Phytochemical Screening and Antibacterial Activity of Commercially Available Essential Oils Combinations with Conventional Antibiotics against Gram-Positive and Gram-Negative Bacteria
Abstract
:1. Introduction
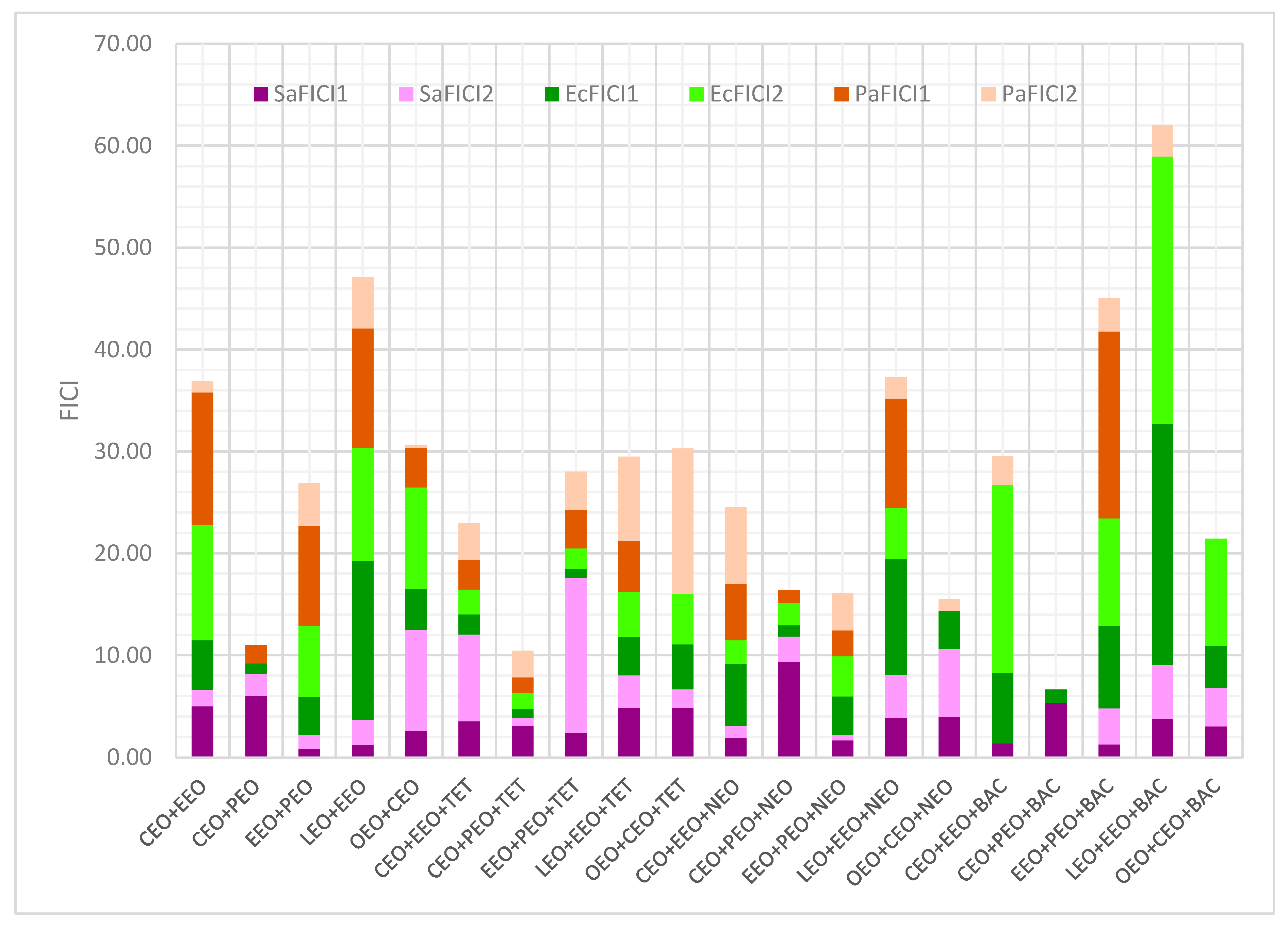
2. Results
2.1. Gas Chromatography–Mass Spectrometry Analysis
2.2. Antibacterial Activity on Gram-Positive and Gram-Negative Bacteria
2.2.1. Antibacterial Activity against S. aureus
| Technique | Cellulose Disc Technique (DDM) | Cylinder Technique (CT) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | S. aureus | |||||||||||
| IZD (mm) | MIC (µg/mL) | FICI | IZD (mm) | MIC (µg/mL) | FICI | |||||||
| Range | Value | Range | Value | Range | Value | Range | Value | |||||
| LEO | ≥35 | 40 | 2.1 | - | - | ≥35 | 40 | 21.2 | - | - | ||
| CEO | 25–34 | 27 | 4.7 | - | - | 25–34 | 25 | 54.3 | - | - | ||
| OEO | ≥35 | 40 | 2.1 | - | - | ≥35 | 40 | 21.2 | - | - | ||
| EEO | 15–24 | 20 | 8.5 | - | - | 25–34 | 25 | 54.3 | - | - | ||
| PEO | 25–34 | 25 | 5.4 | - | - | 15–24 | 15 | 150.9 | - | - | ||
| OEO+CEO | 25–34 | 30 | 3.8 | >1 | ≤4 | 2.60 | 15–24 | 15 | 150.9 | >4 | ≤10 | 9.90 |
| CEO+EEO | 15–24 | 15 | 15.1 | >4 | ≤10 | 5.00 | 25–34 | 27.5 | 44.9 | >1 | ≤4 | 1.60 |
| CEO+PEO | 15–24 | 15 | 15.1 | >4 | ≤10 | 6.00 | 15–24 | 20 | 84.9 | >1 | ≤4 | 2.20 |
| LEO+EEO | ≥35 | 40 | 2.1 | >1 | ≤4 | 1.20 | 25–34 | 30 | 37.7 | >1 | ≤4 | 2.50 |
| EEO+PEO | ≥35 | 35 | 2.8 | >0.5 | <1 | 0.80 | 25–34 | 25 | 54.3 | >1 | ≤4 | 1.40 |
| NEO | <10 | 7 | 86.7 | - | - | 15–24 | 20 | 106.2 | - | - | ||
| OEO+CEO+NEO | 25–34 | 25 | 5.7 | >1 | ≤4 | 3.98 | 15–24 | 20 | 89.2 | >4 | ≤10 | 6.67 |
| CEO+EEO+NEO | 25–34 | 25 | 5.7 | >1 | ≤4 | 1.94 | ≥35 | 37.5 | 25.4 | >1 | ≤4 | 1.15 |
| CEO+PEO+NEO | 10–14 | 12.5 | 22.8 | >4 | ≤10 | 9.33 | 15–24 | 22 | 73.7 | >1 | ≤4 | 2.52 |
| LEO+EEO+NEO | 15–24 | 23.5 | 6.5 | >1 | ≤4 | 3.83 | 25–34 | 25 | 57.1 | >4 | ≤10 | 4.27 |
| EEO+PEO+NEO | 25–34 | 26 | 5.3 | >1 | ≤4 | 1.66 | 15–24 | 15 | 15.9 | >0.5 | <1 | 0.53 |
| TET | 25–34 | 25 | 6.8 | - | - | 25–34 | 29 | 50.5 | - | - | ||
| OEO+CEO+TET | 25–34 | 25 | 5.7 | >4 | ≤10 | 4.87 | ≥35 | 40 | 21.2 | >1 | ≤4 | 1.80 |
| CEO+EEO+TET | 15–24 | 22 | 7.4 | >1 | ≤4 | 3.52 | 15–24 | 15 | 150.9 | >4 | ≤10 | 8.52 |
| CEO+PEO+TET | 25–34 | 25 | 5.7 | >1 | ≤4 | 3.09 | ≥35 | 45 | 16.8 | >0.5 | <1 | 0.74 |
| LEO+EEO+TET | 25–34 | 26 | 5.3 | >4 | ≤10 | 4.83 | 25–34 | 30 | 37.7 | >1 | ≤4 | 3.20 |
| EEO+PEO+TET | 25–34 | 26 | 5.3 | >1 | ≤4 | 2.37 | 10–14 | 10 | 339.7 | >15 | ≤20 | 15.22 |
| BAC | 15–24 | 17 | 14.7 | - | - | 25–34 | 25 | 67.9 | - | - | ||
| OEO+CEO+BAC | 25–34 | 30 | 4.0 | >1 | ≤4 | 3.02 | 25–34 | 30 | 47.2 | >1 | ≤4 | 3.77 |
| CEO+EEO+BAC | 25–34 | 32.5 | 3.4 | >1 | ≤4 | 1.35 | 0 | 0 | - | - | - | |
| CEO+PEO+BAC | 15–24 | 17.5 | 11.6 | >4 | ≤10 | 5.38 | 0 | 0 | - | - | - | |
| LEO+EEO+BAC | 25–34 | 25 | 5.7 | >1 | ≤4 | 3.76 | 25–34 | 27 | 48.9 | >4 | ≤10 | 5.32 |
| EEO+PEO+BAC | 25–34 | 32.5 | 3.4 | >1 | ≤4 | 1.25 | 15–24 | 20 | 89.2 | >1 | ≤4 | 3.54 |
2.2.2. Antibacterial Activity against E. coli
| Technique | Cellulose Disc Technique | Cylinder Technique | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | E. coli | |||||||||||
| IZD (mm) | MIC (µg/mL) | FICI | IZD (mm) | MIC (µg/mL) | FICI | |||||||
| Range | Value | Range | Value | Range | Value | Range | Value | |||||
| LEO | ≥35 | 40 | 2.1 | - | - | ≥35 | 40 | 21.2 | - | - | ||
| CEO | 15–24 | 22 | 7.0 | - | - | 15–24 | 15 | 150.9 | - | - | ||
| OEO | ≥35 | 45 | 1.7 | - | - | ≥35 | 45 | 16.8 | - | - | ||
| EEO | 25–34 | 25 | 5.4 | - | - | 25–34 | 30 | 37.7 | - | - | ||
| PEO | 0 | 0 | - | - | - | 10–14 | 10 | 339.7 | - | - | ||
| OEO+CEO | 25–34 | 25 | 5.4 | >1 | ≤4 | 4.00 | 15–24 | 15 | 150.9 | >4 | ≤10 | 10.00 |
| CEO+EEO | 15–24 | 15 | 15.1 | >4 | ≤10 | 4.90 | 10–14 | 10 | 339.7 | >10 | ≤15 | 11.30 |
| CEO+PEO | 15–24 | 22 | 7.0 | =1 | 1.00 | 0 | 0 | - | - | - | ||
| LEO+EEO | 10–14 | 12 | 23.6 | >15 | ≤20 | 15.60 | 15–24 | 15 | 150.9 | >10 | ≤15 | 11.10 |
| EEO+PEO | 10–14 | 13 | 20.1 | >1 | ≤4 | 3.70 | 10–14 | 12 | 235.9 | >4 | ≤10 | 7.00 |
| NEO | 15–24 | 15 | 18.9 | - | - | 25–34 | 25 | 67.9 | - | - | ||
| OEO+CEO+NEO | 25–34 | 30 | 4.0 | >1 | ≤4 | 3.70 | 0 | 0 | - | - | - | |
| CEO+EEO+NEO | 15–24 | 15 | 15.9 | >4 | ≤10 | 6.05 | 25–34 | 27 | 48.9 | >1 | ≤4 | 2.33 |
| CEO+PEO+NEO | 25–34 | 25 | 5.7 | >1 | ≤4 | 1.11 | 15–24 | 20 | 89.2 | >1 | ≤4 | 2.16 |
| LEO+EEO+NEO | 15–24 | 15 | 15.9 | >10 | ≤15 | 11.35 | 25–34 | 25 | 57.1 | >4 | ≤10 | 5.04 |
| EEO+PEO+NEO | 15–24 | 15 | 15.9 | >1 | ≤4 | 3.78 | 15–24 | 20 | 89.2 | >1 | ≤4 | 3.93 |
| TET | 10–14 | 14 | 21.7 | - | - | 25–34 | 30 | 47.2 | - | - | ||
| OEO+CEO+TET | 25–34 | 25 | 5.7 | >4 | ≤10 | 4.42 | 25–34 | 25 | 57.1 | >4 | ≤10 | 4.96 |
| CEO+EEO+TET | 25–34 | 26 | 5.3 | >1 | ≤4 | 1.97 | 25–34 | 28 | 45.5 | >1 | ≤4 | 2.46 |
| CEO+PEO+TET | 25–34 | 27 | 4.9 | >0.5 | <1 | 0.92 | 25–34 | 26 | 52.8 | >1 | ≤4 | 1.59 |
| LEO+EEO+TET | 25–34 | 26 | 5.3 | >1 | ≤4 | 3.74 | 25–34 | 27.5 | 47.2 | >4 | ≤10 | 4.47 |
| EEO+PEO+TET | 25–34 | 30 | 4.0 | >0.5 | <1 | 0.92 | 25–34 | 30 | 39.6 | >1 | ≤4 | 1.99 |
| BAC | 0 | 0 | - | - | - | 0 | 0 | - | - | - | ||
| OEO+CEO+BAC | 25–34 | 25 | 5.7 | >4 | ≤10 | 4.16 | 15–24 | 15 | 158.5 | >10 | ≤15 | 10.48 |
| CEO+EEO+BAC | 10–14 | 13 | 21.1 | >4 | ≤10 | 6.91 | <10 | 8 | 557.4 | >15 20 | ≤20 | 18.47 |
| CEO+PEO+BAC | 15–24 | 20 | 8.9 | >1 | ≤4 | 1.27 | 0 | 0 | - | - | - | |
| LEO+EEO+BAC | 10–14 | 10 | 35.7 | >20 | 23.61 | 10–14 | 10 | 356.7 | >20 | 26.28 | ||
| EEO+PEO+BAC | <10 | 9 | 44.0 | >4 | ≤10 | 8.14 | 10–14 | 10 | 356.7 | >10 | ≤15 | 10.51 |
2.2.3. Antibacterial Activity against P. aeruginosa
| Technique | Cellulose Disc Technique | Cylinder Technique | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | P. aeruginosa | |||||||||||
| IZD (mm) | MIC (µg/mL) | FICI | IZD (mm) | MIC (µg/mL) | FICI | |||||||
| Range | Value | Range | Value | Range | Value | Range | Value | |||||
| LEO | 10–14 | 11 | 28.1 | - | - | <10 | 7 | 693.2 | - | - | ||
| CEO | 10–14 | 10 | 33.9 | - | - | <10 | 8 | 530.9 | - | - | ||
| OEO | 10–14 | 11 | 28.1 | - | - | 10–14 | 10 | 339.7 | - | - | ||
| EEO | 25–34 | 25 | 5.4 | - | - | ≥35 | 35 | 27.7 | - | - | ||
| PEO | 0 | 0 | - | - | - | 0 | 0 | - | - | - | ||
| OEO+CEO | <10 | 7.5 | 60.6 | >1 | ≤4 | 3.90 | 25–34 | 30 | 37.7 | ≤0.5 | 0.20 | |
| CEO+EEO | <10 | 7.5 | 60.6 | >10 | ≤15 | 13.00 | ≥35 | 35 | 27.7 | >1 | ≤4 | 1.10 |
| CEO+PEO | <10 | 7.5 | 60.6 | >1 | ≤4 | 1.80 | 0 | 0 | - | - | - | |
| LEO+EEO | <10 | 8 | 53.0 | >10 | ≤15 | 11.70 | 15–24 | 16 | 132.7 | >4 | ≤10 | 5.00 |
| EEO+PEO | <10 | 8 | 53.0 | >4 | ≤10 | 9.80 | 15–24 | 17 | 117.5 | >4 | ≤10 | 4.20 |
| NEO | 10–14 | 10 | 42.4 | - | - | 15–24 | 20 | 106.2 | - | - | ||
| OEO+CEO+NEO | 0 | 0 | - | - | - | 15–24 | 22 | 73.7 | >1 | ≤4 | 1.16 | |
| CEO+EEO+NEO | 10–14 | 12.5 | 22.8 | >4 | ≤10 | 5.56 | 15–24 | 15 | 158.5 | >4 | ≤10 | 7.50 |
| CEO+PEO+NEO | 15–24 | 15 | 15.9 | >1 | ≤4 | 1.29 | 0 | 0 | - | - | - | |
| LEO+EEO+NEO | <10 | 9 | 44.0 | >10 | ≤15 | 10.73 | 10–14 | 14 | 182.0 | >1 | ≤4 | 2.05 |
| EEO+PEO+NEO | 15–24 | 17 | 12.3 | >1 | ≤4 | 2.56 | 15–24 | 21 | 80.9 | >1 | ≤4 | 3.68 |
| TET | 15–24 | 15 | 18.9 | - | - | 25–34 | 25 | 67.9 | - | - | ||
| OEO+CEO+TET | 0 | 0 | - | - | - | <10 | 7 | 727.9 | >10 | ≤15 | 14.23 | |
| CEO+EEO+TET | 15–24 | 18 | 11.0 | >1 | ≤4 | 2.93 | 15–24 | 23 | 67.4 | >1 | ≤4 | 3.54 |
| CEO+PEO+TET | 10–14 | 14 | 18.2 | >1 | ≤4 | 1.49 | 15–24 | 15 | 158.5 | >1 | ≤4 | 2.62 |
| LEO+EEO+TET | 10–14 | 14 | 18.2 | >4 | ≤10 | 4.97 | 15–24 | 15 | 158.5 | >4 | ≤10 | 8.27 |
| EEO+PEO+TET | 15–24 | 15 | 15.9 | >1 | ≤4 | 3.78 | 15–24 | 22 | 73.7 | >1 | ≤4 | 3.74 |
| BAC | 0 | 0 | - | - | - | 0 | 0 | - | - | - | ||
| OEO+CEO+BAC | 0 | 0 | - | - | - | 0 | 0 | - | - | - | ||
| CEO+EEO+BAC | 0 | 0 | - | - | - | 15–24 | 22 | 73.7 | >1 | ≤4 | 2.79 | |
| CEO+PEO+BAC | 0 | 0 | - | - | - | 0 | 0 | - | - | - | ||
| LEO+EEO+BAC | 0 | 0 | - | - | - | 15–24 | 21 | 80.9 | >1 | ≤4 | 3.03 | |
| EEO+PEO+BAC | <10 | 6 | 99.1 | >15 | ≤20 | 18.35 | 15–24 | 20 | 89.2 | >1 | ≤4 | 3.22 |
2.3. Data Analysis
3. Materials and Methods
3.1. Materials and Equipment
3.2. GC-MS Analysis
3.3. In Vitro Evaluation of Antibacterial Activity
3.3.1. Bacterial Inoculum
3.3.2. Sample Solutions
3.3.3. Technique
- Q—the quantity (µg) of sample solution applied.
- V—the volume of the environment in which the tested sample diffused and inhibited microbial multiplication.
- π = 3.14 (the circle constant);
- R2—the square of the radius from the mean IZD (mm);
- G—the cylinder generator, respectively, the thickness of the culture medium layer (mm).
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Federal Office of Public Health. Which Are the Main Diseases Due to Bacteria? Available online: https://www.bag.admin.ch/bag/en/home/krankheiten/infektionskrankheiten-bekaempfen/antibiotikaresistenzen/welche-bakteriellen-krankheiten-gibt-es-.html (accessed on 16 March 2024).
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Manion, C.R.; Widder, R.M. Essentials of Essential Oils. Am. J. Health Syst. Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Yeddes, W.; Ouerghemmi, I.; Hammami, M.; Gadhoumi, H.; Affes, T.G.; Mohamed, S.N.; Aidi-Wannes, W.; Witrowa-Rajchert, D.; Saidani-Tounsi, M.; Nowacka, M. Optimizing the Method of Rosemary Essential Oils Extraction by Using Response Surface Methodology (RSM)-Characterization and Toxicological Assessment. Sustainability 2022, 14, 3927. [Google Scholar] [CrossRef]
- Khodaei, N.; Nguyen, M.M.; Mdimagh, A.; Bayen, S.; Karboune, S. Compositional Diversity and Antioxidant Properties of Essential Oils: Predictive Models. LWT 2021, 138, 110684. [Google Scholar] [CrossRef]
- Piatkowska, E.; Rusiecka-Ziółkowska, J. Influence of Essential Oils on Infectious Agents. Adv. Clin. Exp. Med. 2016, 25, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Rhayour, K.; Bouchikhi, T.; Tantaoui-Elaraki, A.; Sendide, K.; Remmal, A. The Mechanism of Bactericidal Action of Oregano and Clove Essential Oils and of Their Phenolic Major Components on Escherichia coli and Bacillus subtilis. J. Essent. Oil Res. 2003, 15, 286–292. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Belmamoun, A.R.; Urcan, A.C.; Bobiş, O.; Lassoued, M.A. 1,8-Cineol (Eucalyptol) Disrupts Membrane Integrity and Induces Oxidative Stress in Methicillin-Resistant Staphylococcus aureus. Antioxidants 2023, 12, 1388. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.; Liang, W.; Mei, J.; Di, Y.; Lan, H.; Yang, Y.; Wang, W.; Luo, Y.; Wang, H. Antibacterial Activity and Mode of Action of Mentha Arvensis Ethanol Extract against Multidrug-Resistant Acinetobacter Baumannii. Trop. J. Pharm. Res. 2015, 14, 2099. [Google Scholar] [CrossRef]
- Tian, L.; Wang, X.; Liu, R.; Zhang, D.; Wang, X.; Sun, R.; Guo, W.; Yang, S.; Li, H.; Gong, G. Antibacterial Mechanism of Thymol against Enterobacter sakazakii. Food Control 2021, 123, 107716. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral, and Cytotoxic Properties—An Overview. Forsch Komplementarmed 2009, 16, 79–90. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- LaLonde, T.; Bowser, T.; Jadeja, R. Essential Oils as Antimicrobials. Madridge J. Food Technol. 2019, 4, 163–169. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Upadhyay, R. Essential Oils: Antimicrobial, Antihelminthic, Antiviral, Anticancer and Antiinsect Properties. J. Appl. Biosci. 2010, 36, 1–22. [Google Scholar]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Influence of Subinhibitory Concentrations of Plant Essential Oils on the Production of Enterotoxins A and B and α-Toxin by Staphylococcus aureus. J. Med. Microbiol. 2004, 53, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Smith-palmer, A.; Stewart, J.; Fyfe, L. Inhibition of Listeriolysin O and Phosphatidylcholine-Specific Production in Listeria Monocytogenes by Subinhibitory Concentrations of Plant Essential Oils. J. Med. Microbiol. 2002, 51, 567–608. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Facchiano, S.; Boscaino, F.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Towards Green Strategies of Food Security: Antibacterial Synergy of Essential Oils from Thymus Vulgaris and Syzygium aromaticum to Inhibit Escherichia coli and Staphylococcus aureus Pathogenic Food Isolates. Microorganisms 2022, 10, 2446. [Google Scholar] [CrossRef] [PubMed]
- Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Aubourg, S.P. Antibacterial, Antiviral and Antifungal Activity of Essential Oils: Mechanisms and Applications. In Antimicrobial Compounds: Current Strategies and New Alternatives; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-40444-3. [Google Scholar]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Neagu, R.; Popovici, V.; Ionescu, L.E.; Ordeanu, V.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Antibacterial and Antibiofilm Effects of Different Samples of Five Commercially Available Essential Oils. Antibiotics 2023, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline Antibiotics and Resistance Mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Deb, J.K. Molecular Understanding of Aminoglycoside Action and Resistance. Appl. Microbiol. Biotechnol. 2006, 70, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yamada, K.; Nagao, M.; Aoki, E.; Matsumoto, M.; Hirayama, T.; Yamamoto, H.; Hiramatsu, R.; Ichiyama, S.; Iinuma, Y. Antimicrobial Ointments and Methicillin-Resistant Staphylococcus Aureus USA300. Emerg. Infect. Dis. 2011, 17, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Vorobets, N.M.; Yavorska, H.V. Modifications of Agar Diffusion Method to DeterMination of the AntiMicrobial Effect of the Herbal Medicinal Products. Ukraïns’kij Bìofarmacevtičnij Žurnal 2016, 2, 80–84. [Google Scholar] [CrossRef]
- Dickert, H.; Machka, K.; Braveny, I. The Uses and Limitations of Disc Diffusion in the Antibiotic Sensitivity Testing of Bacteria. Infection 1981, 9, 18–24. [Google Scholar] [CrossRef]
- Boz, I.; Gille, E.; Necula, R.; Dunca, S.; Zamfirache, M.M. Chemical Composition and Antibacterial Activity of Essential Oils from Five Populations of Thymus pulegioides L. Cellul. Chem. Technol. 2015, 49, 169–174. [Google Scholar]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial Activity and Interactions of Plant Essential Oil Combinations against Gram-Positive and Gram-Negative Bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Tahric, A.; Kolic, H.; Lavic, A.; Latinovic, D.; Pramenkovic, E. Antibacterial Activity of Oregano Essential Oil and Its Effect on Biofilm Formation. J. Pure Appl. Microbiol. 2023, 17, 1205–1213. [Google Scholar] [CrossRef]
- Truong, S.; Mudgil, P. The Antibacterial Effectiveness of Lavender Essential Oil against Methicillin-Resistant Staphylococcus aureus: A Systematic Review. Front. Pharmacol. 2023, 14, 1306003. [Google Scholar] [CrossRef] [PubMed]
- Horváth, P.; Koščová, J. In Vitro Antibacterial Activity of Mentha Essential Oils Against Staphylococcus aureus. Folia Vet. 2017, 61, 71–77. [Google Scholar] [CrossRef]
- Damjanović-Vratnica, B.; Đakov, T.; Šuković, D.; Damjanović, J. Antimicrobial Effect of Essential Oil Isolated from Eucalyptus globulus Labill. from Montenegro. Czech J. Food Sci. 2011, 29, 277–284. [Google Scholar] [CrossRef]
- Pereira, V.; Dias, C.; Vasconcelos, M.C.; Rosa, E.; Saavedra, M.J. Antibacterial Activity and Synergistic Effects between Eucalyptus globulus Leaf Residues (Essential Oils and Extracts) and Antibiotics against Several Isolates of Respiratory Tract Infections (Pseudomonas aeruginosa). Ind. Crops Prod. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Damborg, P.; Pirolo, M.; Schøn Poulsen, L.; Frimodt-Møller, N.; Guardabassi, L. Dogs Can Be Reservoirs of Escherichia coli Strains Causing Urinary Tract Infection in Human Household Contacts. Antibiotics 2023, 12, 1269. [Google Scholar] [CrossRef]
- Batinović, F.; Kujundžić, S.L.; Golec, N.; Sunara, D. Malignant Otitis Externa in an Adult Diabetic Patient Caused by Escherichia Coli: A Case Report. SN Compr. Clin. Med. 2023, 5, 190. [Google Scholar] [CrossRef]
- Ishida, K.; Noborio, M.; Nakamura, M.; Ieki, Y.; Sogabe, T.; Sadamitsu, D. Spontaneous Escherichia Coli Bacterial Meningitis Mimicking Heatstroke in an Adult. Clin. Case Rep. 2016, 4, 323–326. [Google Scholar] [CrossRef]
- Angeletti, S.; Cella, E.; Prosperi, M.; Spoto, S.; Fogolari, M.; De Florio, L.; Antonelli, F.; Dedej, E.; De Flora, C.; Ferraro, E.; et al. Multidrug Resistant Pseudomonas aeruginosa Nosocomial Strains: Molecular Epidemiology and Evolution. Microb. Pathog. 2018, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and Antiplasmid Activities of Essential Oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, S.; Viljoen, A. Plant-Based Antimicrobial Studies—Methods and Approaches to Study the Interaction between Natural Products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-E.; Kim, H.-Y.; Cha, J.-D. Synergistic Effect between Clove Oil and Its Major Compounds and Antibiotics against Oral Bacteria. Arch. Oral Biol. 2011, 56, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.L.; Santos, M.; Rocha, S.M.; Trindade, T. Encapsulation of Essential Oils in SiO2 Microcapsules and Release Behavior of Volatile Compounds. J. Microencapsul. 2014, 31, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial Activity of Essential Oils and Their Major Constituents against Respiratory Tract Pathogens by Gaseous Contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, J.B.; Garner, C.M. A Review of Essential Oils as Antimicrobials in Foods with Special Emphasis on Fresh Produce. J. Food Prot. 2022, 85, 1300–1319. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint Essential Oil: Its Phytochemistry, Biological Activity, Pharmacological Effect and Application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Puvača, N.; Milenković, J.; Galonja Coghill, T.; Bursić, V.; Petrović, A.; Tanasković, S.; Pelić, M.; Ljubojević Pelić, D.; Miljković, T. Antimicrobial Activity of Selected Essential Oils against Selected Pathogenic Bacteria: In Vitro Study. Antibiotics 2021, 10, 546. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.; Buton, N.; Badea, M.; Marutescu, L. Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Clavijo-Romero, A.; Quintanilla-Carvajal, M.X.; Ruiz, Y. Stability and Antimicrobial Activity of Eucalyptus Essential Oil Emulsions. Food Sci. Technol. Int. 2019, 25, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Syafri, S.; Jaswir, I.; Yusof, F.; Rohman, A.; Ahda, M.; Hamidi, D. The Use of Instrumental Technique and Chemometrics for Essential Oil Authentication: A Review. Results Chem. 2022, 4, 100622. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of Oregano, Carvacrol and Thymol on Staphylococcus aureus and Staphylococcus epidermidis Biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Al Qurashi, Y.M.A.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic Effect of Eugenol, Carvacrol, Thymol, p-Cymene and γ-Terpinene on Inhibition of Drug Resistance and Biofilm Formation of Oral Bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The Influence of Phytosociological Cultivation and Fertilization on Polyphenolic Content of Menthae and Melissae Folium and Evaluation of Antioxidant Properties through In Vitro and In Silico Methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef] [PubMed]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. Am. Soc. Microbiol. 2012, 1–13. Available online: https://asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/Kirby-Bauer-Disk-DiffusionSusceptibility-Test-Protocol-pdf.pdf (accessed on 3 March 2024).
- Popovici, V.; Bucur, L.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Schröder, V.; Gîrd, C.E.; Gherghel, D.; Vochita, G.; et al. Elemental Analysis and In Vitro Evaluation of Antibacterial and Antifungal Activities of Usnea barbata (L.) Weber Ex F.H. Wigg from Călimani Mountains, Romania. Plants 2022, 11, 32. [Google Scholar]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Karampelas, O.; Musuc, A.M.; Atkinson, I.; et al. Evaluation of Usnea barbata (L.) Weber Ex F.H. Wigg Extract in Canola Oil Loaded in Bioadhesive Oral Films for Potential Applications in Oral Cavity Infections and Malignancy. Antioxidants 2022, 11, 1601. [Google Scholar] [CrossRef]
- Ivan, I.M.; Popovici, V.; Chițescu, C.L.; Popescu, L.; Luță, E.A.; Ilie, E.I.; Brașoveanu, L.I.; Hotnog, C.M.; Olaru, O.T.; Nițulescu, G.M.; et al. Phytochemical Profile, Antioxidant and Cytotoxic Potential of Capsicum annuum (L.) Dry Hydro-Ethanolic Extract. Pharmaceutics 2024, 16, 245. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Popescu, A.; Matei, E.; Caraiane, A.; Botnarciuc, M. Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea barbata (L.) Weber Ex F.H. Wigg from Călimani Mountains, Romania. Pharmaceuticals 2022, 15, 829. [Google Scholar] [CrossRef] [PubMed]
- Luță, E.-A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Popescu, L.; Bejenaru, L.E.; Bejenaru, C.; Popovici, V.; Olaru, O.T.; et al. Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 11670. [Google Scholar] [CrossRef] [PubMed]












| RT [min] | Essential Oil | CEO | EEO | LEO | PEO | OEO |
|---|---|---|---|---|---|---|
| Compound Name | Quantity (%) | |||||
| 45.79 | Eugenol | 86.2272 ± 4.8956 | - | - | - | - |
| 47.29 | Caryophyllene | 6.8733 ± 0.4562 | - | - | 1.6170 ± 0.2035 | 1.4581 ± 0.2668 |
| 56.44 | Eugenol acetate | 5.7515 ± 0.6057 | - | - | - | - |
| 21.00 | Eucalyptol | - | 83.7478 ± 4.8364 | 0.7360 ± 0.1087 | 5.0452 ± 0.2877 | 0.8904 ± 0.0880 |
| 21.77 | γ-Terpinene | - | 2.2315 ± 0.2701 | - | 0.2864 ± 0.0166 | 2.9827 ± 0.3837 |
| 31.34 | Isomenthone | - | - | - | 26.5289 ± 1.5690 | - |
| 26.16 | Linalool | - | 0.0102 ± 0.0019 | 52.9348 ± 7.4084 | 0.0670 ± 0.0036 | 1.4072 ± 0.1055 |
| 31.48 | Camphor | - | 0.0138 ± 0.0009 | 1.3463 ± 0.2607 | - | - |
| 32.23 | Neoisomenthol | - | - | - | 55.0951 ± 7.7417 | - |
| 43.29 | p-Thymol | - | - | - | 0.0896 ± 0.0147 | 72.0862 ± 9.3764 |
| 35.69 | Linalyl acetate | - | - | 32.3146 ± 2.5124 | - | 0.0152 ± 0.0021 |
| 42.95 | Nerol | - | - | 2.1132 ± 0.1975 | - | - |
| 20.10 | Limonene | - | 5.4506 ± 1.0435 | 1.1219 ± 0.1812 | 2.0857 ± 0.2091 | 0.6877 ± 0.0737 |
| 20.42 | o-Cymene | - | 4.1323 ± 0.5402 | 0.2470 ± 0.0253 | 0.1803 ± 0.0320 | 16.2692 ± 1.4297 |
| 14.79 | α-Pinene | - | 2.8165 ± 0.2594 | 3.1612 ± 0.2142 | 0.8279 ± 0.1647 | 2.3971 ± 0.3353 |
| 17.46 | β-Pinene | - | 0.2631 ± 0.0227 | 0.0478 ± 0.0055 | 0.8802 ± 0.0767 | 1.3942 ± 0.2326 |
| 30.16 | Menthofuran | - | - | - | 2.4656 ± 0.3486 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, R.; Popovici, V.; Ionescu, L.-E.; Ordeanu, V.; Biță, A.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Phytochemical Screening and Antibacterial Activity of Commercially Available Essential Oils Combinations with Conventional Antibiotics against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2024, 13, 478. https://doi.org/10.3390/antibiotics13060478
Neagu R, Popovici V, Ionescu L-E, Ordeanu V, Biță A, Popescu DM, Ozon EA, Gîrd CE. Phytochemical Screening and Antibacterial Activity of Commercially Available Essential Oils Combinations with Conventional Antibiotics against Gram-Positive and Gram-Negative Bacteria. Antibiotics. 2024; 13(6):478. https://doi.org/10.3390/antibiotics13060478
Chicago/Turabian StyleNeagu, Răzvan, Violeta Popovici, Lucia-Elena Ionescu, Viorel Ordeanu, Andrei Biță, Diana Mihaela Popescu, Emma Adriana Ozon, and Cerasela Elena Gîrd. 2024. "Phytochemical Screening and Antibacterial Activity of Commercially Available Essential Oils Combinations with Conventional Antibiotics against Gram-Positive and Gram-Negative Bacteria" Antibiotics 13, no. 6: 478. https://doi.org/10.3390/antibiotics13060478






