Combination of Chromatographic Analysis and Chemometric Methods with Bioactivity Evaluation of the Antibacterial Properties of Helichrysum italicum Essential Oil
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
2.2. Antimicrobial Potential of H. italicum Essential Oils
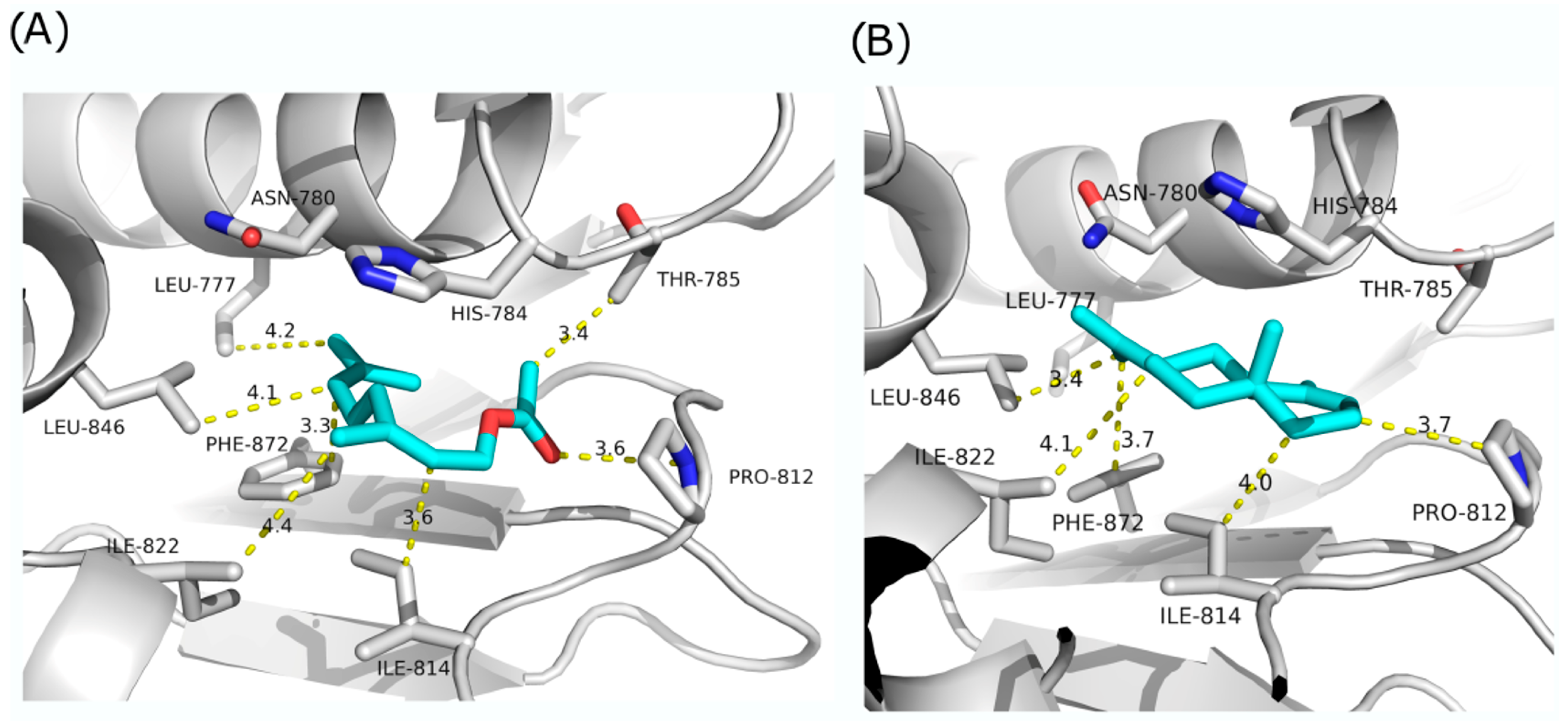
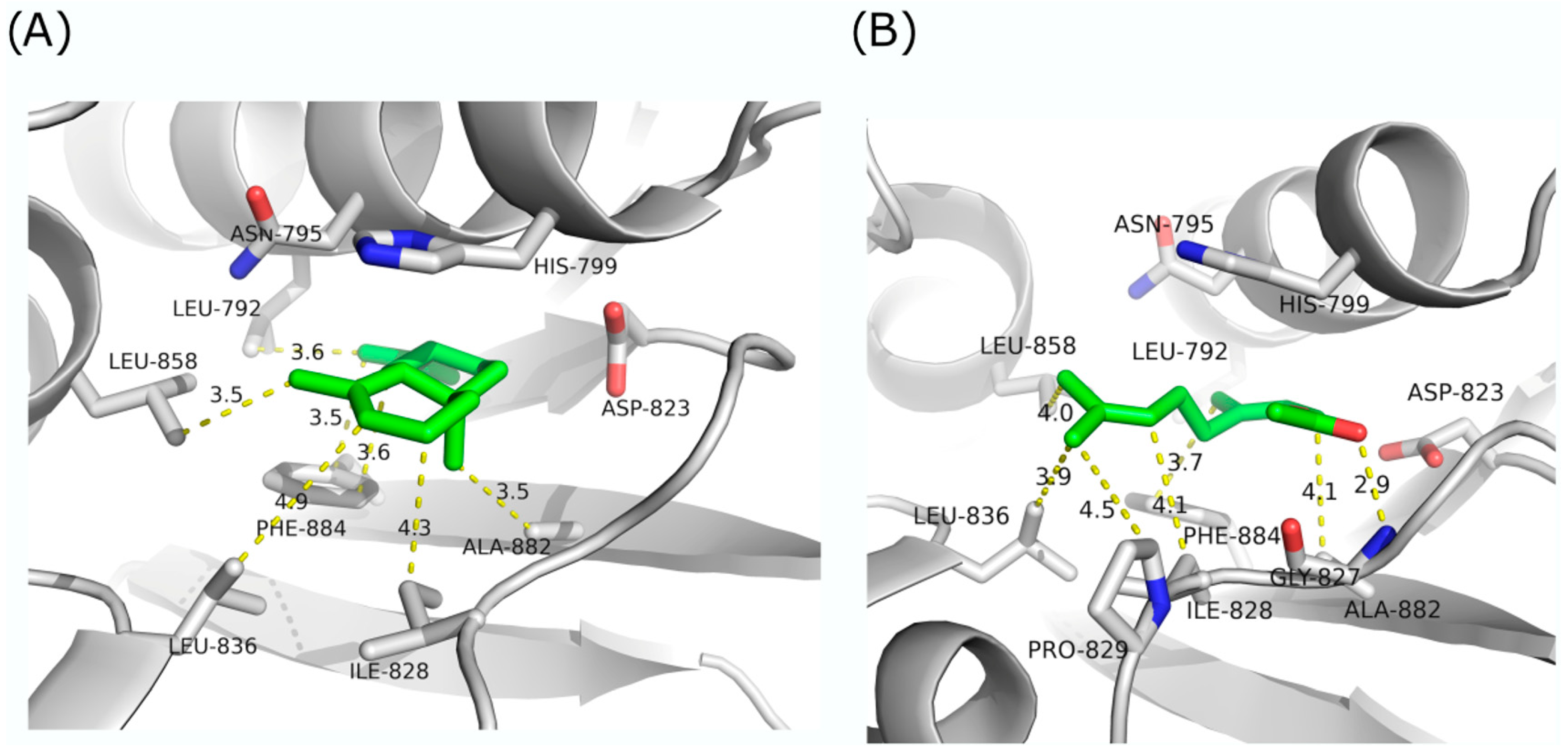
2.3. In Silico Molecular Simulation Model
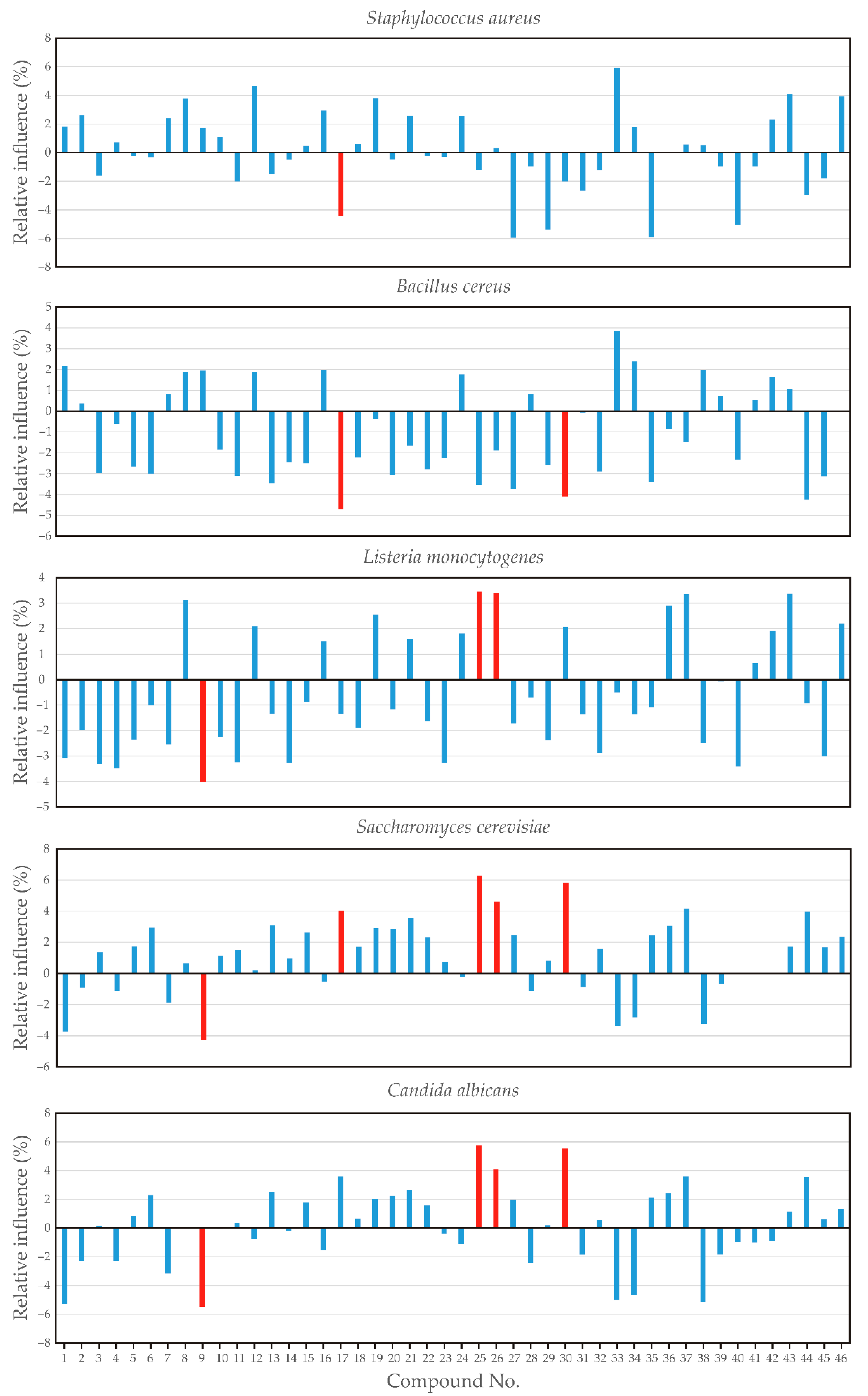
2.4. Artificial Neural Network Modeling
3. Discussion
Global Sensitivity Analysis
4. Materials and Methods
4.1. Essential Oil Samples
4.2. Chromatographic Analysis of Essential Oil Samples
4.3. Antimicrobial Potential
- The prokaryotes were streaked from −80 °C glycerol stock onto Müller–Hinton Agar (HiMedia, Mumbai, India) and incubated at 37 °C for 24 h.
- The eukaryotes were streaked from −80 °C glycerol stock onto Sabouraud Maltose Agar (HiMedia, Mumbai, India) and incubated at 25 °C for 5 days (A. brasiliensis and P. aurantiogriseum), 30 °C for 48 h (S. cerevisiae), and 37 °C for 48 h (C. albicans).
4.4. In Silico Molecular Modeling Simulations
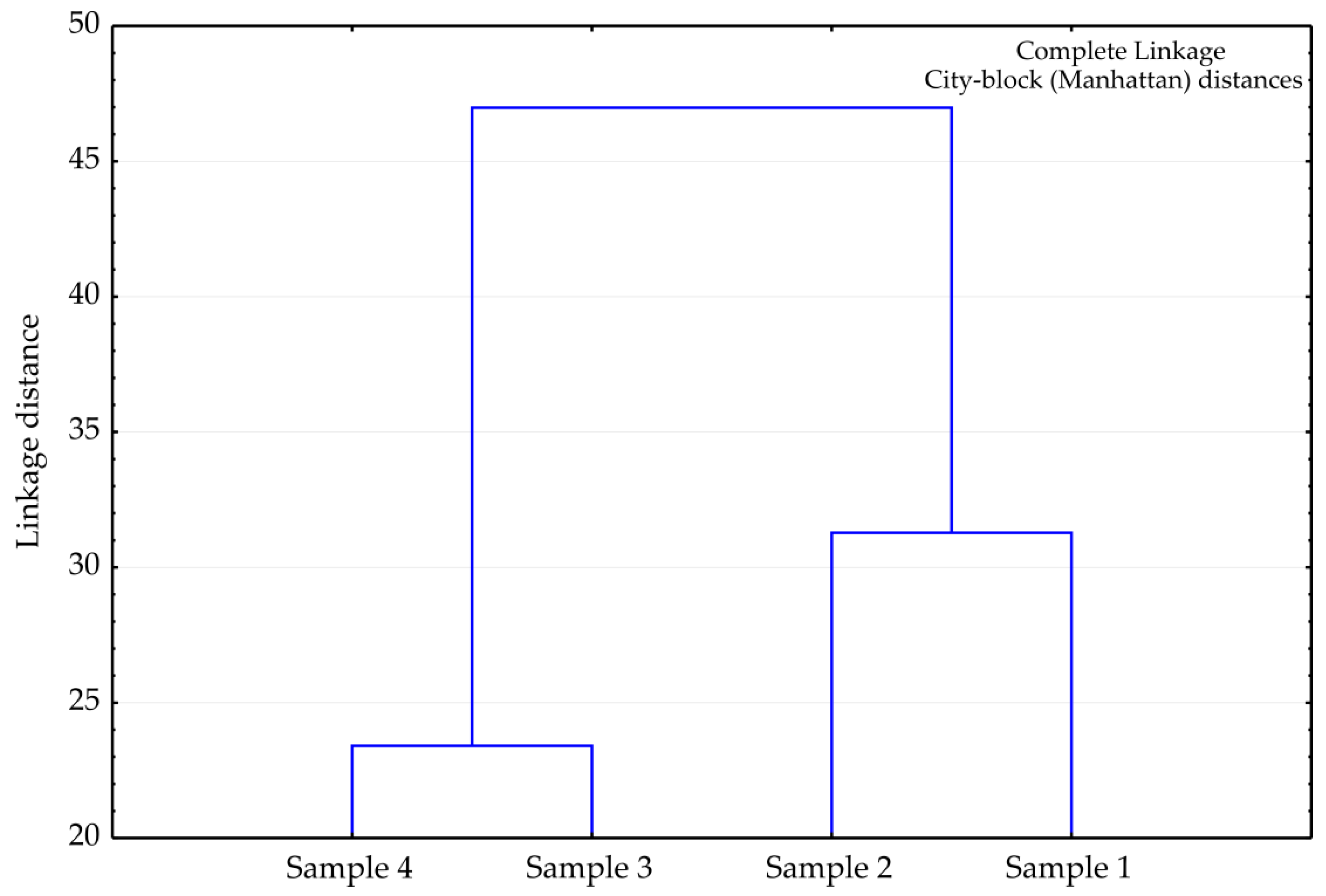
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, Y.; Harmon, P.F.; Treadwell, D.D.; Carrillo, D.; Sarkhosh, A.; Brecht, J.K. Biocontrol potential of essential oils in organic horticulture systems: From farm to fork. Front. Nutr. 2022, 8, 805138. [Google Scholar] [CrossRef] [PubMed]
- Winska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents-Myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Tit, D.M.; Bungau, S.G. Antioxidant activity of essential oils. Antioxidants 2023, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martin-Belloso, O. Antimicrobial activity of essential oils on Salmonella Enteritidis, Escherichia coli, and Listeria innocua in fruit juices. J. Food Prot. 2006, 69, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, F.; Huang, J.; Li, Q.; Yang, Q.; Ju, J. Application of essential oils as slow-release antimicrobial agents in food preservation: Preparation strategies, release mechanisms and application cases. Crit. Rev. Food Sci. Nutr. 2023, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dreger, M.; Wielgus, K. Application of essential oils as natural cosmetic preservatives. Herba Pol. 2013, 59, 142–156. [Google Scholar] [CrossRef]
- Shkreli, R.; Terziu, R.; Memushaj, L.; Dhamo, K.; Malaj, L. Selected essential oils as natural ingredients in cosmetic emulsions: Development, stability testing and antimicrobial activity. Indian J. Pharm. Educ. Res. 2023, 57, 125–133. [Google Scholar] [CrossRef]
- Edris, A. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Bogdanović, A.; Skala, D. Application of Plant Essential Oils in Pharma and Aroma Industries. In Plant Essential Oils; Prakash, B., Dubey, N.K., Freitas Brilhante de São José, J., Eds.; Springer: Singapore, 2024; pp. 177–203. [Google Scholar] [CrossRef]
- Aćimović, M.; Lončar, B. Aromatherapy: Therapy with fragrances of lavender essential oil. In Plant Specialized Metabolites; Reference Series in, Phytochemistry; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2023; pp. 1–43. [Google Scholar] [CrossRef]
- Miljković, A.; Aćimović, M.; Božanić Tanjga, B.; Lončar, B.; Raičević, V.; Šovljanski, O.; Travičić, V.; Pezo, M.; Pezo, L. Inhalation and topical application of rose essential oil—A systematic overview of Rosa damascena aromatherapy. J. Agron. Technol. Eng. Manag. 2024, 7, 998–1020. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant essential oils as biopesticides: Applications, mechanisms, innovations, and constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Catani, L.; Grassi, E.; di Montanara, A.C.; Guidi, L.; Sandulli, R.; Manachini, B.; Semprucci, F. Essential oils and their applications in agriculture and agricultural products: A literature analysis through VOSviewer. Biocatal. Agric. Biotechnol. 2022, 45, 102502. [Google Scholar] [CrossRef]
- Thoma, J.L.; Cantrell, C.L.; Zheljazkov, V.D. Evaluation of essential oils as sprout suppressants for potato (Solanum tuberosum) at room temperature storage. Plants 2022, 11, 3055. [Google Scholar] [CrossRef]
- Furlan, V.; Bren, U. Helichrysum italicum: From extraction, distillation, and encapsulation techniques to beneficial health effects. Foods 2023, 12, 802. [Google Scholar] [CrossRef]
- Akinfenwa, A.O.; Sagbo, I.J.; Makhaba, M.; Mabusela, W.T.; Hussein, A.A. Helichrysum genus and compound activities in the management of diabetes mellitus. Plants 2022, 11, 1386. [Google Scholar] [CrossRef]
- Jažo, Z.; Glumac, M.; Drventić, I.; Žilić, L.; Dujmović, T.; Bajić, D.; Vučemilo, M.; Ivić, E.; Bektić, S.; Anačkov, G.; et al. The essential oil composition of Helichrysum italicum (Roth) G. Don: Influence of steam, hydro and microwave-assisted distillation. Separations 2022, 9, 280. [Google Scholar] [CrossRef]
- Tzanova, M.; Grozeva, N.; Gerdzhikova, M.; Atanasov, V.; Terzieva, S.; Prodanova, R. Biochemical composition of essential oil of Corsican Helichrysum italicum (Roth) G. Don, introduced and cultivated in South Bulgaria. Bulg. J. Agric. Sci. 2018, 24, 1071–1077. [Google Scholar]
- Aćimović, M.; Ljujić, J.; Vulić, J.; Zheljazkov, V.D.; Pezo, L.; Varga, A.; Tumbas Šaponjac, V. Helichrysum italicum (Roth) G. Don essential oil from Serbia: Chemical composition, classification and biological activity—May it be a suitable new crop for Serbia? Agronomy 2021, 11, 1282. [Google Scholar] [CrossRef]
- Erbas, S.; Erdogan, U.; Mutlucan, M. The scent compounds of immortelle ecotypes (Helichrysum italicum (Roth) G. Don.) grown in Turkiye and its new products (absolute and concrete). S. Afr. J. Bot. 2023, 158, 301–311. [Google Scholar] [CrossRef]
- Chinou, I.; Roussis, V.; Perdetzoglou, D.; Loukis, A. Chemical and biological studies on two Helichrysum species of Greek origin. Planta Med. 1996, 62, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Melito, S.; Sias, A.; Petretto, G.; Chessa, M.; Pintore, G.; Porceddu, A. Genetic and metabolite diversity of Sardinian populations of Helichrysum italicum. PLoS ONE 2013, 8, e79043. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Formisano, C.; Senatore, F.; Picente, S.; Pagano, E.; Capasso, R.; Borrelli, F.; Izzo, A. Intestinal antispasmotic effects of Helichrysum italicum (Roth) Don ssp. italicum and chemical identification of the active ingredients. J. Ethnoph. 2013, 150, 901–906. [Google Scholar] [CrossRef]
- Aćimović, M. Corsica vs Balkan immortelle essential oil—Comparison of chemical profiles. Altern. Crops Cultivat. Prac. 2023, 5, 1–6. [Google Scholar]
- Saint-Lary, L.; Minaglou, F.; Escriva, C.; Beyls, A.S.; Badie, F. Helichrysum italicum D.C. essential oil from Balkans. Perfum. Flavor. 2018, 43, 52–66. [Google Scholar]
- Elsharif, S.A.; Buettner, A. Structure–odor relationship study on geraniol, nerol, and their synthesized oxygenated derivatives. J. Agric. Food Chem. 2016, 66, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Mancianti, F.; Ebani, V.V. Biological activity of essential oils. Molecules 2020, 25, 678. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Genčić, M.; Aksić, J.; Živković Stošić, M.; Ranđelović, P.; Stojanović, N.; Stojanović-Radić, Z.; Radulović, N. Linking the antimicrobial and anti-inflammatory effects of immortelle essential oil with its chemical composition—The interplay between the major and minor constituents. Food Chem. Toxicol. 2021, 158, 112666. [Google Scholar] [CrossRef]
- Andreani, S.; Uehara, A.; Blagojević, P.; Radulović, N.; Muselli, A.; Baldovini, N. Key odorants of industrially-produced Helichrysum italicum subsp. italicum essential oil. Ind. Crops. Prod. 2019, 132, 275–282. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, J.; Stanojević, L.; Bulatović, V.; Zvezdanić, J.; Milenković, A.; Simonović, N.; Cvetković, D. Chemical composition and antioxidant activity of immortelle (Helichrysum italicum) (Roth) G. Don) and yarrow (Achillea millefolium L.) essential oils. Adv. Technol. 2022, 11, 93–103. [Google Scholar] [CrossRef]
- Manitto, P.; Monti, D.; Colombo, E. Two new β-diketones from Helichrysum italicum. Phytochemistry 1972, 11, 2112–2114. [Google Scholar] [CrossRef]
- Tira, S.; Di Modica, G. New β-diketones from Helichrysum italicum G. Don. Tetrahedron Lett. 1967, 2, 143–148. [Google Scholar] [CrossRef]
- Bianchini, A.; Santoni, F.; Paolini, J.; Bernardini, A.F.; Mouillot, D.; Costa, J. Partitioning the relative contributions of inorganic plant composition and soil characteristics to the quality of Helichrysum italicum subsp. italicum (Roth) G. Don fil. essential oil. Chem. Biodivers. 2009, 6, 1014–1033. [Google Scholar] [CrossRef] [PubMed]
- Guinoiseau, E.; Lorenzi, V.; Luciani, A.; Muselli, A.; Costa, J.; Casanova, J.; Berti, L. Biological properties and resistance reversal effect of Helichrysum italicum (Roth) G. Don. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 2, pp. 1073–1080. [Google Scholar]
- Weglarz, Z.; Kosakowska, O.; Pioro-Jabrucka, E.; Przybyl, J.; Gniewosz, M.; Krasniewska, K.; Szyndel, M.; Costa, R.; Barbara Baczek, K. Antioxidant and antimicrobial activity of Helichrysum italicum (Roth) G. Don. From Central Europe. Pharmaceuticals 2022, 15, 735. [Google Scholar] [CrossRef]
- Bezek, K.; Kramberger, K.; Barlič-Maganja, D. Antioxidant and antimicrobial properties of Helichrysum italicum (Roth) G. Don hydrosol. Antibiotics 2022, 11, 1017. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Lin, L. Antibacterial activity of Helichrysum italicum oil on vegetables and its mechanism of action. J. Food Process. Preserv. 2015, 39, 2663–2672. [Google Scholar] [CrossRef]
- Borges, M.F.d.A.; Lacerda, R.d.S.; Correia, J.P.d.A.; de Melo, T.R.; Ferreira, S.B. Potential antibacterial action of α-pinene. Med. Sci. Forum 2022, 12, 11. [Google Scholar] [CrossRef]
- Gevrenova, R.; Kostadinova, I.; Stefanova, A.; Balabanova, V.; Zengin, G.; Zheleva-Dimitrova, D.; Momekov, G. Phytochemical Profiling, Antioxidant and Cognitive-Enhancing Effect of Helichrysum italicum ssp. italicum (Roth) G. Don (Asteraceae). Plants 2023, 12, 2755. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef]
- Issa, D.; Najjar, A.; Greige-Gerges, H.; Nehme, H. Screening of some essential oil constituents as potential inhibitors of the ATP synthase of Escherichia coli. J. Food Sci. 2019, 84, 138–146. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef]
- Su, F.; Yang, G.; Hu, D.; Ruan, C.; Wang, J.; Zhang, Y.; Zhu, Q. Chemical composition, antibacterial and antioxidant activities of essential oil from Centipeda minima. Molecules 2023, 28, 824. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holley, R.A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006, 111, 170–174. [Google Scholar] [CrossRef]
- Kishii, R.; Falzon, L.; Yoshida, T.; Kobayashi, H.; Inouye, M. Structural and functional studies of the HAMP domain of EnvZ, an osmosensing transmembrane histidine kinase in Escherichia coli. J. Biol. Chem. 2007, 282, 26401–26408. [Google Scholar] [CrossRef]
- Khorchid, A.; Inouye, M.; Ikura, M. Structural characterization of Escherichia coli sensor histidine kinase EnvZ: The periplasmic C-terminal core domain is critical for homodimerization. Biochem. J. 2005, 385, 255–264. [Google Scholar] [CrossRef]
- Maruca, A.; Lanzillotta, D.; Rocca, R.; Lupia, A.; Costa, G.; Catalano, R.; Moraca, F.; Gaudio, E.; Ortuso, F.; Artese, A.; et al. Multi-targeting bioactive compounds extracted from essential oils as kinase inhibitors. Molecules 2020, 25, 2174. [Google Scholar] [CrossRef]
- Aćimović, M.; Zeremski, T.; Šovljanski, O.; Lončar, B.; Pezo, L.; Zheljazkov, V.D.; Pezo, M.; Šuput, D.; Kurunci, Z. Seasonal variations in essential oil composition of immortelle cultivated in Serbia. Horticulturae 2022, 8, 1183. [Google Scholar] [CrossRef]
- Aćimović, M.; Šovljanski, O.; Pezo, L.; Travičić, V.; Tomić, A.; Zheljazkov, V.D.; Cetković, G.; Švarc-Gajić, J.; Brezo-Borjan, T.; Sofrenić, I. Variability in biological activities of Satureja montana subsp. montana and subsp. variegata based on different extraction methods. Antibiotics 2022, 11, 1235. [Google Scholar] [CrossRef]
- AlphaFold Database. Available online: https://alphafold.ebi.ac.uk/ (accessed on 15 January 2024).
- UniProt Database. Available online: https://www.uniprot.org/ (accessed on 15 January 2024).
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, J.; Li, Y.; Wang, W.; Houa, T.; Wangb, J.; Lia, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods: I. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Comput. Sci. 2011, 51, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Casino, P.; Miguel-Romero, L.; Marina, A. Visualizing autophosphorylation in histidine kinases. Nat. Commun. 2014, 5, 3258. [Google Scholar] [CrossRef]
- Chen, H.; Yu, C.; Wu, H.; Li, G.; Li, C.; Hong, W.; Yang, X.; Wang, H.; You, X. Recent advances in histidine kinase-targeted antimicrobial agents. Front. Chem. 2022, 10, 866392. [Google Scholar] [CrossRef]
- Ochoa-Martínez, C.I.; Ayala-Aponte, A.A. Prediction of mass transfer kinetics during osmotic dehydration of apples using neural networks. LWT—Food Sci. Technol. 2007, 40, 638. [Google Scholar] [CrossRef]
- Yoon, Y.; Swales, G.; Margavio, T.M. A Comparison of Discriminant Analysis versus Artificial Neural Networks. J. Oper. Res. Soc. 1993, 44, 51. [Google Scholar] [CrossRef]
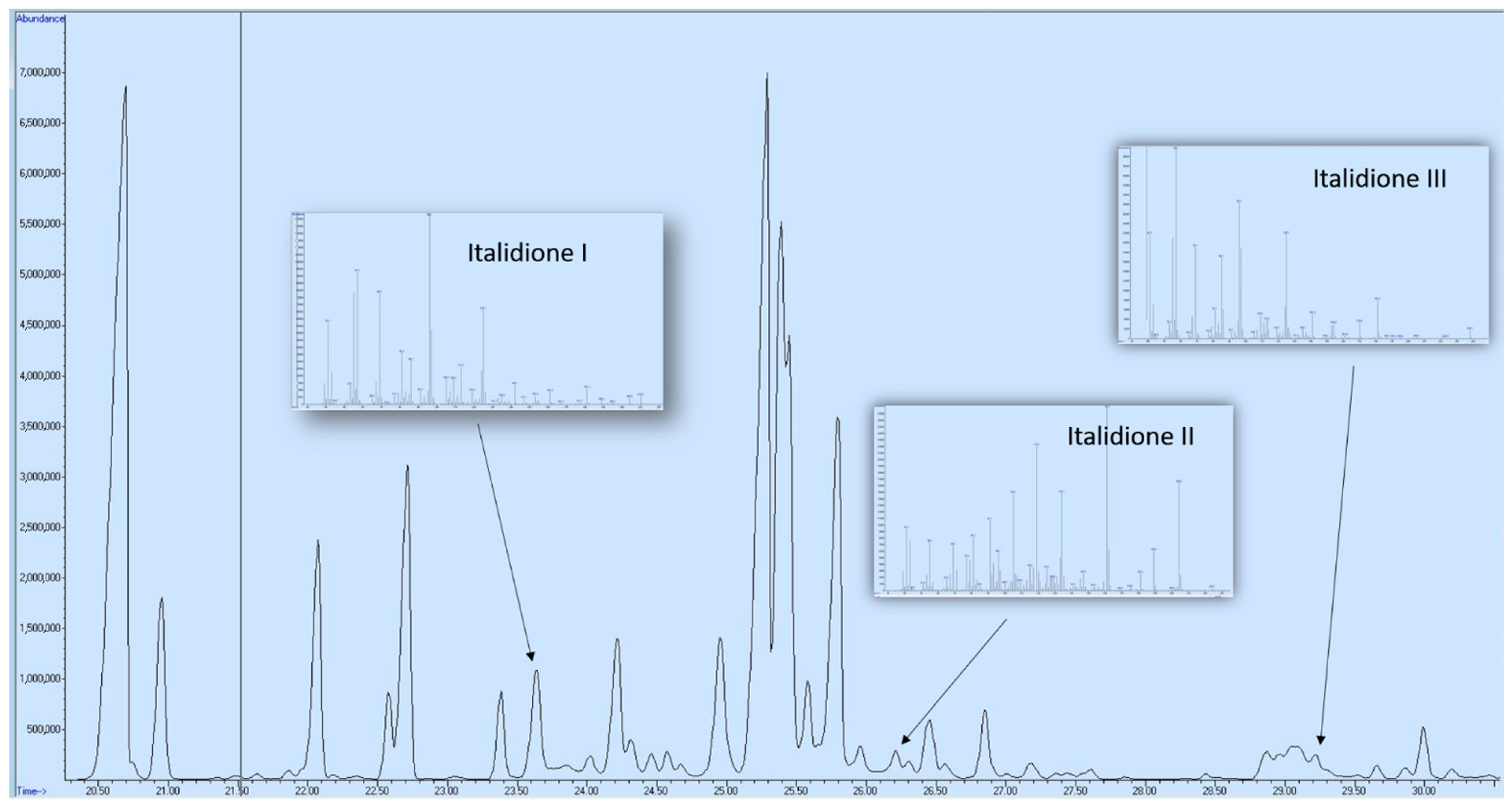


| Compound | Class | RT (min) | Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
|---|---|---|---|---|---|---|---|
| 1 | α-pinene | Monoterpene | 4.935 | 14.45 | 9.75 | 11.65 | 11.21 |
| 2,3 | α-fenchene + camphene | Monoterpene | 5.191 | 0.53 | 0.38 | 0.33 | 0.18 |
| 4 | β-pinene | Monoterpene | 5.932 | 0.54 | 0.57 | 0.19 | 0.30 |
| 5 | myrcene | Monoterpene | 6.281 | 0.20 | 0.12 | nd | nd |
| 6 | α-terpinene | Monoterpene | 7.043 | 0.20 | 0.21 | 0.16 | 0.16 |
| 7 | p-cymene | Monoterpene | 7.285 | 0.27 | 0.32 | 0.22 | 0.21 |
| 8 | limonene | Monoterpene | 7.427 | 5.29 | 3.30 | 2.98 | 1.96 |
| 9 | 1,8-cineole | Monoterpene | 7.484 | 0.31 | 0.29 | 0.56 | 0.32 |
| 10 | trans-β-ocimene | Monoterpene | 8.032 | 0.16 | nd | nd | nd |
| 11 | isobutyl angelate | Ester | 8.132 | 0.45 | 0.45 | 0.29 | 0.24 |
| 12 | γ-terpinene | Monoterpene | 8.388 | 0.48 | 0.50 | 0.33 | 0.39 |
| 13 | terpinolene | Monoterpene | 9.412 | 0.21 | 0.18 | 0.29 | 0.15 |
| 14 | linalool | Monoterpene | 9.839 | 1.35 | 2.24 | 0.47 | 0.70 |
| 15 | 2-methyl butyl-2-methyl butyrate | Ester | 9.979 | 0.23 | 0.23 | 0.13 | 0.14 |
| 16 | 2,4, dimethyloctan, 3-5dion (Mw 170) | Ketone | 11.878 | 1.32 | 1.53 | 1.07 | 0.92 |
| 17 | borneol | Monoterpene | 12.312 | 0.10 | nd | 0.27 | 0.10 |
| 18 | terpinene-4-ol | Monoterpene | 12.790 | 0.30 | 0.39 | 0.26 | 0.34 |
| 19 | 3,4 octan dione | Ketone | 13.196 | 0.73 | 0.79 | 0.43 | 0.35 |
| 20 | α-terpineole | Monoterpene | 13.339 | 0.39 | 0.44 | 0.48 | 0.32 |
| 21 | nerol | Monoterpene | 14.877 | 1.22 | 1.82 | 0.54 | 0.48 |
| 22 | 3-Butyl-6-methylpiperazine-2,5-dione Mw 184, dione | Ketone | 17.291 | 0.30 | 0.55 | 0.43 | nd |
| 23 | neryl acetate | Ester | 20.318 | 7.42 | 9.38 | 3.45 | 3.10 |
| 24 | α-ylangene | Sesquterpene | 20.618 | 9.84 | 9.61 | 3.53 | 4.00 |
| 25 | α-copaene | Sesquterpene | 20.960 | 2.64 | 1.67 | 4.94 | 3.04 |
| 26 | isoitalicene | Sesquterpene | 21.864 | 0.11 | 0.18 | 0.18 | 0.16 |
| 27 | italicene | Sesquterpene | 22.085 | 3.42 | 4.50 | 5.01 | 4.26 |
| 28 | cis-α-bergamotene | Sesquterpene | 22.605 | 1.11 | 1.39 | 0.99 | 1.72 |
| 29 | trans-caryophyllene | Sesquterpene | 22.748 | 4.82 | 3.59 | 5.55 | 6.00 |
| 30 | trans-α-bergamotene | Sesquterpene | 23.396 | 1.17 | 1.19 | 1.03 | 1.52 |
| 31 | italidione I (Mw 210) | Ketone | 23.681 | 1.90 | 3.84 | 2.96 | 2.89 |
| 32 | α-humulene | Sesquterpene | 24.037 | 0.24 | 0.17 | 0.26 | 0.36 |
| 33 | neryl propanoate | Ester | 24.215 | 2.12 | 2.36 | 0.60 | 0.94 |
| 34 | trans-β-farnesene | Sesquterpene | 24.314 | 0.47 | nd | 0.46 | nd |
| 35 | γ-selinene | Sesquterpene | 24.963 | 2.46 | 1.05 | 2.56 | 2.22 |
| 36 | γ-curcumene | Sesquterpene | 25.362 | 13.11 | 15.81 | 13.34 | 19.98 |
| 37 | ar-curcumene | Sesquterpene | 25.447 | 5.91 | 9.08 | 14.40 | 11.74 |
| 38 | β-selinene | Sesquterpene | 25.646 | 0.68 | 2.10 | 3.07 | 1.90 |
| 39 | α-selinene | Sesquterpene | 25.853 | 5.72 | 2.61 | 5.17 | 5.38 |
| 40 | α-muurolene | Sesquterpene | 25.995 | 0.35 | nd | 0.74 | 0.86 |
| 41 | italdione II (Mw 224) | Ketone | 26.238 | 0.26 | 0.18 | nd | 0.49 |
| 42 | γ-cadinene | Sesquterpene | 26.466 | 0.95 | 0.78 | 1.36 | 1.42 |
| 43 | δ-cadinene | Sesquterpene | 26.879 | 0.97 | 0.51 | 2.33 | 1.34 |
| 44 | italdione III (Mw 238) | Ketone | 28.880 | 0.54 | 0.61 | 0.98 | 0.47 |
| 45 | guaiol | Sesquterpene | 29.671 | 0.19 | 0.36 | 0.11 | 0.20 |
| 46 | rosifoliol | Sesquterpene | 30.005 | 0.73 | 0.80 | 0.33 | 0.47 |
| 47 | α-eudesmol | Sesquterpene | 31.544 | 0.25 | 0.30 | 0.38 | 0.11 |
| Total monoterpenes | 26.00 | 20.51 | 18.73 | 16.82 | |||
| Total esters | 10.22 | 12.42 | 4.47 | 4.42 | |||
| Total ketones | 5.05 | 7.50 | 5.87 | 5.12 | |||
| Total sesquterpenes | 55.14 | 55.70 | 65.74 | 66.68 | |||
| TOTAL IDENTIFIED | 96.41 | 97.13 | 94.81 | 93.04 |
| Samples | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Positive Control for Bacteria | Positive Control for Yeast and Fungi | Negative Control | ||||||
| ATCC | Microbial Strain | 1 | 2 | 3 | 4 | Cefotaxime + Clavulanic Acid | Actidione | Water |
| 25922 | Escherichia coli | nd | nd | nd | nd | 27.00 | / | nd |
| 27853 | Pseudomonas aeruginosa | nd | nd | nd | nd | 21.00 | / | nd |
| 13311 | Salmonella Typhimurium | nd | nd | nd | nd | 29.00 | / | nd |
| 25923 | Staphylococcus aureus | 17.67 | 12.00 | 19.67 | 10.00 | 28.33 | / | nd |
| 11778 | Bacillus cereus | 11.00 | nd | 12.67 | 7.33 | 27.00 | / | nd |
| 19115 | Listeria monocytogenes | 7.33 | 13.00 | 17.00 | 13.00 | 29.00 | / | nd |
| 9763 | Saccharomyces cerevisiae | 13.67 | 18.67 | 15.33 | 14.67 | / | 34.00 | nd |
| 10231 | Candida albicans | 7.67 | 13.67 | 11.33 | 10.33 | / | 37.00 | nd |
| 16025 | Penicillium aurantiogriseum | nd | nd | nd | nd | / | 26.33 | nd |
| 16404 | Aspergillus brasiliensis | nd | nd | nd | nd | / | 27.00 | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeremski, T.; Šovljanski, O.; Vukić, V.; Lončar, B.; Rat, M.; Perković Vukčević, N.; Aćimović, M.; Pezo, L. Combination of Chromatographic Analysis and Chemometric Methods with Bioactivity Evaluation of the Antibacterial Properties of Helichrysum italicum Essential Oil. Antibiotics 2024, 13, 499. https://doi.org/10.3390/antibiotics13060499
Zeremski T, Šovljanski O, Vukić V, Lončar B, Rat M, Perković Vukčević N, Aćimović M, Pezo L. Combination of Chromatographic Analysis and Chemometric Methods with Bioactivity Evaluation of the Antibacterial Properties of Helichrysum italicum Essential Oil. Antibiotics. 2024; 13(6):499. https://doi.org/10.3390/antibiotics13060499
Chicago/Turabian StyleZeremski, Tijana, Olja Šovljanski, Vladimir Vukić, Biljana Lončar, Milica Rat, Nataša Perković Vukčević, Milica Aćimović, and Lato Pezo. 2024. "Combination of Chromatographic Analysis and Chemometric Methods with Bioactivity Evaluation of the Antibacterial Properties of Helichrysum italicum Essential Oil" Antibiotics 13, no. 6: 499. https://doi.org/10.3390/antibiotics13060499
APA StyleZeremski, T., Šovljanski, O., Vukić, V., Lončar, B., Rat, M., Perković Vukčević, N., Aćimović, M., & Pezo, L. (2024). Combination of Chromatographic Analysis and Chemometric Methods with Bioactivity Evaluation of the Antibacterial Properties of Helichrysum italicum Essential Oil. Antibiotics, 13(6), 499. https://doi.org/10.3390/antibiotics13060499










