Is It Still Beneficial to Monitor the Trough Concentration of Vancomycin? A Quantitative Meta-Analysis of Nephrotoxicity and Efficacy
Abstract
:1. Introduction
2. Results
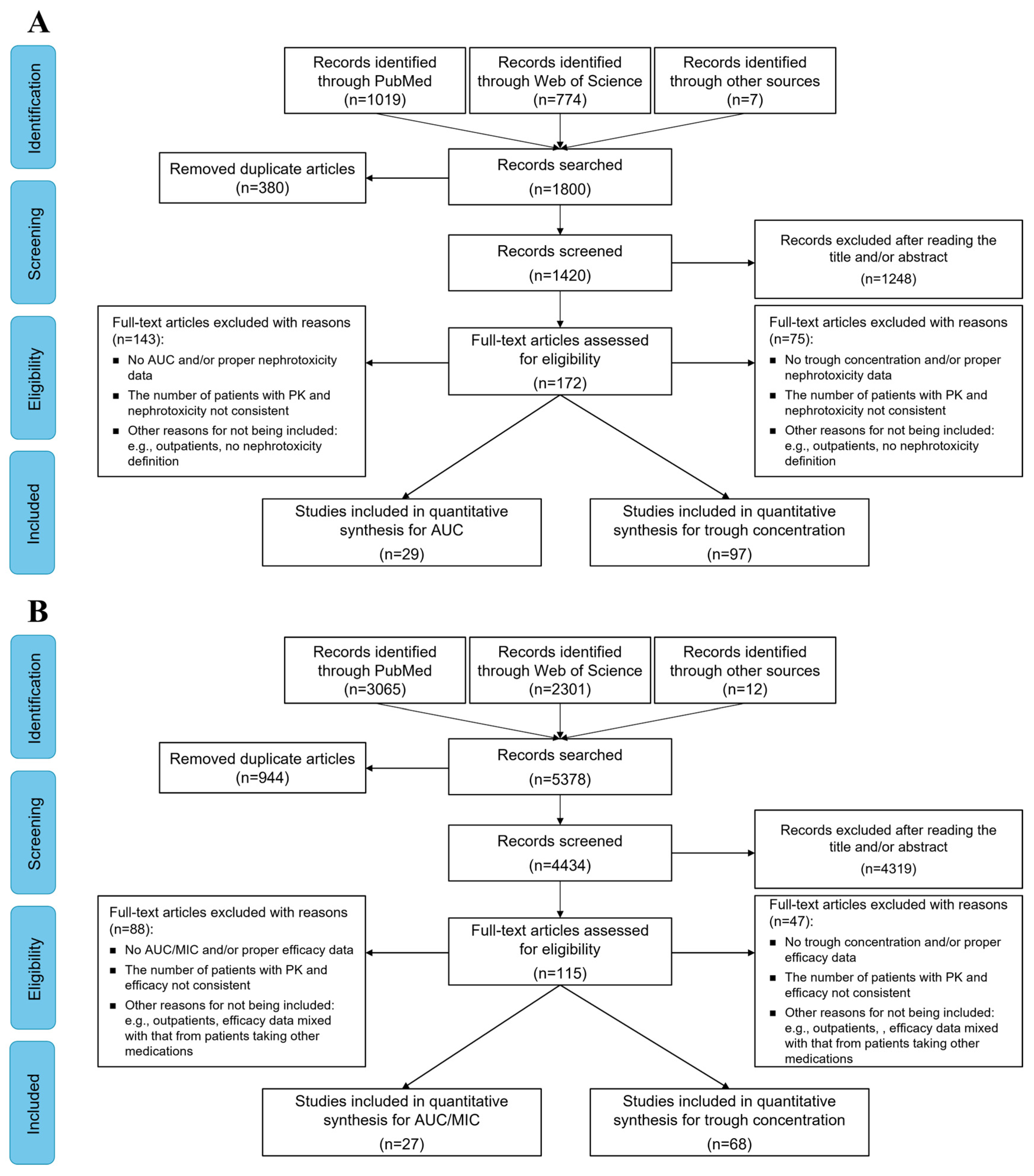
2.1. Characteristics of the Included Studies
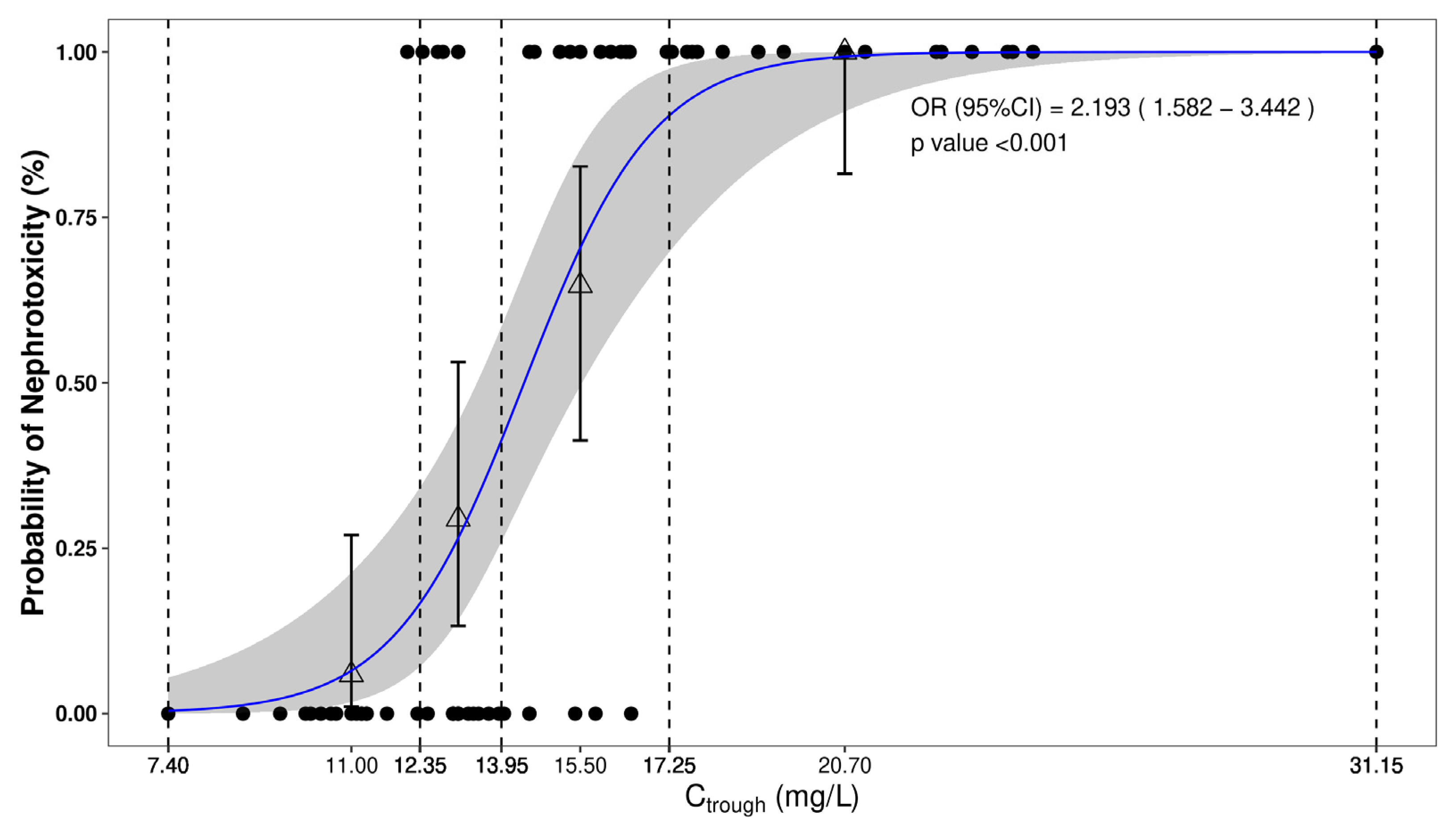
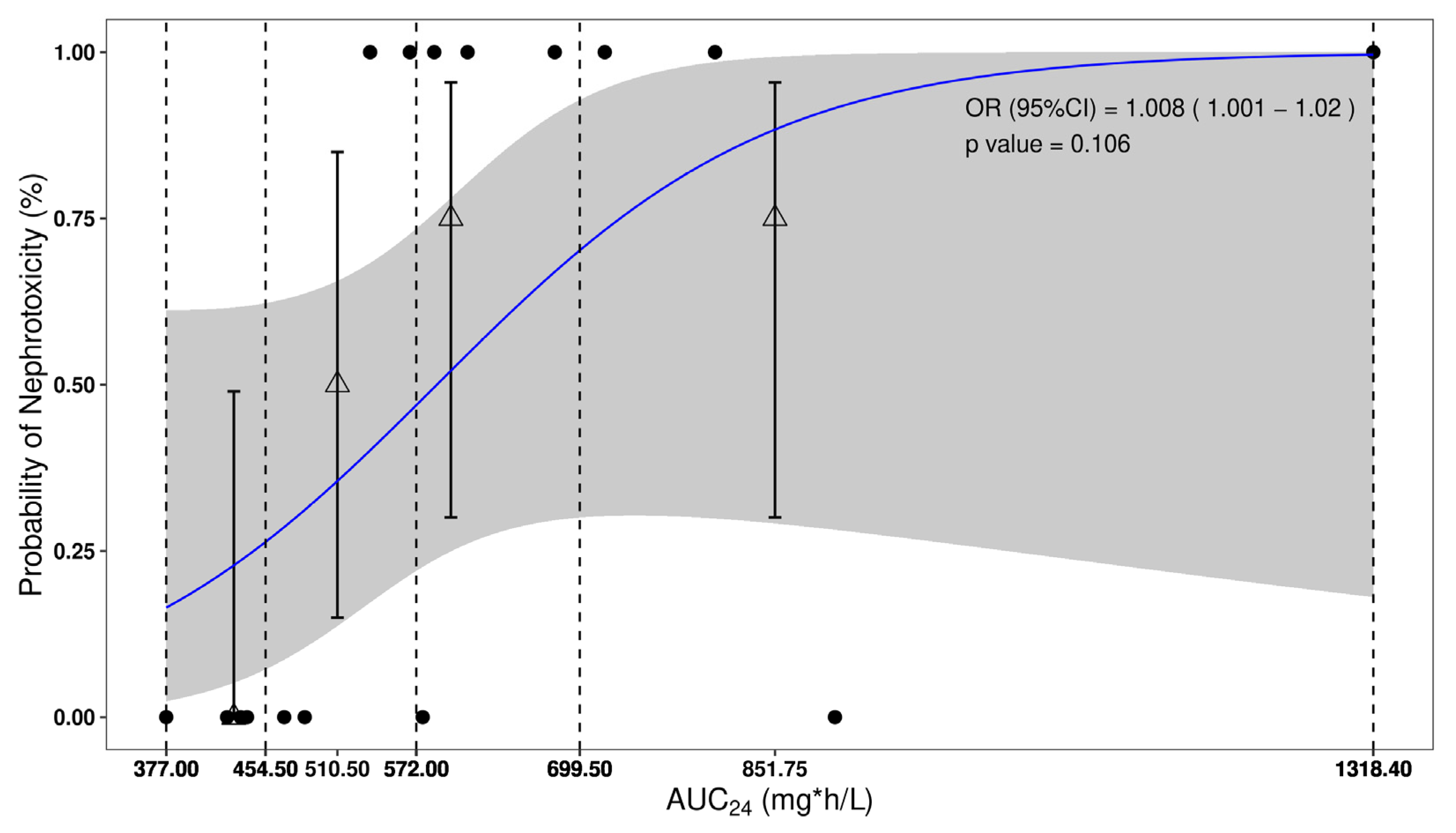
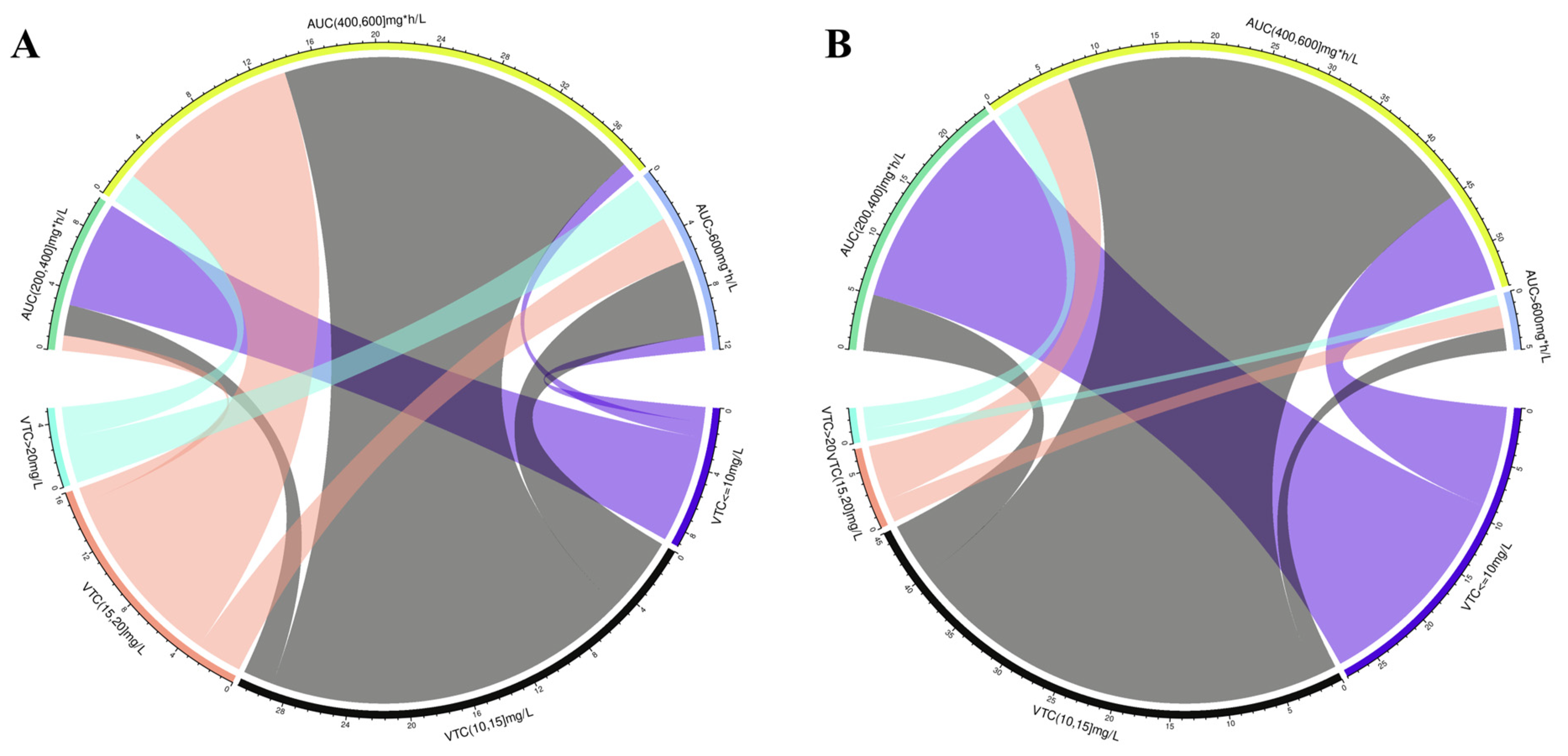
2.2. Nephrotoxicity
2.3. Efficacy
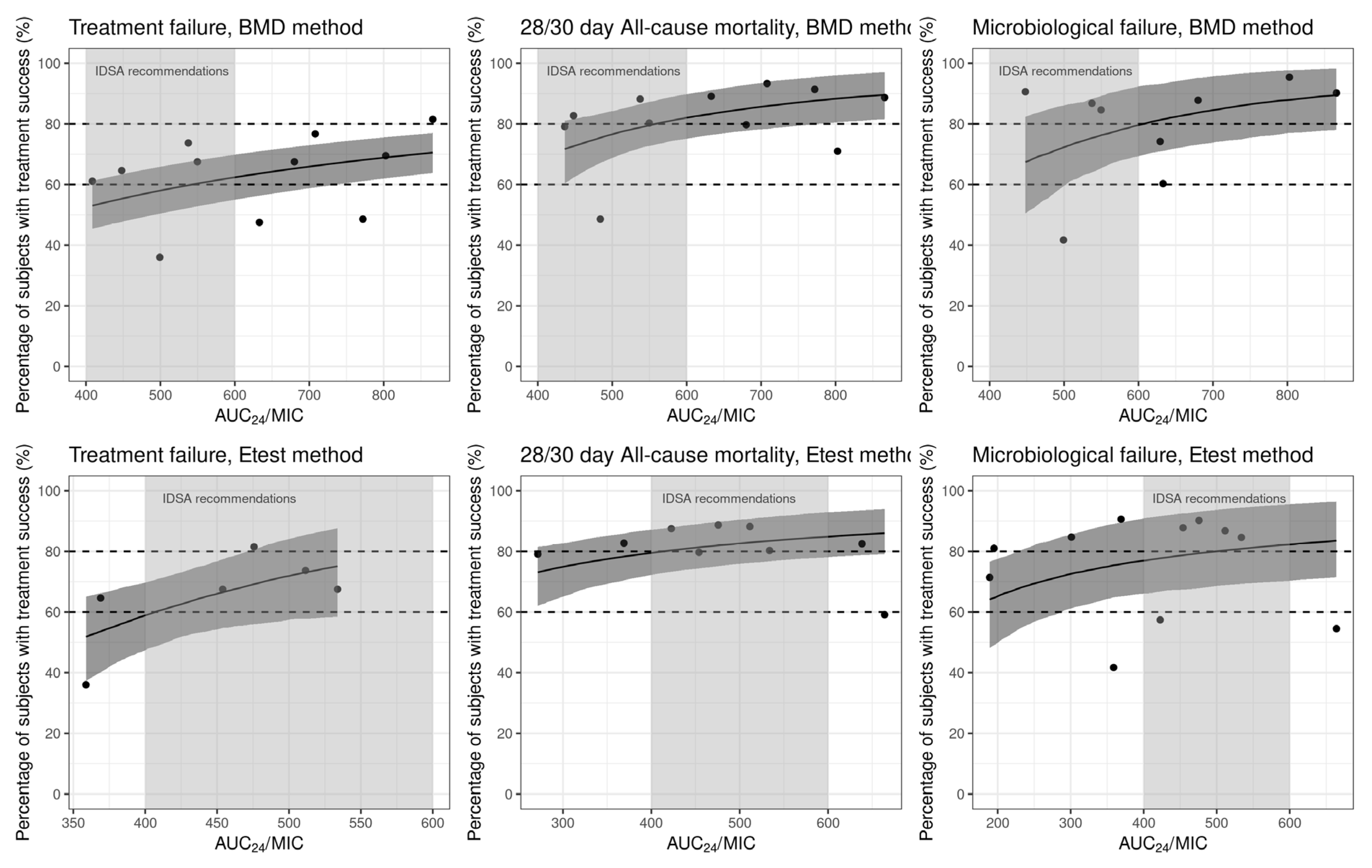
2.3.1. Treatment Failure
2.3.2. All-Cause Mortality
2.3.3. Microbiologic Failure
2.4. Relationship of Vancomycin Mean AUC24 and Ctrough
3. Discussion
4. Methods
4.1. Search Strategy
4.2. Inclusion Criteria and Outcomes
4.3. Data Extraction
4.4. Data Handling
4.5. Analytical Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006, 42 (Suppl. S1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 2003, 17, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Tsutsuura, M.; Moriyama, H.; Kojima, N.; Mizukami, Y.; Tashiro, S.; Osa, S.; Enoki, Y.; Taguchi, K.; Oda, K.; Fujii, S.; et al. The monitoring of vancomycin: A systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect. Dis. 2021, 21, 153. [Google Scholar] [CrossRef]
- Oda, K.; Jono, H.; Nosaka, K.; Saito, H. Reduced nephrotoxicity with vancomycin therapeutic drug monitoring guided by area under the concentration-time curve against a trough 15–20 mug/mL concentration. Int. J. Antimicrob. Agents 2020, 56, 106109. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.R.; Carr, D.R.; Trienski, T.; Buchanan, C.; White, K.; Bremmer, D.N. Outpatient vancomycin therapy: Acute kidney injury in individualized AUC-based goal trough ranges versus traditional trough dosing. J. Am. Pharm. Assoc. (2003) 2022, 62, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Finch, N.A.; Zasowski, E.J.; Murray, K.P.; Mynatt, R.P.; Zhao, J.J.; Yost, R.; Pogue, J.M.; Rybak, M.J. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob. Agents Chemother. 2017, 61, e01293-17. [Google Scholar] [CrossRef] [PubMed]
- Linder, A.; Fjell, C.; Levin, A.; Walley, K.R.; Russell, J.A.; Boyd, J.H. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am. J. Respir. Crit. Care Med. 2014, 189, 1075–1081. [Google Scholar] [CrossRef]
- Lee, B.V.; Fong, G.; Bolaris, M.; Neely, M.; Minejima, E.; Kang, A.; Lee, G.; Gong, C.L. Cost-benefit analysis comparing trough, two-level AUC and Bayesian AUC dosing for vancomycin. Clin. Microbiol. Infect. 2021, 27, 1346.e1–1346.e7. [Google Scholar] [CrossRef]
- Aljefri, D.M.; Avedissian, S.N.; Rhodes, N.J.; Postelnick, M.J.; Nguyen, K.; Scheetz, M.H. Vancomycin Area under the curve and acute kidney injury: A meta-analysis. Clin. Infect. Dis. 2019, 69, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Neely, M.; Rodvold, K.A.; Lodise, T.P. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv. Drug Deliv. Rev. 2014, 77, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.N.; Youn, G.; Jones, B.; Jelliffe, R.W.; Drusano, G.L.; Rodvold, K.A.; Lodise, T.P. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob. Agents Chemother. 2014, 58, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.; Skrupky, L.P.; Servais, R.; Brummitt, C.F.; Dilworth, T.J. Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented Staphylococcal infections. Ther. Drug Monit. 2019, 41, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Bel Kamel, A.; Bourguignon, L.; Marcos, M.; Ducher, M.; Goutelle, S. Is Trough concentration of vancomycin predictive of the area under the curve? A clinical study in elderly patients. Ther. Drug Monit. 2017, 39, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Drusano, G. Vancomycin area under the curve-guided dosing and monitoring for adult and pediatric patients with suspected or documented serious methicillin-resistant Staphylococcus aureus infections: Putting the safety of our patients first. Clin. Infect. Dis. 2021, 72, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar] [PubMed]
- Matsumoto, K.; Oda, K.; Shoji, K.; Hanai, Y.; Takahashi, Y.; Fujii, S.; Hamada, Y.; Kimura, T.; Mayumi, T.; Ueda, T.; et al. Clinical practice guidelines for therapeutic drug monitoring of vancomycin in the framework of model-informed precision dosing: A consensus review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Pharmaceutics 2022, 14, 489. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.R.; Rajakumar, I.; Langevin, A.; Ondro, C.; Sabuda, D.; Griener, T.P.; Dersch-Mills, D.; Rennert-May, E. Vancomycin area under the curve to minimum inhibitory concentration ratio predicting clinical outcome: A systematic review and meta-analysis with pooled sensitivity and specificity. Clin. Microbiol. Infect. 2020, 26, 436–446. [Google Scholar] [CrossRef]
- Bellos, I.; Daskalakis, G.; Pergialiotis, V. Relationship of vancomycin trough levels with acute kidney injury risk: An exposure-toxicity meta-analysis. J. Antimicrob. Chemother. 2020, 75, 2725–2734. [Google Scholar] [CrossRef]
- Lodise, T.P.; Patel, N.; Lomaestro, B.M.; Rodvold, K.A.; Drusano, G.L. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 2009, 49, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, J.; Yu, H.; Zhou, L.; Zhao, Y.; Zhong, L.; Zhu, J.; Liang, G.; Yang, Y.; Zheng, Y.; et al. Should the trough concentration of vancomycin be abandoned in therapeutic drug monitoring? A multicentre, retrospective study of critically ill patients without any form of dialysis. Int. J. Antimicrob. Agents 2023, 61, 106812. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Su, S.; Ye, Z.; Du, G.; He, B.; Li, D.; Liu, Y.; Yang, K.; Zhang, X.; Zhang, Y.; et al. Evidence-based Guideline for Therapeutic Drug Monitoring of Vancomycin: 2020 Update by the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Clin. Infect. Dis. 2020, 71, S363–S371. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Stocker, S.L.; Alffenaar, J.C.; Baldelli, S.; Cattaneo, D.; Jones, G.; Koch, B.C.P.; Kocic, D.; Mathew, S.K.; Molinaro, M.; et al. Optimal practice for vancomycin therapeutic drug monitoring: Position statement from the Anti-infectives Committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther. Drug Monit. 2022, 44, 121–132. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Acute Kidney Injury, N. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef]
- Sohn, Y.; Rim, J.H.; Cho, Y.; Hyun, J.; Baek, Y.; Kim, M.; Kim, J.H.; Seong, H.; Ahn, J.Y.; Lee, S.G.; et al. Association of vancomycin trough concentration on the treatment outcome of patients with bacteremia caused by Enterococcus species. BMC Infect. Dis. 2021, 21, 1099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marko, R.; Hajjar, J.; Nzeribe, V.; Pittman, M.; Deslandes, V.; Sant, N.; Cowan, J.; Kyermentang, K.; Ramsay, T.; Zelenitsky, S.; et al. Therapeutic Drug Monitoring of Vancomycin in Adult Patients with Methicillin-Resistant Staphylococcus aureus Bacteremia or Pneumonia. Can. J. Hosp. Pharm. 2021, 74, 334–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katip, W.; Oberdorfer, P. A Monocentric Retrospective Study of AUC/MIC Ratio of Vancomycin Associated with Clinical Outcomes and Nephrotoxicity in Patients with Enterococcal Infections. Pharmaceutics 2021, 13, 1378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Sulaiti, F.K.; Nader, A.M.; Saad, M.O.; Shaukat, A.; Parakadavathu, R.; Elzubair, A.; Al-Badriyeh, D.; Elewa, H.; Awaisu, A. Clinical and Pharmacokinetic Outcomes of Peak-Trough-Based Versus Trough-Based Vancomycin Therapeutic Drug Monitoring Approaches: A Pragmatic Randomized Controlled Trial. Eur. J. Drug Metab. Pharmacokinet 2019, 44, 639–652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wan, M.; Walker, S.A.N.; Martin, E.; Elligsen, M.; Palmay, L.; Leis, J.A. The impact of vancomycin trough concentrations on outcomes in non-deep seated infections: A retrospective cohort study. BMC Pharmacol. Toxicol. 2018, 19, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mogle, B.T.; Steele, J.M.; Seabury, R.W.; Dang, U.J.; Kufel, W.D. Implementation of a two-point pharmacokinetic AUC-based vancomycin therapeutic drug monitoring approach in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Int. J. Antimicrob. Agents 2018, 52, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Jumah, M.T.B.; Vasoo, S.; Menon, S.R.; De, P.P.; Neely, M.; Teng, C.B. Pharmacokinetic/Pharmacodynamic Determinants of Vancomycin Efficacy in Enterococcal Bacteremia. Antimicrob. Agents Chemother. 2018, 62, e01602-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukumori, S.; Tsuji, Y.; Mizoguchi, A.; Kasai, H.; Ishibashi, T.; Iwamura, N.; To, H. Association of the clinical efficacy of vancomycin with the novel pharmacokinetic parameter area under the trough level (AUTL) in elderly patients with hospital-acquired pneumonia. J. Clin. Pharm. Ther. 2016, 41, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tokimatsu, I.; Morinaga, Y.; Sato, Y.; Takano, K.; Kohno, K.; Ogata, M.; Hiramatsu, K.; Itoh, H.; Kadota, J. A retrospective analysis to estimate target trough concentration of vancomycin for febrile neutropenia in patients with hematological malignancy. Clin. Chim. Acta. 2015, 440, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, D. The characteristics and impact indicator of vancomycin pharmacokinetics in cancer patients complicated with severe pneumonia. J. Infect. Chemother. 2020, 26, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Perin, N.; Roger, C.; Marin, G.; Molinari, N.; Evrard, A.; Lavigne, J.P.; Barbar, S.; Claret, P.G.; Boutin, C.; Muller, L.; et al. Vancomycin Serum Concentration after 48 h of Administration: A 3-Years Survey in an Intensive Care Unit. Antibiotics 2020, 9, 793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yahav, D.; Abbas, M.; Nassar, L.; Ghrayeb, A.; Shepshelovich, D.; Kurnik, D.; Leibovici, L.; Paul, M. Attention to age: Similar dosing regimens lead to different vancomycin levels among older and younger patients. Age Ageing 2019, 49, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Frazee, E.; Rule, A.D.; Lieske, J.C.; Kashani, K.B.; Barreto, J.N.; Virk, A.; Kuper, P.J.; Dierkhising, R.A.; Leung, N. Cystatin C-Guided Vancomycin Dosing in Critically Ill Patients: A Quality Improvement Project. Am. J. Kidney Dis. 2017, 69, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kawasaki, K.; Sato, Y.; Tokimatsu, I.; Itoh, H.; Hiramatsu, K.; Takeyama, M.; Kadota, J. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chemotherapy 2012, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Delatour, F.; Faurisson, F.; Rauss, A.; Pean, Y.; Misset, B.; Thomas, F.; Timsit, J.F.; Similowski, T.; Mentec, H. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: Prospective multicenter randomized study. Antimicrob. Agents Chemother. 2001, 45, 2460–2467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gawronski, K.M.; Goff, D.A.; Brown, J.; Khadem, T.M.; Bauer, K.A. A stewardship program’s retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. Clin. Ther. 2013, 35, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Duszynska, W.; Taccone, F.S.; Hurkacz, M.; Wiela-Hojenska, A.; Kübler, A. Continuous vs. intermittent vancomycin therapy for Gram-positive infections not caused by methicillin-resistant Staphylococcus aureus. Minerva Anestesiol. 2016, 82, 284–293. [Google Scholar] [PubMed]
- Mizokami, F.; Shibasaki, M.; Yoshizue, Y.; Noro, T.; Mizuno, T.; Furuta, K. Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin-resistant Staphylococcus aureus hospital-acquired pneumonia. Clin. Interv. Aging 2013, 8, 1015–1021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zasowski, E.J.; Murray, K.P.; Trinh, T.D.; Finch, N.A.; Pogue, J.M.; Mynatt, R.P.; Rybak, M.J. Identification of Vancomycin Exposure-Toxicity Thresholds in Hospitalized Patients Receiving Intravenous Vancomycin. Antimicrob. Agents Chemother. 2017, 62, e01684-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.L.; Xue, M.; Wang, H.F.; Huang, L.L.; Li, Q.; Xu, J.Y.; Xie, J.F.; Huang, Y.Z. An area under curve-based nomogram to predicts vancomycin-associated nephrotoxicity in critically ill patients: A retrospective cohort study. Zhonghua Nei Ke Za Zhi 2022, 61, 291–297. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Takesue, Y.; Nakajima, K.; Ichiki, K.; Ishikawa, K.; Yamada, K.; Tsuchida, T.; Otani, N.; Takahashi, Y.; Ishihara, M.; et al. Validation of Vancomycin Area under the Concentration-Time Curve Estimation by the Bayesian Approach Using One-Point Samples for Predicting Clinical Outcomes in Patients with Methicillin-Resistant Staphylococcus aureus Infections. Antibiotics 2022, 11, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yasu, T.; Konuma, T.; Oiwa-Monna, M.; Kato, S.; Isobe, M.; Takahashi, S.; Tojo, A. Lower vancomycin trough levels in adults undergoing unrelated cord blood transplantation. Leuk Lymphoma. 2021, 62, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, N.; Wei, W.; Jiang, C. Outcomes and Nephrotoxicity Associated with Vancomycin Treatment in Patients 80 Years and Older. Clin. Interv. Aging 2021, 16, 1023–1035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Zhang, Y.; Xu, X.; Wu, B.; Ni, J.; Li, T.; Xing, C.; Mao, H. Comparative Prevalence of Acute Kidney Injury in Chinese Patients Receiving Vancomycin with Concurrent β-Lactam Antibiotics: A Retrospective Cohort Study. Clin. Ther. 2021, 43, e319–e351. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.M.; Huang, V.; Hall, S.T.; Buckley, M.S.; Bikin, D.; Barletta, J.F. Optimizing outcomes using vancomycin therapeutic drug monitoring in patients with MRSA bacteremia: Trough concentrations or area under the curve? Diagn Microbiol. Infect. Dis. 2021, 101, 115442. [Google Scholar] [CrossRef] [PubMed]
- Al Sulaiman, K.; Alshaya, A.; Aljuhani, O.; Alsaeed, A.; Alshehri, N.; Vishwakarma, R.; Alzahrani, H.; Althewaibi, S.; Alghamdi, N.; Alhelal, K.; et al. The impact of early target attainment of vancomycin in critically ill patients with confirmed Gram-positive infection: A retrospective cohort study. BMC Infect. Dis. 2021, 21, 1182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Wang, T.; Zhang, D.; You, H.; Dong, Y.; Liu, Y.; Du, Q.; Sun, D.; Zhang, T.; Dong, Y. Therapeutic Drug Monitoring Coupled With Bayesian Forecasting Could Prevent Vancomycin-Associated Nephrotoxicity in Renal Insufficiency Patients: A Prospective Study and Pharmacoeconomic Analysis. Ther. Drug Monit. 2020, 42, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Sanematsu, E.; Furuya, Y.; Shinohara, Y.; Murakami, Y.; Miyazaki, A.; Sakamoto, Y.; Nakashima, M.N.; Nakashima, M. Relationship between vancomycin-associated nephrotoxicity and the number of combined nephrotoxic agents. Pharmazie 2020, 75, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Tsoi, M.F.; Zhao, X.; Zhang, L.; Qi, Z.; Cheung, B.M.Y. Vancomycin-associated acute kidney injury in Hong Kong in 2012–2016. BMC Nephrol. 2020, 21, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, C.; Wen, A.; Li, X.; Li, D.; Zhang, Y.; Liao, Y.; Ren, Y.; Shen, S. Development and Validation of a Risk Prediction Model of Vancomycin-Associated Nephrotoxicity in Elderly Patients: A Pilot Study. Clin. Transl. Sci. 2020, 13, 491–497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mcgrady, K.A.; Benton, M.; Tart, S.; Bowers, R. Evaluation of traditional initial vancomycin dosing versus utilizing an electronic AUC/MIC dosing program. Pharm. Pract. 2020, 18, 2024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, N.H.; Walker, S.A.N.; Elligsen, M.; Kiss, A.; Palmay, L.; Ho, G.; Powis, J.; Bansal, V.; Leis, J.A. Retrospective multicentre matched cohort study comparing safety and efficacy outcomes of intermittent-infusion versus continuous-infusion vancomycin. J. Antimicrob. Chemother. 2020, 75, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Takekuma, Y.; Kashiwagi, H.; Miyai, T.; Kobayashi, M.; Iseki, K.; Sugawara, M. Validation of the usefulness of artificial neural networks for risk prediction of adverse drug reactions used for individual patients in clinical practice. PLoS ONE 2020, 15, e0236789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brunetti, L.; Song, J.H.; Suh, D.; Kim, H.J.; Seong, Y.H.; Lee, D.S.; Lee, S.M.; Suh, D.C. The risk of vancomycin toxicity in patients with liver impairment. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Truong, J.; Smith, S.R.; Veillette, J.J.; Forland, S.C. Individualized Pharmacokinetic Dosing of Vancomycin Reduces Time to Therapeutic Trough Concentrations in Critically Ill Patients. J. Clin. Pharmacol. 2018, 58, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Abbas, M.; Nassar, L.; Ghrayeb, A.; Kurnik, D.; Shepshelovich, D.; Leibovici, L.; Paul, M. The association of vancomycin trough levels with outcomes among patients with methicillin-resistant Staphylococcus aureus (MRSA) infections: Retrospective cohort study. PLoS ONE 2019, 14, e0214309. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, C.D.C.; Simões E Silva, A.C.; de Queiroz Oliveira, J.A.; Batista, I.S.F.; Pereira, F.H.; Gonçalves, J.E.; Nobre, V.; Martins, M.A.P. Vancomycin-associated nephrotoxicity in non-critically ill patients admitted in a Brazilian public hospital: A prospective cohort study. PLoS ONE 2019, 14, e0222095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakashima, T.; Koido, K.; Baba, H.; Otsuka, R.; Okinaka, K.; Sano, T.; Nishigaki, R.; Hashimoto, H.; Otsuka, T.; Esaki, M.; et al. Contribution of pharmacists with expertise in infectious diseases to appropriate individualized vancomycin dosing. Pharmazie 2018, 73, 422–424. [Google Scholar] [CrossRef] [PubMed]
- May, C.C.; Erwin, B.L.; Childress, M.; Cortopassi, J.; Curtis, G.; Kilpatrick, T.; Taylor, J.; Vance, B.; Wylie, D. Assessment of acute kidney injury in neurologically and traumatically injured intensive care patients receiving large vancomycin doses. Int. J. Crit. Illn. Inj. Sci. 2018, 8, 194–200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, X.; Fan, Y.; Yang, M.; Zhang, J.; Wu, J.; Yu, J.; Tao, J.; Lu, G.; Zhang, H.; Wang, R.; et al. A Prospective Multicenter Clinical Observational Study on Vancomycin Efficiency and Safety with Therapeutic Drug Monitoring. Clin. Infect. Dis. 2018, 67 (Suppl. S2), S249–S255. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Pettit, N.N.; Landon, E.M.; Brielmaier, B.D. Impact of Pharmacy Practice Model Expansion on Pharmacokinetic Services: Optimization of Vancomycin Dosing and Improved Patient Safety. Hosp Pharm. 2017, 52, 273–279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chavada, R.; Ghosh, N.; Sandaradura, I.; Maley, M.; Van Hal, S.J. Establishment of an AUC0-24 Threshold for Nephrotoxicity Is a Step towards Individualized Vancomycin Dosing for Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2017, 61, e02535-16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, C.W.; Cazares, K.S.; Lustik, M.B.; Patel, S.M.; Denunzio, T.M. Vancomycin vs. Vancomycin/Piperacillin-Tazobactam-Associated Acute Kidney Injury in Noncritically Ill Patients at a Tertiary Care Military Treatment Facility. Mil. Med. 2017, 182, e1773–e1778. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, K.; Brimacombe, M.; Yu, A.; Goodloe, N.; Haidar, W.; El Atrouni, W. Vancomycin Trough and Acute Kidney Injury: A Large Retrospective, Cohort Study. Am. J. Nephrol. 2016, 44, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, T.P.; Kotapati, C.; Roberts, M.J.; Rowland, J.; Lipman, J.; Roberts, J.A.; Udy, A. Factors associated with vancomycin nephrotoxicity in the critically ill. Anaesth Intensive Care 2015, 43, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.H.; Wang, J.W.; Wu, Y.; Chen, B.Y.; Yu, M.; Wen, A.D. Evaluation of body weight-based vancomycin therapy and the incidence of nephrotoxicity: A retrospective study in the northwest of China. Int. J. Infect. Dis. 2015, 37, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, T.P.; Harlow, G.; Hutchinson, J.; Dulhunty, J.M.; Lipman, J.; Whitehouse, T.; Roberts, J.A. Vancomycin-associated nephrotoxicity in the critically ill: A retrospective multivariate regression analysis*. Crit. Care Med. 2014, 42, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.G., 2nd; Blaszczyk, A.T.; Thompson, K.A.; Brouse, S.D.; Giuliano, C.A.; Frei, C.R.; Forcade, N.A.; Mortensen, E.M.; Bell, T.; Bedimo, R.J.; et al. Impact of empiric weight-based vancomycin dosing on nephrotoxicity and mortality in geriatric patients with methicillin-resistant Staphylococcus aureus bacteraemia. J. Clin. Pharm. Ther. 2014, 39, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Burgess, L.D.; Drew, R.H. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy 2014, 34, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Mizokami, F.; Fukami, K.; Ito, K.; Shibasaki, M.; Nagamatsu, T.; Furuta, K. The influence of severe hypoalbuminemia on the half-life of vancomycin in elderly patients with methicillin-resistant Staphylococcus aureus hospital-acquired pneumonia. Clin. Interv. Aging 2013, 8, 1323–1328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horey, A.; Mergenhagen, K.A.; Mattappallil, A. The Relationship of nephrotoxicity to vancomycin trough serum concentrations in a veteran’s population: A retrospective analysis. Ann. Pharmacother. 2012, 46, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, L.K.; Hsu, D.I.; Quist, R.; Shriner, K.A.; Wong-Beringer, A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: Efficacy and toxicity. Arch Intern Med 2006, 166, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Flannery, A.H.; Delozier, N.L.; Effoe, S.A.; Wallace, K.L.; Cook, A.M.; Burgess, D.S. First-Dose Vancomycin Pharmacokinetics Versus Empiric Dosing on Area-Under-the-Curve Target Attainment in Critically Ill Patients. Pharmacotherapy 2020, 40, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Hanada, K.; Kanno, A.; Akashi, M.; Itoh, T. Risk factors for vancomycin nephrotoxicity and time course of renal function during vancomycin treatment. Eur. J. Clin. Pharmacol. 2019, 75, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Chuma, M.; Azuma, M.; Nakamura, S.; Miki, H.; Hamano, H.; Goda, M.; Takechi, K.; Zamami, Y.; Abe, M.; et al. Effect of serum concentration and concomitant drugs on vancomycin-induced acute kidney injury in haematologic patients: A single-centre retrospective study. Eur. J. Clin. Pharmacol. 2019, 75, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.; Jiménez, C.; Borobia, A.M.; Tong, H.Y.; Medrano, N.; Krauel-Bidwell, L.; Carcas, A.J.; Selgas, R.; Frías, J. Vancomycin-induced acute kidney injury detected by a prospective pharmacovigilance program from laboratory signals. Ther. Drug Monit. 2013, 35, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Molina, K.C.; Barletta, J.F.; Hall, S.T.; Yazdani, C.; Huang, V. The Risk of Acute Kidney Injury in Critically Ill Patients Receiving Concomitant Vancomycin with Piperacillin-Tazobactam or Cefepime. J. Intensive Care Med. 2020, 35, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Braekevelt, K.; Kale-Pradhan, P.; Szpunar, S.; Khatib, R. Are Blacks at Higher Risk for Vancomycin-Related Acute Kidney Injury? J. Pharm. Pract. 2020, 33, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Hays, W.B.; Tillman, E. Vancomycin-Associated Acute Kidney Injury in Critically Ill Adolescent and Young Adult Patients. J. Pharm. Pract. 2020, 33, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Covvey, J.R.; Erickson, O.; Fiumara, D.; Mazzei, K.; Moszczenski, Z.; Slipak, K.; Nemecek, B.D.; Zimmerman, D.E.; Guarascio, A.J. Comparison of Vancomycin Area-Under-the-Curve Dosing Versus Trough Target-Based Dosing in Obese and Nonobese Patients with Methicillin-Resistant Staphylococcus aureus Bacteremia. Ann. Pharmacother. 2020, 54, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.E.; Katona, B.G.; Plaisance, K.I. Association of vancomycin serum concentrations with outcomes in patients with gram-positive bacteremia. Pharmacotherapy 1995, 15, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Brumer, E.; Dubrovskaya, Y.; Scipione, M.R.; Aberle, C.; Rahimian, J.; Papadopoulos, J. Evaluation of Treatment Courses When Vancomycin Is Given Every 8 Hours in Adult Patients. J. Pharm. Pract. 2015, 28, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; Hutchison, A.M.; McAtee, A.M.; Gaillard, P.R.; Childress, D.T. Allometric versus consensus guideline dosing in achieving target vancomycin trough concentrations. Am. J. Health Syst. Pharm. 2017, 74, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Cano, E.L.; Haque, N.Z.; Welch, V.L.; Cely, C.M.; Peyrani, P.; Scerpella, E.G.; Ford, K.D.; Zervos, M.J.; Ramirez, J.A.; Kett, D.H. Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Study Group. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: Retrospective analysis of the IMPACT-HAP Database. Clin. Ther. 2012, 34, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Cappelletty, D.; Jablonski, A.; Jung, R. Risk factors for acute kidney injury in adult patients receiving vancomycin. Clin. Drug Investig. 2014, 34, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, B.; Ber Ce, P.; Szabo, A.; Chhabra, S.; D’Souza, A. Correlates and Outcomes of Early Acute Kidney Injury after Hematopoietic Cell Transplantation. Am. J. Med. Sci. 2021, 362, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, H.; Zhou, J.; Xu, M.; Zhou, S. Efficacy of Vancomycin on Gram-Positive Bacterial Infection in Elderly Critical Patients and Risk Factors Associated with Nephrotoxicity. Arch. Iran. Med. 2018, 21, 349–355. [Google Scholar] [PubMed]
- Higashi, T.; Tsukamoto, H.; Kodawara, T.; Igarashi, T.; Watanabe, K.; Yano, R.; Iwasaki, H.; Goto, N. Evaluation of risk factors for nephrotoxicity associated with high-dose vancomycin in Japanese patients. Pharmazie 2021, 76, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Fodero, K.E.; Horey, A.L.; Krajewski, M.P.; Ruh, C.A.; Sellick, J.A., Jr.; Mergenhagen, K.A. Impact of an Antimicrobial Stewardship Program on Patient Safety in Veterans Prescribed Vancomycin. Clin. Ther. 2016, 38, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Golenia, B.S.; Levine, A.R.; Moawad, I.M.; Yeh, D.D.; Arpino, P.A. Evaluation of a vancomycin dosing nomogram based on the Modification of Diet in Renal Disease equation in intensive care unit patients. J. Crit. Care 2013, 28, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Hale, C.M.; Seabury, R.W.; Steele, J.M.; Darko, W.; Miller, C.D. Are Vancomycin Trough Concentrations of 15 to 20 mg/L Associated with Increased Attainment of an AUC/MIC ≥ 400 in Patients with Presumed MRSA Infection? J. Pharm. Pract. 2017, 30, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.F.; Athans, V.; Wanek, M.R.; Wang, L.; Estep, J.D.; Williams, B. Evaluation of a hospital-wide vancomycin-dosing nomogram in patients with continuous-flow left ventricular assist devices. Int. J. Artif. Organs 2021, 44, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Moh’d, H.; Kheir, F.; Kong, L.; Du, P.; Farag, H.; Mohamad, A.; Zurlo, J.J. Incidence and predictors of vancomycin-associated nephrotoxicity. S. Med. J. 2014, 107, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K.; An, H.; Shin, K.H.; Shin, D.; Lee, S.H.; Kim, J.H.; Cho, S.H.; Kang, H.R.; Jang, I.J.; Yu, K.S.; et al. Trough concentration over 12.1 mg/L is a major risk factor of vancomycin-related nephrotoxicity in patients with therapeutic drug monitoring. Ther. Drug Monit. 2014, 36, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Haruki, Y.; Hagiya, H.; Haruki, M.; Inoue, Y.; Sugiyama, T. Concomitant vancomycin and piperacillin/tazobactam treatment is associated with an increased risk of acute kidney injury in Japanese patients. J. Infect. Chemother. 2020, 26, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, E.D.; Hanson, M.; Sankaranarayanan, J.; Stoner, J.A.; Florescu, M.C.; Rupp, M.E. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin. Drug Saf. 2010, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.T.; Goolsby, T.A.; Sherman, D.S.; Mueller, S.W.; Reynolds, P.; Cava, L.; Neumann, R.; Kiser, T.H. Continuous infusion vs. intermittent vancomycin in neurosurgical intensive care unit patients. J. Crit. Care 2015, 30, 1153.e1–1153.e6. [Google Scholar] [CrossRef] [PubMed]
- Ghehi, M.T.; Rezaee, S.; Hayatshahi, A.; Hadjibabaie, M.; Gholami, K.; Javadi, M.; Khoee, S.H.; Radfar, M.; Esfandbod, M.; Ghavamzadeh, A. Vancomycin Pharmacokinetic Parameters in Patients Undergoing Hematopoietic Stem Cell Transplantation (HSCT). Int. J. Hematol. Oncol. Stem. Cell Res. 2013, 7, 1–9. [Google Scholar] [PubMed] [PubMed Central]
- Imai, S.; Yamada, T.; Kasashi, K.; Kobayashi, M.; Iseki, K. Usefulness of a decision tree model for the analysis of adverse drug reactions: Evaluation of a risk prediction model of vancomycin-associated nephrotoxicity constructed using a data mining procedure. J. Eval. Clin. Pract. 2017, 23, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Yamada, T.; Kasashi, K.; Niinuma, Y.; Kobayashi, M.; Iseki, K. Construction of a risk prediction model of vancomycin-associated nephrotoxicity to be used at the time of initial therapeutic drug monitoring: A data mining analysis using a decision tree model. J. Eval. Clin. Pract. 2019, 25, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Harada, M.Y.; Barmparas, G.; Jay, J.; Sun, B.J.; Chen, E.; Mehrzadi, D.; Patel, B.; Mason, R.; Ley, E.J. Reducing acute kidney injury due to vancomycin in trauma patients. J. Trauma Acute Care Surg. 2016, 81, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Leonard, S.N.; Davis, S.L.; Delgado, G., Jr.; Pogue, J.M.; Wahby, K.A.; Falcione, B.; Rybak, M.J. Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15–20 mg/L suggested by the vancomycin consensus guidelines. Pharmacotherapy 2011, 31, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Davis, S.L.; Taylor, T.N.; Kaye, K.S.; Rybak, M.J. Effects of targeting higher vancomycin trough levels on clinical outcomes and costs in a matched patient cohort. Pharmacotherapy 2012, 32, 195–201, Erratum in Pharmacotherapy 2012, 32, 869. [Google Scholar] [CrossRef] [PubMed]
- Ley, E.J.; Liou, D.Z.; Singer, M.B.; Mirocha, J.; Srour, M.; Bukur, M.; Margulies, D.R.; Salim, A. Supratherapeutic vancomycin levels after trauma predict acute kidney injury and mortality. J. Surg. Res. 2013, 184, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, Y.; Liu, X.Z.; Yao, H.J.; Li, L.X.; Chen, J.H.; Chen, T.; Lu, X.T.; Bu, S.H.; Zhang, J. Retrospective Analysis of Vancomycin Nephrotoxicity in Elderly Chinese Patients. Pharmacology 2015, 95, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Maiguma, T.; Komoto, A.; Haruki, Y.; Sugiyama, T.; Kondo, S.; Teshima, D. Impact of pharmacist intervention on preventing nephrotoxicity from vancomycin. Int. J. Clin. Pharmacol. Ther. 2015, 53, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wong, T.; Huang, S.; Mui, E.; Nguyen, V.; Espinosa, G.; Desai, J.; Holubar, M.; Deresinski, S. Conversion from Vancomycin Trough Concentration-Guided Dosing to Area under the Curve-Guided Dosing Using Two Sample Measurements in Adults: Implementation at an Academic Medical Center. Pharmacotherapy 2019, 39, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Muklewicz, J.D.; Steuber, T.D.; Edwards, J.D. Evaluation of area under the concentration-time curve-guided vancomycin dosing with or without piperacillin-tazobactam on the incidence of acute kidney injury. Int. J. Antimicrob. Agents 2021, 57, 106234. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lim, N.R.; Park, H.J.; Yang, J.W.; Kim, M.J.; Kim, K.; In, Y.W.; Lee, Y.M. Evaluation of risk factors for vancomycin-induced nephrotoxicity. Int. J. Clin. Pharm. 2018, 40, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Prabaker, K.K.; Tran, T.P.; Pratummas, T.; Goetz, M.B.; Graber, C.J. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J. Hosp. Med. 2012, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, L.; Baker, C.; Leggett, J.; Sehdev, P.; Brown, A.; Bayley, K.B. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am. J. Med. 2010, 123, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Du, G.; Weng, C.; Zhou, H.; Zhou, X. Evaluation of the variability and safety of serum trough concentrations of vancomycin in patients admitted to the intensive care unit. Int. J. Infect. Dis. 2017, 60, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.C.; Waite, L.H.; Alexander, D.P.; DeRyke, C.A. Performance of a vancomycin dosage regimen developed for obese patients. Am. J. Health Syst. Pharm. 2012, 69, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.D.; Li, C.; Hammond, D.A.; Dickey, T.A. Incidence of Acute Kidney Injury Among Patients Receiving the Combination of Vancomycin with Piperacillin-Tazobactam or Meropenem. Pharmacotherapy 2018, 38, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Albrecht, L.M.; Boike, S.C.; Chandrasekar, P.H. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J. Antimicrob. Chemother. 1990, 25, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Sazanami, K.; Inose, R.; Dote, S.; Horiuchi, N.; Kobayashi, Y.; Muraki, Y. Combination therapy of vancomycin and piperacillin/tazobactam in adult febrile neutropenia patients with haematopoietic malignancies increases the risk of acute kidney injury regardless of vancomycin trough concentration. J. Chemother. 2021, 33, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Alosaimy, S.; Murray, K.P.; Zasowski, E.J.; Morrisette, T.; Lagnf, A.M.; Lodise, T.P.; Rybak, M.J. Vancomycin Area under the Curve to Predict Timely Clinical Response in the Treatment of Methicillin-resistant Staphylococcus aureus Complicated Skin and Soft Tissue Infections. Clin. Infect. Dis. 2021, 73, e4560–e4567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, Y.X.; Chen, M.T.; Li, N.Y.; Liu, X.F.; Yang, M.J.; Chen, Y.C.; Liang, X.Y.; Wu, J.F.; Guo, B.N.; Song, S.C.; et al. Sequence Type 5 (ST5) as a Possible Predictor of Bacterial Persistence in Adult Patients with Methicillin-Resistant Staphylococcus aureus Pneumonia Treated with Vancomycin. Microbiol. Spectr. 2022, 10, e0134822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, J.; Hou, Y.; Li, J.; Gao, Y.; Li, R.; Jin, X.; Zhang, J.; Wang, X.; Wang, G. An evaluation on the association of vancomycin trough concentration with mortality in critically ill patients: A multicenter retrospective study. Clin. Transl. Sci. 2021, 14, 1780–1790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lines, J.; Burchette, J.; Kullab, S.M.; Lewis, P. Evaluation of a trough-only extrapolated area under the curve vancomycin dosing method on clinical outcomes. Int. J. Clin. Pharm. 2021, 43, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ren, J.; Li, J.; Jin, X.; Gao, Y.; Li, R.; Zhang, J.; Wang, X.; Li, X.; Wang, G. Relationship Between Mean Vancomycin Trough Concentration and Mortality in Critically Ill Patients: A Multicenter Retrospective Study. Front Pharmacol. 2021, 12, 690157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lodise, T.P.; Rosenkranz, S.L.; Finnemeyer, M.; Evans, S.; Sims, M.; Zervos, M.J.; Creech, C.B.; Patel, P.C.; Keefer, M.; Riska, P.; et al. The Emperor’s New Clothes: PRospective Observational Evaluation of the Association Between Initial VancomycIn Exposure and Failure Rates among Adult HospitalizEd Patients With Methicillin-resistant Staphylococcus aureus Bloodstream Infections (PROVIDE). Clin. Infect. Dis. 2020, 70, 1536–1545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chattaweelarp, T.; Changpradub, D.; Punyawudho, B.; Thunyaharn, S.; Santimaleeworagun, W. Is Early Monitoring Better? Impact of Early Vancomycin Exposure on Treatment Outcomes and Nephrotoxicity in Patients with Methicillin-Resistant Staphylococcus aureus Infections. Antibiotics 2020, 9, 672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makmor-Bakry, M.; Ahmat, A.; Shamsuddin, A.; Lau, C.L.; Ramli, R. Association between single trough-based area under the curve estimation of vancomycin and treatment outcome among methicillin-resistant Staphylococcus aureus bacteremia patients. Anaesthesiol. Intensive Ther. 2019, 51, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Yang, M.; Fan, Y.; Liang, X.; Chen, Y.; Wu, J.; Yu, J.; Zhang, H.; Wang, R.; Zhang, F.; et al. Model-based Evaluation of the Clinical and Microbiological Efficacy of Vancomycin: A Prospective Study of Chinese Adult In-house Patients. Clin. Infect. Dis. 2018, 67 (Suppl. S2), S256–S262. [Google Scholar] [CrossRef] [PubMed]
- Komoto, A.; Maiguma, T.; Teshima, D.; Sugiyama, T.; Haruki, Y. Effects of pharmacist intervention in Vancomycin treatment for patients with bacteremia due to Methicillin-resistant Staphylococcus aureus. PLoS ONE 2018, 13, e0203453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, C.F.; Huang, J.D.; Wang, J.T.; Lin, S.W.; Wu, C.C. The ratio of pre-dialysis vancomycin trough serum concentration to minimum inhibitory concentration is associated with treatment outcomes in methicillin-resistant Staphylococcus aureus bacteremia. PLoS ONE 2018, 13, e0193585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moise, P.A.; Culshaw, D.L.; Wong-Beringer, A.; Bensman, J.; Lamp, K.C.; Smith, W.J.; Bauer, K.; Goff, D.A.; Adamson, R.; Leuthner, K.; et al. Comparative Effectiveness of Vancomycin Versus Daptomycin for MRSA Bacteremia With Vancomycin MIC > 1 mg/L: A Multicenter Evaluation. Clin. Ther. 2016, 38, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Kim, H.K.; Kim, S.K.; Lee, W.; Sung, H.; Chun, S.; Kim, M.N.; Min, W.K. Vancomycin AUC24 /MIC Ratio in Patients with Methicillin-Resistant Staphylococcus aureus Pneumonia. J. Clin. Lab. Anal. 2016, 30, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, S.; Tang, W.; Cho, Y.; Mudge, D.W.; Hawley, C.M.; Badve, S.V.; Johnson, D.W. The role of monitoring vancomycin levels in patients with peritoneal dialysis-associated peritonitis. Perit. Dial. Int. 2015, 35, 222–228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, K.H.; Kim, H.B.; Kim, H.S.; Lee, M.J.; Jung, Y.; Kim, G.; Hwang, J.H.; Kim, N.H.; Kim, M.; Kim, C.J.; et al. Impact of area under the concentration-time curve to minimum inhibitory concentration ratio on vancomycin treatment outcomes in methicillin-resistant Staphylococcus aureus bacteraemia. Int. J. Antimicrob. Agents 2015, 46, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Casapao, A.M.; Lodise, T.P.; Davis, S.L.; Claeys, K.C.; Kullar, R.; Levine, D.P.; Rybak, M.J. Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob. Agents Chemother. 2015, 59, 2978–2985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, G.; Liang, X.; Zhang, J.; Zhou, Y.; Wu, J.; Zhang, Y.; Chen, Y.; Huang, J.; Liu, X.; Yu, J. Vancomycin serum trough concentration vs. clinical outcome in patients with gram-positive infection: A retrospective analysis. J. Clin. Pharm. Ther. 2015, 40, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Drusano, G.L.; Zasowski, E.; Dihmess, A.; Lazariu, V.; Cosler, L.; McNutt, L.A. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: How much is enough? Clin. Infect. Dis. 2014, 59, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jiang, Z.; Chen, J.; Ouyang, B.; Chen, M.; Guan, X. Clinical research for trough value of serum vancomycin in critical patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014, 26, 473–477. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Song, K.H.; Cho, J.; Kim, H.S.; Kim, N.H.; Kim, T.S.; Choe, P.G.; Chung, J.Y.; Park, W.B.; Bang, J.H.; et al. Area under the concentration-time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin-resistant Staphylococcus aureus bacteraemia. Int. J. Antimicrob. Agents 2014, 43, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Chavada, R.; Maley, M.; van Hal, S.J. Impact of source of infection and vancomycin AUC0-24/MICBMD targets on treatment failure in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect. 2014, 20, O1098–O1105. [Google Scholar] [CrossRef] [PubMed]
- Zelenitsky, S.; Rubinstein, E.; Ariano, R.; Iacovides, H.; Dodek, P.; Mirzanejad, Y.; Kumar, A. Cooperative Antimicrobial Therapy of Septic Shock-CATSS Database Research Group. Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int. J. Antimicrob. Agents 2013, 41, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.E.; Turnidge, J.D.; Munckhof, W.J.; Robinson, J.O.; Korman, T.M.; O’Sullivan, M.V.; Anderson, T.L.; Roberts, S.A.; Warren, S.J.; Gao, W.; et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2013, 57, 1654–1663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, C.L.; Osaki-Kiyan, P.; Haque, N.Z.; Perri, M.B.; Donabedian, S.; Zervos, M.J. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: A case-control study. Clin. Infect. Dis. 2012, 54, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.Y.; Makmor-Bakry, M.; Lau, C.L.; Abdul Rahman, R. The relationship between trough concentration of vancomycin and effect on methicillin-resistant Staphylococcus aureus in critically ill patients. S. Afr. Med. J. 2012, 102, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Brown, K.; Forrest, A. Vancomycin AUC24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant Staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob. Agents Chemother. 2012, 56, 634–638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clemens, E.C.; Chan, J.D.; Lynch, J.B.; Dellit, T.H. Relationships between vancomycin minimum inhibitory concentration, dosing strategies, and outcomes in methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol. Infect. Dis. 2011, 71, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Oh, J.M.; Cho, E.M.; Jang, H.J.; Hong, S.B.; Lim, C.M.; Koh, Y.S. Optimal dose of vancomycin for treating methicillin-resistant Staphylococcus aureus pneumonia in critically ill patients. Anaesth Intensive Care 2011, 39, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.D.; Pham, T.N.; Wong, J.; Hessel, M.; Cuschieri, J.; Neff, M.; Dellit, T.H. Clinical outcomes of linezolid vs vancomycin in methicillin-resistant Staphylococcus aureus ventilator-associated pneumonia: Retrospective analysis. J. Intensive Care Med. 2011, 26, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Davis, S.L.; Levine, D.P.; Rybak, M.J. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: Support for consensus guidelines suggested targets. Clin. Infect. Dis. 2011, 52, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Mohammedi, I.; Descloux, E.; Argaud, L.; Le Scanff, J.; Robert, D. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int. J. Antimicrob. Agents 2006, 27, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Jeffres, M.N.; Isakow, W.; Doherty, J.A.; McKinnon, P.S.; Ritchie, D.J.; Micek, S.T.; Kollef, M.H. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: Specific evaluation of vancomycin pharmacokinetic indices. Chest 2006, 130, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet 2004, 43, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Vuagnat, A.; Stern, R.; Lotthe, A.; Schuhmacher, H.; Duong, M.; Hoffmeyer, P.; Bernard, L. High dose vancomycin for osteomyelitis: Continuous vs. intermittent infusion. J. Clin. Pharm. Ther. 2004, 29, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Moise, P.A.; Forrest, A.; Bhavnani, S.M.; Birmingham, M.C.; Schentag, J.J. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am. J. Health Syst. Pharm. 2000, 57 (Suppl. S2), S4–S9, Erratum in Am. J. Health Syst. Pharm. 2001, 58, 78. [Google Scholar] [CrossRef] [PubMed]
- Karam, C.M.; McKinnon, P.S.; Neuhauser, M.M.; Rybak, M.J. Outcome assessment of minimizing vancomycin monitoring and dosing adjustments. Pharmacotherapy 1999, 19, 257–266, Erratum in Pharmacotherapy 1999, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.; Bunsow, E.; Muñoz, P.; Cercenado, E.; Rodríguez-Créixems, M.; Bouza, E. Vancomycin MICs do not predict the outcome of methicillin-resistant Staphylococcus aureus bloodstream infections in correctly treated patients. J. Antimicrob. Chemother. 2012, 67, 1760–1768. [Google Scholar] [CrossRef] [PubMed]

| Outcome | Endpoint | PK/PD Parameter | Emax (%) (CV%) | EC50 (mg/L) (CV%) | γ (CV%) |
|---|---|---|---|---|---|
| Nephrotoxicity | 2009 Consensus | Ctrough (mg/L) (n = 90) | 32.5 (43.7%) | 18.8 (78.4) | 1.0 (FIX) |
| AKIN | Ctrough (mg/L) (n = 22) | 42.7 (37.6) | 21.4 (86.2) | 1.51 (148) | |
| KDIGO | Ctrough (mg/L) (n = 24) | 100 (FIX) | 22.7 (19.8) | 4.15 (45.1) | |
| RIFLE | Ctrough (mg/L) (n = 29) | 100 (FIX) | 51.1 (25.1) | 1.47 (23.1) | |
| Efficacy | Treatment failure | AUC24/MICBMD (n = 11) | 100 (FIX) | 367 (20.0) | 1.0 FIX |
| AUC24/MICEtest (n = 6) | 100 (FIX) | 335 (36.6) | 2.65 (59.2) | ||
| 30- or 28-day all-cause mortality | AUC24/MICBMD (n = 8) | 100 (FIX) | 123 (19.4) | 1.0 FIX | |
| AUC24/MICEtest (n = 9) | 100 (FIX) | 96.7 (62.3) | 1.03 (54.4) | ||
| Microbiologic failure | AUC24/MICBMD (n = 9) | 100 (FIX) | 296 (36.0) | 2.39 (64.9) | |
| AUC24/MICEtest (n = 11) | 100 (FIX) | 99.8 (60.8) | 1.01 (79.2) |
| Analysis, n (%/%) | Ctrough (mg/L) | ||||
|---|---|---|---|---|---|
| Nephrotoxicity (n = 61) | AUC24 (mg·h/L) | ≤10 (n = 9) | 10–15 (n = 31) | 15–20 (n = 16) | >20 (n = 5) |
| ≤200 (n = 0) | 0 (0/0) | 0 (0/0) | 0 (0/0) | 0 (0/0) | |
| 200–400 (n = 10) | 7 (70.0/77.8) | 2 (20.0/6.5) | 1 (10.0/6.2) | 0 (0/0) | |
| 400–600 (n = 39) | 1 (2.6/11.1) | 24 (61.5/77.4) | 12 (30.8/75.0) | 2 (5.1/40.0) | |
| >600 (n = 12) | 1 (8.3/11.1) | 5 (41.7/16.1) | 3 (25.0/18.8) | 3 (25.0/60.0) | |
| Efficacy (n = 83) | AUC24 (mg·h/L) | ≤10 (n = 28) | 10–15 (n = 45) | 15–20 (n = 7) | >20 (n = 3) |
| ≤200 (n = 0) | 0 (0/0) | 0 (0/0) | 0 (0/0) | 0 (0/0) | |
| 200–400 (n = 24) | 19 (79.2/67.9) | 5 (20.8/11.1) | 0 (0/0) | 0 (0/0) | |
| 400–600 (n = 54) | 9 (16.7/32.1) | 38 (70.4/84.4) | 5 (9.3/71.4) | 2 (3.7/66.7) | |
| >600 (n = 5) | 0 (0/0) | 2 (40.0/4.4) | 2 (40.0/28.6) | 1 (20.0/33.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Zhang, K.; Chen, Y.; Fan, Y.; Zhang, J. Is It Still Beneficial to Monitor the Trough Concentration of Vancomycin? A Quantitative Meta-Analysis of Nephrotoxicity and Efficacy. Antibiotics 2024, 13, 497. https://doi.org/10.3390/antibiotics13060497
Yang W, Zhang K, Chen Y, Fan Y, Zhang J. Is It Still Beneficial to Monitor the Trough Concentration of Vancomycin? A Quantitative Meta-Analysis of Nephrotoxicity and Efficacy. Antibiotics. 2024; 13(6):497. https://doi.org/10.3390/antibiotics13060497
Chicago/Turabian StyleYang, Wanqiu, Kaiting Zhang, Yuancheng Chen, Yaxin Fan, and Jing Zhang. 2024. "Is It Still Beneficial to Monitor the Trough Concentration of Vancomycin? A Quantitative Meta-Analysis of Nephrotoxicity and Efficacy" Antibiotics 13, no. 6: 497. https://doi.org/10.3390/antibiotics13060497





