Antibiofilm Strategies in Neonatal and Pediatric Infections
Abstract
:1. Introduction
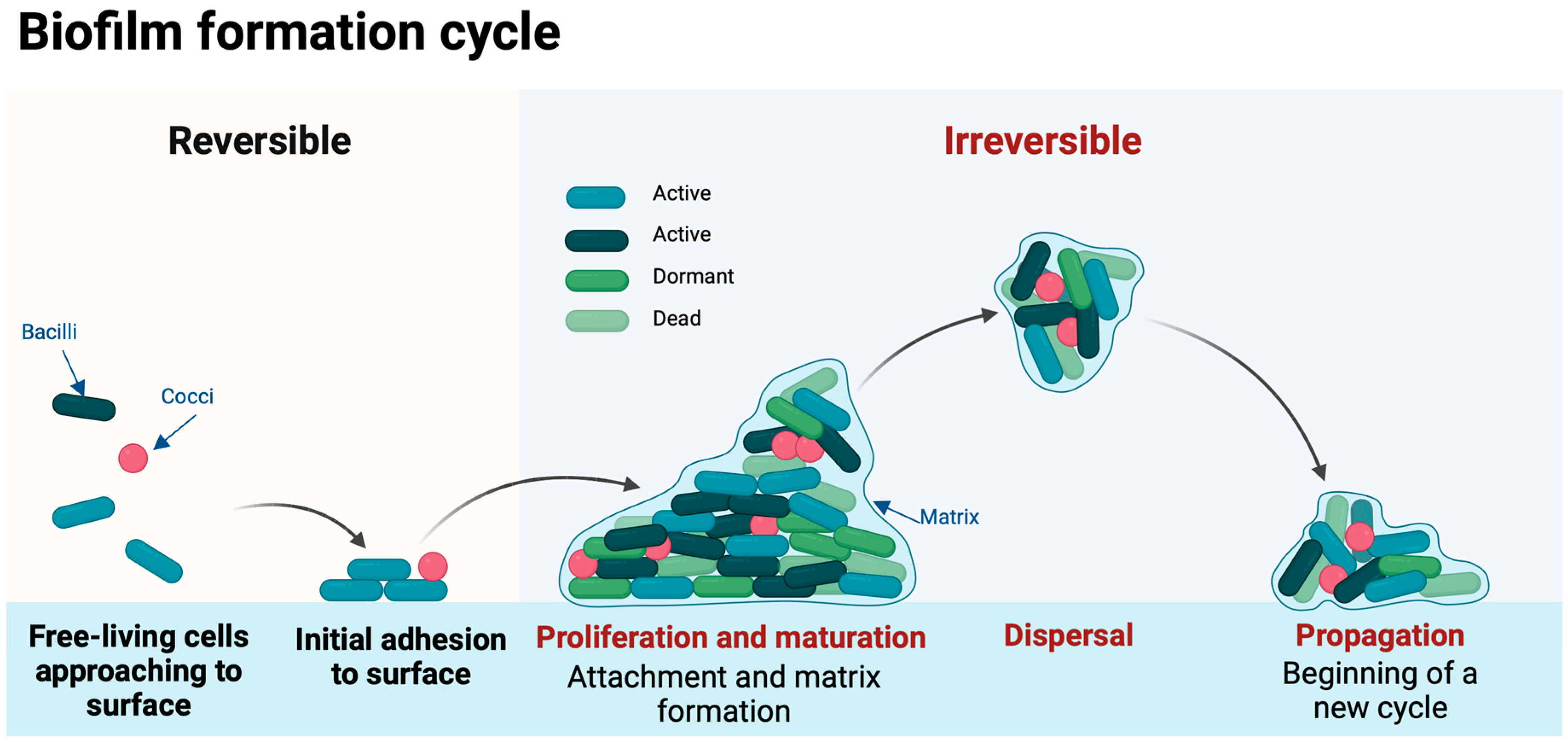
2. Biofilm Formation
3. Biofilm Infections in Children and in Neonates—Clinical Manifestations and Diagnosis
3.1. Medical Device-Associated Biofilm Infections
3.2. Biofilm Formation in Childhood Diseases
3.3. Biofilm Formation in Neonates
4. Biofilm Formation Prevention
5. Treatment of Biofilm Infections
5.1. Antibiotics
5.2. Foreign Body Removal
5.3. Novel Approaches
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shrestha, L.; Kayama, S.; Sasaki, M.; Kato, F.; Hisatsune, J.; Tsuruda, K.; Koizumi, K.; Tatsukawa, N.; Yu, L.; Takeda, K.; et al. Inhibitory effects of antibiofilm compound 1 against Staphylococcus aureus biofilms. Microbiol. Immunol. 2016, 60, 148–159. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Westerbeek, E.A.; van den Berg, A.; Lafeber, H.N.; Knol, J.; Fetter, W.P.; van Elburg, R.M. The intestinal bacterial colonisation in preterm infants: A review of the literature. Clin. Nutr. 2006, 25, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Parsek, M.R. New insight into the early stages of biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 4317–4319. [Google Scholar] [CrossRef]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.; Fischer, W.; Krokotsch, A.; Leopold, K.; Hartmann, R.; Egge, H.; Laufs, R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: Purification and structural analysis. J. Bacteriol. 1996, 178, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.; Rumbaugh, K. The Consequences of Biofilm Dispersal on the Host. Sci. Rep. 2018, 8, 10738. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Highmore, C.J.; Webb, J.S.; Faust, S.N. Diagnosis and treatment of biofilm infections in children. Curr. Opin. Infect. Dis. 2019, 32, 505–509. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- García-Betancur, J.C.; Lopez, D. Cell Heterogeneity in Staphylococcal Communities. J. Mol. Biol. 2019, 431, 4699–4711. [Google Scholar] [CrossRef] [PubMed]
- Dunne, W.M.; Mason, E.O.; Kaplan, S.L. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 1993, 37, 2522–2526. [Google Scholar] [CrossRef]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef]
- Fluit, A.C.; Visser, M.R.; Schmitz, F.-J. Molecular Detection of Antimicrobial Resistance. Clin. Microbiol. Rev. 2001, 14, 836–871. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.E.; Christensen, H.; Aarestrup, F.M. Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. J. Antimicrob. Chemother. 2006, 57, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist. Infect. Control 2020, 9, 162. [Google Scholar] [CrossRef]
- Le, K.Y.; Park, M.D.; Otto, M. Immune Evasion Mechanisms of Staphylococcus epidermidis Biofilm Infection. Front. Microbiol. 2018, 9, e02192. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Stoodley, P.; Kathju, S.; Høiby, N.; Moser, C.; Costerton, J.W.; Moter, A.; Bjarnsholt, T. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol. Med Microbiol. 2012, 65, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21 (Suppl. S1), S1–S25. [Google Scholar] [CrossRef]
- Silva, N.B.S.; Marques, L.A.; Röder, D.D.B. Diagnosis of biofilm infections: Current methods used, challenges and perspectives for the future. J. Appl. Microbiol. 2021, 131, 2148–2160. [Google Scholar] [CrossRef]
- Mooney, J.A.; Pridgen, E.M.; Manasherob, R.; Suh, G.; Blackwell, H.E.; Barron, A.E.; Bollyky, P.L.; Goodman, S.B.; Amanatullah, D.F. Periprosthetic bacterial biofilm and quorum sensing. J. Orthop. Res. 2018, 36, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, P.; Elbourne, A.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A review of methods for the detection of pathogenic microorganisms. Analyst 2019, 144, 396–411. [Google Scholar] [CrossRef]
- Ordonez, A.; Sellmyer, M.; Gowrishankar, G.; Ruiz-Bedoya, C.; Tucker, E.; Palestro, C. Molecular imaging of bacterial infections: Overcoming the barriers to clinical translation. Sci. Transl. Med. 2019, 11, eaax8251. [Google Scholar] [CrossRef]
- Gaudreau, A.M.; Labrie, J.; Goetz, C.; Dufour, S.; Jacques, M. Evaluation of MALDI-TOF mass spectrometry for the identification of bacteria growing as biofilms. J. Microbiol. Methods 2018, 145, 79–81. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Tan, L.P.; Mat Yazid, K.A.; Saeed, S.I.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Chivu, A.; Gibson, A.J. Targeting the bacterial protective armour; challenges and novel strategies in the treatment of microbial biofilm. Materials 2018, 11, 1705. [Google Scholar] [CrossRef]
- Percival, S.L. Importance of biofilm formation in surgical infection. Br. J. Surg. 2017, 104, e85–e94. [Google Scholar] [CrossRef]
- Galli, J.; Calo, L.; Meucci, D.; Giuliani, M.; Lucidi, D.; Paludetti, G.; Torelli, R.; Sanguinetti, M.; Parrilla, C. Biofilm in voice prosthesis: A prospective cohort study and laboratory tests using sonication and SEM analysis. Clin. Otolaryngol. 2018, 43, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.M.; Johani, K.; Melo, D.S.; Lopes, L.K.O.; Lopes Lima, L.K.O.; Tipple, A.F.V.; Hu, H.; Vickery, K. Biofilm contamination of high-touched surfaces in intensive care units: Epidemiology and potential impacts. Lett. Appl. Microbiol. 2019, 68, 269–276. [Google Scholar] [CrossRef]
- Hurrell, E.; Kucerova, E.; Loughlin, M.; Caubilla-Barron, J.; Hilton, A.; Armstrong, R.; Smith, C.; Grant, J.; Shoo, S.; Forsythe, S. Neonatal enteral feeding tubes as loci for colonisation by members of the Enterobacteriaceae. BMC Infect. Dis. 2009, 9, 146. [Google Scholar] [CrossRef]
- Juma, N.A.; Forsythe, S.J. Microbial biofilm development on neonatal enteral feeding tubes. Adv. Exp. Med. Biol. 2015, 830, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014, 3, 23. [Google Scholar] [CrossRef]
- Ares, G.; Hunter, C.J. Central venous access in children: Indications, devices, and risks. Curr. Opin. Pediatr. 2017, 29, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Raad, I.; Hanna, H.; Maki, D. Intravascular catheter-related infections: Advances in diagnosis, prevention, and management. Lancet Infect. Dis. 2007, 7, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.H.; Langley, J.M.; Kuhle, S.; Kirkland, S. Risk Factors for Central Venous Catheter-Associated Bloodstream Infection in Pediatric Patients: A Cohort Study. Infect. Control. Hosp. Epidemiol. 2016, 37, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; O’Horo, J.C.; Rogers, M.A.M.; Maki, D.G.; Safdar, N. The Risk of Bloodstream Infection Associated with Peripherally Inserted Central Catheters Compared with Central Venous Catheters in Adults: A Systematic Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2013, 34, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Niu, J.; He, Z.; Fu, W.; Huang, Q.; Guan, L.; Zhou, L.; Chen, R. Prophylactic antibiotics for preventing ventilator-associated pneumonia: A pairwise and Bayesian network meta-analysis. Eur. J. Med Res. 2023, 28, 348. [Google Scholar] [CrossRef] [PubMed]
- Prusseit, J.; Simon, M.; von der Brelie, C.; Heep, A.; Molitor, E.; Volz, S.; Simon, A. Epidemiology, prevention and management of ventriculoperitoneal shunt infections in children. Pediatr. Neurosurg. 2009, 45, 325–336. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Zaas, A.; Fuchs, H.E.; George, T.M.; Kaye, K.; Sexton, D.J. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin. Infect. Dis. 2003, 36, 858–862. [Google Scholar] [CrossRef]
- Yakut, N.; Soysal, A.; Kepenekli Kadayifci, E.; Dalgic, N.; Yılmaz Ciftdogan, D.; Karaaslan, A.; Akkoc, G.; Ocal Demir, S.; Cagan, E.; Celikboya, E.; et al. Ventriculoperitoneal shunt infections and re-infections in children: A multicentre retrospective study. Br. J. Neurosurg. 2018, 32, 196–200. [Google Scholar] [CrossRef]
- Hartl, D.; Gaggar, A.; Bruscia, E.; Hector, A.; Marcos, V.; Jung, A.; Greene, C.; McElvaney, G.; Mall, M.; Döring, G. Innate immunity in cystic fibrosis lung disease. J. Cyst. Fibros. 2012, 11, 363–382. [Google Scholar] [CrossRef]
- Lee, B.; Schjerling, C.K.; Kirkby, N.; Hoffmann, N.; Borup, R.; Molin, S.; Høiby, N.; Ciofu, O. Mucoid Pseudomonas aeruginosa isolates maintain the biofilm formation capacity and the gene expression profiles during the chronic lung infection of CF patients. Apmis 2011, 119, 263–274. [Google Scholar] [CrossRef]
- Gloag, E.S.; German, G.K.; Stoodley, P.; Wozniak, D.J. Viscoelastic properties of Pseudomonas aeruginosa variant biofilms. Sci. Rep. 2018, 8, 9691. [Google Scholar] [CrossRef]
- Singh, S.B.; McLearn-Montz, A.J.; Milavetz, F.; Gates, L.K.; Fox, C.; Murry, L.T.; Sabus, A.; Porterfield, H.S.; Fischer, A.J. Pathogen acquisition in patients with cystic fibrosis receiving ivacaftor or lumacaftor/ivacaftor. Pediatr. Pulmonol. 2019, 54, 1200–1208. [Google Scholar] [CrossRef]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E. Management of Neonates Born at ≥35 0/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182894. [Google Scholar] [CrossRef]
- Klingenberg, C.; Aarag, E.; Rønnestad, A.; Sollid, J.E.; Abrahamsen, T.G.; Kjeldsen, G.; Flægstad, T. Coagulase-Negative Staphylococcal Sepsis in Neonates: Association Between Antibiotic Resistance, Biofilm Formation and the Host Inflammatory Response. Pediatr. Infect. Dis. J. 2005, 24, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, Y.; Cameron, D.R.; Easton, C.D.; Zhu, X.; Zhu, M.; Salwiczek, M.; Muir, B.W.; Thissen, H.; Daley, A.; et al. Hyperosmotic Infusion and Oxidized Surfaces Are Essential for Biofilm Formation of Staphylococcus capitis From the Neonatal Intensive Care Unit. Front. Microbiol. 2020, 11, 920. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Mittal, S.; Chaudhary, U. Role of Non Albicans Candida Spp. and Biofilm in Neonatal ICU. Infect. Disord.-Drug Targets 2016, 16, 192–198. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Li, Y.; Chen, X.; Ding, C.; Liu, Y. Risk factors and biofilm formation analyses of hospital-acquired infection of Candida pelliculosa in a neonatal intensive care unit. BMC Infect. Dis. 2021, 21, 620. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef] [PubMed]
- Marschall, J.; Mermel, L.A.; Fakih, M.; Hadaway, L.; Kallen, A.; O’Grady, N.P.; Pettis, A.M.; Rupp, M.E.; Sandora, T.; Maragakis, L.L.; et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect. Control. Hosp. Epidemiol. 2014, 35, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Daley, A.J.; Istivan, T.S.; Garland, S.M.; Deighton, M.A. Antibiotic susceptibility of coagulase-negative staphylococci isolated from very low birth weight babies: Comprehensive comparisons of bacteria at different stages of biofilm formation. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- França, A. The Role of Coagulase-Negative Staphylococci Biofilms on Late-Onset Sepsis: Current Challenges and Emerging Diagnostics and Therapies. Antibiotics 2023, 12, 554. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.H.; Monk, I.R.; Gonçalves da Silva, A.; Seemann, T.; Chua, K.Y.L.; Kearns, A.; Hill, R.; Woodford, N.; Bartels, M.D.; Strommenger, B.; et al. Global spread of three multidrug-resistant lineages of Staphylococcus epidermidis. Nat. Microbiol. 2018, 3, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Multidrug tolerance of biofilms and persister cells. Curr. Top. Microbiol. Immunol. 2008, 322, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef]
- Muñoz-Egea, M.C.; García-Pedrazuela, M.; Mahillo-Fernandez, I.; Esteban, J. Effect of Antibiotics and Antibiofilm Agents in the Ultrastructure and Development of Biofilms Developed by Nonpigmented Rapidly Growing Mycobacteria. Microb. Drug Resist. 2016, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Egea, M.C.; García-Pedrazuela, M.; Mahillo, I.; Esteban, J. Effect of ciprofloxacin in the ultrastructure and development of biofilms formed by rapidly growing mycobacteria. BMC Microbiol. 2015, 15, 18. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Gilbert, R.; Brown, M.; Rainford, N.; Donohue, C.; Fraser, C.; Sinha, A.; Dorling, J.; Gray, J.; McGuire, W.; Gamble, C.; et al. Antimicrobial-impregnated central venous catheters for prevention of neonatal bloodstream infection (PREVAIL): An open-label, parallel-group, pragmatic, randomised controlled trial. Lancet Child Adolesc. Health 2019, 3, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Subhadra, B.; Kim, D.H.; Woo, K.; Surendran, S.; Choi, C.H. Control of Biofilm Formation in Healthcare: Recent Advances Exploiting Quorum-Sensing Interference Strategies and Multidrug Efflux Pump Inhibitors. Materials 2018, 11, 1676. [Google Scholar] [CrossRef]
- Dror, N.; Mandel, M.; Hazan, Z.; Lavie, G. Advances in microbial biofilm prevention on indwelling medical devices with emphasis on usage of acoustic energy. Sensors 2009, 9, 2538–2554. [Google Scholar] [CrossRef]
- Jansen, B.; Kohnen, W. Prevention of biofilm formation by polymer modification. J. Ind. Microbiol. 1995, 15, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Boelens, J.J.; Tan, W.F.; Dankert, J.; Zaat, S.A. Antibacterial activity of antibiotic-soaked polyvinylpyrrolidone-grafted silicon elastomer hydrocephalus shunts. J. Antimicrob. Chemother. 2000, 45, 221–224. [Google Scholar] [CrossRef]
- John, T.; Rajpurkar, A.; Smith, G.; Fairfax, M.; Triest, J. Antibiotic pretreatment of hydrogel ureteral stent. J. Endourol. 2007, 21, 1211–1216. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef]
- Li, X.H.; Lee, J.H. Antibiofilm agents: A new perspective for antimicrobial strategy. J. Microbiol. 2017, 55, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef]
- Barraud, N.; Schleheck, D.; Klebensberger, J.; Webb, J.S.; Hassett, D.J.; Rice, S.A.; Kjelleberg, S. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 2009, 191, 7333–7342. [Google Scholar] [CrossRef]
- Ravindran, D.; Ramanathan, S.; Arunachalam, K.; Jeyaraj, G.P.; Shunmugiah, K.P.; Arumugam, V.R. Phytosynthesized silver nanoparticles as antiquorum sensing and antibiofilm agent against the nosocomial pathogen Serratia marcescens: An in vitro study. J. Appl. Microbiol. 2018, 124, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. BioMed Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Q.; Sun, H. Novel strategies for the prevention and treatment of biofilm related infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef] [PubMed]
| Common Biofilm Infections in Neonates and in Children | |
|---|---|
Device-related infections
| Tissue infections
|
| Methods to Combat Biofilms |
|---|
Preventive strategies
|
Treatment of biofilm-related infections
|
| Foreign Body Removal |
Novel approaches
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmeri, C.; Giapros, V.; Serbis, A.; Balomenou, F.; Baltogianni, M. Antibiofilm Strategies in Neonatal and Pediatric Infections. Antibiotics 2024, 13, 509. https://doi.org/10.3390/antibiotics13060509
Kosmeri C, Giapros V, Serbis A, Balomenou F, Baltogianni M. Antibiofilm Strategies in Neonatal and Pediatric Infections. Antibiotics. 2024; 13(6):509. https://doi.org/10.3390/antibiotics13060509
Chicago/Turabian StyleKosmeri, Chrysoula, Vasileios Giapros, Anastasios Serbis, Foteini Balomenou, and Maria Baltogianni. 2024. "Antibiofilm Strategies in Neonatal and Pediatric Infections" Antibiotics 13, no. 6: 509. https://doi.org/10.3390/antibiotics13060509






