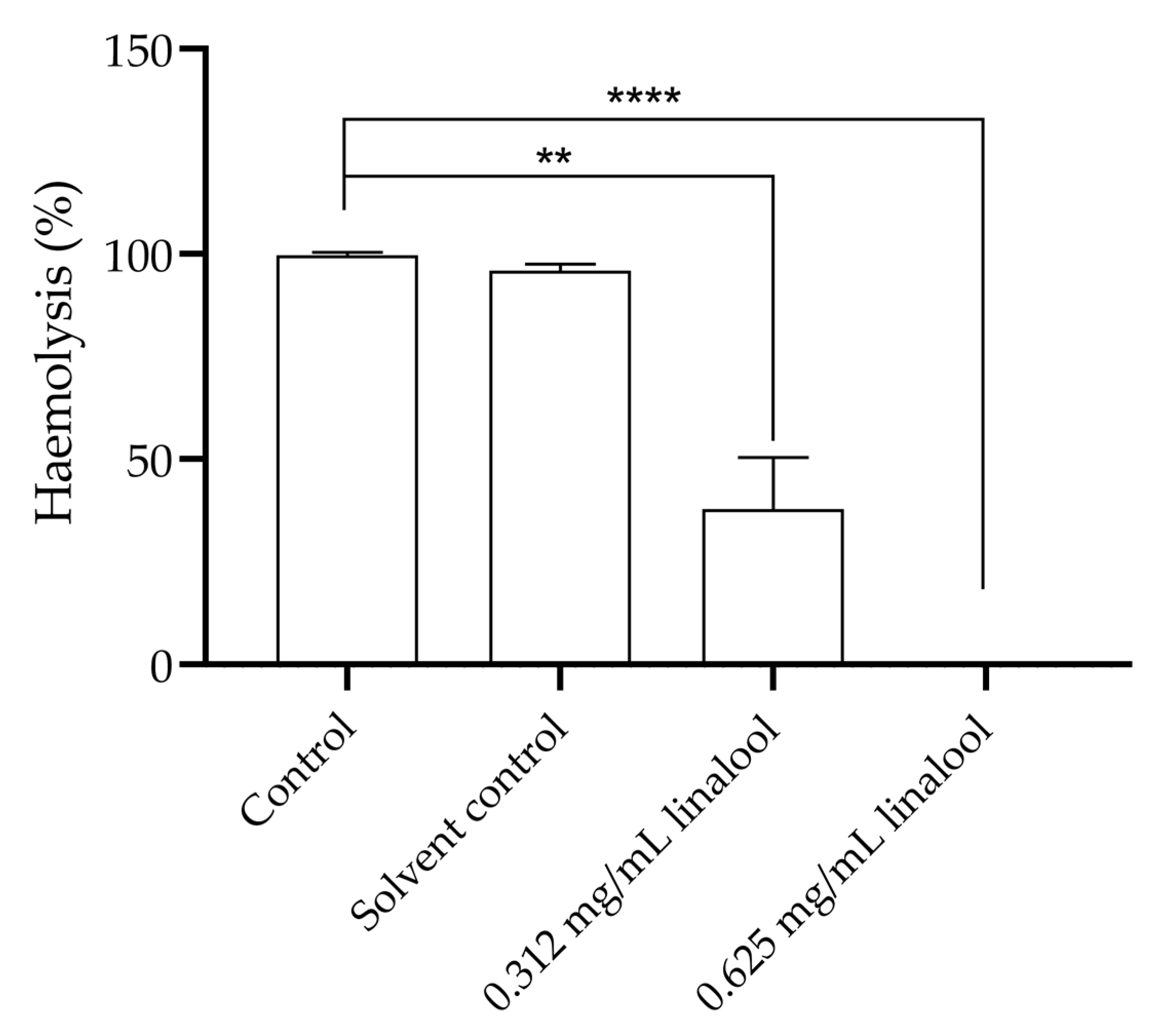
2.3. Study of the Haemolysis Capacity of Linalool in Humans’ Erythrocytes and Study of the Effect of Linalool in Haemolysis Induced by Listeria monocytogenes
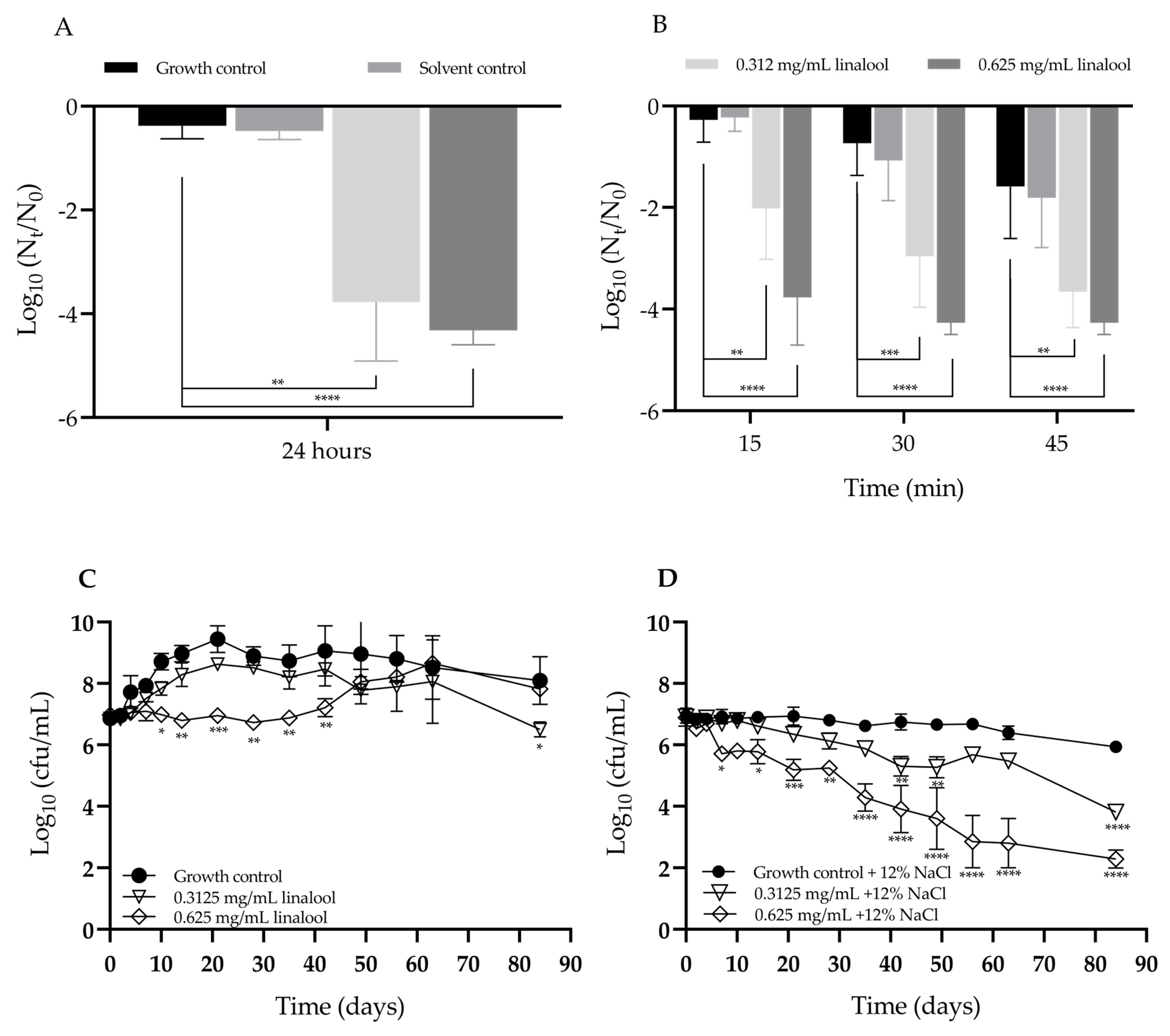
2.4. Evaluation of the Effect of Linalool in the Tolerance to Adverse Conditions
3. Discussion
4. Materials and Methods
4.1. Linalool, Bacterial Strain and Growth Conditions
4.2. Growth Curves Determination in Presence of Linalool
4.3. Quorum-Sensing Inhibition by Linalool
4.4. Evaluation of Listeria monocytogenes Motility When Exposed to Linalool
4.5. Inhibition of Listeria monocytogenes Biofilm Formation in the Presence of Linalool
4.6. Haemolytic Activity of Linalool in Humans’ Erytrocytes
4.7. Study of the Inhibitory Capabilities of Linalool on Human Erythrocytes Haemolysis Caused by Listeria monocytogenes
4.8. Evaluation of Linalool on the Tolerance of Listeria monocytogenes to Adverse Conditions
- (i)
- To assess the effect on osmotic stress, the tubes were supplemented with NaCl at 12% (w/v). The tubes were incubated for 24 h and measurements were made at 0 and 24 h.
- (ii)
- The effect of a high temperature was evaluated by incubating tubes at 55 °C, and samples collected at 0, 15, 30 and 45 min.
- (iii)
- The effect of low temperature was assessed also in absence or presence of osmotic stress; thus, the experiment was conducted using TSB or TSB with 12% NaCl (w/v), respectively; in this case, in a final volume of 1 mL. The tubes were incubated at a low storage temperature of 4 °C for 84 days. The viable count measurements were performed by drop-plate methods, and the assays repeated at least two independent times.
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 27 November 2023).
- Shamloo, E.; Hosseini, H.; Moghadam, A.Z.; Larsen, H.M.; Haslberger, A.; Alebouyeh, M. Importance of Listeria monocytogenes in food safety: A review of its prevalence, detection, and antibiotic resistance. Iran. J. Vet. Res. 2019, 20, 241. [Google Scholar] [PubMed]
- Kannan, S.; Balakrishnan, J.; Govindasamy, A. Listeria monocytogens—Amended understanding of its pathogenesis with a complete picture of its membrane vesicles, quorum sensing, biofilm and invasion. Microb. Pathog. 2020, 149, 104575. [Google Scholar] [CrossRef]
- Freitag, N.E.; Port, G.C.; Miner, M.D. Listeria monocytogenes—From saprophyte to intracellular pathogen. Nat. Rev. Microbiol. 2009, 7, 623–628. [Google Scholar] [CrossRef] [PubMed]
- McDougal, C.E.; Sauer, J.D. Listeria monocytogenes: The Impact of Cell Death on Infection and Immunity. Pathogens 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, M.R.; Massaro, C.; Arrigo, I.; Diquattro, O.; Di Bernardo, F.; Galia, E.; Palermo, M.; Fasciana, T.; Giammanco, A. Characterization of Listeria monocytogenes Strains Isolated in Palermo (Sicily and Italy) during the Years 2018–2020 from Severe Cases of Listeriosis. Antibiotics 2024, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, S.; Nucera, D.; Filipello, V. The evolution and epidemiology of Listeria monocytogenes in Europe and the United States. Infect. Genet. Evol. 2015, 35, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Milian, A.; Payeras-Cifre, A. What is new in Listeriosis? Biomed. Res. Int. 2014, 2014, 358051. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.C.D.; Notermans, S. Surveillance of listeriosis and its causative pathogen, Listeria monocytogenes. Food Control 2011, 22, 1484–1490. [Google Scholar] [CrossRef]
- Duze, S.T.; Marimani, M.; Patel, M. Tolerance of Listeria monocytogenes to biocides used in food processing environments. Food Microbiol. 2021, 97, 103758. [Google Scholar] [CrossRef]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J. Vet. Res. 2020, 87, 20. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [PubMed]
- Gao, Z.; Zhong, W.; Liu, T.; Zhao, T.; Guo, J. Global proteomic analysis of Listeria monocytogenes’ response to linalool. Foods 2021, 10, 2449. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Coimbra, A.T.; Silva, L.; Duarte, A.P.; Ferreira, S. Melissa officinalis essential oil as an antimicrobial agent against Listeria monocytogenes in watermelon juice. Food Microbiol. 2023, 109, 104105. [Google Scholar] [CrossRef] [PubMed]
- Fasciana, T.; Gargano, M.L.; Serra, N.; Galia, E.; Arrigo, I.; Tricoli, M.R.; Diquattro, O.; Graceffa, G.; Vieni, S.; Venturella, G.; et al. Potential activity of albino Grifola frondosa mushroom extract against biofilm of meticillin-resistant Staphylococcus aureus. J. Fungi 2021, 7, 551. [Google Scholar] [CrossRef]
- Gargano, M.L.; Zervakis, G.I.; Isikhuemhen, O.S.; Venturella, G.; Calvo, R.; Giammanco, A.; Fasciana, T.; Ferraro, V. Ecology, Phylogeny, and Potential Nutritional and Medicinal Value of a Rare White “Maitake” Collected in a Mediterranean Forest. Diversity 2020, 12, 230. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; El-Dougdoug, N.K. Controlling foodborne pathogens with natural antimicrobials by biological control and antivirulence strategies. Heliyon 2020, 6, e05020. [Google Scholar] [CrossRef]
- Lu, G.; Xu, L.; Zhang, P.; Dou, X.; Yu, J.; Feng, H. Betulin efficiently suppresses the process of an experimental Listeria monocytogenes infection as an antagonist against listeriolysin O. Fitoterapia 2019, 139, 104409. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, S.; Sivaranjani, M.; Kamaladevi, A.; Ravi, A.V.; Balamurugan, K.; Karutha Pandian, S. Cyclic dipeptide cyclo(l-leucyl-l-prolyl) from marine Bacillus amyloliquefaciens mitigates biofilm formation and virulence in Listeria monocytogenes. Pathog. Dis. 2016, 74, ftw017. [Google Scholar] [CrossRef]
- Liu, M.; Lv, Q.; Xu, J.; Liu, B.; Zhou, Y.; Zhang, S.; Shen, X.; Wang, L. Isoflavone glucoside genistin, an inhibitor targeting Sortase A and Listeriolysin O, attenuates the virulence of Listeria monocytogenes in vivo and in vitro. Biochem. Pharmacol. 2023, 209, 115447. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Vongkamjan, K.; Voravuthikunchai, S.P. Antioxidant Properties and Antibacterial Effects of Eucalyptus camaldulensis Ethanolic Leaf Extract on Biofilm Formation, Motility, Hemolysin Production, and Cell Membrane of the Foodborne Pathogen Listeria monocytogenes. Foodborne Pathog. Dis. 2019, 16, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Teng, Z.; Li, M.; Niu, X.; Wang, J.; Deng, X. Epigallocatechin gallate inhibits Streptococcus pneumoniae virulence by simultaneously targeting pneumolysin and sortase A. J. Cell. Mol. Med. 2017, 21, 2586–2598. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, J.; Tan, W.; Zhang, Y.; Wang, H.; Zhou, X.; Liu, S.; Feng, H.; Li, W.; Niu, X.; et al. Fisetin Inhibits Listeria monocytogenes Virulence by Interfering with the Oligomerization of Listeriolysin O. J. Infect. Dis. 2015, 211, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.; Carvalho, F.; Duarte, A.P.; Ferreira, S. Antimicrobial activity of Thymus zygis essential oil against Listeria monocytogenes and its application as food preservative. Innov. Food Sci. Emerg. Technol. 2022, 80, 103077. [Google Scholar] [CrossRef]
- Zhang, D.; Gan, R.Y.; Zhang, J.R.; Farha, A.K.; Li, H.B.; Zhu, F.; Wang, X.H.; Corke, H. Antivirulence properties and related mechanisms of spice essential oils: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1018–1055. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.H.R.; Parmar, A.; Ali, M.R.; El-Mogy, M.M.; Abdelgawad, K.F. Extending the shelf-life of fresh-cut green bean pods by ethanol, ascorbic acid, and essential oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- CFR—Code of Federal Regulations Title 21. Food and Drugs, Chapter I-Food and Drug Administration, Department of Health and Human Services, Subchapter B—Food for Human Consumption, Part 182-Substances Generally Recognized as Safe, Sec. 182.60 Synthetic. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=182.60&SearchTerm=linalool (accessed on 27 November 2023).
- Mączka, W.; Duda-Madej, A.; Grabarczyk, M.; Wińska, K. Natural Compounds in the Battle against Microorganisms—Linalool. Molecules 2022, 27, 6928. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Ren, J.N.; Li, X.; Fan, G.; Qu, S.S.; Song, Y.; Li, Y.; Pan, S.Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.; Duarte, A.; Sousa, S.; Domingues, F.C. Study of the major essential oil compounds of Coriandrum sativum against Acinetobacter baumannii and the effect of linalool on adhesion, biofilms and quorum sensing. Biofouling 2016, 32, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.K.; Lee, J.H.; Kim, Y.G.; Kim, S.-I.; Lee, J. Inhibitory effects of the essential oils α-longipinene and linalool on biofilm formation and hyphal growth of Candida albicans. Biofouling 2017, 33, 143–155. [Google Scholar] [CrossRef]
- Gao, Z.; Van Nostrand, J.D.; Zhou, J.; Zhong, W.; Chen, K.; Guo, J. Anti-listeria Activities of Linalool and Its Mechanism Revealed by Comparative Transcriptome Analysis. Front. Microbiol. 2019, 10, 496012. [Google Scholar]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Luz, L.; Mendonça, M.; Fogaça, B.; Kipnis, A.; Bhunia, A.K.; Bührer-Sékula, S.; Mendonc¸a, M.; Bernardes Fogaça, M.; Kipnis, A.E.; B€ Uhrer-S Ekula, S. Listeria monocytogenes: Review of pathogenesis and virulence determinants-targeted immunological assays. Crit. Rev. Microbiol. 2021, 47, 647–666. [Google Scholar] [CrossRef]
- Matereke, L.T.; Okoh, A.I. Listeria monocytogenes Virulence, Antimicrobial Resistance and Environmental Persistence: A Review. Pathogens 2020, 9, 528. [Google Scholar] [CrossRef]
- He, R.; Zhong, Q.; Chen, W.; Zhang, M.; Pei, J.; Chen, H.; Chen, W. Transcriptomic and proteomic investigation of metabolic disruption in Listeria monocytogenes triggered by linalool and its application in chicken breast preservation. LWT 2023, 176, 114492. [Google Scholar] [CrossRef]
- He, R.; Chen, W.; Chen, H.; Zhong, Q.; Zhang, H.; Zhang, M.; Chen, W. Antibacterial mechanism of linalool against L. monocytogenes, a metabolomic study. Food Control 2022, 132, 108533. [Google Scholar] [CrossRef]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. MBio 2018, 9, e02331-17. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, S.; Mukherjee, D.; Joshi, S.J.; Ray, R.R. Antibiofilm and anti-quorum sensing activities of eugenol and linalool from Ocimum tenuiflorum against Pseudomonas aeruginosa biofilm. J. Appl. Microbiol. 2021, 131, 2821–2837. [Google Scholar] [CrossRef] [PubMed]
- Kanekar, S.; Devasya, R.P. Growth-phase specific regulation of cviI/R based quorum sensing associated virulence factors in Chromobacterium violaceum by linalool, a monoterpenoid. World J Microbiol Biotechnol. 2022, 38, 23. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Huang, X.; Yang, H.; Qiu, Z.; Zou, L.; Liang, Q.; Shi, Y.; Wu, Y.; Wu, S.; et al. Study on Antibacterial and Quorum-Sensing Inhibition Activities of Cinnamomum camphora Leaf Essential Oil. Molecules 2019, 24, 3792. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Essential oil components inhibit biofilm formation in Erwinia carotovora and Pseudomonas fluorescens via anti-quorum sensing activity. LWT 2018, 92, 133–139. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sowndarya, J.; Farisa Banu, S.; Madhura, G.; Yuvalakshmi, P.; Rubini, D.; Bandeira Junior, G.; Baldisserotto, B.; Vadivel, V.; Nithyanand, P. Agro food by-products and essential oil constituents curtail virulence and biofilm of Vibrio harveyi. Microb. Pathog. 2019, 135, 103633. [Google Scholar] [CrossRef] [PubMed]
- Ngome, M.T.; Alves, J.G.; de Oliveira, A.C.; da Silva Machado, P.; Mondragón-Bernal, O.L.; Piccoli, R.H. Linalool, citral, eugenol and thymol: Control of planktonic and sessile cells of Shigella flexneri. AMB Express 2018, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lai, W.L.; Chuang, K.C.; Lee, M.H.; Tsai, Y.C. The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med. Mycol. 2013, 51, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, J.H.; Kim, S.I.; Baek, K.H.; Lee, J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int. J. Food Microbiol. 2015, 195, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Schnupf, P.; Portnoy, D.A. Listeriolysin O: A phagosome-specific lysin. Microbes Infect. 2007, 9, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Vadia, S.; Arnett, E.; Haghighat, A.C.; Wilson-Kubalek, E.M.; Tweten, R.K.; Seveau, S. The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of L. monocytogenes into Human Hepatocytes. PLoS Pathog. 2011, 7, e1002356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Meng, R.; Zhao, X.; Shi, C.; Zhang, X.; Zhang, Y.; Guo, N. Inhibition effect of tea tree oil on Listeria monocytogenes growth and exotoxin proteins listeriolysin O and p60 secretion. Lett. Appl. Microbiol. 2016, 63, 450–457. [Google Scholar] [CrossRef]
- Arioli, S.; Montanari, C.; Magnani, M.; Tabanelli, G.; Patrignani, F.; Lanciotti, R.; Mora, D.; Gardini, F. Modelling of Listeria monocytogenes Scott A after a mild heat treatment in the presence of thymol and carvacrol: Effects on culturability and viability. J. Food Eng. 2019, 240, 73–82. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to Stress Conditions Encountered in Food and food processing environments. Front. Microbiol. 2018, 9, 417209. [Google Scholar]
- Tasara, T.; Stephan, R. Cold Stress Tolerance of Listeria monocytogenes: A Review of Molecular Adaptive Mechanisms and Food Safety Implications. J. Food Prot. 2006, 69, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, P.; Trigo, M.J.; Ferreira, R.B.; Brito, L. Differences in the Expression of Cold Stress–Related Genes and in the Swarming Motility Among Persistent and Sporadic Strains of Listeria monocytogenes. Foodborne Pathog. Dis. 2015, 12, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Asensio, C.M.; Quiroga, P.R.; Al-Gburi, A.; Huang, Q.; Grosso, N.R. Rheological Behavior, Antimicrobial and Quorum Sensig Inhibition Study of an Argentinean Oregano Essential Oil Nanoemulsion. Front. Nutr. 2020, 7, 569913. [Google Scholar] [PubMed]
- Stepanović, S.; Ćirković, I.; Ranin, L.; Švabić-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.F.; Ferreira, S.; Oliveira, R.; Domingues, F.C. Effect of Coriander Oil (Coriandrum sativum) on Planktonic and Biofilm Cells of Acinetobacter baumannii. Nat. Prod. Commun. 2013, 8, 673–678. [Google Scholar] [CrossRef]
- Du, W.; Zhou, M.; Liu, Z.; Chen, Y.; Li, R. Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes. Food Control 2018, 85, 119–126. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Domingues, F.C.; Ferreira, S. The influence of resveratrol adaptation on resistance to antibiotics, benzalkonium chloride, heat and acid stresses of Staphylococcus aureus and Listeria monocytogenes. Food Control 2017, 73, 1420–1425. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, J.P.; Domingues, F.C.; Ferreira, S. Linalool Reduces Virulence and Tolerance to Adverse Conditions of Listeria monocytogenes. Antibiotics 2024, 13, 474. https://doi.org/10.3390/antibiotics13060474
Dias JP, Domingues FC, Ferreira S. Linalool Reduces Virulence and Tolerance to Adverse Conditions of Listeria monocytogenes. Antibiotics. 2024; 13(6):474. https://doi.org/10.3390/antibiotics13060474
Chicago/Turabian StyleDias, Joel P., Fernanda C. Domingues, and Susana Ferreira. 2024. "Linalool Reduces Virulence and Tolerance to Adverse Conditions of Listeria monocytogenes" Antibiotics 13, no. 6: 474. https://doi.org/10.3390/antibiotics13060474
APA StyleDias, J. P., Domingues, F. C., & Ferreira, S. (2024). Linalool Reduces Virulence and Tolerance to Adverse Conditions of Listeria monocytogenes. Antibiotics, 13(6), 474. https://doi.org/10.3390/antibiotics13060474







