Deciphering the Antibacterial Mechanisms of 5-Fluorouracil in Escherichia coli through Biochemical and Transcriptomic Analyses
Abstract
:1. Introduction
2. Results
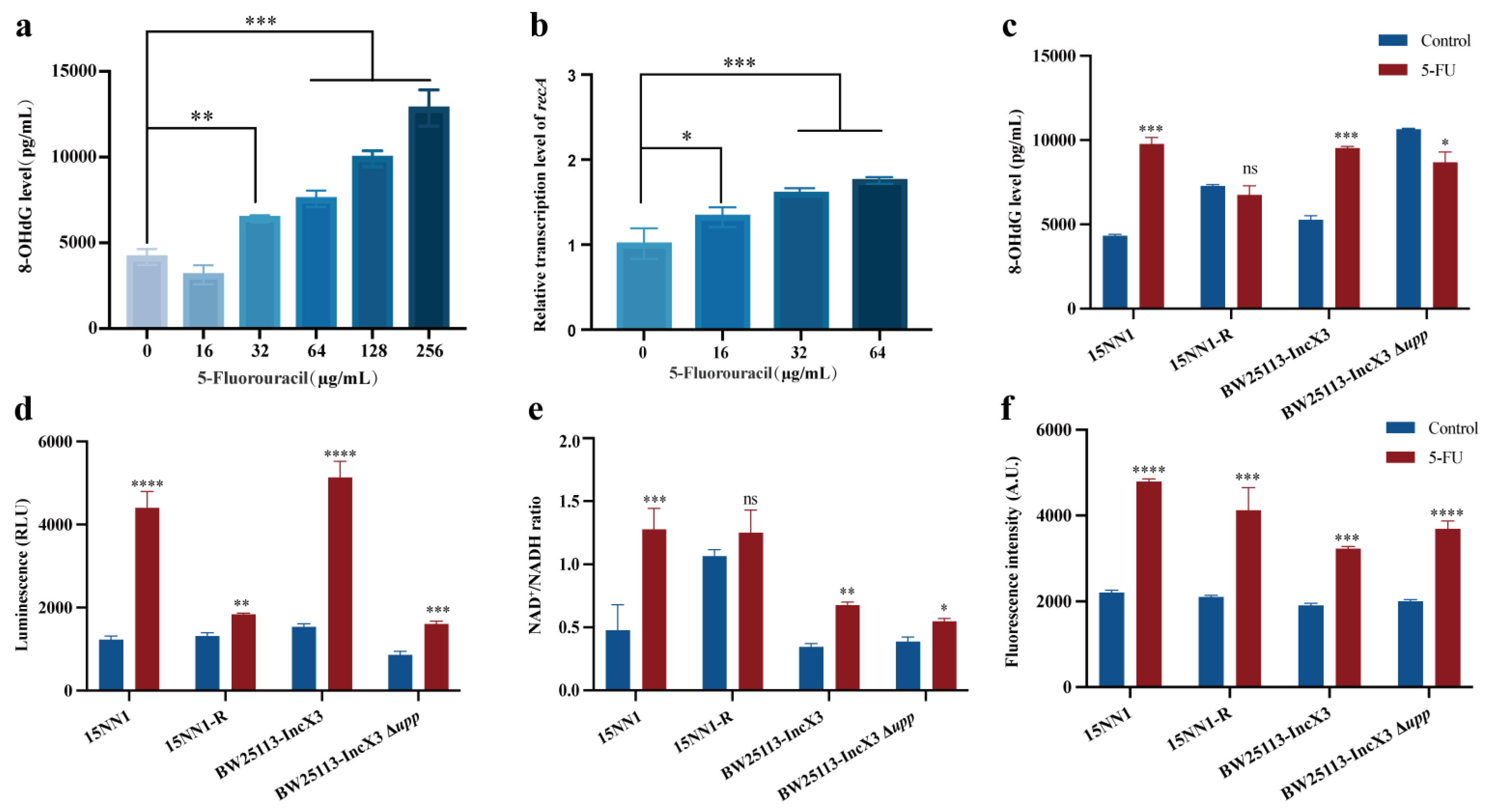
2.1. Mutation in upp Confers Resistance to 5-FU
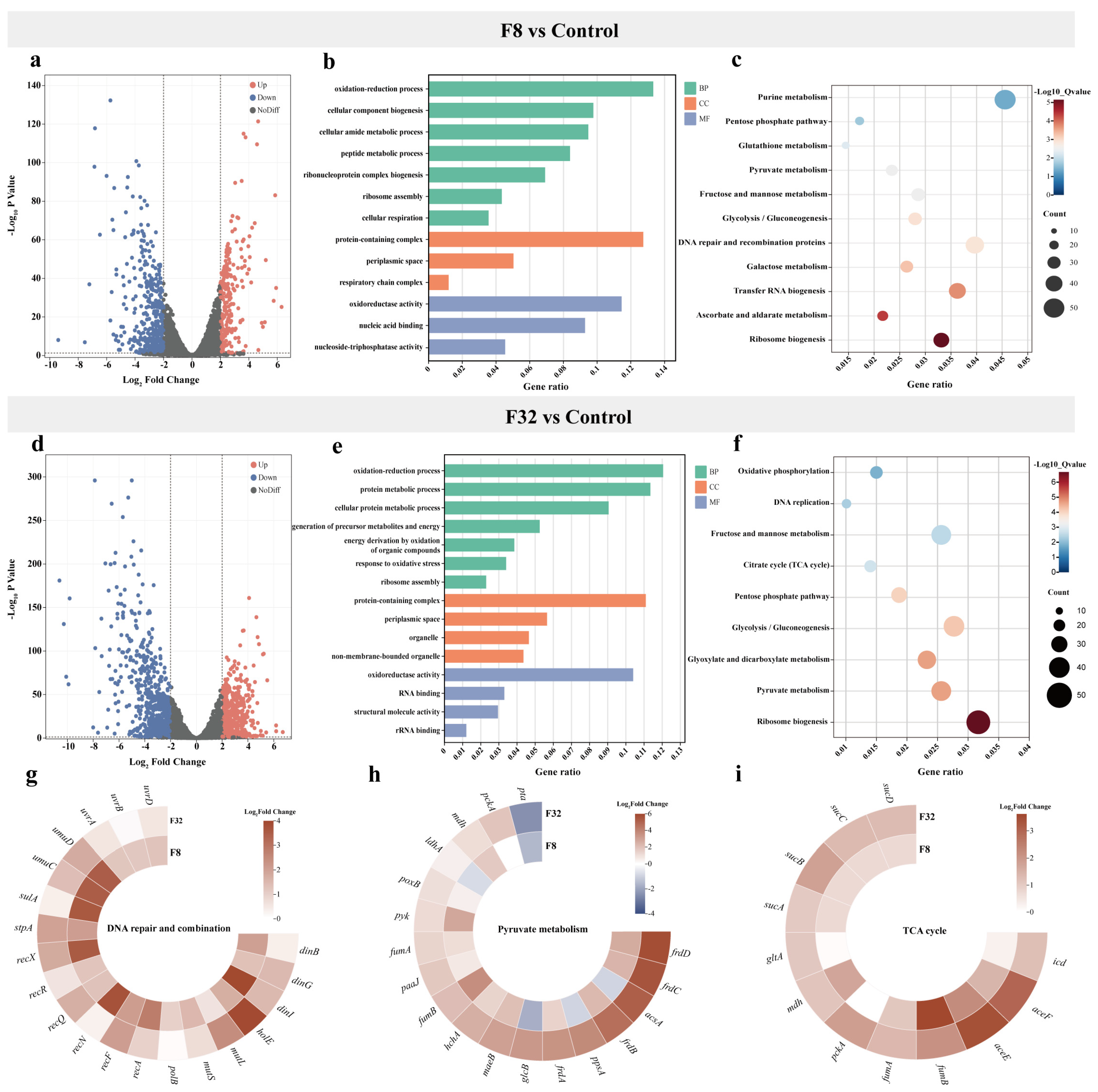
2.2. Transcriptomics Profile of 5-FU Treatment
2.3. 5-FU Induced DNA Damage and Enhanced Bacteria Metabolism
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Chemical Reagents
4.2. Growth Curves of Bacteria
4.3. Serial Passaging Assay to Evolve Resistance
4.4. Construction of Gene Knockout Strain
4.5. Transcriptomic Sequencing and Analysis
4.6. RT-PCR Assay
4.7. 8-OHdG Level Determination
4.8. NAD+/NADH Determination
4.9. ATP Measurement
4.10. Measurement of ROS Activity
4.11. Time-Dependent Killing Curves
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Rev. Infect. Dis. 1980, 2, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Maria, C.; de Matos, A.M.; Rauter, A.P. Recent antibacterial carbohydrate-based prodrugs, drugs and delivery systems to overcome antimicrobial resistance. Curr. Opin. Chem. Biol. 2024, 78, 102419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Z.; Shi, J.; Li, R.; Upton, M.; Wang, Z. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics 2021, 11, 4910–4928. [Google Scholar] [CrossRef] [PubMed]
- Foletto, V.S.; da Rosa, T.F.; Serafin, M.B.; Bottega, A.; Hörner, R. Repositioning of non-antibiotic drugs as an alternative to microbial resistance: A systematic review. Int. J. Antimicrob. Agents 2021, 58, 106380. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ma, R.; Reeves, T.; Katz, A.J.; Levi, N. Repurposing farnesol for combating drug-resistant and persistent single and polymicrobial biofilms. Antibiotics 2024, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Li, Z.; Huang, P.; Tan, H. Engineering nucleoside antibiotics toward the development of novel antimicrobial agents. J. Antibiot. 2019, 72, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Brecik, M.; Mukherjee, R.; Evans, J.C.; Svetlíková, Z.; Blaško, J.; Surade, S.; Blackburn, J.; Warner, D.F.; Mikušová, K.; et al. The Complex mechanism of antimycobacterial action of 5-fluorouracil. Chem. Biol. 2015, 22, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Yssel, A.E.J.; Vanderleyden, J.; Steenackers, H.P. Repurposing of nucleoside- and nucleobase-derivative drugs as antibiotics and biofilm inhibitors. J. Antimicrob. Chemother. 2017, 72, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.S.; Flaks, J.G.; Barner, H.D.; Loeb, M.R.; Lichtenstein, J. The mode of action of 5-fluorouracil and its derivatives. Proc. Natl. Acad. Sci. USA 1958, 44, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, C.; Chaudhuri, N.K.; Danneberg, P.; Mooren, D.; Griesbach, L.; Duschinsky, R.; Schnitzer, R.J.; Pleven, E.; Scheiner, J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Quan, Y.; Li, J.; Li, Y.; Song, D.; Li, X.; Wang, Y.; Yi, L.; Wang, Y. Tackling antibiotic resistance: Exploring 5-fluorouracil as a promising antimicrobial strategy for the treatment of Streptococcus suis infection. Animals 2024, 14, 1286. [Google Scholar] [CrossRef] [PubMed]
- Gieringer, J.H.; Wenz, A.F.; Just, H.M.; Daschner, F.D. Effect of 5-fluorouracil, mitoxantrone, methotrexate, and vincristine on the antibacterial activity of ceftriaxone, ceftazidime, cefotiam, piperacillin, and netilmicin. Chemotherapy 1986, 32, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Nyhlén, A.; Ljungberg, B.; Nilsson-Ehle, I.; Odenholt, I. Bactericidal effect of combinations of antibiotic and antineoplastic agents against Staphylococcus aureus and Escherichia coli. Chemotherapy 2002, 48, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Collins, C.; Hastings, J.G.; White, P.J. Radiochemical assay to measure the biofilm produced by coagulase-negative Staphylococci on solid surfaces and its use to quantitate the effects of various antibacterial compounds on the formation of the biofilm. J. Med. Microbiol. 1992, 37, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Attila, C.; Ueda, A.; Wood, T.K. 5-Fluorouracil reduces biofilm formation in Escherichia coli K-12 through global regulator AriR as an antivirulence compound. Appl. Microbiol. Biotechnol. 2009, 82, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Walz, J.M.; Avelar, R.L.; Longtine, K.J.; Carter, K.L.; Mermel, L.A.; Heard, S.O.; 5-FU Catheter Study Group. Anti-infective external coating of central venous catheters: A randomized, noninferiority trial comparing 5-fluorouracil with chlorhexidine/silver sulfadiazine in preventing catheter colonization. Crit. Care Med. 2010, 38, 2095–2102. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Pan, Z.; Liao, X.; Zhong, Y.; Guo, J.; Pang, R.; Chen, X.; Ye, G.; Su, Y. Uracil restores susceptibility of methicillin-resistant Staphylococcus aureus to aminoglycosides through metabolic reprogramming. Front. Pharmacol. 2023, 14, 1133685. [Google Scholar] [CrossRef] [PubMed]
- Kitzenberg, D.A.; Lee, J.S.; Mills, K.B.; Kim, J.-S.; Liu, L.; Vázquez-Torres, A.; Colgan, S.P.; Kao, D.J. Adenosine awakens metabolism to enhance growth-independent killing of tolerant and persister bacteria across multiple classes of antibiotics. mBio 2022, 13, e0048022. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, Y.; Ou, D.; Yang, H.; Feng, H.; Song, H.; Xie, N.; Niu, X.; Deng, X.; Sun, M.; et al. Potent synergistic efficacy of 2-methoxy-1,4-naphthoquinone derived from quinones against drug-resistant bacteria. One Health Adv. 2024, 2, 1. [Google Scholar] [CrossRef]
- Song, H.; Wang, X.; Zhang, M.; Zou, Z.; Yang, S.; Yi, T.; Wang, J.; Liu, D.; Shen, Y.; Dai, C.; et al. Dual effects of feed-additive-derived chelerythrine in combating mobile colistin resistance. Engineering 2023, 32, 163–173. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Wang, Z. Thymine sensitizes gram-negative pathogens to antibiotic killing. Front. Microbiol. 2021, 12, 622798. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Rigol, S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 2018, 71, 153–184. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; Brown, E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Imperi, F.; Massai, F.; Facchini, M.; Frangipani, E.; Visaggio, D.; Leoni, L.; Bragonzi, A.; Visca, P. Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc. Natl. Acad. Sci. USA 2013, 110, 7458–7463. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gong, Y.; Ji, P.; Xie, Y.; Jiang, Y.-Z.; Liu, G. Targeting nucleotide metabolism: A promising approach to enhance cancer immunotherapy. J. Hematol. Oncol. 2022, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Lin, K.; Liao, W.; Xie, Y.; Yu, G.; Shao, Y.; Dai, M.; Sun, F. Transcriptomic analyses reveal the potential antibacterial mechanism of citral against Staphylococcus aureus. Front. Microbiol. 2023, 14, 1171339. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, R.; Li, X.; Addo, K.A.; Fang, M.; Zhang, Y.; Yu, Y. Antibacterial mechanism of Kojic acid and Tea polyphenols against Escherichia coli O157:H7 through transcriptomic analysis. Food Sci. Hum. Wellness 2024, 13, 736–747. [Google Scholar] [CrossRef]
- Lopatkin, A.J.; Bening, S.C.; Manson, A.L.; Stokes, J.M.; Kohanski, M.A.; Badran, A.H.; Earl, A.M.; Cheney, N.J.; Yang, J.H.; Collins, J.J. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science 2021, 371, eaba0862. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial metabolism and antibiotic efficacy. Cell Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Bacterial metabolism-inspired molecules to modulate antibiotic efficacy. J. Antimicrob. Chemother. 2019, 74, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Wright, S.N.; Hamblin, M.; McCloskey, D.; Alcantar, M.A.; Schrübbers, L.; Lopatkin, A.J.; Satish, S.; Nili, A.; Palsson, B.O.; et al. A white-box machine learning approach for revealing antibiotic mechanisms of action. Cell 2019, 177, 1649–1661.e9. [Google Scholar] [CrossRef] [PubMed]


| Bacteria ID | Mutation | Annotation | Description |
|---|---|---|---|
| BW25113 | C→T | Q65* (CAG→TAG) | Uracil phosphoribosyltransferase |
| G→A | W106* (TGG→TAG) | Melibiose operon regulatory protein MelR, AraC family | |
| T→A | D105V (GAT→GTT) | Thymidine kinase | |
| DH5α | A→G | Q36Q (CAA→CAG) | c-di-GMP phosphodiesterase |
| G→T | E105* (GAA→TAA) | Uracil phosphoribosyltransferase | |
| T→G | K13Q (AAG→CAG) | Small inner membrane protein, | |
| 15NN1 | 2 bp → CA | Coding (218–219/726 nt) | Uridine monophosphate kinase |
| C→A | L29F (TTG→TTT) | Chromosomal replication initiator protein DnaA | |
| T→C | I49T (ATC→ACC) | S-adenosylmethionine decarboxylase proenzyme, prokaryotic class 1A | |
| 1DM10 | T→G | K14Q (AAG→CAG) | Uracil phosphoribosyltransferase |
| G→A | Q185* (CAG→TAG) | Uracil phosphoribosyltransferase | |
| A→C | D158E (GAT→GAG) | Phage tail fiber protein | |
| G→A | P182P (CCC→CCT) | Molybdenum ABC transporter permease protein ModB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Song, H.; Yang, S.; Zhang, Y.; Tian, Y.; Wang, Y.; Liu, D. Deciphering the Antibacterial Mechanisms of 5-Fluorouracil in Escherichia coli through Biochemical and Transcriptomic Analyses. Antibiotics 2024, 13, 528. https://doi.org/10.3390/antibiotics13060528
Zhang M, Song H, Yang S, Zhang Y, Tian Y, Wang Y, Liu D. Deciphering the Antibacterial Mechanisms of 5-Fluorouracil in Escherichia coli through Biochemical and Transcriptomic Analyses. Antibiotics. 2024; 13(6):528. https://doi.org/10.3390/antibiotics13060528
Chicago/Turabian StyleZhang, Muchen, Huangwei Song, Siyuan Yang, Yan Zhang, Yunrui Tian, Yang Wang, and Dejun Liu. 2024. "Deciphering the Antibacterial Mechanisms of 5-Fluorouracil in Escherichia coli through Biochemical and Transcriptomic Analyses" Antibiotics 13, no. 6: 528. https://doi.org/10.3390/antibiotics13060528





